Functional Networks of Reward and Punishment Processing and Their Molecular Profiles Predicting the Severity of Young Adult Drinking
Abstract
1. Introduction
1.1. Reward and Punishment Processing in Alcohol Misuse
1.2. Functional Connectomics of Individual Traits and Neuropsychiatric Diagnoses
1.3. Molecular Profiles of Functional Brain Networks
1.4. The Present Study
2. Materials and Methods
2.1. Dataset and Demographics
2.2. Clinical Measures
2.3. Neuroimaging Data Acquisition
2.4. Functional Connectivity and Connectome-Based Predictive Modeling (CPM)
2.5. Correlation with Neurotransmitters
3. Results
3.1. Drinking Severity PC1 Related Regional Activations
3.2. Predicting Drinking Severity PC1: Loss Processing
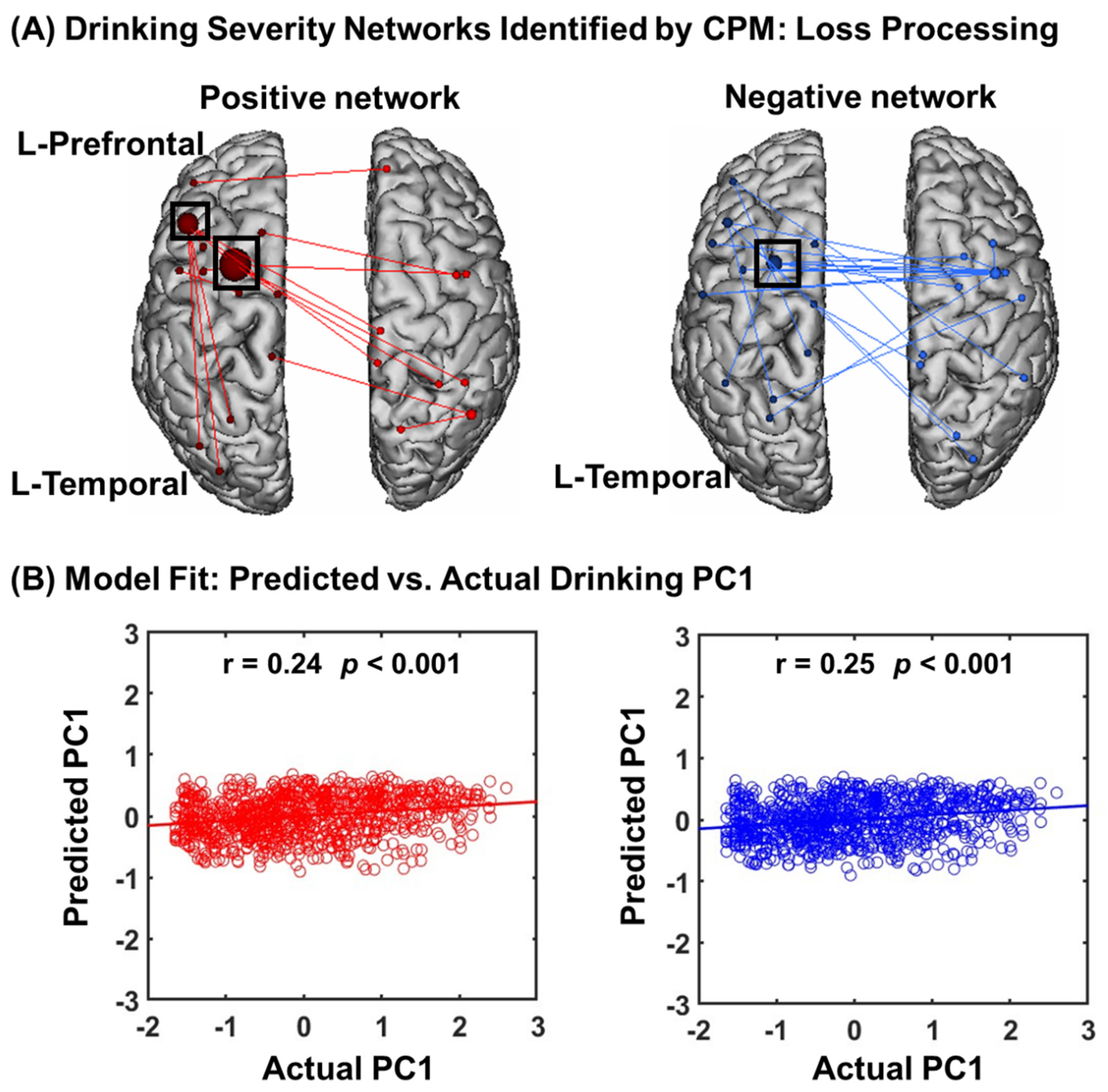
3.2.1. CPM of Loss Processing
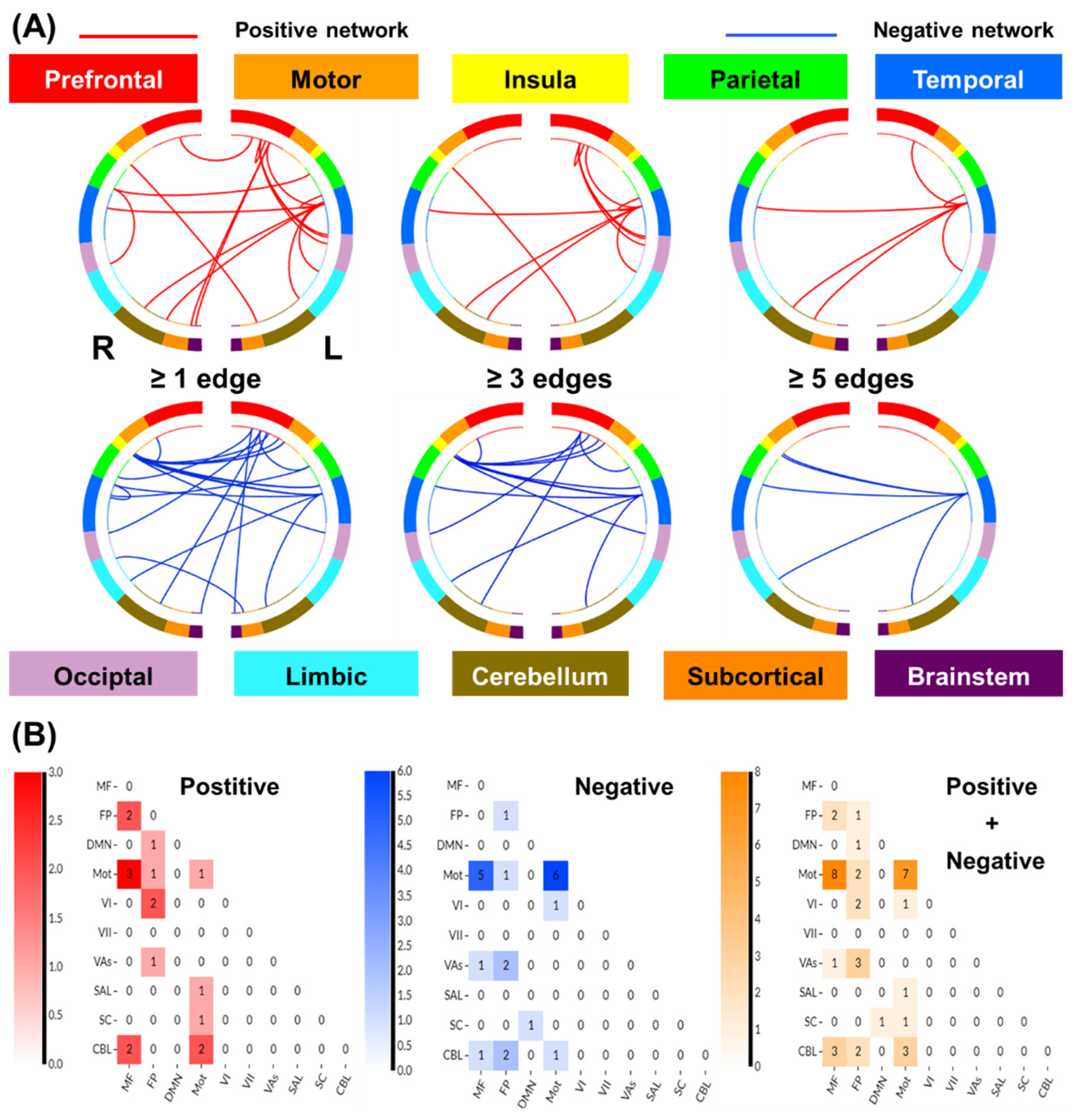
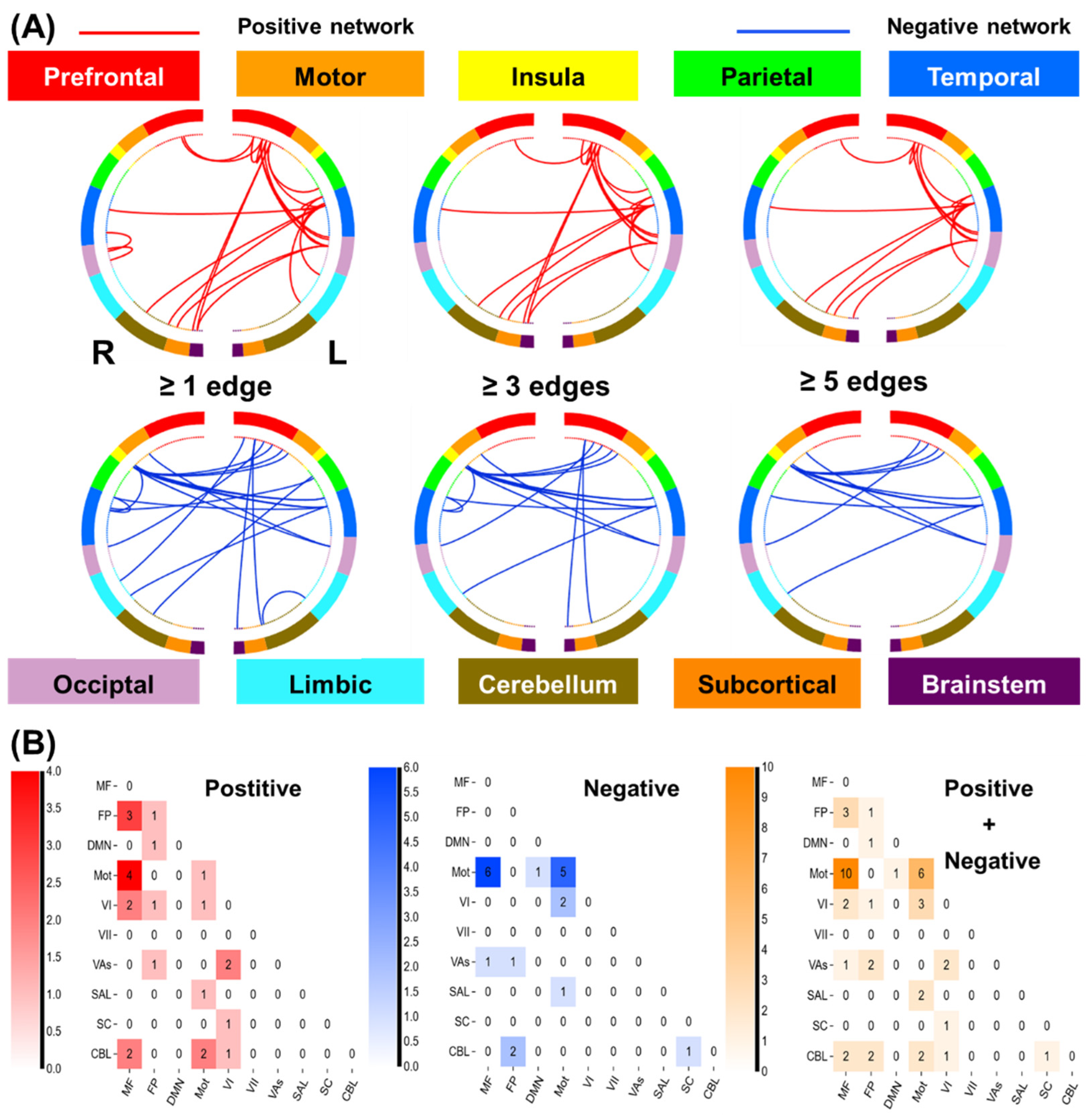
3.2.2. Network Anatomy of Loss Processing
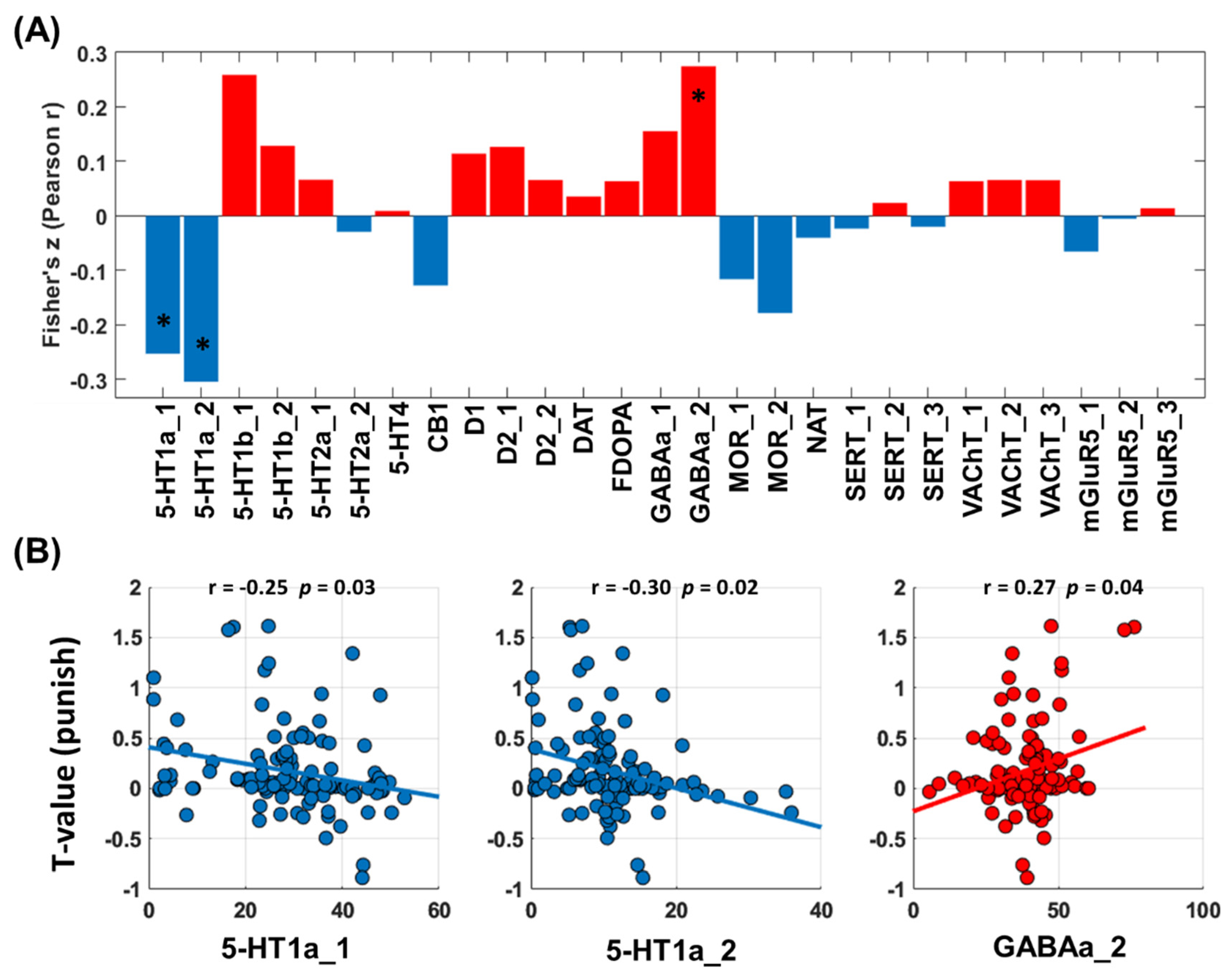
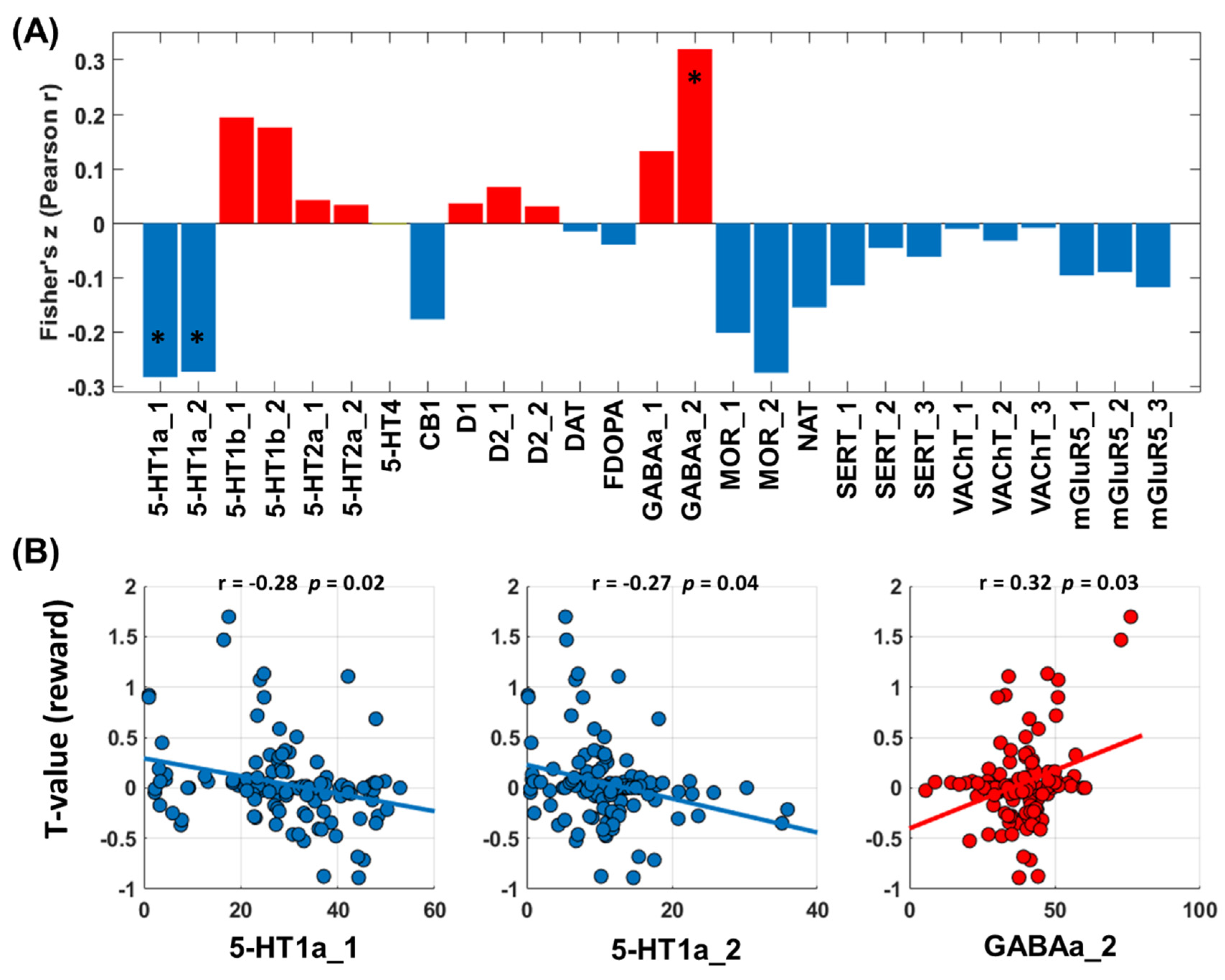
3.2.3. Neurotransmitters Associated with Network Predictors of Alcohol Use Severity: Loss Processing
3.3. Predicting Drinking Severity: Win Processing
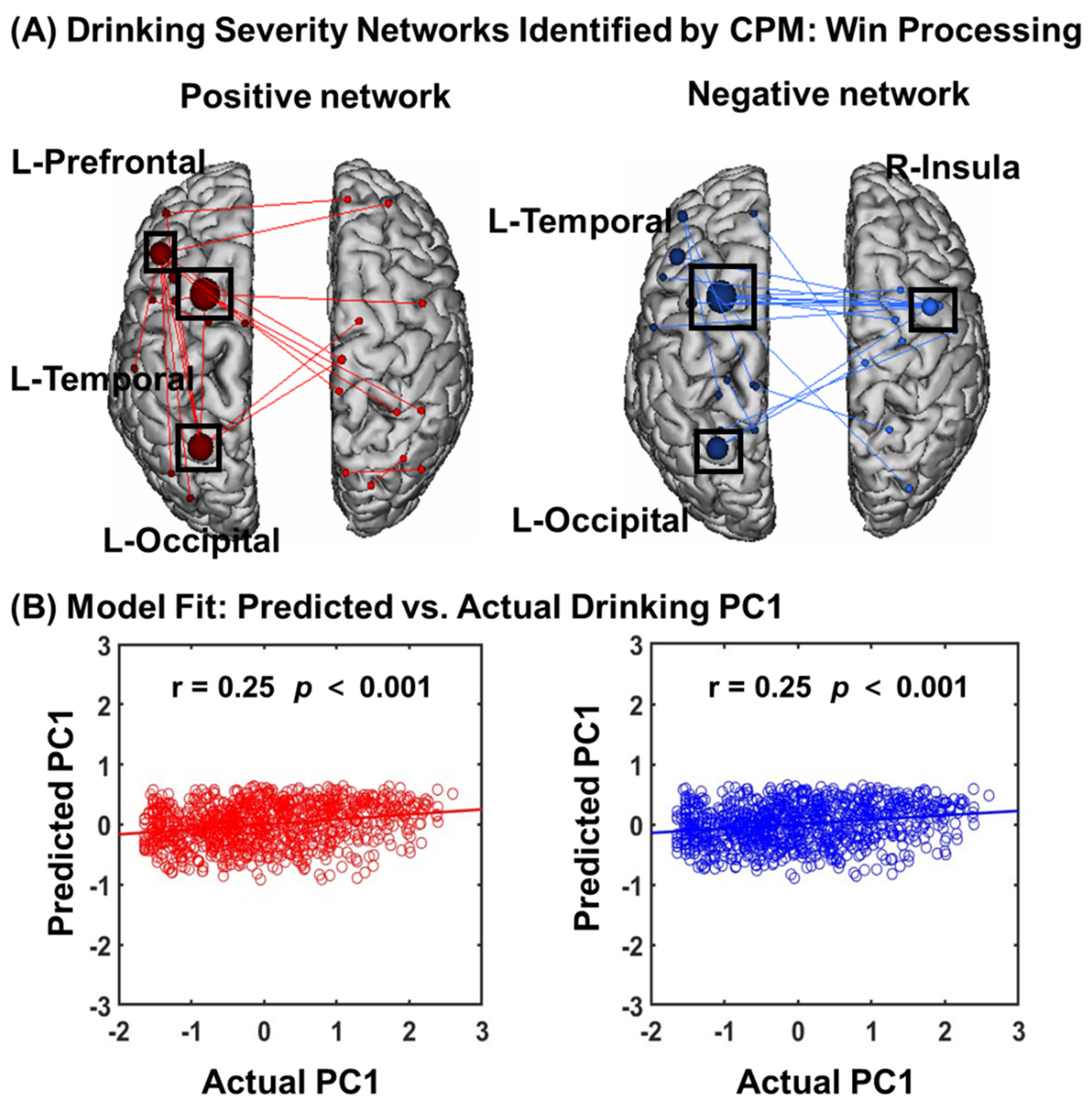
3.3.1. CPM of Win Processing
3.3.2. Network Anatomy of Win Processing
3.3.3. Neurotransmitters Associated with Network Predictors of Alcohol Use Severity: Win Processing
4. Discussion
4.1. Connectivity Features That Predict Drinking Severity
4.2. Molecular Profiles of the Connectivity Networks
4.3. Speculation on Research and Clinical Implications
4.4. Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, C.-S.R. Appetitive and aversive cue reactivities differentiate neural subtypes of alcohol drinkers. Addict. Neurosci. 2023, 7, 100089. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.E.; Chiu, P.H.; Deater-Deckard, K.; Hochgraf, A.K.; King-Casas, B.; Kim-Spoon, J. The Interaction Between Punishment Sensitivity and Effortful Control for Emerging Adults’ Substance Use Behaviors. Subst. Use Misuse 2018, 53, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Moreno Padilla, M.; O’Halloran, L.; Bennett, M.; Cao, Z.; Whelan, R. Impulsivity and Reward Processing Endophenotypes in Youth Alcohol Misuse. Curr. Addict. Rep. 2017, 4, 350–363. [Google Scholar] [CrossRef]
- Cheng, W.; Rolls, E.T.; Robbins, T.W.; Gong, W.; Liu, Z.; Lv, W.; Du, J.; Wen, H.; Ma, L.; Quinlan, E.B.; et al. Decreased brain connectivity in smoking contrasts with increased connectivity in drinking. Elife 2019, 8, e40765. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Y.; Chaudhary, S.; Tang, X.; Li, C.-S.R. Loss and Frontal Striatal Reactivities Characterize Alcohol Use Severity and Rule-Breaking Behavior in Young Adult Drinkers. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Le, T.M.; Zhornitsky, S.; Wang, W.; Ide, J.; Zhang, S.; Li, C.R. Posterior Cingulate Cortical Response to Active Avoidance Mediates the Relationship between Punishment Sensitivity and Problem Drinking. J. Neurosci. 2019, 39, 6354–6364. [Google Scholar] [CrossRef] [PubMed]
- Finn, E.S.; Shen, X.; Scheinost, D.; Rosenberg, M.D.; Huang, J.; Chun, M.M.; Papademetris, X.; Constable, R.T. Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Finn, E.S.; Scheinost, D.; Rosenberg, M.D.; Chun, M.M.; Papademetris, X.; Constable, R.T. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat. Protoc. 2017, 12, 506–518. [Google Scholar] [CrossRef]
- Feng, C.; Yuan, J.; Geng, H.; Gu, R.; Zhou, H.; Wu, X.; Luo, Y. Individualized prediction of trait narcissism from whole-brain resting-state functional connectivity. Hum. Brain Mapp. 2018, 39, 3701–3712. [Google Scholar] [CrossRef]
- Rosenberg, M.D.; Finn, E.S.; Scheinost, D.; Papademetris, X.; Shen, X.; Constable, R.T.; Chun, M.M. A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 2016, 19, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mummaneni, A.; Kardan, O.; Stier, A.J.; Chamberlain, T.A.; Chao, A.F.; Berman, M.G.; Rosenberg, M.D. Functional brain connectivity predicts sleep duration in youth and adults. Hum. Brain Mapp. 2023, 44, 6293–6307. [Google Scholar] [CrossRef] [PubMed]
- Beaty, R.E.; Kenett, Y.N.; Christensen, A.P.; Rosenberg, M.D.; Benedek, M.; Chen, Q.; Fink, A.; Qiu, J.; Kwapil, T.R.; Kane, M.J.; et al. Robust prediction of individual creative ability from brain functional connectivity. Proc. Natl. Acad. Sci. USA 2018, 115, 1087–1092. [Google Scholar] [CrossRef]
- Antons, S.; Yip, S.W.; Lacadie, C.M.; Dadashkarimi, J.; Scheinost, D.; Brand, M.; Potenza, M.N. Connectome-based prediction of craving in gambling disorder and cocaine use disorder. Dialogues Clin. Neuro 2023, 25, 33–42. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, C.; Guan, X.; Bai, X.; Guo, T.; Wu, J.; Chen, J.; Wen, J.; Wu, C.; Cao, Z.; et al. Functional connectomes of akinetic-rigid and tremor within drug-naïve Parkinson’s disease. CNS Neurosci. Ther. 2023, 29, 3507–3517. [Google Scholar] [CrossRef]
- Tong, T.T.; Vaidya, J.G.; Kramer, J.R.; Kuperman, S.; Langbehn, D.R.; O’Leary, D.S. Impact of binge drinking during college on resting state functional connectivity. Drug Alcohol. Depend. 2021, 227, 108935. [Google Scholar] [CrossRef]
- Dukart, J.; Holiga, S.; Rullmann, M.; Lanzenberger, R.; Hawkins, P.C.T.; Mehta, M.A.; Hesse, S.; Barthel, H.; Sabri, O.; Jech, R.; et al. JuSpace: A tool for spatial correlation analyses of magnetic resonance imaging data with nuclear imaging derived neurotransmitter maps. Hum. Brain Mapp. 2021, 42, 555–566. [Google Scholar] [CrossRef]
- Premi, E.; Dukart, J.; Mattioli, I.; Libri, I.; Pengo, M.; Gadola, Y.; Cotelli, M.; Manenti, R.; Binetti, G.; Gazzina, S.; et al. Unravelling neurotransmitters impairment in primary progressive aphasias. Hum. Brain Mapp. 2023, 44, 2245–2253. [Google Scholar] [CrossRef] [PubMed]
- Pengo, M.; Mattioli, I.; Cantoni, V.; Dukart, J.; Gasparotti, R.; Buratti, E.; Todd, E.G.; Bouzigues, A.; Cash, D.M.; Russell, L.L.; et al. Early neurotransmitters changes in prodromal frontotemporal dementia: A GENFI study. Neurobiol. Dis. 2023, 179, 106068. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Xue, K.; Han, S.; Wang, C.; Wen, B.; Cheng, J. The interaction between first-episode drug-naïve schizophrenia and age based on gray matter volume and its molecular analysis: A multimodal magnetic resonance imaging study. Psychopharmacology 2023, 240, 813–826. [Google Scholar] [CrossRef]
- Ren, J.; Yan, L.; Zhou, H.; Pan, C.; Xue, C.; Wu, J.; Liu, W. Unraveling neurotransmitter changes in de novo GBA-related and idiopathic Parkinson’s disease. Neurobiol. Dis. 2023, 185, 106254. [Google Scholar] [CrossRef] [PubMed]
- Fiore, A.; Preziosa, P.; Tedone, N.; Margoni, M.; Vizzino, C.; Mistri, D.; Gueye, M.; Rocca, M.A.; Filippi, M. Correspondence among gray matter atrophy and atlas-based neurotransmitter maps is clinically relevant in multiple sclerosis. Mol. Psychiatry 2023, 28, 1770–1782. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Jiang, P.; Cheng, Y.; Cai, H.; Zhu, J.; Yu, Y. Molecular mechanisms underlying resting-state brain functional correlates of behavioral inhibition. Neuroimage 2023, 283, 120415. [Google Scholar] [CrossRef] [PubMed]
- Hirjak, D.; Schmitgen, M.M.; Werler, F.; Wittemann, M.; Kubera, K.M.; Wolf, N.D.; Sambataro, F.; Calhoun, V.D.; Reith, W.; Wolf, R.C. Multimodal MRI data fusion reveals distinct structural, functional and neurochemical correlates of heavy cannabis use. Addict. Biol. 2022, 27, e13113. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Ren, P.; Ma, K.; Li, S.; Wang, X.; Guan, Y.; Zhou, J.; Li, T.; Liang, X.; Luan, G. The correspondence between morphometric MRI and metabolic profile in Rasmussen’s encephalitis. Neuroimage-Clin. 2022, 33, 102918. [Google Scholar] [CrossRef] [PubMed]
- Dugre, J.R.; Potvin, S. The origins of evil: From lesions to the functional architecture of the antisocial brain. Front. Psychiatry 2022, 13, 969206. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, D.C.; Ugurbil, K.; Auerbach, E.; Barch, D.; Behrens, T.E.J.; Bucholz, R.; Chang, A.; Chen, L.; Corbetta, M.; Curtiss, S.W.; et al. The Human Connectome Project: A data acquisition perspective. NeuroImage 2012, 62, 2222–2231. [Google Scholar] [CrossRef]
- Ide, J.S.; Li, H.T.; Chen, Y.; Le, T.M.; Li, C.S.P.; Zhornitsky, S.; Li, C.R. Gray matter volumetric correlates of behavioral activation and inhibition system traits in children: An exploratory voxel-based morphometry study of the ABCD project data. Neuroimage 2020, 220, 117085. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, S.; Le, T.M.; Tang, X.; Li, C.-S.R. Neural Responses to Reward in a Gambling Task: Sex Differences and Individual Variation in Reward-Driven Impulsivity. Cereb. Cortex Commun. 2020, 1, tgaa025. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.; Wang, W.; Dhingra, I.; Zhornitsky, S.; Tang, X.; Li, C.-S.R. Sex Differences in Neural Responses to the Perception of Social Interactions. Front. Hum. Neurosci. 2020, 14, 565132. [Google Scholar] [CrossRef]
- Li, G.; Le, T.M.; Wang, W.; Zhornitsky, S.; Chen, Y.; Chaudhary, S.; Zhu, T.; Zhang, S.; Bi, J.; Tang, X.; et al. Perceived stress, self-efficacy, and the cerebral morphometric markers in binge-drinking young adults. NeuroImage Clin. 2021, 32, 102866. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Y.; Le, T.M.; Zhornitsky, S.; Wang, W.; Dhingra, I.; Zhang, S.; Tang, X.; Li, C.-S.R. Perceived friendship and binge drinking in young adults: A study of the Human Connectome Project data. Drug Alcohol. Depend. 2021, 224, 108731. [Google Scholar] [CrossRef]
- Barch, D.M.; Burgess, G.C.; Harms, M.P.; Petersen, S.E.; Schlaggar, B.L.; Corbetta, M.; Glasser, M.F.; Curtiss, S.; Dixit, S.; Feldt, C.; et al. Function in the human connectome: Task-fMRI and individual differences in behavior. Neuroimage 2013, 80, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Zhang, Z.; Chen, Y.; Li, B.; Hao, D.; Yang, L.; Yang, Y.; Li, X.; Li, C.R. Sex differences in externalizing and internalizing traits and ventral striatal responses to monetary loss. J. Psychiatry Res. 2023, 162, 11–20. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Chen, Y.; Wang, W.; Bi, J.; Tang, X.; Li, C.-S.R. Cognitive challenges are better in distinguishing binge from nonbinge drinkers: An exploratory deep-learning study of fMRI data of multiple behavioral tasks and resting state. J. Magn. Reson. Imaging 2022, 57, 856–868. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.; Chaudhary, S.; Li, C.S.; Hao, D.; Yang, L.; Li, C.R. Sleep dysfunction mediates the relationship between hypothalamic-insula connectivity and anxiety-depression symptom severity bidirectionally in young adults. Neuroimage 2023, 279, 120340. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.; Tang, X.; Li, C.-S.R. Alcohol use severity and the neural correlates of the effects of sleep disturbance on sustained visual attention. J. Psychiatry Res. 2021, 142, 302–311. [Google Scholar] [CrossRef]
- Li, G.; Zhong, D.; Li, B.; Chen, Y.; Yang, L.; Li, C.R. Sleep Deficits Inter-Link Lower Basal Forebrain-Posterior Cingulate Connectivity and Perceived Stress and Anxiety Bidirectionally in Young Men. Int. J. Neuropsychopharmacol. 2023, 26, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Shen, X.; Tokoglu, F.; Papademetris, X.; Constable, R.T. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage 2013, 82, 403–415. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, S.; Chao, H.H.; Krystal, J.H.; Li, C.-S.R. Association of Drinking Problems and Duration of Alcohol Use to Inhibitory Control in Nondependent Young Adult Social Drinkers. Alcohol. Clin. Exp. Res. 2016, 40, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Hornoiu, I.L.; Lee, A.M.; Tan, H.; Nakovics, H.; Bach, P.; Mann, K.; Kiefer, F.; Sommer, W.H.; Vollstädt-Klein, S. The Role of Unawareness, Volition, and Neural Hyperconnectivity in Alcohol Use Disorder: A Functional Magnetic Resonance Imaging Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 660–671. [Google Scholar] [CrossRef]
- Rapuano, K.M.; Rosenberg, M.D.; Maza, M.T.; Dennis, N.J.; Dorji, M.; Greene, A.S.; Horien, C.; Scheinost, D.; Todd Constable, R.; Casey, B.J. Corrigendum to “Behavioral and brain signatures of substance use vulnerability in childhood” [Developmental Cognitive Neuroscience 46 (December) (2020) 100878]. Dev. Cogn. Neurosci. 2021, 47, 100891. [Google Scholar] [CrossRef]
- Yao, G.; Wei, L.; Jiang, T.; Dong, H.; Baeken, C.; Wu, G.R. Neural mechanisms underlying empathy during alcohol abstinence: Evidence from connectome-based predictive modeling. Brain Imaging Behav. 2022, 16, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhu, X.; Zhou, W.; Zhang, Z.; Li, P.; Dong, G.; Meng, S.; Deng, J.; Lu, L. Connectome-based predictive modelling of smoking severity in smokers. Addict. Biol. 2022, 27, e13242. [Google Scholar] [CrossRef]
- Lichenstein, S.D.; Scheinost, D.; Potenza, M.N.; Carroll, K.M.; Yip, S.W. Dissociable neural substrates of opioid and cocaine use identified via connectome-based modelling. Mol. Psychiatry 2021, 26, 4383–4393. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.W.; Scheinost, D.; Potenza, M.N.; Carroll, K.M. Connectome-Based Prediction of Cocaine Abstinence. Am. J. Psychiatry 2019, 176, 156–164. [Google Scholar] [CrossRef]
- Anton-Toro, L.F.; Shpakivska-Bilan, D.; Del Cerro-Leon, A.; Bruna, R.; Uceta, M.; Garcia-Moreno, L.M.; Maestu, F. Longitudinal change of inhibitory control functional connectivity associated with the development of heavy alcohol drinking. Front. Psychol. 2023, 14, 1069990. [Google Scholar] [CrossRef] [PubMed]
- Davies, M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J. Psychiatry Neurosci. JPN 2003, 28, 263–274. [Google Scholar]
- Maccioni, P.; Colombo, G. Potential of GABA(B) Receptor Positive Allosteric Modulators in the Treatment of Alcohol Use Disorder. CNS Drugs 2019, 33, 107–123. [Google Scholar] [CrossRef]
- Logge, W.B.; Morley, K.C.; Haber, P.S. GABA(B) Receptors and Alcohol Use Disorders: Clinical Studies. Curr. Top. Behav. Neurosci. 2022, 52, 195–212. [Google Scholar] [CrossRef]
- Andersen, K.A.A.; Carhart-Harris, R.; Nutt, D.J.; Erritzoe, D. Therapeutic effects of classic serotonergic psychedelics: A systematic review of modern-era clinical studies. Acta Psychiatry Scand. 2021, 143, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Hyytia, P. Alcohol preference and consumption are controlled by the caudal linear nucleus in alcohol-preferring rats. Eur. J. Neurosci. 2016, 43, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Ashton, M.K.; Rueda, A.V.L.; Ho, A.M.; Noor Aizin, N.; Sharma, H.; Dodd, P.R.; Stadlin, A.; Camarini, R. Sex differences in GABA(A) receptor subunit transcript expression are mediated by genotype in subjects with alcohol-related cirrhosis of the liver. Genes Brain Behav. 2022, 21, e12785. [Google Scholar] [CrossRef] [PubMed]
- Belmer, A.; Depoortere, R.; Beecher, K.; Newman-Tancredi, A.; Bartlett, S.E. Neural serotonergic circuits for controlling long-term voluntary alcohol consumption in mice. Mol. Psychiatry 2022, 27, 4599–4610. [Google Scholar] [CrossRef] [PubMed]
- Belmer, A.; Patkar, O.L.; Lanoue, V.; Bartlett, S.E. 5-HT1A receptor-dependent modulation of emotional and neurogenic deficits elicited by prolonged consumption of alcohol. Sci. Rep. 2018, 8, 2099. [Google Scholar] [CrossRef]
- McKenzie-Quirk, S.D.; Miczek, K.A. 5-HT1A agonists: Alcohol drinking in rats and squirrel monkeys. Psychopharmacology 2003, 167, 145–152. [Google Scholar] [CrossRef]
- Hillmer, A.T.; Wooten, D.W.; Tudorascu, D.L.; Barnhart, T.E.; Ahlers, E.O.; Resch, L.M.; Larson, J.A.; Converse, A.K.; Moore, C.F.; Schneider, M.L.; et al. The effects of chronic alcohol self-administration on serotonin-1A receptor binding in nonhuman primates. Drug Alcohol. Depend. 2014, 144, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zaso, M.J.; Desalu, J.M.; Park, A. Interaction between the 5-hydroxytryptamine transporter-linked polymorphic region (5-HTTLPR) and negative life events in adolescent heavy drinking. J. Stud. Alcohol. Drugs 2020, 81, 566–574. [Google Scholar] [CrossRef]
- White, A.M.; Matthews, D.B.; Best, P.J. Ethanol, memory, and hippocampal function: A review of recent findings. Hippocampus 2000, 10, 88–93. [Google Scholar] [CrossRef]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G.; Anderson, E.; Lynch, G.S.; Baudry, M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature 1986, 319, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.E.; Castellanos-Ryan, N.; Schumann, G.; Cattrell, A.; Flor, H.; Nees, F.; Banaschewski, T.; Bokde, A.; Whelan, R.; Buechel, C.; et al. Modulation of orbitofrontal-striatal reward activity by dopaminergic functional polymorphisms contributes to a predisposition to alcohol misuse in early adolescence. Psychol. Med. 2019, 49, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Ridge, J.P.; Ho, A.M.C.; Dodd, P.R. Sex Differences in NMDA Receptor Expression in Human Alcoholics. Alcohol Alcohol. 2009, 44, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Marron Fernandez de Velasco, E.; Tipps, M.E.; Haider, B.; Souders, A.; Aguado, C.; Rose, T.R.; Vo, B.N.; DeBaker, M.C.; Luján, R.; Wickman, K. Ethanol-Induced Suppression of G Protein–Gated Inwardly Rectifying K+–Dependent Signaling in the Basal Amygdala. Biol. Psychiatry 2023, 94, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Weiss, F. Neuropharmacology of Cocaine and Ethanol Dependence. Recent. Dev. Alcohol. 1992, 10, 201–233. [Google Scholar] [CrossRef] [PubMed]
- Li, T.K. Pharmacogenetics of responses to alcohol and genes that influence alcohol drinking. J. Stud. Alcohol. 2000, 61, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.A. Emerging neurochemical concepts in the actions of ethanol at ligand-gated ion channels. Behav. Pharmacol. 1994, 5, 383–404. [Google Scholar] [CrossRef] [PubMed]
- Diana, M.; Brodie, M.; Muntoni, A.; Puddu, M.C.; Pillolla, G.; Steffensen, S.; Spiga, S.; Little, H.J. Enduring Effects of Chronic Ethanol in the CNS: Basis for Alcoholism. Alcohol. Clin. Exp. Res. 2003, 27, 354–361. [Google Scholar] [CrossRef]
- Olsen, R.W. Extrasynaptic GABAA receptors in the nucleus accumbens are necessary for alcohol drinking. Proc. Natl. Acad. Sci. USA 2011, 108, 4699–4700. [Google Scholar] [CrossRef]
- Roh, S.; Matsushita, S.; Hara, S.; Maesato, H.; Matsui, T.; Suzuki, G.; Miyakawa, T.; Ramchandani, V.A.; Li, T.-K.; Higuchi, S. Role of GABRA2 in Moderating Subjective Responses to Alcohol. Alcohol. Clin. Exp. Res. 2011, 35, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Sanacora, G.; Krystal, J.H. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019, 102, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Kraus, C.; Castrén, E.; Kasper, S.; Lanzenberger, R. Serotonin and neuroplasticity–Links between molecular, functional and structural pathophysiology in depression. Neurosci. Biobehav. Rev. 2017, 77, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Akil, H.; Gordon, J.; Hen, R.; Javitch, J.; Mayberg, H.; McEwen, B.; Meaney, M.J.; Nestler, E.J. Treatment resistant depression: A multi-scale, systems biology approach. Neurosci. Biobehav. Rev. 2018, 84, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Koohsari, S.; Sadabad, F.E.; Pittman, B.; Gallezot, J.D.; Carson, R.E.; van Dyck, C.H.; Li, C.R.; Potenza, M.N.; Matuskey, D. Relationships of in vivo brain norepinephrine transporter and age, BMI, and gender. Synapse 2023, 77, e22279. [Google Scholar] [CrossRef] [PubMed]
- Angarita, G.A.; Worhunsky, P.D.; Naganawa, M.; Toyonaga, T.; Nabulsi, N.B.; Li, C.R.; Esterlis, I.; Skosnik, P.D.; Radhakrishnan, R.; Pittman, B.; et al. Lower prefrontal cortical synaptic vesicle binding in cocaine use disorder: An exploratory (11) C-UCB-J positron emission tomography study in humans. Addict. Biol. 2022, 27, e13123. [Google Scholar] [CrossRef] [PubMed]
- Li, C.S.; Potenza, M.N.; Lee, D.E.; Planeta, B.; Gallezot, J.D.; Labaree, D.; Henry, S.; Nabulsi, N.; Sinha, R.; Ding, Y.S.; et al. Decreased norepinephrine transporter availability in obesity: Positron Emission Tomography imaging with (S,S)-[(11)C]O-methylreboxetine. Neuroimage 2014, 86, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Li, H.; Cui, B.; Liang, P.; Wu, Y.; Han, X.; Li, C.R.; Li, K.; Wang, Z. Altered multimodal magnetic resonance parameters of basal nucleus of Meynert in Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2020, 7, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jia, X.; Qi, Z.; Fan, X.; Ma, T.; Ni, H.; Li, C.R.; Li, K. Altered Functional Connectivity of the Basal Nucleus of Meynert in Mild Cognitive Impairment: A Resting-State fMRI Study. Front. Aging Neurosci. 2017, 9, 127. [Google Scholar] [CrossRef]
- Wang, W.; Zhornitsky, S.; Zhang, S.; Li, C.R. Noradrenergic correlates of chronic cocaine craving: Neuromelanin and functional brain imaging. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2021, 46, 851–859. [Google Scholar] [CrossRef]
- Peterson, A.C.; Zhang, S.; Hu, S.; Chao, H.H.; Li, C.R. The Effects of Age, from Young to Middle Adulthood, and Gender on Resting State Functional Connectivity of the Dopaminergic Midbrain. Front. Hum. Neurosci. 2017, 11, 52. [Google Scholar] [CrossRef]
- Zalesky, A.; Fornito, A.; Bullmore, E.T. Network-based statistic: Identifying differences in brain networks. NeuroImage 2010, 53, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, H.; Shi, F.; Shen, D. Hierarchical Graph Convolutional Network Built by Multiscale Atlases for Brain Disorder Diagnosis Using Functional Connectivity. IEEE Trans. Neural Networks Learn. Syst. 2023, 1–13. [Google Scholar] [CrossRef]
- Zhou, W.R.; Wang, Y.M.; Wang, M.; Wang, Z.L.; Zheng, H.; Wang, M.J.; Potenza, M.N.; Dong, G.H. Connectome-based prediction of craving for gaming in internet gaming disorder. Addict. Biol. 2022, 27, e13076. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.L.; Thornton, L.K.; Hiles, S.; Hides, L.; Lubman, D.I. Psychological interventions for alcohol misuse among people with co-occurring depression or anxiety disorders: A systematic review. J. Affect. Disord. 2012, 139, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Debell, F.; Fear, N.T.; Head, M.; Batt-Rawden, S.; Greenberg, N.; Wessely, S.; Goodwin, L. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc. Psychiatry Psychiatry Epidemiol. 2014, 49, 1401–1425. [Google Scholar] [CrossRef]
- Gazula, H.; Rootes-Murdy, K.; Holla, B.; Basodi, S.; Zhang, Z.; Verner, E.; Kelly, R.; Murthy, P.; Chakrabarti, A.; Basu, D.; et al. Federated Analysis in COINSTAC Reveals Functional Network Connectivity and Spectral Links to Smoking and Alcohol Consumption in Nearly 2000 Adolescent Brains. Neuroinform 2023, 21, 287–301. [Google Scholar] [CrossRef]
- Galinowski, A.; Miranda, R.; Lemaitre, H.; Artiges, E.; Paillère Martinot, M.L.; Filippi, I.; Penttilä, J.; Grimmer, Y.; Noort, B.M.; Stringaris, A.; et al. Heavy drinking in adolescents is associated with change in brainstem microstructure and reward sensitivity. Addict. Biol. 2020, 25, e12781. [Google Scholar] [CrossRef]
- Harper, J.; Malone, S.M.; Wilson, S.; Hunt, R.H.; Thomas, K.M.; Iacono, W.G. The Effects of Alcohol and Cannabis Use on the Cortical Thickness of Cognitive Control and Salience Brain Networks in Emerging Adulthood: A Co-twin Control Study. Biol. Psychiatry 2021, 89, 1012–1022. [Google Scholar] [CrossRef]
- Logtenberg, E.; Overbeek, M.F.; Pasman, J.A.; Abdellaoui, A.; Luijten, M.; van Holst, R.J.; Vink, J.M.; Denys, D.; Medland, S.E.; Verweij, K.J.H.; et al. Investigating the causal nature of the relationship of subcortical brain volume with smoking and alcohol use. Br. J. Psychiatry 2022, 221, 377–385. [Google Scholar] [CrossRef]
- Rane, R.P.; de Man, E.F.; Kim, J.; Goergen, K.; Tschorn, M.; Rapp, M.A.; Banaschewski, T.; Bokde, A.L.W.; Desrivieres, S.; Flor, H.; et al. Structural differences in adolescent brainscan predict alcohol misuse. Elife 2022, 11, e77545. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhornitsky, S.; Le, T.M.; Zhang, S.; Li, C.-S.R. Heart Rate Variability, Cue-Evoked Ventromedial Prefrontal Cortical Response, and Problem Alcohol Use in Adult Drinkers. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhornitsky, S.; Le, T.M.; Li, C.R. Hypothalamic Responses to Cocaine and Food Cues in Individuals with Cocaine Dependence. Int. J. Neuropsychopharmacol. 2019, 22, 754–764. [Google Scholar] [CrossRef]
- Zhornitsky, S.; Zhang, S.; Ide, J.S.; Chao, H.H.; Wang, W.; Le, T.M.; Leeman, R.F.; Bi, J.; Krystal, J.H.; Li, C.R. Alcohol Expectancy and Cerebral Responses to Cue-Elicited Craving in Adult Nondependent Drinkers. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 493–504. [Google Scholar] [CrossRef] [PubMed]

| Region | Cluster Size | Peak Voxel (T) | Cluster FWE p-Value | MNI Coordinates (mm) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| “reward-baseline” | ||||||
| Calcarine | 228 | 4.20 | 0.000 | 4 | −64 | 14 |
| “punishment-baseline” | ||||||
| L lingual | 138 | 4.83 | 0.001 | −16 | −80 | −8 |
| R angular | 76 | 4.81 | 0.031 | 44 | −52 | 36 |
| R frontal middle cortex | 69 | 4.77 | 0.046 | 24 | 38 | 26 |
| R calcarine | 77 | 4.40 | 0.029 | 14 | −88 | 10 |
| R calcarine | 398 | 4.25 | 0.000 | 4 | −64 | 14 |
| R frontal middle cortex | 71 | 4.18 | 0.041 | 30 | 24 | 32 |
| “reward-punishment” | ||||||
| None | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, L.; Hao, D.; Chen, Y.; Ye-Lin, Y.; Li, C.-S.R.; Li, G. Functional Networks of Reward and Punishment Processing and Their Molecular Profiles Predicting the Severity of Young Adult Drinking. Brain Sci. 2024, 14, 610. https://doi.org/10.3390/brainsci14060610
Li Y, Yang L, Hao D, Chen Y, Ye-Lin Y, Li C-SR, Li G. Functional Networks of Reward and Punishment Processing and Their Molecular Profiles Predicting the Severity of Young Adult Drinking. Brain Sciences. 2024; 14(6):610. https://doi.org/10.3390/brainsci14060610
Chicago/Turabian StyleLi, Yashuang, Lin Yang, Dongmei Hao, Yu Chen, Yiyao Ye-Lin, Chiang-Shan Ray Li, and Guangfei Li. 2024. "Functional Networks of Reward and Punishment Processing and Their Molecular Profiles Predicting the Severity of Young Adult Drinking" Brain Sciences 14, no. 6: 610. https://doi.org/10.3390/brainsci14060610
APA StyleLi, Y., Yang, L., Hao, D., Chen, Y., Ye-Lin, Y., Li, C.-S. R., & Li, G. (2024). Functional Networks of Reward and Punishment Processing and Their Molecular Profiles Predicting the Severity of Young Adult Drinking. Brain Sciences, 14(6), 610. https://doi.org/10.3390/brainsci14060610






