Sensitivity to and Control of Distraction: Distractor-Entrained Oscillation and Frontoparietal EEG Gamma Synchronization †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethics
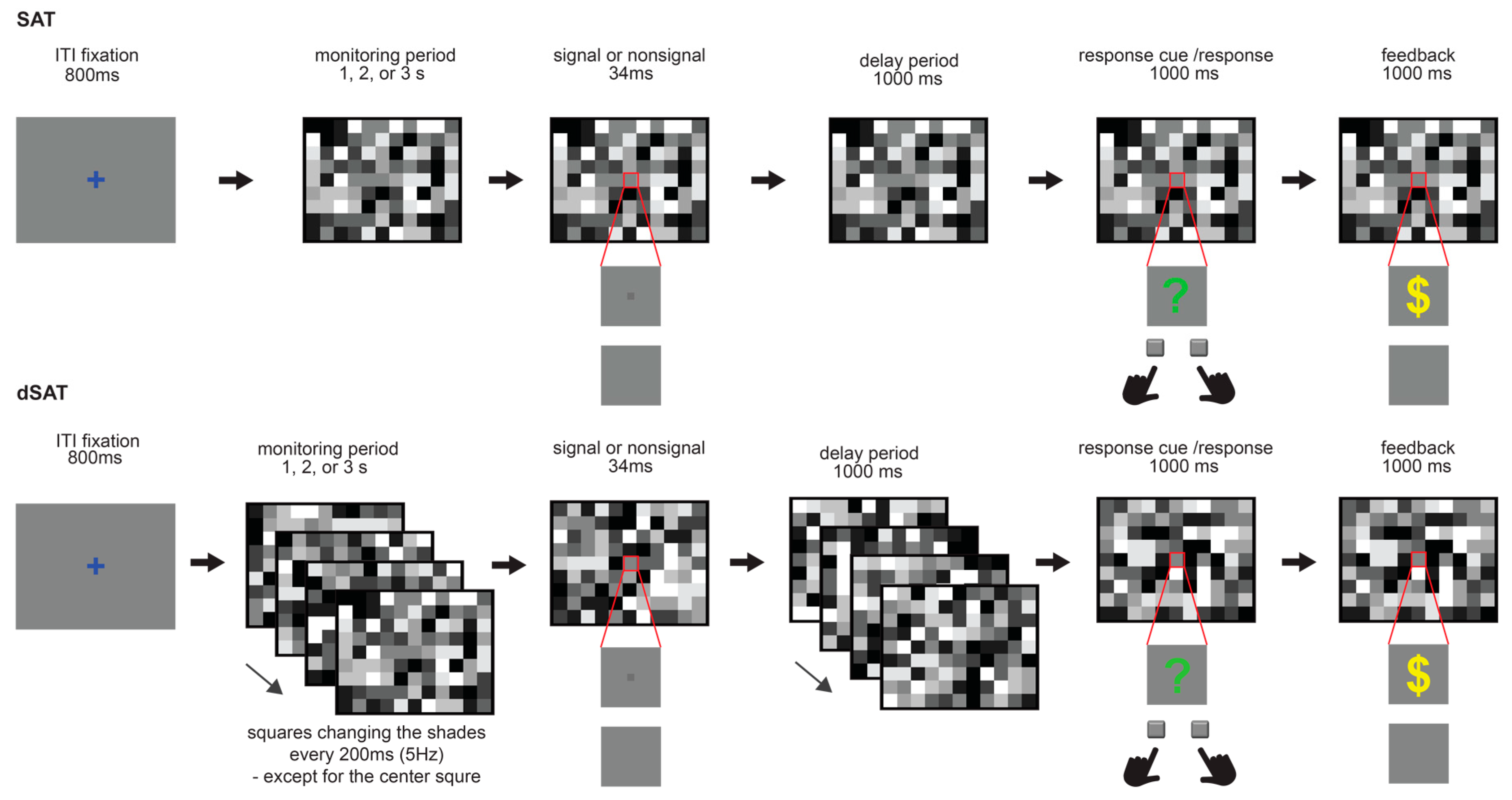
2.3. Modified Distractor Condition Sustained Attention Task (dSAT)
2.4. Procedure
2.5. EEG Recording and Preprocessing
2.6. EEG Data Analyses
2.6.1. Local Gamma Oscillation
2.6.2. Trial-by-Trial Variations of Gamma Oscillation and Signal Detection Performance
2.6.3. Inter-Trial Coherence on the Distractor-Evoked 5 Hz Oscillations
2.7. Statistical Analysis
3. Results
3.1. Behavioral Results
3.2. EEG Results
3.2.1. Gamma Power and Variability during SAT Performance (Hypotheses 1 and 2)
3.2.2. Changes in Gamma Power and Variability Related to Distraction (Hypotheses 3 and 4)
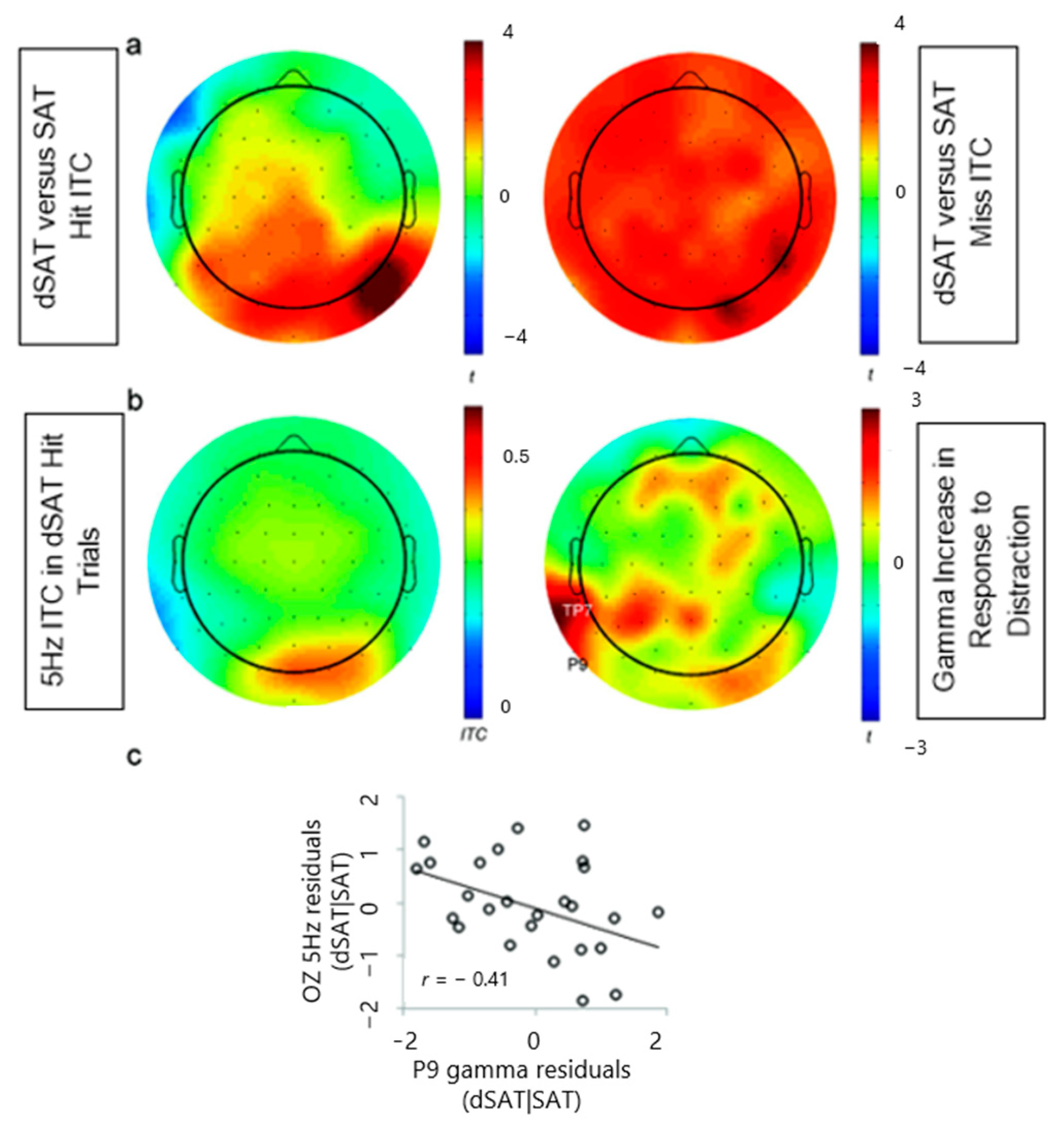
3.2.3. Distractor-Entrained Oscillation: Inter-Trial Coherence (ITC) (Hypotheses 5 and 6)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scolari, M.; Seidl-Rathkopf, K.N.; Kastner, S. Functions of the human frontoparietal attention network: Evidence from neuroimaging. Curr. Opin. Behav. Sci. 2015, 1, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Hopfinger, J.B.; Buonocore, M.H.; Mangun, G.R. The neural mechanisms of top-down attentional control. Nat. Neurosci. 2000, 3, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; D’Esposito, M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 90–103. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. USA 2008, 105, 12569–12574. [Google Scholar] [CrossRef] [PubMed]
- Spielberg, J.M.; Miller, G.A.; Heller, W.; Banich, M.T. Flexible brain network reconfiguration supporting inhibitory control. Proc. Natl. Acad. Sci. USA 2015, 112, 10020–10025. [Google Scholar] [CrossRef]
- Depue, B.E.; Orr, J.M.; Smolker, H.R.; Naaz, F.; Banich, M.T. The Organization of Right Prefrontal Networks Reveals Common Mechanisms of Inhibitory Regulation Across Cognitive, Emotional, and Motor Processes. Cereb. Cortex 2016, 26, 1634–1646. [Google Scholar] [CrossRef]
- Langner, R.; Eickhoff, S.B. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychol. Bull. 2013, 139, 870–900. [Google Scholar] [CrossRef]
- Berry, A.S.; Sarter, M.; Lustig, C. Distinct Frontoparietal Networks Underlying Attentional Effort and Cognitive Control. J. Cogn. Neurosci. 2017, 29, 1212–1225. [Google Scholar] [CrossRef]
- Springer, S.D.; Okelberry, H.J.; Willett, M.P.; Johnson, H.J.; Meehan, C.E.; Schantell, M.; Embury, C.M.; Rempe, M.P.; Wilson, T.W. Age-related alterations in the oscillatory dynamics serving verbal working memory processing. Aging 2023, 15, 14574–14590. [Google Scholar] [CrossRef]
- Lustig, C.; Sarter, M. Attention and the Cholinergic System: Relevance to Schizophrenia. Transl. Neuropsychopharmacol. 2016, 28, 327–362. [Google Scholar] [CrossRef] [PubMed]
- Reuter-Lorenz, P.A.; Lustig, C. Brain aging: Reorganizing discoveries about the aging mind. Curr. Opin. Neurobiol. 2005, 15, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Reuter-Lorenz, P.A.; Cappell, K.A. Neurocognitive Aging and the Compensation Hypothesis. Curr. Dir. Psychol. Sci. 2008, 17, 177–182. [Google Scholar] [CrossRef]
- Talamonti, D.; Montgomery, C.A.; Clark, D.P.A.; Bruno, D. Age-related prefrontal cortex activation in associative memory: An fNIRS pilot study. Neuroimage 2020, 222, 117223. [Google Scholar] [CrossRef]
- Panico, F.; De Marco, S.; Sagliano, L.; D’Olimpio, F.; Grossi, D.; Trojano, L. Brain hemodynamic response in Examiner-Examinee dyads during spatial short-term memory task: An fNIRS study. Exp. Brain Res. 2021, 239, 1607–1616. [Google Scholar] [CrossRef] [PubMed]
- Callicott, J.H.; Mattay, V.S.; Bertolino, A.; Finn, K.; Coppola, R.; Frank, J.A.; Goldberg, T.E.; Weinberger, D.R. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb. Cortex 1999, 9, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Cappell, K.A.; Gmeindl, L.; Reuter-Lorenz, P.A. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 2010, 46, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Garces, N.J.; Gordon, B.A.; Brumback-Peltz, C.R.; Shin, E.; Lee, Y.; Sutton, B.P.; Maclin, E.L.; Gratton, G.; Fabiani, M. Span, CRUNCH, and Beyond: Working Memory Capacity and the Aging Brain. J. Cogn. Neurosci. 2010, 22, 655–669. [Google Scholar] [CrossRef]
- Sarter, M.; Lustig, C.; Blakely, R.D.; Koshy Cherian, A. Cholinergic genetics of visual attention: Human and mouse choline transporter capacity variants influence distractibility. J. Physiol. 2016, 110, 10–18. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Sarter, M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology 2011, 36, 52–73. [Google Scholar] [CrossRef]
- Sarter, M.; Lustig, C.; Howe, W.M.; Gritton, H.; Berry, A.S. Deterministic functions of cortical acetylcholine. Eur. J. Neurosci. 2014, 39, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Gehring, W.J.; Kozak, R. More attention must be paid: The neurobiology of attentional effort. Brain Res. Rev. 2006, 51, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Raizada, R.D.S.; Poldrack, R.A. Selective Amplification of Stimulus Differences during Categorical Processing of Speech. Neuron 2007, 56, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.B.; Arvaneh, M.; Harty, S.; Maguire, T.; O’Connell, R.; Robertson, I.H.; Dockree, P.M. Prefrontal Modulation of Visual Processing and Sustained Attention in Aging, a tDCS–EEG Coregistration Approach. J. Cogn. Neurosci. 2018, 30, 1630–1645. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.S.; Blakely, R.D.; Sarter, M.; Lustig, C. Cholinergic capacity mediates prefrontal engagement during challenges to attention: Evidence from imaging genetics. NeuroImage 2015, 108, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Demeter, E.; Guthrie, S.K.; Taylor, S.F.; Sarter, M.; Lustig, C. Increased distractor vulnerability but preserved vigilance in patients with schizophrenia: Evidence from a translational Sustained Attention Task. Schizophr. Res. 2013, 144, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Demeter, E.; Sarter, M.; Lustig, C. Rats and humans paying attention: Cross-species task development for translational research. Neuropsychology 2008, 22, 787–799. [Google Scholar] [CrossRef]
- Gritton, H.J.; Howe, W.M.; Mallory, C.S.; Hetrick, V.L.; Berke, J.D.; Sarter, M. Cortical cholinergic signaling controls the detection of cues. Proc. Natl. Acad. Sci. USA 2016, 113, E1089–E1097. [Google Scholar] [CrossRef] [PubMed]
- Sapountzis, P. Neural signatures of attention insights from decoding population activity patterns. Front. Biosci. 2018, 23, 221–246. [Google Scholar] [CrossRef]
- Buschman, T.J.; Miller, E.K. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 2007, 315, 1860–1862. [Google Scholar] [CrossRef]
- Misselhorn, J.; Friese, U.; Engel, A.K. Frontal and parietal alpha oscillations reflect attentional modulation of cross-modal matching. Sci. Rep. 2019, 9, 5030. [Google Scholar] [CrossRef] [PubMed]
- Beldzik, E.; Domagalik, A.; Beres, A.; Marek, T. Linking visual gamma to task-related brain networks—A simultaneous EEG-fMRI study. Psychophysiology 2019, 56, e13462. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.P.; Werner, P.; Steinbrink, J.; Fries, P.; Obrig, H. Stimulus-induced and state-dependent sustained gamma activity is tightly coupled to the hemodynamic response in humans. J. Neurosci. 2009, 29, 13962–13970. [Google Scholar] [CrossRef] [PubMed]
- Niessing, J.; Ebisch, B.; Schmidt, K.E.; Niessing, M.; Singer, W.; Galuske, R.A.W. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 2005, 309, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Greening, S.G.; Ueno, T.; Clewett, D.; Ponzio, A.; Sakaki, M.; Mather, M. Arousal increases neural gain via the locus coeruleus–noradrenaline system in younger adults but not in older adults. Nat. Hum. Behav. 2018, 2, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. The costs and benefits of brain dopamine for cognitive control. WIREs Cogn. Sci. 2016, 7, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Thankachan, S.; McKenna, J.T.; McNally, J.M.; Yang, C.; Choi, J.H.; Chen, L.; Kocsis, B.; Deisseroth, K.; Strecker, R.E.; et al. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc. Natl. Acad. Sci. USA 2015, 112, 3535–3540. [Google Scholar] [CrossRef] [PubMed]
- Doesburg, S.M.; Roggeveen, A.B.; Kitajo, K.; Ward, L.M. Large-scale gamma-band phase synchronization and selective attention. Cereb. Cortex 2008, 18, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Howe, W.M.; Gritton, H.J.; Lusk, N.A.; Roberts, E.A.; Hetrick, V.L.; Berke, J.D.; Sarter, M. Acetylcholine Release in Prefrontal Cortex Promotes Gamma Oscillations and Theta–Gamma Coupling during Cue Detection. J. Neurosci. 2017, 37, 3215–3230. [Google Scholar] [CrossRef]
- Rodriguez, R.; Kallenbach, U.; Singer, W.; Munk, M.H.J. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J. Neurosci. 2004, 24, 10369–10378. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef] [PubMed]
- Fries, P. Neuronal Gamma-Band Synchronization as a Fundamental Process in Cortical Computation. Annu. Rev. Neurosci. 2009, 32, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Fries, P. Rhythms for Cognition: Communication through Coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.M.; McCormick, D.A. Chattering Cells: Superficial Pyramidal Neurons Contributing to the Generation of Synchronous Oscillations in the Visual Cortex. Science 1996, 274, 109–113. [Google Scholar] [CrossRef] [PubMed]
- McGaughy, J.; Sarter, M. Behavioral vigilance in rats: Task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology 1995, 117, 340–357. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Rothlein, D.; Kucyi, A.; Valera, E.M.; Esterman, M. Brain state-based detection of attentional fluctuations and their modulation. NeuroImage 2021, 236, 118072. [Google Scholar] [CrossRef] [PubMed]
- Vaurio, R.G.; Simmonds, D.J.; Mostofsky, S.H. Increased intra-individual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia 2009, 47, 2389–2396. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.T.; Hansen, J.C.; Hillyard, S.A. Selective attention to stimulus location modulates the steady-state visual evoked potential. Proc. Natl. Acad. Sci. USA 1996, 93, 4770–4774. [Google Scholar] [CrossRef]
- Andersen, S.K.; Muller, M.M.; Hillyard, S.A. Attentional Selection of Feature Conjunctions Is Accomplished by Parallel and Independent Selection of Single Features. J. Neurosci. 2015, 35, 9912–9919. [Google Scholar] [CrossRef]
- Singer, J.L.; Antrobus, J.S. A factor-analytic study of daydreaming and conceptually-related cognitive and personality variables. Percept. Mot. Ski. 1963, 17, 187–209. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Gratton, G.; Coles, M.G.; Donchin, E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y. The Mystery 40 Hz: Unraveling the Efficacy of Rhythmic Stimulation in Alzheimer’s Disease. Neurosci. Bull. 2024, 40, 831–834. [Google Scholar] [CrossRef]
- Gulbinaite, R.; Roozendaal, D.H.M.; VanRullen, R. Attention differentially modulates the amplitude of resonance frequencies in the visual cortex. NeuroImage 2019, 203, 116146. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice; The MIT Press: Cambridge, MA, USA, 2014. [Google Scholar] [CrossRef]
- Tallon-Baudry, C.; Bertrand, O.; Delpuech, C.; Pernier, J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J. Neurosci. 1996, 16, 4240–4249. [Google Scholar] [CrossRef] [PubMed]
- Bardouille, T.; Ross, B. MEG imaging of sensorimotor areas using inter-trial coherence in vibrotactile steady-state responses. NeuroImage. 2008, 42, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Haenschel, C.; Linden, D. Exploring intermediate phenotypes with EEG: Working memory dysfunction in schizophrenia. Behav. Brain Res. 2011, 216, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.D. Detection of Influential Observation in Linear Regression. Technometrics 1977, 19, 15–18. [Google Scholar] [CrossRef]
- Atkinson, A.C.; Cook, R.D.; Weisberg, S. Residuals and Influence in Regression. Biometrics 1983, 39, 818. [Google Scholar] [CrossRef]
- Bollen, K.A.; Jackman, R.W. Regression Diagnostics: An Expository Treatment of Outliers and Influential Cases. Sociol. Methods Res. 1985, 13, 510–542. [Google Scholar] [CrossRef]
- Macmillan, N.A.; Creelman, C.D. Detection Theory, 2nd ed.; Psychology Press: London, UK, 2004. [Google Scholar] [CrossRef]
- St Peters, M.; Demeter, E.; Lustig, C.; Bruno, J.P.; Sarter, M. Enhanced Control of Attention by Stimulating Mesolimbic-Corticopetal Cholinergic Circuitry. J. Neurosci. 2011, 31, 9760–9771. [Google Scholar] [CrossRef] [PubMed]
- Demeter, E.; Hernandez-Garcia, L.; Sarter, M.; Lustig, C. Challenges to attention: A continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. NeuroImage 2011, 54, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.K.; Fries, P.; Singer, W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2001, 2, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Womelsdorf, T.; Fries, P. The role of neuronal synchronization in selective attention. Curr. Opin. Neurobiol. 2007, 17, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Kaiser, J.; Lachaux, J.P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Tallon-Baudry, C.; Bertrand, O.; Hénaff, M.A.; Isnard, J.; Fischer, C. Attention Modulates Gamma-band Oscillations Differently in the Human Lateral Occipital Cortex and Fusiform Gyrus. Cereb. Cortex. 2005, 15, 654–662. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Singh, K.D. Visual gamma oscillations: The effects of stimulus type, visual field coverage and stimulus motion on MEG and EEG recordings. NeuroImage 2013, 69, 223–230. [Google Scholar] [CrossRef]
- Schadow, J.; Lenz, D.; Dettler, N.; Fründ, I.; Herrmann, C.S. Early gamma-band responses reflect anticipatory top-down modulation in the auditory cortex. NeuroImage 2009, 47, 651–658. [Google Scholar] [CrossRef]
- Debener, S.; Herrmann, C.S.; Kranczioch, C.; Gembris, D.; Engel, A.K. Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport 2003, 14, 683–686. [Google Scholar] [CrossRef]
- Bauer, M.; Oostenveld, R.; Peeters, M.; Fries, P. Tactile Spatial Attention Enhances Gamma-Band Activity in Somatosensory Cortex and Reduces Low-Frequency Activity in Parieto-Occipital Areas. J. Neurosci. 2006, 26, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Brovelli, A.; Lachaux, J.P.; Kahane, P.; Boussaoud, D. High gamma frequency oscillatory activity dissociates attention from intention in the human premotor cortex. NeuroImage 2005, 28, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Medendorp, W.P.; Kramer, G.F.I.; Jensen, O.; Oostenveld, R.; Schoffelen, J.M.; Fries, P. Oscillatory Activity in Human Parietal and Occipital Cortex Shows Hemispheric Lateralization and Memory Effects in a Delayed Double-Step Saccade Task. Cereb. Cortex 2007, 17, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Van Der Werf, J.; Jensen, O.; Fries, P.; Medendorp, W.P. Neuronal Synchronization in Human Posterior Parietal Cortex during Reach Planning. J. Neurosci. 2010, 30, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- ElShafei, H.A.; Fornoni, L.; Masson, R.; Bertrand, O.; Bidet-Caulet, A. Age-related modulations of alpha and gamma brain activities underlying anticipation and distraction. PLoS ONE 2020, 15, e0229334. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Müller, M.L.T.M.; Bohnen, N.I.; Sarter, M.; Lustig, C. Thalamic cholinergic innervation makes a specific bottom-up contribution to signal detection: Evidence from Parkinson’s disease patients with defined cholinergic losses. NeuroImage 2017, 149, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Seeburger, D.T.; Xu, N.; Ma, M.; Larson, S.; Godwin, C.; Keilholz, S.D.; Schumacher, E.H. Time-varying functional connectivity predicts fluctuations in sustained attention in a serial tapping task. Cogn. Affect. Behav. Neurosci. 2024, 24, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Hallenbeck, G.E.; Sprague, T.C.; Rahmati, M.; Sreenivasan, K.K.; Curtis, C.E. Working memory representations in visual cortex mediate distraction effects. Nat. Commun. 2021, 12, 4714. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, R.L.; Chunharas, C.; Serences, J.T. Coexisting representations of sensory and mnemonic information in human visual cortex. Nat. Neurosci. 2019, 22, 1336–1344. [Google Scholar] [CrossRef]
- Bettencourt, K.C.; Xu, Y. Decoding the content of visual short-term memory under distraction in occipital and parietal areas. Nat. Neurosci. 2016, 19, 150–157. [Google Scholar] [CrossRef]
- Touroutoglou, A.; Andreano, J.; Dickerson, B.C.; Barrett, L.F. The tenacious brain: How the anterior mid-cingulate contributes to achieving goals. Cortex 2020, 123, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Frank, M.J. Frontal theta as a mechanism for cognitive control. Trends Cogn. Sci. 2014, 18, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex: One decade on. Trends Cogn. Sci. 2014, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Banich, M.T.; Depue, B.E. Recent advances in understanding neural systems that support inhibitory control. Curr. Opin. Behav. Sci. 2015, 1, 17–22. [Google Scholar] [CrossRef]
- Chatham, C.H.; Claus, E.D.; Kim, A.; Curran, T.; Banich, M.T.; Munakata, Y. Cognitive Control Reflects Context Monitoring, Not Motoric Stopping, in Response Inhibition. PLoS ONE 2012, 7, e31546. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Chamberlain, S.R.; Monti, M.M.; Duncan, J.; Owen, A.M. The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage 2010, 50, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Goard, M.; Dan, Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci. 2009, 12, 1444–1449. [Google Scholar] [CrossRef]
- Pinto, L.; Goard, M.J.; Estandian, D.; Xu, M.; Kwan, A.C.; Lee, S.-H.; Harrison, T.C.; Feng, G.; Dan, Y. Fast modulation of visual perception by basal forebrain cholinergic neurons. Nat. Neurosci. 2013, 16, 1857–1863. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Luo, M. Optogenetic Activation of Basal Forebrain Cholinergic Neurons Modulates Neuronal Excitability and Sensory Responses in the Main Olfactory Bulb. J. Neurosci. 2012, 32, 10105–10116. [Google Scholar] [CrossRef] [PubMed]
- Broussard, J.I.; Karelina, K.; Sarter, M.; Givens, B. Cholinergic optimization of cue-evoked parietal activity during challenged attentional performance. Eur. J. Neurosci. 2009, 29, 1711–1722. [Google Scholar] [CrossRef]
- Sarter, M.; Givens, B.; Bruno, J.P. The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Res. Rev. 2001, 35, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gritton, H.; Sarter, M.; Aton, S.J.; Booth, V.; Zochowski, M. Theta-gamma coupling emerges from spatially heterogeneous cholinergic neuromodulation. PLoS Comput. Biol. 2021, 17, e1009235. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Lutzenberger, W. Induced gamma-band activity and human brain function. Neuroscientist 2003, 9, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Lutzenberger, W. Human gamma-band activity: A window to cognitive processing. Neuroreport 2005, 16, 207–211. [Google Scholar] [CrossRef]
- Deco, G.; Thiele, A. Attention: Oscillations and neuropharmacology. Eur. J. Neurosci. 2009, 30, 347–354. [Google Scholar] [CrossRef]




| SAT | dSAT | |||
|---|---|---|---|---|
| m | SD | m | SD | |
| hit rate | 0.73 | 0.12 | 0.80 | 0.14 |
| hit response time (ms) | 326.01 | 54.13 | 374.22 | 61.20 |
| correct rejection rate | 0.96 | 0.03 | 0.86 | 0.09 |
| correct rejection response time (ms) | 505.72 | 61.00 | 542.53 | 62.64 |
| d’ | 2.57 | 0.70 | 2.20 | 0.96 |
| beta | 6.00 | 4.64 | 1.34 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, T.; Kim, K.; Gehring, W.J.; Lustig, C.; Bohnen, N.I. Sensitivity to and Control of Distraction: Distractor-Entrained Oscillation and Frontoparietal EEG Gamma Synchronization. Brain Sci. 2024, 14, 609. https://doi.org/10.3390/brainsci14060609
Brown T, Kim K, Gehring WJ, Lustig C, Bohnen NI. Sensitivity to and Control of Distraction: Distractor-Entrained Oscillation and Frontoparietal EEG Gamma Synchronization. Brain Sciences. 2024; 14(6):609. https://doi.org/10.3390/brainsci14060609
Chicago/Turabian StyleBrown, Taylor, Kamin Kim, William J. Gehring, Cindy Lustig, and Nicolaas I. Bohnen. 2024. "Sensitivity to and Control of Distraction: Distractor-Entrained Oscillation and Frontoparietal EEG Gamma Synchronization" Brain Sciences 14, no. 6: 609. https://doi.org/10.3390/brainsci14060609
APA StyleBrown, T., Kim, K., Gehring, W. J., Lustig, C., & Bohnen, N. I. (2024). Sensitivity to and Control of Distraction: Distractor-Entrained Oscillation and Frontoparietal EEG Gamma Synchronization. Brain Sciences, 14(6), 609. https://doi.org/10.3390/brainsci14060609






