Automated Pupillometry Is Able to Discriminate Patients with Acute Stroke from Healthy Subjects: An Observational, Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Sample
3.2. Descriptive Analysis of Pupillometric Parameters
3.3. Potential Predictors of Stroke
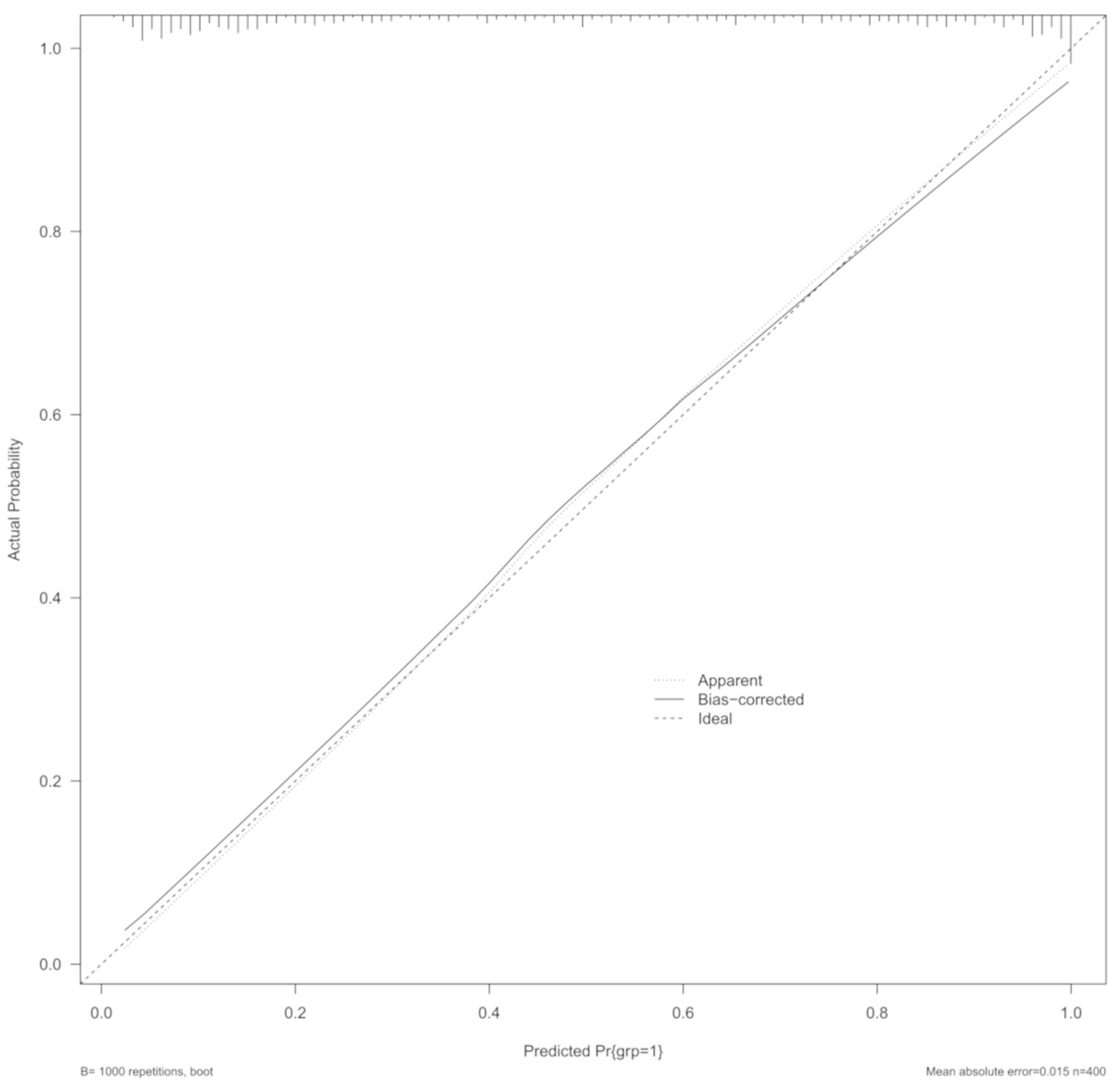
3.4. Predictive Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hultborn, H.; Mori, K.; Tsukahara, N. The neuronal pathway subserving the pupillary light reflex. Brain Res. 1978, 159, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Bouffard, M.A. The Pupil. Continuum 2019, 25, 1194–1214. [Google Scholar] [CrossRef] [PubMed]
- Keil, V.; Hepach, R.; Vierrath, S.; Caffier, D.; Tuschen-Caffier, B.; Klein, C.; Schmitz, J. Children with social anxiety disorder show blunted pupillary reactivity and altered eye contact processing in response to emotional faces: Insights from pupillometry and eye movements. J. Anxiety Disord. 2018, 58, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Mestanikova, A.; Ondrejka, I.; Mestanik, M.; Cesnekova, D.; Visnovcova, Z.; Bujnakova, I.; Oppa, M.; Calkovska, A.; Tonhajzerova, I. Pupillary light reflex is altered in adolescent depression. Physiol. Res. 2017, 66, S277–S284. [Google Scholar] [CrossRef] [PubMed]
- Strauch, C.; Wang, C.A.; Einhauser, W.; Van der Stigchel, S.; Naber, M. Pupillometry as an integrated readout of distinct attentional networks. Trends Neurosci. 2022, 45, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Sharshar, T.; Citerio, G.; Andrews, P.J.; Chieregato, A.; Latronico, N.; Menon, D.K.; Puybasset, L.; Sandroni, C.; Stevens, R.D. Neurological examination of critically ill patients: A pragmatic approach. Report of an ESICM expert panel. Intensive Care Med. 2014, 40, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Stevens, R.D.; Shoykhet, M.; Cadena, R. Emergency Neurological Life Support: Intracranial Hypertension and Herniation. Neurocrit. Care 2015, 23 (Suppl. S2), S76–S82. [Google Scholar] [CrossRef] [PubMed]
- Litvan, I.; Saposnik, G.; Maurino, J.; Gonzalez, L.; Saizar, R.; Sica, R.E.; Bartko, J.J. Pupillary diameter assessment: Need for a graded scale. Neurology 2000, 54, 530–531. [Google Scholar] [CrossRef]
- NeurOptics. NeurOptics—The Leader in the Science of Pupillometry. Available online: https://neuroptics.com (accessed on 9 May 2023).
- Luz Teixeira, T.; Peluso, L.; Banco, P.; Njimi, H.; Abi-Khalil, L.; Chanchay Pillajo, M.; Schuind, S.; Creteur, J.; Bouzat, P.; Taccone, F.S. Early Pupillometry Assessment in Traumatic Brain Injury Patients: A Retrospective Study. Brain Sci. 2021, 11, 1657. [Google Scholar] [CrossRef]
- Peluso, L.; Ferlini, L.; Talamonti, M.; Ndieugnou Djangang, N.; Gouvea Bogossian, E.; Menozzi, M.; Annoni, F.; Macchini, E.; Legros, B.; Severgnini, P.; et al. Automated Pupillometry for Prediction of Electroencephalographic Reactivity in Critically Ill Patients: A Prospective Cohort Study. Front. Neurol. 2022, 13, 867603. [Google Scholar] [CrossRef]
- Bellavia, S.; Scala, I.; Luigetti, M.; Brunetti, V.; Gabrielli, M.; Zileri Dal Verme, L.; Servidei, S.; Calabresi, P.; Frisullo, G.; Della Marca, G. Instrumental Evaluation of COVID-19 Related Dysautonomia in Non-Critically-Ill Patients: An Observational, Cross-Sectional Study. J. Clin. Med. 2021, 10, 5861. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Guglielmino, V.; Di Paolantonio, A.; Bisogni, G.; Sabatelli, M.; Della Marca, G.; Minnella, A.M.; Maceroni, M.; Bellavia, S.; Scala, I.; et al. Pupillometric findings in ATTRv patients and carriers: Results from a single-centre experience. Amyloid 2022, 29, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Scala, I.; Rizzo, P.A.; Bellavia, S.; Brunetti, V.; Colo, F.; Broccolini, A.; Della Marca, G.; Calabresi, P.; Luigetti, M.; Frisullo, G. Autonomic Dysfunction during Acute SARS-CoV-2 Infection: A Systematic Review. J. Clin. Med. 2022, 11, 3883. [Google Scholar] [CrossRef] [PubMed]
- Vespa, S.; Stumpp, L.; Liberati, G.; Delbeke, J.; Nonclercq, A.; Mouraux, A.; El Tahry, R. Characterization of vagus nerve stimulation-induced pupillary responses in epileptic patients. Brain Stimul. 2022, 15, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.Y.; Balogun, O.O.; Prescott, B.R.; Saglam, H.; Olson, D.M.; Speir, K.; Stutzman, S.E.; Schneider, N.; Aguilera, V.; Lussier, B.L.; et al. Quantitative pupillometry and radiographic markers of intracranial midline shift: A pilot study. Front. Neurol. 2022, 13, 1046548. [Google Scholar] [CrossRef] [PubMed]
- Kossel, C.S.; Kobus, F.; Borutta, M.C.; Kartner, M.; Kuramatsu, J.B.; Engelhorn, T.; Schwab, S.; Koehn, J. Pupillometry in the follow-up of patients undergoing EVT—Prediction of space-occupying hemispheric infarction. J. Neurol. 2023, 270, 4507–4517. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; Stutzman, S.E.; Atem, F.; Olson, D.; Hicks, A.D.; Ortega-Perez, S.; Aoun, S.G.; Salem, A.; Aiyagari, V. Correlation of Objective Pupillometry to Midline Shift in Acute Stroke Patients. J. Stroke Cerebrovasc. Dis. 2019, 28, 1902–1910. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, V.; Vollono, C.; Testani, E.; Pilato, F.; Della Marca, G. Autonomic Nervous System Modifications During Wakefulness and Sleep in a Cohort of Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Miglis, M.G.; Muppidi, S. Autonomic dysfunction predicts poor outcome in stroke: Updates on recent autonomic research. Clin. Auton. Res. 2018, 28, 9–11. [Google Scholar] [CrossRef]
- Larson, M.D.; Behrends, M. Portable infrared pupillometry: A review. Anesth. Analg. 2015, 120, 1242–1253. [Google Scholar] [CrossRef]
- Lussier, B.L.; Olson, D.M.; Aiyagari, V. Automated Pupillometry in Neurocritical Care: Research and Practice. Curr. Neurol. Neurosci. Rep. 2019, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Birschel, P.; Ellul, J.; Barer, D. Progressing stroke: Towards an internationally agreed definition. Cerebrovasc. Dis. 2004, 17, 242–252. [Google Scholar] [CrossRef]
- Steffen Moritz, L.F.; Nowak, G.; Welsh, A.H.; O’Neill, T.J. imputeR: A General Multivariate Imputation Framework. R Package Version 2.2. 2020. Available online: https://CRAN.R-project.org/package=imputeR (accessed on 30 May 2023).
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef]
- Bache, S.; Wickham, H. magrittr: A Forward-Pipe Operator for R. R Package Version 2.0.3. 2022. Available online: https://CRAN.R-project.org/package=magrittr (accessed on 30 May 2023).
- Frank and HarrellJr. rms: Regression Modeling Strategies. R package version 6.3-0. 2022. Available online: https://CRAN.R-project.org/package=rms (accessed on 30 May 2023).
- Sadatsafavi, M.; Safari, A. predtools: Prediction Model Tools. R package Version 0.0.2. 2021. Available online: https://CRAN.R-project.org/package=predtools (accessed on 30 May 2023).
- Steyerberg, E.W. Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Hu, L.T.; Bentler, P.M. Cutoff Criteria for Fit Indexes in Covariance Structure Analysis: Conventional Criteria versus New Alternatives. Struct. Equ. Model. A Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Chang, W.; Cheng, J.; Allaire, J.; Sievert, C.; Schloerke, B.; Xie, Y.; Allen, J.; McPherson, J.; Dipert, A.; Borges, B. shiny: Web Application Framework for R. 2023. Available online: https://github.com/rstudio/shiny (accessed on 30 May 2023).
- Dowlati, E.; Sarpong, K.; Kamande, S.; Carroll, A.H.; Murray, J.; Wiley, A.; Peterson, B.; Mai, J.C.; Chang, J.J.; Aulisi, E.F.; et al. Abnormal neurological pupil index is associated with malignant cerebral edema after mechanical thrombectomy in large vessel occlusion patients. Neurol. Sci. 2021, 42, 5139–5148. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Park, S.H.; Jeong, H.B.; Ha, E.J.; Cho, W.S.; Kang, H.S.; Kim, J.E.; Ko, S.B. Neurological Pupil Index as an Indicator of Neurological Worsening in Large Hemispheric Strokes. Neurocrit. Care 2020, 33, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Sabourdin, N.; Diarra, C.; Wolk, R.; Piat, V.; Louvet, N.; Constant, I. Pupillary Pain Index Changes After a Standardized Bolus of Alfentanil Under Sevoflurane Anesthesia: First Evaluation of a New Pupillometric Index to Assess the Level of Analgesia During General Anesthesia. Anesth. Analg. 2019, 128, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Peluso, L.; Oddo, M.; Minini, A.; Citerio, G.; Horn, J.; Di Bernardini, E.; Rundgren, M.; Cariou, A.; Payen, J.F.; Storm, C.; et al. Neurological pupil index and its association with other prognostic tools after cardiac arrest: A post hoc analysis. Resuscitation 2022, 179, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Gouvea Bogossian, E.; Blandino Ortiz, A.; Esposito, V.; Caricato, A.; Righy Shinotsuka, C.; Monleon Lopez, B.; Gianni, G.; Macchini, E.; de Pablo Sanchez, R.; Pisapia, L.; et al. Neurological Pupil Index and Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: A Retrospective Multicentric Study. Neurocrit. Care 2023, 39, 116–124. [Google Scholar] [CrossRef]
- Jahns, F.P.; Miroz, J.P.; Messerer, M.; Daniel, R.T.; Taccone, F.S.; Eckert, P.; Oddo, M. Quantitative pupillometry for the monitoring of intracranial hypertension in patients with severe traumatic brain injury. Crit. Care 2019, 23, 155. [Google Scholar] [CrossRef]
- Peinkhofer, C.; Martens, P.; Grand, J.; Truelsen, T.; Knudsen, G.M.; Kjaergaard, J.; Kondziella, D. Influence of Strategic Cortical Infarctions on Pupillary Function. Front. Neurol. 2018, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Gold, J.I. Pupil Size as a Window on Neural Substrates of Cognition. Trends Cogn. Sci. 2020, 24, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, L.A.; Miyamichi, K.; Gao, X.J.; Beier, K.T.; Weissbourd, B.; DeLoach, K.E.; Ren, J.; Ibanes, S.; Malenka, R.C.; Kremer, E.J.; et al. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 2015, 524, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Szabadi, E. Modulation of physiological reflexes by pain: Role of the locus coeruleus. Front. Integr. Neurosci. 2012, 6, 94. [Google Scholar] [CrossRef]
- Marshall, M.; Deo, R.; Childs, C.; Ali, A. Feasibility and Variability of Automated Pupillometry Among Stroke Patients and Healthy Participants: Potential Implications for Clinical Practice. J. Neurosci. Nurs. 2019, 51, 84–88. [Google Scholar] [CrossRef]

| AIS Group | HS Group | p | |
|---|---|---|---|
| (n = 200) | (n = 200) | ||
| Demographics | |||
| Age (years) | 73 (61–81) | 58 (50–67) | <0.001 |
| Sex (male) | 120 (60) | 116 (58) | 0.760 |
| Comorbidities | |||
| Diabetes | 48 (24) | 15 (7.5) | <0.001 |
| Hypertension | 156 (78) | 54 (27) | <0.001 |
| Dyslipidemia | 86 (43) | 45 (22.5) | <0.001 |
| Previous stroke | 42 (21) | 4 (2) | <0.001 |
| Atrial fibrillation | 50 (25) | 8 (4) | <0.001 |
| Cancer | 32 (16) | 28 (14) | 0.675 |
| Hepatopathy | 7 (3.5) | 7 (3.5) | 1.000 |
| Respiratory disease | 29 (14.5) | 10 (5) | 0.002 |
| Obesity | 33 (16.5) | 41 (20.5) | 0.367 |
| Pharmacological data | |||
| Beta blockers | 87 (43.5) | 22 (11) | <0.001 |
| Alpha blockers | 37 (18.5) | 9 (4.5) | <0.001 |
| ACE inhibitors | 103 (51.5) | 30 (15) | <0.001 |
| Sartans | 48 (24) | 22 (11) | 0.001 |
| Calcium channel blockers | 74 (37) | 17 (8.5) | <0.001 |
| Antidepressants | 12 (6) | 5 (2.5) | 0.135 |
| AIS Group | HS Group | p | |
|---|---|---|---|
| (n = 200) | (n = 200) | ||
| Pupillometry parameters | |||
| NPi | |||
| Overall | 4.50 (4.25–4.70) | 4.36 (4.17–4.53) | <0.001 |
| Absolute difference | 0.10 (0.10–0.30) | 0.10 (0.03–0.14) | <0.001 |
| Baseline Pupil Diameter (mm) | |||
| Overall | 3.34 (2.80–3.87) | 3.50 (3.14–3.93) | 0.028 |
| Absolute difference | 0.29 (0.12–0.53) | 0.20 (0.10–0.35) | <0.001 |
| Minimum Pupil Diameter (mm) | |||
| Overall | 2.39 (2.01–2.66) | 2.54 (2.31–2.78) | <0.001 |
| Absolute difference | 0.16 (0.07–0.31) | 0.09 (0.04–0.17) | <0.001 |
| Percentage of Constriction (%) | |||
| Overall | 29.18 (7.16) | 27.41 (5.67) | 0.006 |
| Absolute difference | 4.0 (2.0–8.0) | 2.0 (1.0–4.0) | <0.001 |
| Average Constriction Velocity (mm/s) | |||
| Overall | 1.98 (0.71) | 2.11 (0.62) | 0.003 |
| Absolute difference | 0.32 (0.15–0.58) | 0.21 (0.10–0.42) | <0.001 |
| Maximum Constriction Velocity (mm/s) | |||
| Overall | 3.03 (2.32–3.61) | 3.01 (2.50–3.65) | 0.313 |
| Absolute difference | 0.50 (0.19–0.80) | 0.28 (0.14–0.56) | <0.001 |
| Reflex Latency (s) | |||
| Overall | 0.23 (0.21–0.27) | 0.24 (0.22–0.26) | 0.493 |
| Absolute difference | 0.03 (0.00–0.04) | 0.01 (0.01–0.02) | <0.001 |
| Dilation Velocity (mm/s) | |||
| Overall | 0.91 (0.29) | 0.89 (0.23) | 0.651 |
| Absolute difference | 0.15 (0.07–0.26) | 0.10 (0.04–0.18) | <0.001 |
| OR (95%CI) | p | |
|---|---|---|
| Demographics | ||
| Age | 1.07 (1.05;1.09) | <0.001 |
| Male Sex | 1.09 (0.73; 1.62) | 0.684 |
| Comorbidities | ||
| Diabetes | 3.89 (2.10;7.23) | <0.001 |
| Hypertension | 9.59 (6.07;15.15) | <0.001 |
| Dyslipidemia | 2.60 (1.68;4.01) | <0.001 |
| Previous stroke | 13.0 (4.57;37.09) | <0.001 |
| Atrial fibrillation | 8.0 (3.68;17.39) | <0.001 |
| Cancer | 1.17 (0.67;2.03) | 0.576 |
| Hepatopathy | 1.00 (0.34;2.90) | 1.000 |
| Respiratory disease | 3.22 (1.52;6.81) | 0.002 |
| Obesity | 0.77 (0.46;1.27) | 0.304 |
| Concomitant Medications | ||
| Beta blockers | 6.23 (3.69;10.52) | <0.001 |
| Alpha blockers | 4.82 (2.26;10.28) | <0.001 |
| ACE inhibitors | 6.02 (3.73;9.69) | <0.001 |
| Sartans | 2.55 (1.48;4.42) | 0.001 |
| Calcium channel blockers | 6.32 (3.56;11.22) | <0.001 |
| Pupillometry parameters | ||
| NPi | ||
| Overall | 1.58 (0.94;2.67) | 0.082 |
| Absolute difference | 114.13 (19.77;658.66) | <0.001 |
| Baseline Pupil Diameter | ||
| Overall | 0.80 (0.61;1.06) | 0.121 |
| Absolute difference | 6.57 (2.85;15.14) | <0.001 |
| Minimum Pupil Diameter | ||
| Overall | 0.45 (0.28;0.71) | 0.001 |
| Absolute difference | 56.39 (12.07;263.43) | <0.001 |
| Percentage of Constriction | ||
| Overall | 1.04 (1.01;1.08) | 0.007 |
| Absolute difference | 1.27 (1.18;1.38) | <0.001 |
| Average Constriction Velocity | ||
| Overall | 0.64 (0.47;0.86) | 0.003 |
| Absolute difference | 4.97 (2.30;10.71) | <0.001 |
| Maximum Constriction Velocity | ||
| Overall | 0.90 (0.73;1.10) | 0.313 |
| Absolute difference | 3.51 (2.05;6.01) | <0.001 |
| Reflex Latency | ||
| Overall | 0.07 (0.00;31.38) | 0.389 |
| Absolute difference | Inf^ (Inf^;Inf^) | <0.001 |
| Dilation Velocity | ||
| Overall | 1.19 (0.57;2.49) | 0.650 |
| Absolute difference | 65.71 (10.23;421.95) | <0.001 |
| Model 3 | |
|---|---|
| OR (95%CI); p | |
| Demographics | |
| Age | 1.05 (1.03;1.08); <0.001 |
| Comorbidities | |
| Previous stroke | 9.70 (2.69;34.92); 0.001 |
| Atrial fibrillation | 3.53 (1.36;9.17); 0.010 |
| Concomitant medications | |
| ACE inhibitors | 4.34 (2.27;8.32); <0.001 |
| Sartans | 2.11 (0.98;4.53); 0.056 |
| CCBs | 2.23 (1.06;4.65); 0.033 |
| Pupillometry parameters | |
| Overall NPi | 0.37 (0.10;1.28); 0.116 |
| BPD absolute diff. | 2.39 (0.73;7.88); 0.151 |
| Overall CH | 1.21 (1.11;1.33); <0.001 |
| CH absolute diff. | 1.13 (1.01;1.26); 0.036 |
| Overall CV | 0.26 (0.11;0.61); 0.002 |
| CV absolute diff. | 4.75 (1.64;13.73); 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scala, I.; Miccoli, M.; Pafundi, P.C.; Rizzo, P.A.; Vitali, F.; Bellavia, S.; Giovanni, J.D.; Colò, F.; Marca, G.D.; Guglielmi, V.; et al. Automated Pupillometry Is Able to Discriminate Patients with Acute Stroke from Healthy Subjects: An Observational, Cross-Sectional Study. Brain Sci. 2024, 14, 616. https://doi.org/10.3390/brainsci14060616
Scala I, Miccoli M, Pafundi PC, Rizzo PA, Vitali F, Bellavia S, Giovanni JD, Colò F, Marca GD, Guglielmi V, et al. Automated Pupillometry Is Able to Discriminate Patients with Acute Stroke from Healthy Subjects: An Observational, Cross-Sectional Study. Brain Sciences. 2024; 14(6):616. https://doi.org/10.3390/brainsci14060616
Chicago/Turabian StyleScala, Irene, Massimo Miccoli, Pia Clara Pafundi, Pier Andrea Rizzo, Francesca Vitali, Simone Bellavia, Jacopo Di Giovanni, Francesca Colò, Giacomo Della Marca, Valeria Guglielmi, and et al. 2024. "Automated Pupillometry Is Able to Discriminate Patients with Acute Stroke from Healthy Subjects: An Observational, Cross-Sectional Study" Brain Sciences 14, no. 6: 616. https://doi.org/10.3390/brainsci14060616
APA StyleScala, I., Miccoli, M., Pafundi, P. C., Rizzo, P. A., Vitali, F., Bellavia, S., Giovanni, J. D., Colò, F., Marca, G. D., Guglielmi, V., Brunetti, V., Broccolini, A., Di Iorio, R., Monforte, M., Calabresi, P., & Frisullo, G. (2024). Automated Pupillometry Is Able to Discriminate Patients with Acute Stroke from Healthy Subjects: An Observational, Cross-Sectional Study. Brain Sciences, 14(6), 616. https://doi.org/10.3390/brainsci14060616






