A Hybrid EEG-Based Stress State Classification Model Using Multi-Domain Transfer Entropy and PCANet
Abstract
:1. Introduction
- (1)
- By ingeniously leveraging the flexibility of fractional Fourier transform in varying the transform order, we achieved the representation of EEG signals in the time domain, frequency domain, and time–frequency domain. This established a multi-domain transfer entropy-based representation scheme for EEG signals, allowing the fusion of temporal–spectral information and better revealing the hidden details in EEG signals.
- (2)
- We introduced deep PCANet, which automatically learns features from low-level and high-level EEG patterns within a supervised learning framework, instead of relying on manually selected features. This effectively avoids the subjectivity introduced by manual feature selection and enhances the model’s generalization capability and robustness.
- (3)
- We designed a well-defined stress induction paradigm and collected EEG data from multiple participants. The proposed algorithm was validated on actual stress-inducing EEG data, and the experimental results demonstrated its effectiveness in the automatic detection of stress states based on EEG signals.
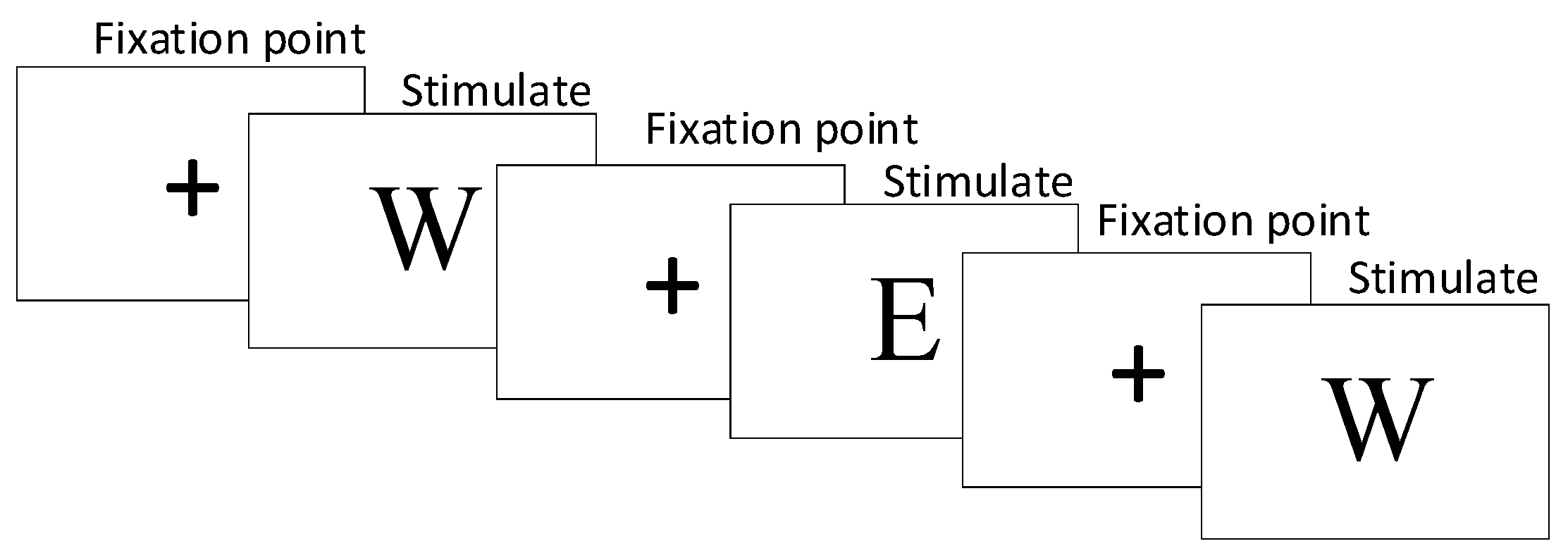
2. Data Collection Paradigms and Schemes
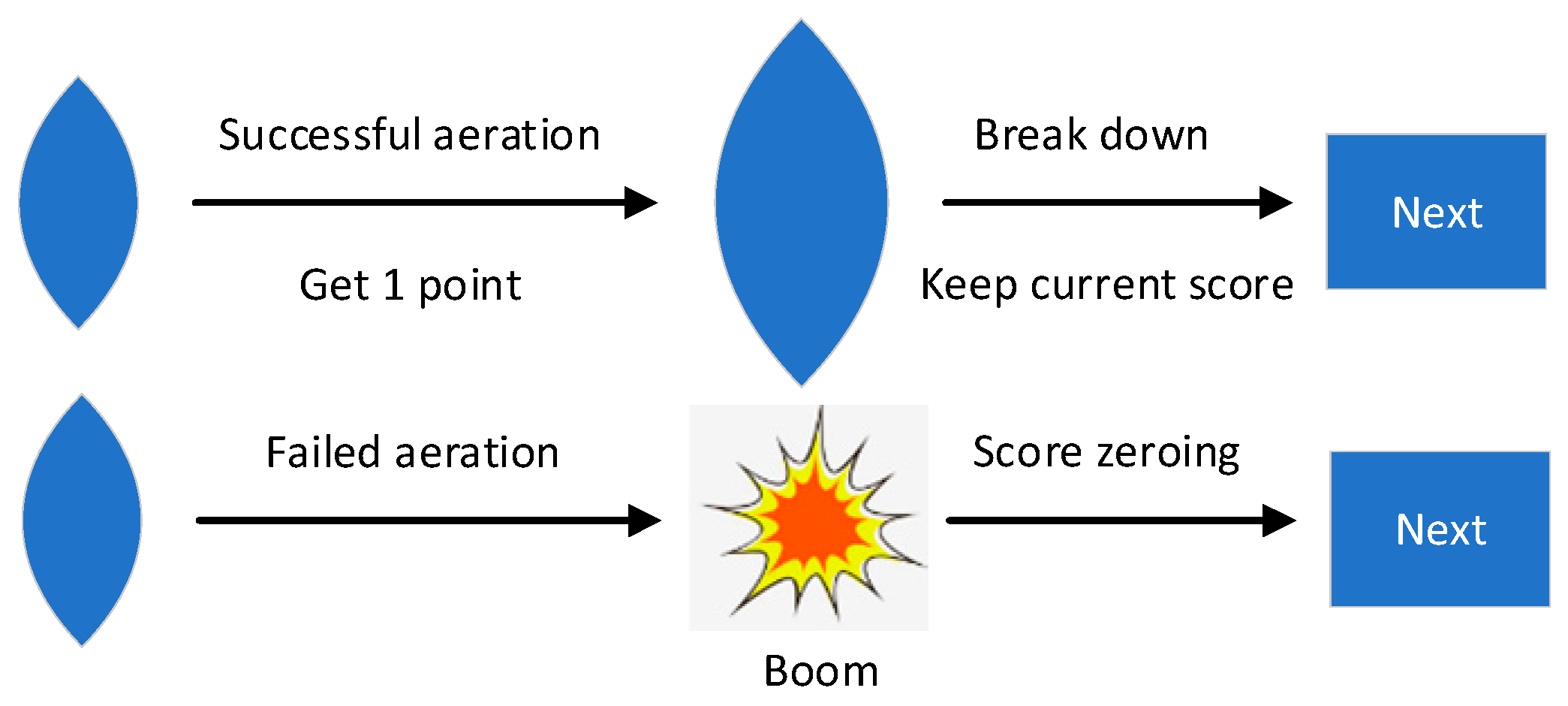
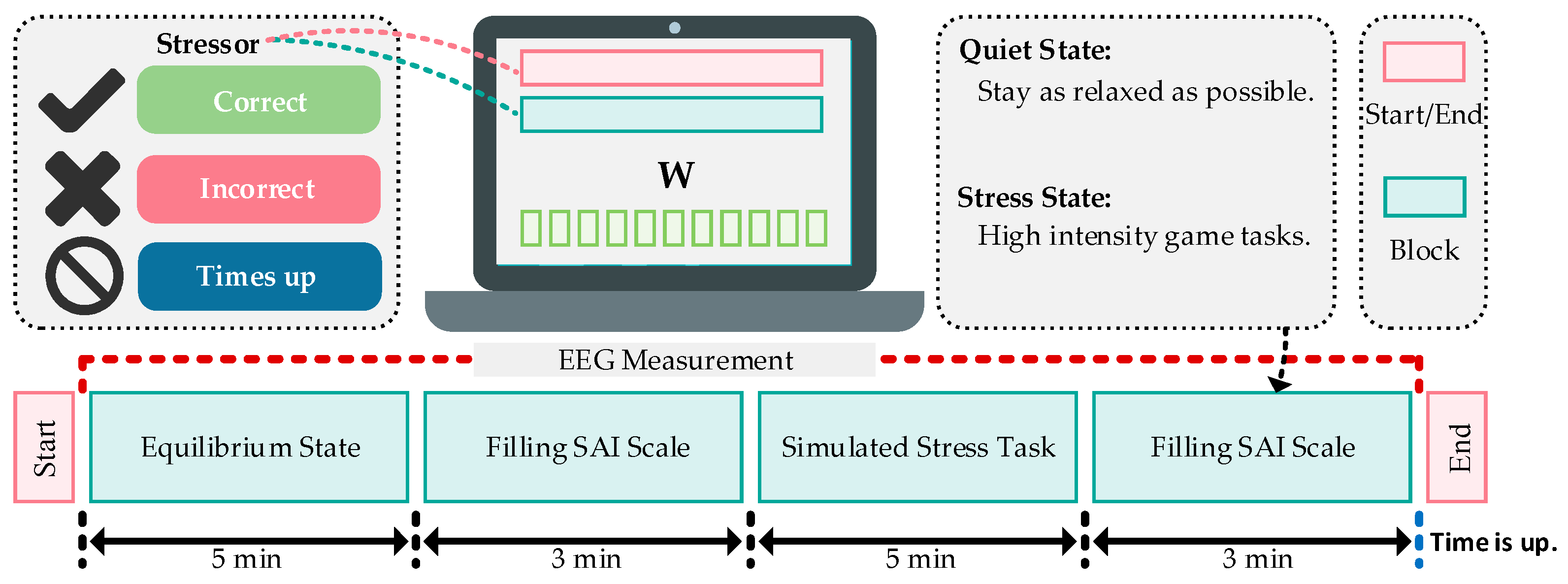
2.1. Stress Induction Paradigm
2.2. Stress Induction Protocol
- (1)
- Experimental Setup: The experiment takes place in a quiet environment. Prior to the formal experiment, participants receive instructions regarding the experimental procedures and game controls. They are advised to maintain a calm state of mind. Before stress induction, their pre-stress EEG signals are recorded. After EEG data collection, participants complete the Subjective Anxiety Inventory (SAI) to assess their pre-stress emotional state.
- (2)
- Stress Task Commencement: Participants engage in a game task under time constraints and negative feedback. During the game, instances of errors prompt real-time negative feedback such as “game failed” or “No points,” accompanied by a time penalty of a 10% reduction in the allocated task time. In order to increase the sense of stress, we will suddenly inform the participant before the task that the results of this task will be ranked and announced, and the final results will affect the final bonus amount.
- (3)
- Stress Task Completion: After the stress task, participants again fill out the SAI to assess their post-stress emotional state.
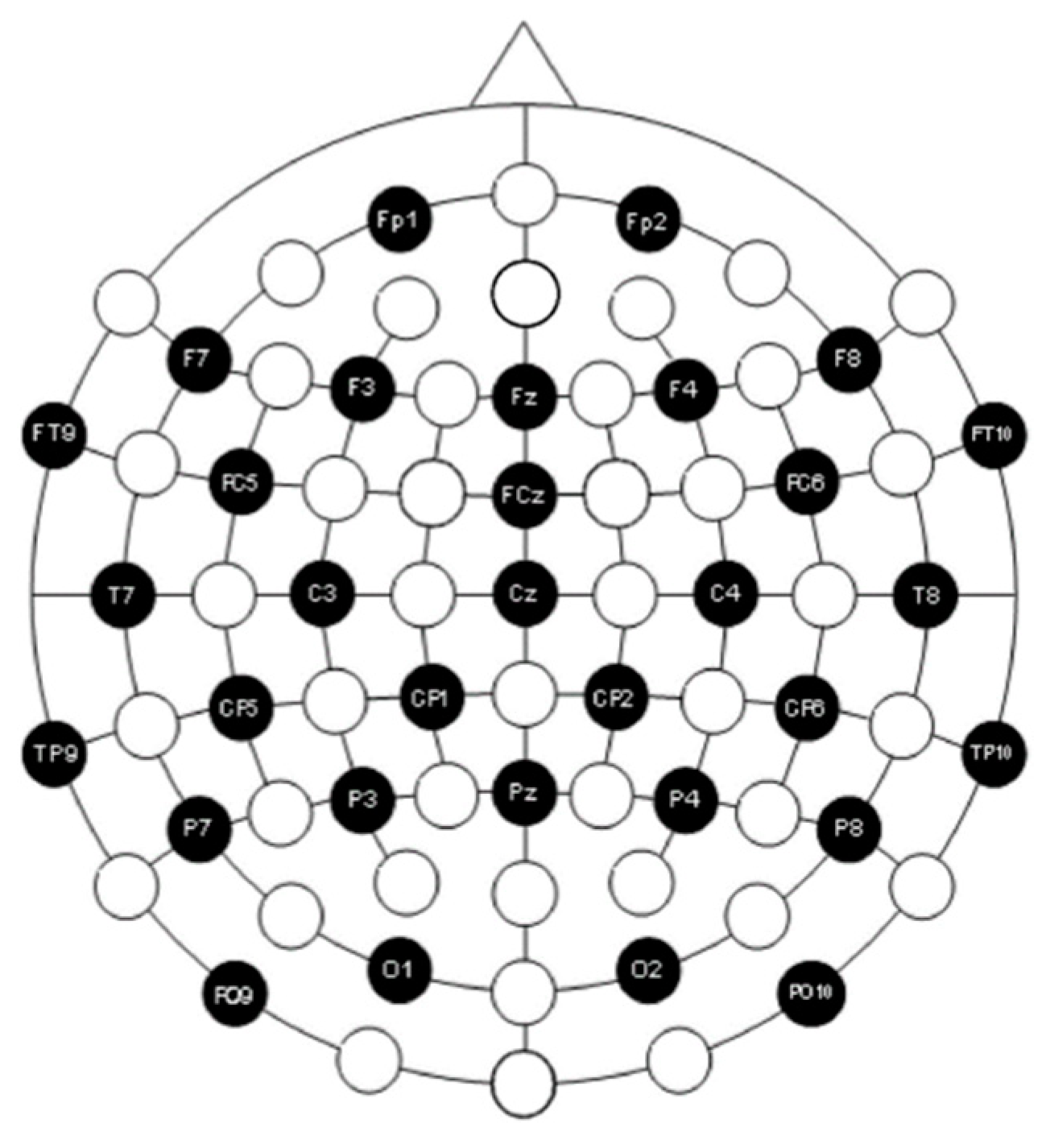
2.3. Data Collection and Preprocessing
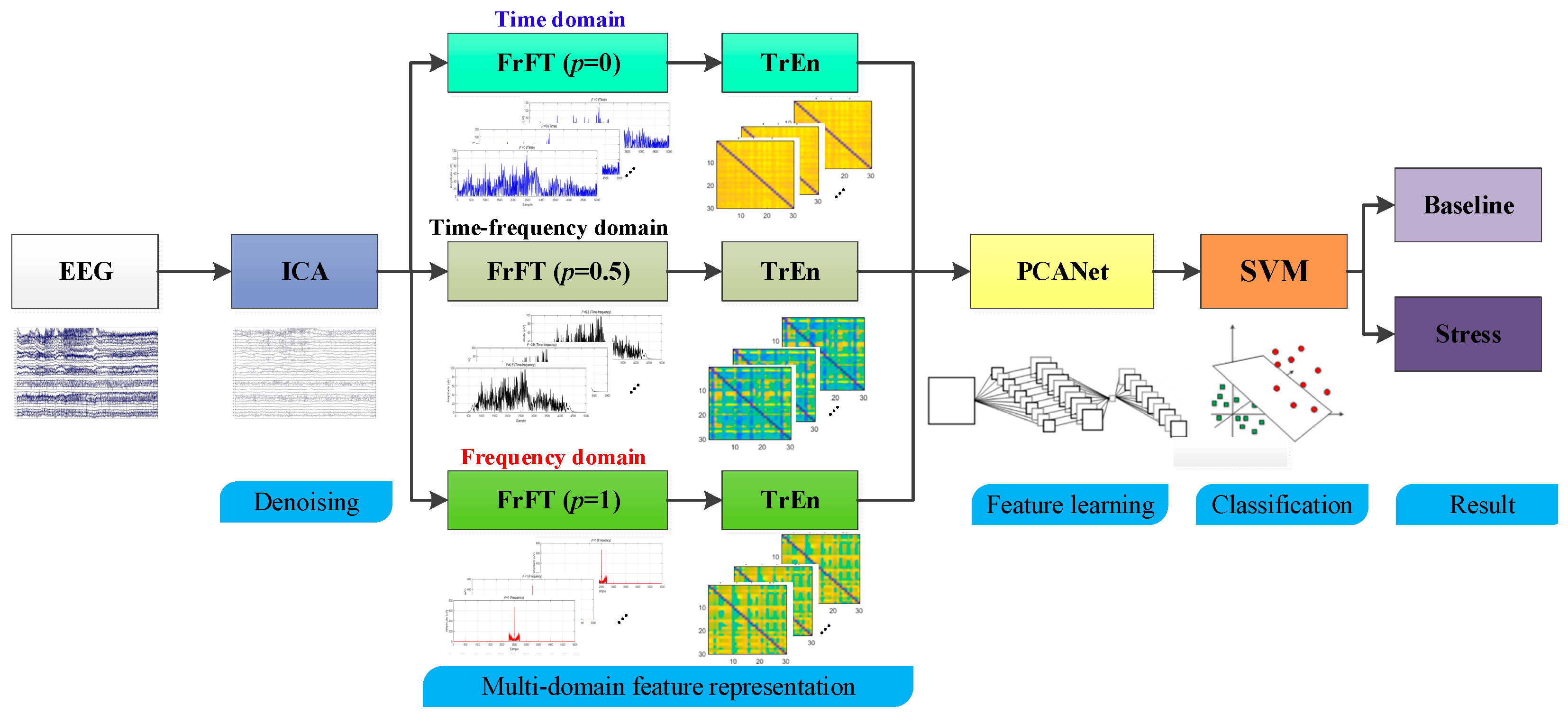
3. Method
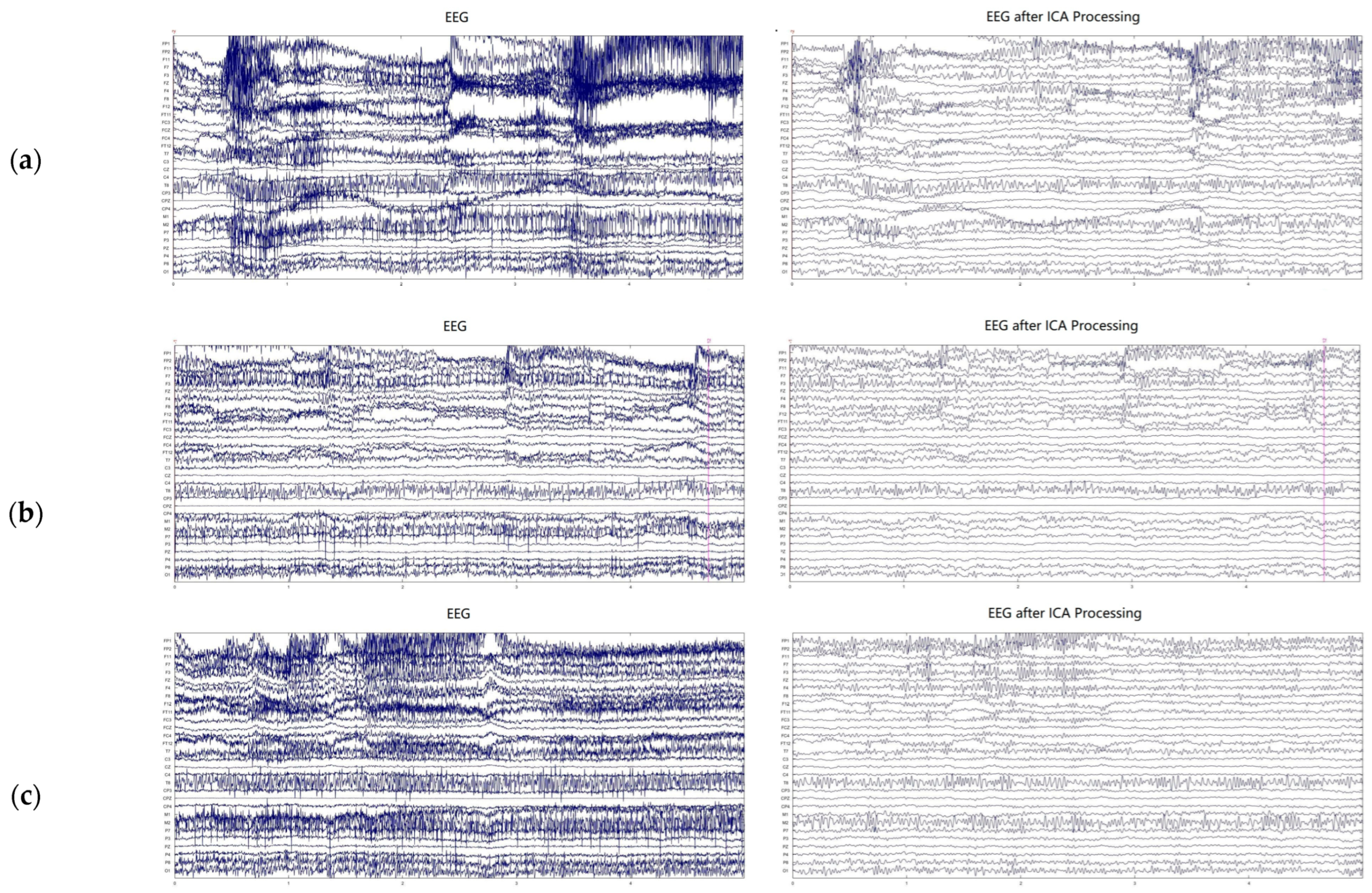
3.1. Independent Component Analysis (ICA)
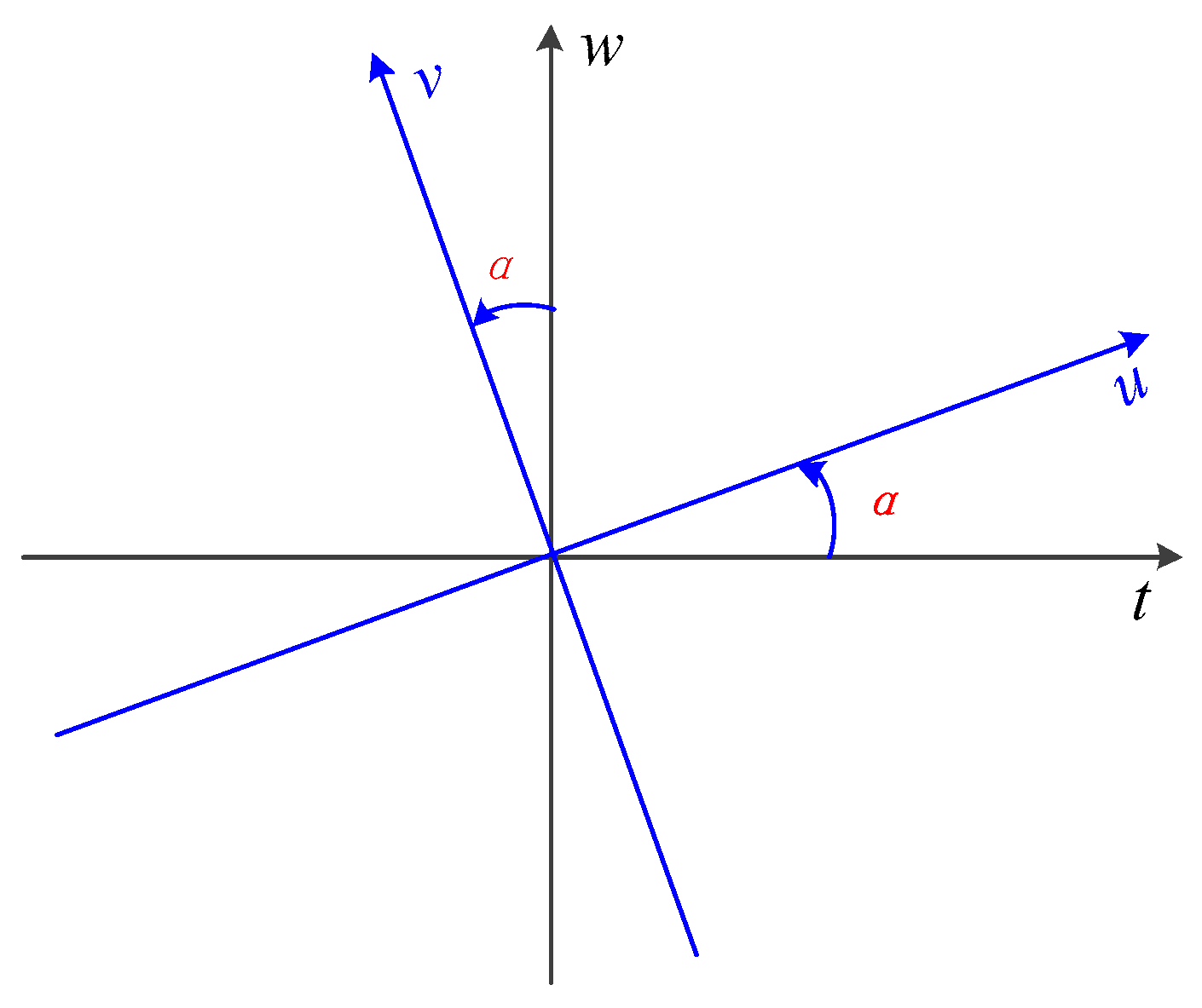
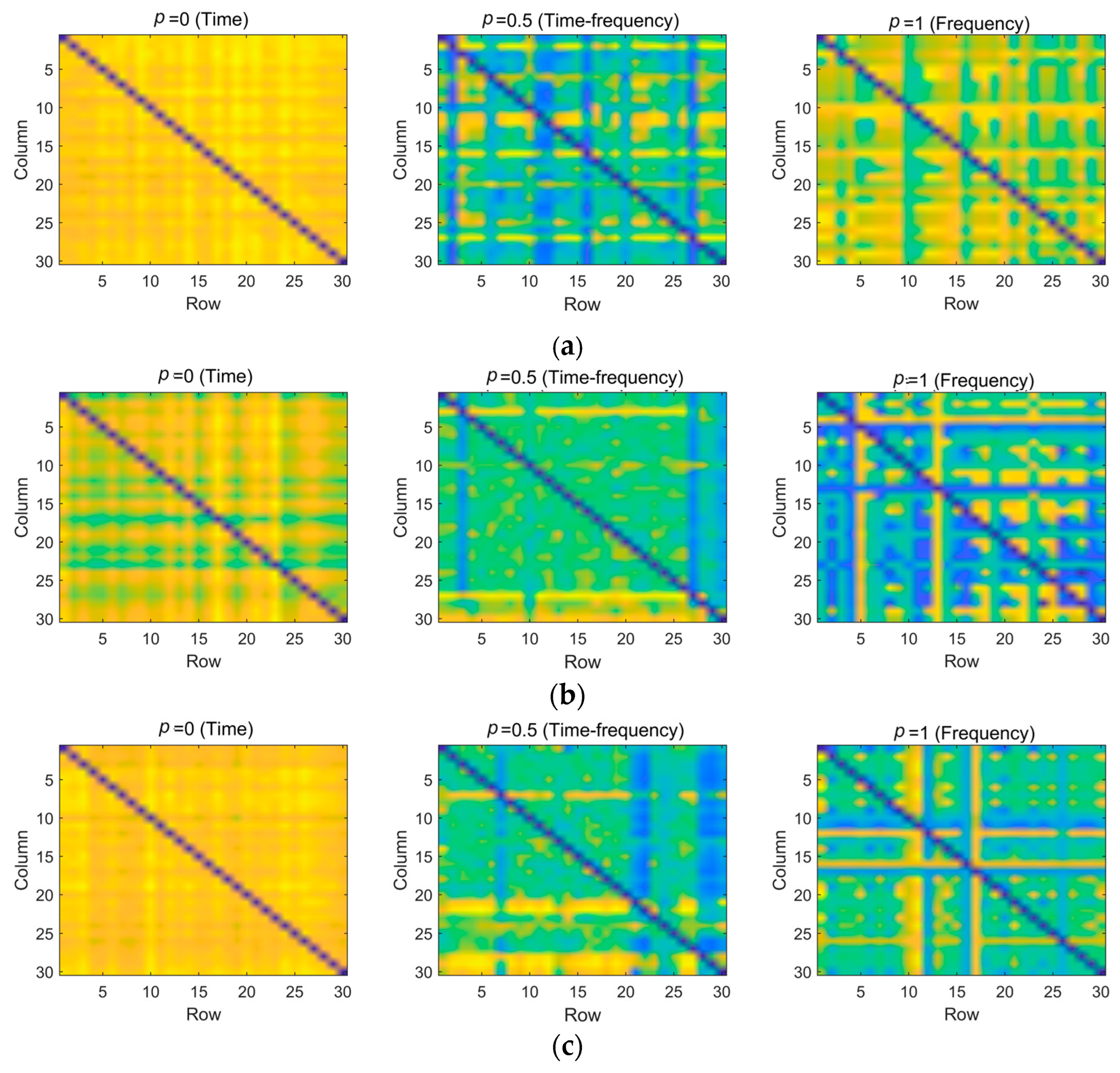
3.2. Fractional Fourier Transform (FrFT)
3.3. Transfer Entropy (TrEn)
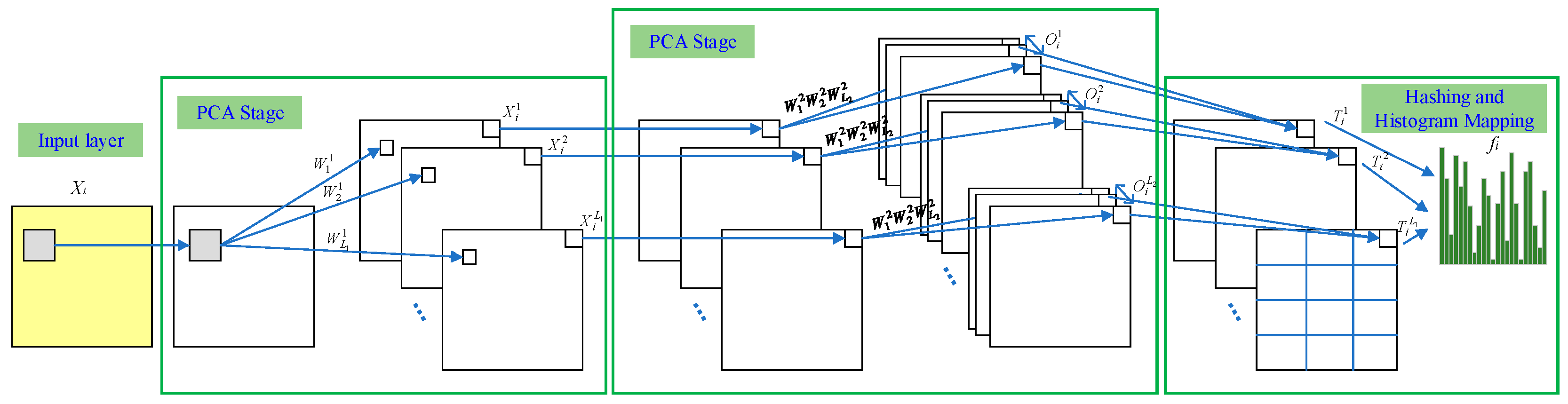
3.4. Principal Component Analysis Network (PCANet)
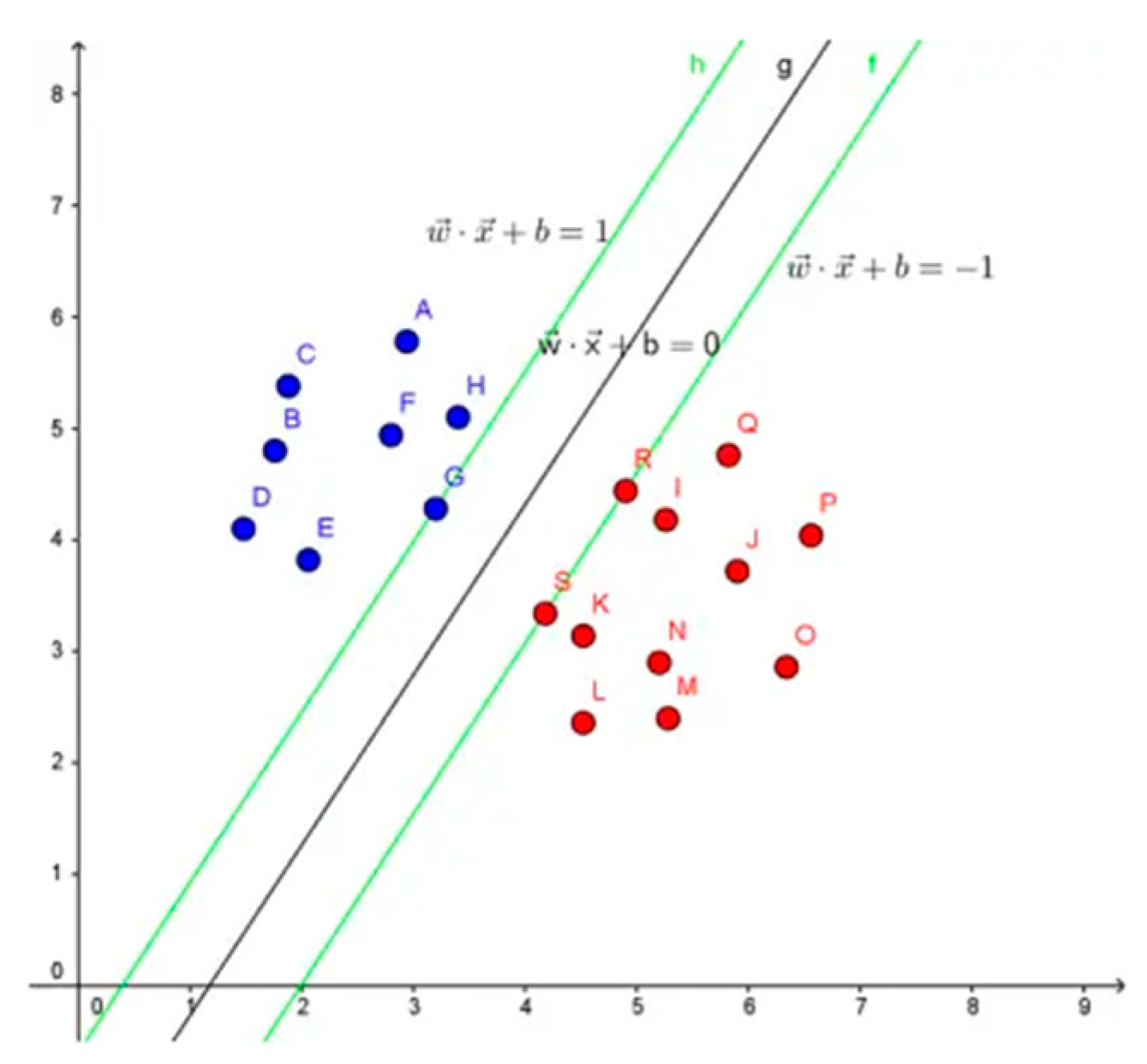
3.5. Support Vector Machine (SVM)
4. Experimental Results and Analysis
4.1. ICA Denoising Analysis
4.2. Multi-Domain Representation Based on FrFT and TrEn
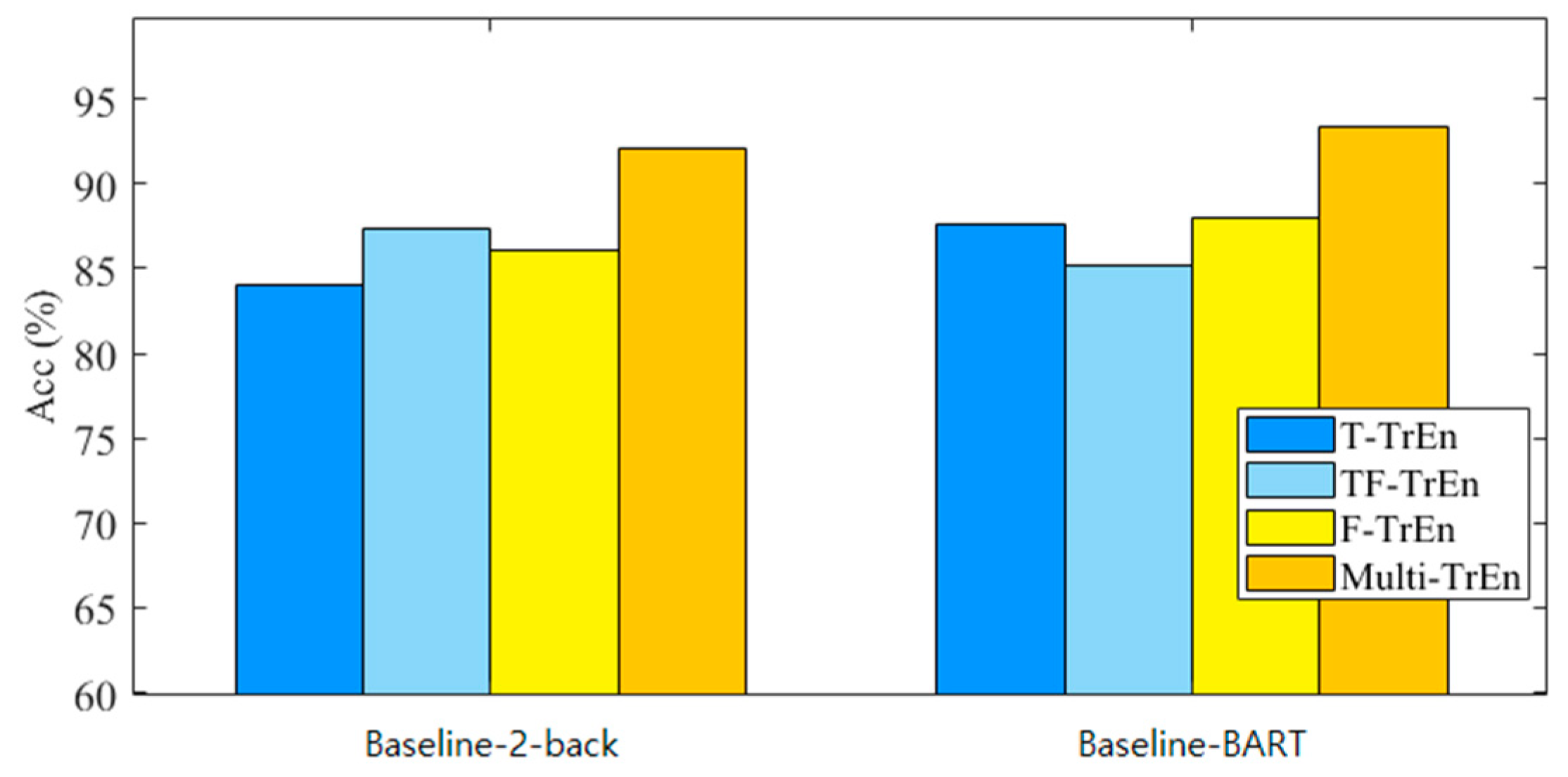
4.3. Classification Based on PCANet
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jackson, E.D.; Payne, J.D.; Nadel, L.; Jacobs, W.J. Stress differentially modulates fear conditioning in healthy men and women. Biol. Psychiatry 2006, 59, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Gao, H.; Guan, L.; Liu, G.; Yang, J. Subjective stress, salivary cortisol, and electrophysiological responses to psychological stress. Front. Psychol. 2016, 7, 174421. [Google Scholar] [CrossRef] [PubMed]
- Kruse, O.; León, I.T.; Stalder, T.; Stark, R.; Klucken, T. Altered reward learning and hippocampal connectivity following psychosocial stress. NeuroImage 2018, 171, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Kumar, M.; Yadav, A.; Yadav, D. FERNET: An Integrated Hybrid DCNN Model for Driver Stress Monitoring via Facial Expressions. Int. J. Pattern Recognit. Artif. Intell. 2023, 37, 2357002. [Google Scholar] [CrossRef]
- Chu, A.M.Y.; Lam, B.S.Y.; Tsang, J.T.Y.; Tiwari, A.; Yuk, H.; Chan, J.N.L.; So, M.K.P. An automatic speech analytics program for digital assessment of stress burden and psychosocial health. Ment. Health Res. 2023, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Pichandi, S.; Balasubramanian, G.; Chakrapani, V. Stress Level Based Emotion Classification Using Hybrid Deep Learning Algorithm. KSII Trans. Internet Inf. Syst. 2023, 17, 3099–3120. [Google Scholar]
- Djemili, R.; Djemili, I. Nonlinear and chaos features over EMD/VMD decomposition methods for ictal EEG signals detection. Comput. Methods Biomech. Biomed. Eng. 2023, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, M.; Feng, G. Study on driver cross-subject emotion recognition based on raw multi-channels EEG data. Electronics 2023, 12, 2359. [Google Scholar] [CrossRef]
- Zayed, A. Two-dimensional fractional Fourier transform and some of its properties. Integral Transform. Spec. Funct. 2018, 29, 553–570. [Google Scholar] [CrossRef]
- Yuan, Y.; Leung, A.W.; Duan, H.; Zhang, L.; Zhang, K.; Wu, J.; Qin, S. The effects of long-term stress on neural dynamics of working memory processing: An investigation using ERP. Sci. Rep. 2016, 6, 23217. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Chen, P.; Lei, Y.; Yan, F.; Yang, Z.; Yang, L.; Wang, L. Effects of acute stress on risky decision-making are related to neuroticism: An fMRI study of the Balloon Analogue Risk Task. J. Affect. Disord. 2023, 340, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Lighthall, N.R.; Mather, M.; Gorlick, M.A. Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PLoS ONE 2009, 4, e6002. [Google Scholar] [CrossRef] [PubMed]
- Gündogdu, S.; Dogan, E.A.; Gülbetekin, E.; Çolak, Ö.H.; Polat, Ö. Evaluation of the EEG Signals and Eye Tracker Data for Working Different N-back Modes. Trait. Du Signal 2019, 36. [Google Scholar]
- Deng, Y.; Wang, M.; Rao, H. Risk-taking research based on the Balloon Analog Risk Task. Adv. Psychol. Sci. 2022, 30, 1377–1392. [Google Scholar] [CrossRef]
- Fatma, N.; Singh, P.; Siddiqui, M.K. Epileptic seizure detection in EEG signal using optimized convolutional neural network with selected feature set. Int. J. Artif. Intell. Tools 2023, 32, 2350045. [Google Scholar] [CrossRef]
- Grifanti, L.; Salimi-Khorshidi, G.; Beckmann, C.F.; Auerbach, E.J.; Douaud, G.; Sexton, C.E.; Zsoldos, E.; Ebmeier, K.P.; Filippini, N.; Mackay, C.E.; et al. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage 2014, 95, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Rai, H.M.; Chatterjee, K. Hybrid adaptive algorithm based on wavelet transform and independent component analysis for denoising of MRI images. Measurement 2019, 144, 72–82. [Google Scholar] [CrossRef]
- Nolan, H.; Whelan, R.; Reilly, R.B. FASTER: Fully automated statistical thresholding for EEG artifact rejection. J. Neurosci. Methods 2010, 192, 152–162. [Google Scholar] [CrossRef]
- Delorme, A.; Palmer, J.; Onton, J.; Oostenveld, R.; Makeig, S. Independent EEG sources are dipolar. PLoS ONE 2012, 7, e30135. [Google Scholar] [CrossRef] [PubMed]
- Urigüen, J.A.; Garcia-Zapirain, B. EEG artifact removal-state-of-the-art and guidelines. J. Neural Eng. 2015, 12, 031001. [Google Scholar] [CrossRef]
- Chaumon, M.; Bishop, D.V.M.; Busch, N.A. A practical guide to the selection of independent components of the electroencephalogram for artifact correction. J. Neurosci. Methods 2015, 250, 47–63. [Google Scholar] [CrossRef]
- Winkler, I.; Debener, S.; Müller, K.R.; Tangermann, M. On the influence of high-pass filtering on ICA-based artifact reduction in EEG-ERP. In Proceedings of the 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 4101–4105. [Google Scholar]
- Pontifex, M.B.; Miskovic, V.; Laszlo, S. Evaluating the efficacy of fully automated approaches for the selection of eyeblink ICA components. Psychophysiology 2017, 54, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Fares, K.; Amine, K.; Salah, E. A robust blind color image watermarking based on Fourier transform domain. Optik 2020, 208, 164562. [Google Scholar] [CrossRef]
- You, Y.; Zhong, X.; Liu, G.; Yang, Z. Automatic sleep stage classification: A light and efficient deep neural network model based on time, frequency and fractional Fourier transform domain features. Artif. Intell. Med. 2022, 127, 102279. [Google Scholar] [CrossRef]
- Li, M.; Chen, W.; Zhang, T. FuzzyEn-based features in FrFT-WPT domain for epileptic seizure detection. Neural Comput. Appl. 2019, 31, 9335–9348. [Google Scholar] [CrossRef]
- Stefan, S.; Schorr, B.; Lopez-Rolon, A.; Kolassa, I.T.; Shock, J.P.; Rosenfelder, M.; Heck, S.; Bender, A. Consciousness indexing and outcome prediction with resting-state EEG in severe disorders of consciousness. Brain Topogr. 2018, 31, 848–862. [Google Scholar] [CrossRef] [PubMed]
- Ursino, M.; Ricci, G.; Magosso, E. Transfer entropy as a measure of brain connectivity: A critical analysis with the help of neural mass models. Front. Comput. Neurosci. 2020, 14, 45. [Google Scholar] [CrossRef]
- Chan, T.H.; Jia, K.; Gao, S.; Lu, J.; Zeng, Z.; Ma, Y. PCANet: A simple deep learning baseline for image classification. IEEE Trans. Image Process. 2015, 24, 5017–5032. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, J. Two-dimensional principal component analysis-based convolutional autoencoder for wafer map defect detection. IEEE Trans. Ind. Electron. 2020, 68, 8789–8797. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, B.; Li, R.; Wang, C.; Wang, J.; She, Q.; Luo, Z.; Zhang, Y. Driving fatigue detection from EEG using a modified PCANet method. Comput. Intell. Neurosci. 2019, 2019, 4721863. [Google Scholar] [CrossRef]
- Li, M.; Chen, W. FFT-based deep feature learning method for EEG classification. Biomed. Signal Process. Control 2021, 66, 102492. [Google Scholar] [CrossRef]
- Aly, W.; Aly, S.; Almotairi, S. User-independent American sign language alphabet recognition based on depth image and PCANet features. IEEE Access 2019, 7, 123138–123150. [Google Scholar] [CrossRef]
- Abdelbaky, A.; Aly, S. Human action recognition using short-time motion energy template images and PCANet features. Neural Comput. Appl. 2020, 32, 12561–12574. [Google Scholar] [CrossRef]
- Soon, F.C.; Khaw, H.Y.; Chuah, J.H.; Kanesa, J. PCANet-based convolutional neural network architecture for a vehicle model recognition system. IEEE Trans. Intell. Transp. Syst. 2018, 20, 749–759. [Google Scholar] [CrossRef]
- Chapelle, O.; Haffner, P.; Vapnik, V.N. Support vector machines for histogram-based image classification. IEEE Trans. Neural Netw. 1999, 10, 1055–1064. [Google Scholar] [CrossRef]
- Richhariya, B.; Tanveer, M. EEG signal classification using universum support vector machine. Expert Syst. Appl. 2018, 106, 169–182. [Google Scholar] [CrossRef]
- Sharma, L.D.; Bohat, V.K.; Habib, M.; Al-Zoubi, A.M.; Faris, H.; Alijarah, I. Evolutionary inspired approach for mental stress detection using EEG signal. Expert Syst. Appl. 2022, 197, 116634. [Google Scholar] [CrossRef]
- Tsai, Y.-H.; Wu, S.-K.; Yu, S.-S.; Tsai, M.-H. Analyzing brain waves of table tennis players with machine learning for stress classification. Appl. Sci. 2022, 12, 8052. [Google Scholar] [CrossRef]
- Subhani, A.R.; Mumtaz, W.; Saad, M.N.B.M.; Kamel, N.; Malik, A.S. Machine learning framework for the detection of mental stress at multiple levels. IEEE Access 2017, 5, 13545–13555. [Google Scholar] [CrossRef]

| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Layer numbers | 2 | Filter numbers (L1 × L2) | 9 × 9 |
| Input matrix size (m × n) | 32 × 96 | Block size | 32 × 32 |
| Patch size (k1 × k2) | 3 × 3 | Block overlap ratio | 0.5 |
| Group | BART | 2-Back | ||||
|---|---|---|---|---|---|---|
| Acc (%) | Sen (%) | Spe (%) | Acc (%) | Sen (%) | Spe (%) | |
| 1 | 94.76 | 95.35 | 93.66 | 90.33 | 93.10 | 87.66 |
| 2 | 94.65 | 96.60 | 91.00 | 93.22 | 90 | 96.33 |
| 3 | 88.37 | 89.82 | 85.66 | 90.50 | 88.27 | 92.66 |
| 4 | 93.48 | 96.60 | 87.66 | 98.64 | 97.93 | 99.33 |
| 5 | 93.48 | 95.71 | 89.33 | 92.88 | 96.20 | 89.66 |
| 6 | 94.53 | 97.5 | 89 | 94.06 | 97.93 | 90.33 |
| 7 | 88.72 | 93.75 | 89.33 | 92.71 | 95.51 | 90 |
| 8 | 92.55 | 86 | 88.33 | 95.59 | 96.20 | 95 |
| 9 | 90.69 | 91.96 | 88.33 | 94.40 | 93.79 | 95 |
| 10 | 93.13 | 95.53 | 88.66 | 90.84 | 90.68 | 91 |
| 11 | 90 | 93.39 | 83.66 | 92.54 | 92.41 | 92.66 |
| 12 | 91.97 | 96.07 | 84.33 | 98.30 | 97.93 | 98.66 |
| 13 | 89.53 | 93.57 | 82 | 92.71 | 89.31 | 96 |
| 14 | 93.72 | 95.35 | 90.67 | 94.23 | 92.41 | 96 |
| 15 | 92.55 | 96.60 | 85 | 88.64 | 89.31 | 88 |
| Ave | 92.14 | 94.25 | 87.11 | 93.31 | 93.40 | 93.22 |
| Groups | Acc (%) | Sen (%) | Spe (%) |
|---|---|---|---|
| 1 | 94.80 | 98.95 | 88.33 |
| 2 | 98.27 | 99.47 | 97.22 |
| 3 | 88.70 | 97.76 | 91.58 |
| 4 | 93.21 | 99.32 | 96.32 |
| 5 | 91.71 | 95.41 | 86.19 |
| 6 | 91.89 | 98.35 | 82.86 |
| 7 | 90.85 | 96.59 | 81.43 |
| 8 | 90.35 | 94.47 | 87.00 |
| 9 | 98.87 | 91.53 | 91.67 |
| 10 | 87.13 | 95.41 | 82.67 |
| 11 | 94.35 | 95.18 | 83.33 |
| 12 | 86.96 | 85.18 | 85.00 |
| 13 | 86.00 | 92.59 | 81.00 |
| 14 | 91.04 | 95.53 | 82.67 |
| 15 | 93.04 | 92.47 | 86.67 |
| Ave | 91.81 | 95.21 | 86.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Xu, L.; Zheng, J.; Wu, D.; Li, H.; Shao, Y.; Shi, G.; Fu, W. A Hybrid EEG-Based Stress State Classification Model Using Multi-Domain Transfer Entropy and PCANet. Brain Sci. 2024, 14, 595. https://doi.org/10.3390/brainsci14060595
Dong Y, Xu L, Zheng J, Wu D, Li H, Shao Y, Shi G, Fu W. A Hybrid EEG-Based Stress State Classification Model Using Multi-Domain Transfer Entropy and PCANet. Brain Sciences. 2024; 14(6):595. https://doi.org/10.3390/brainsci14060595
Chicago/Turabian StyleDong, Yuefang, Lin Xu, Jian Zheng, Dandan Wu, Huanli Li, Yongcong Shao, Guohua Shi, and Weiwei Fu. 2024. "A Hybrid EEG-Based Stress State Classification Model Using Multi-Domain Transfer Entropy and PCANet" Brain Sciences 14, no. 6: 595. https://doi.org/10.3390/brainsci14060595




