Modeling the Interictal Epileptic State for Therapeutic Development with Tetanus Toxin
Abstract
1. Introduction
2. Interictal Spiking Has Highly Reproducible Propagation Patterns and Adversely Affects Cognition and Behavior
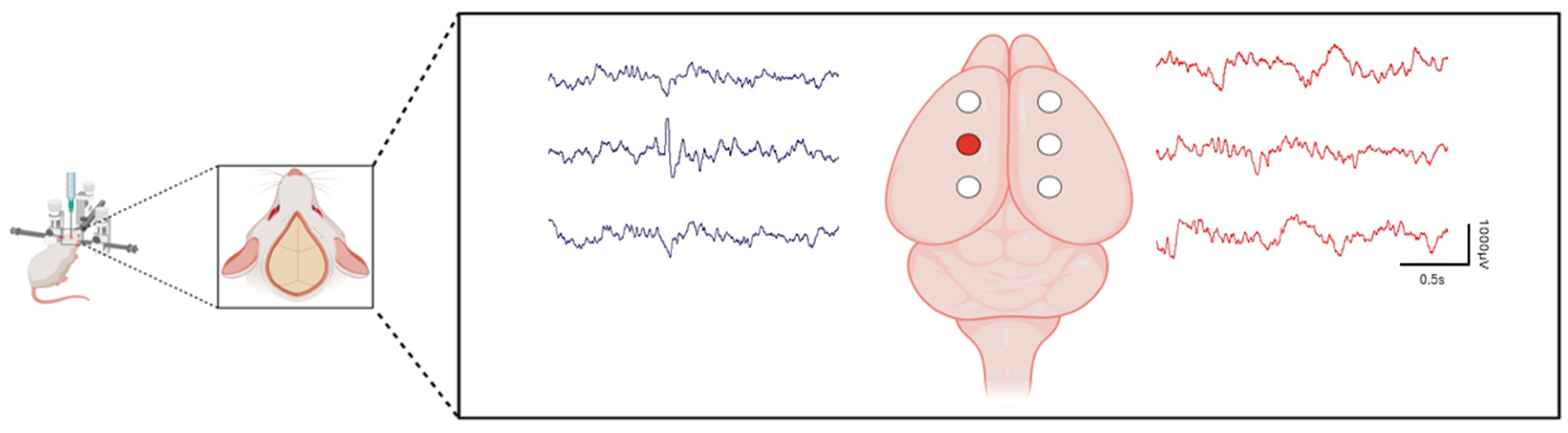
3. Tetanus Toxin: An Ideal Model to Study the Development of Focal Epilepsy and for Drug Development
4. Identification of Novel Therapeutics against Interictal Spiking from Human Epileptic Tissues
5. Testing Potential Therapeutics That Target Interictal Spiking Using the TeNT Model
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pillai, J.; Sperling, M.R. Interictal EEG and the diagnosis of epilepsy. Epilepsia 2006, 47, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Ji, T.; Liu, T.; Liu, J.; Chen, Y.; Li, Z.; Lu, N.; Li, Q. Efficacy and safety of six new antiseizure medications for adjunctive treatment of focal epilepsy and epileptic syndrome: A systematic review and network meta-analysis. Epilepsy Behav. 2024, 152, 109653. [Google Scholar] [CrossRef] [PubMed]
- Sundqvist, A. Epilepsy: A clinical diagnostic overview. Eur. J. Pain 2002, 6, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Korzeniewska, A.; Cervenka, M.C.; Jouny, C.C.; Perilla, J.R.; Harezlak, J.; Bergey, G.K.; Franaszczuk, P.J.; Crone, N.E. Ictal propagation of high frequency activity is recapitulated in interictal recordings: Effective connectivity of epileptogenic networks recorded with intracranial EEG. Neuroimage 2014, 101, 96–113. [Google Scholar] [CrossRef]
- Maharathi, B.; Wlodarski, R.; Bagla, S.; Asano, E.; Hua, J.; Patton, J.; Loeb, J.A. Interictal spike connectivity in human epileptic neocortex. Clin. Neurophysiol. 2019, 130, 270–279. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Avanzini, G. Interictal spikes in focal epileptogenesis. Prog. Neurobiol. 2001, 63, 541–567. [Google Scholar] [CrossRef] [PubMed]
- Salinsky, M.; Kanter, R.; Dasheiff, R.M. Effectiveness of multiple EEGs in supporting the diagnosis of epilepsy: An operational curve. Epilepsia 1987, 28, 331–334. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, H.K.; Lee, S.K.; Chu, K.; Chung, C.K. Extent of neocortical resection and surgical outcome of epilepsy: Intracranial EEG analysis. Epilepsia 2010, 51, 1010–1017. [Google Scholar] [CrossRef]
- Inui, K.; Motomura, E.; Okushima, R.; Kaige, H.; Inoue, K.; Nomura, J. Electroencephalographic findings in patients with DSM-IV mood disorder, schizophrenia, and other psychotic disorders. Biol. Psychiatry 1998, 43, 69–75. [Google Scholar] [CrossRef]
- Tharp, B.R. Epileptic encephalopathies and their relationship to developmental disorders: Do spikes cause autism? Ment. Retard. Dev. Disabil. Res. Rev. 2004, 10, 132–134. [Google Scholar] [CrossRef]
- Hughes, J.R. A review of the usefulness of the standard EEG in psychiatry. Clin. Electroencephalogr. 1996, 27, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Bridgers, S.L. Epileptiform abnormalities discovered on electroencephalographic screening of psychiatric inpatients. Arch. Neurol. 1987, 44, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Donnelly, J.H.; Tournay, A.E.; Book, T.M.; Filipek, P. Absence of seizures despite high prevalence of epileptiform EEG abnormalities in children with autism monitored in a tertiary care center. Epilepsia 2006, 47, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Richer, L.P.; Shevell, M.I.; Rosenblatt, B.R. Epileptiform abnormalities in children with attention-deficit-hyperactivity disorder. Pediatr. Neurol. 2002, 26, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Volnova, A.; Tsytsarev, V.; Ganina, O.; Vélez-Crespo, G.E.; Alves, J.M.; Ignashchenkova, A.; Inyushin, M. The anti-epileptic effects of carbenoxolone in vitro and in vivo. Int. J. Mol. Sci. 2022, 23, 663. [Google Scholar] [CrossRef]
- Temkin, N.R. Preventing and treating posttraumatic seizures: The human experience. Epilepsia 2009, 50, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Crunelli, V.; Lőrincz, M.L.; McCafferty, C.; Lambert, R.C.; Leresche, N.; Di Giovanni, G.; David, F. Clinical and experimental insight into pathophysiology, comorbidity and therapy of absence seizures. Brain 2020, 143, 2341–2368. [Google Scholar] [CrossRef]
- Rusina, E.; Bernard, C.; Williamson, A. The kainic acid models of temporal lobe epilepsy. Eneuro 2021, 8, ENEURO.0337-20.2021. [Google Scholar] [CrossRef]
- Lévesque, M.; Avoli, M. The kainic acid model of temporal lobe epilepsy. Neurosci. Biobehav. Rev. 2013, 37, 2887–2899. [Google Scholar] [CrossRef]
- Covolan, L.; Mello, L. Temporal profile of neuronal injury following pilocarpine or kainic acid-induced status epilepticus. Epilepsy Res. 2000, 39, 133–152. [Google Scholar] [CrossRef]
- Jefferys, J.G. Models and mechanisms of experimental epilepsies. Epilepsia 2003, 44, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Pitkänen, A.; Sutula, T.P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002, 1, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Couturier, N.H.; Durand, D.M. Comparison of fiber tract low frequency stimulation to focal and ANT stimulation in an acute rat model of focal cortical seizures. Brain Stimul. 2020, 13, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Mellanby, J.; George, G.; Robinson, A.; Thompson, P. Epileptiform syndrome in rats produced by injecting tetanus toxin into the hippocampus. J. Neurol. Neurosurg. Psychiatry 1977, 40, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Williamson, P.D.; Darcey, T.M. Chronic focal epilepsy induced by microinjection of tetanus toxin into the cat motor cortex. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Jefferys, J.; Evans, B.; Hughes, S.; Williams, S. Neuropathology of the chronic epileptic syndrome induced by intrahippocampal tetanus toxin in rat: Preservation of pyramidal cells and incidence of dark cells. Neuropathol. Appl. Neurobiol. 1992, 18, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Brace, H.; Jefferys, J.; Mellanby, J. Long-term changes in hippocampal physiology and learning ability of rats after intrahippocampal tetanus toxin. J. Physiol. 1985, 368, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, A.; Dachet, F.; Loeb, J.A. Identifying targets for preventing epilepsy using systems biology of the human brain. Neuropharmacology 2020, 168, 107757. [Google Scholar] [CrossRef]
- Dachet, F.; Bagla, S.; Keren-Aviram, G.; Morton, A.; Balan, K.; Saadat, L.; Valyi-Nagy, T.; Kupsky, W.; Song, F.; Dratz, E. Predicting novel histopathological microlesions in human epileptic brain through transcriptional clustering. Brain 2015, 138, 356–370. [Google Scholar] [CrossRef]
- Maharathi, B.; Loeb, J.A.; Patton, J. Central sulcus is a barrier to causal propagation in epileptic networks. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 2555–2559. [Google Scholar]
- Maharathi, B.; Patton, J.; Serafini, A.; Slavin, K.; Loeb, J.A. Highly consistent temporal lobe interictal spike networks revealed from foramen ovale electrodes. Clin. Neurophysiol. 2021, 132, 2065–2074. [Google Scholar] [CrossRef]
- Nicolai, J.; Trenité, D.K.-N. Interictal discharges and cognition. Epilepsy Behav. 2011, 22, 134–136. [Google Scholar] [CrossRef]
- Holmes, G.L.; Lenck-Santini, P.-P. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006, 8, 504–515. [Google Scholar] [CrossRef]
- Ung, H.; Cazares, C.; Nanivadekar, A.; Kini, L.; Wagenaar, J.; Becker, D.; Krieger, A.; Lucas, T.; Litt, B.; Davis, K.A. Interictal epileptiform activity outside the seizure onset zone impacts cognition. Brain 2017, 140, 2157–2168. [Google Scholar] [CrossRef] [PubMed]
- Nirkko, A.C.; Bernasconi, C.; von Allmen, A.; Liechti, C.; Mathis, J.; Krestel, H. Virtual car accidents of epilepsy patients, interictal epileptic activity, and medication. Epilepsia 2016, 57, 832–840. [Google Scholar] [CrossRef]
- Kleen, J.K.; Scott, R.C.; Holmes, G.L.; Roberts, D.W.; Rundle, M.M.; Testorf, M.; Lenck-Santini, P.-P.; Jobst, B.C. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology 2013, 81, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Dinkelacker, V.; Xin, X.; Baulac, M.; Samson, S.; Dupont, S. Interictal epileptic discharge correlates with global and frontal cognitive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 2016, 62, 197–203. [Google Scholar] [CrossRef]
- Meekes, J.; Jennekens-Schinkel, A. Effects of interictal epileptiform discharges on cognition. J. Pediatr. Epilepsy 2018, 7, 82–88. [Google Scholar]
- Cheng, D.; Yan, X.; Xu, K.; Zhou, X.; Chen, Q. The effect of interictal epileptiform discharges on cognitive and academic performance in children with idiopathic epilepsy. BMC Neurol. 2020, 20, 233. [Google Scholar] [CrossRef]
- Verrotti, A.; Filippini, M.; Matricardi, S.; Agostinelli, M.F.; Gobbi, G. Memory impairment and Benign Epilepsy with centrotemporal spike (BECTS): A growing suspicion. Brain Cogn. 2014, 84, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Trenité, D.K.N.; Siebelink, B.; Berends, S.; Van Strien, J.; Meinardi, H. Lateralized effects of subclinical epileptiform EEG discharges on scholastic performance in children. Epilepsia 1990, 31, 740–746. [Google Scholar] [CrossRef]
- Landi, S.; Petrucco, L.; Sicca, F.; Ratto, G.M. Transient cognitive impairment in epilepsy. Front. Mol. Neurosci. 2019, 11, 458. [Google Scholar] [CrossRef] [PubMed]
- Shatskikh, T.N.; Raghavendra, M.; Zhao, Q.; Cui, Z.; Holmes, G.L. Electrical induction of spikes in the hippocampus impairs recognition capacity and spatial memory in rats. Epilepsy Behav. 2006, 9, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, C.E. Interictal spikes: Memories forsaken. Epilepsy Curr. 2010, 10, 135–136. [Google Scholar] [CrossRef]
- Kleen, J.K.; Scott, R.C.; Holmes, G.L.; Lenck-Santini, P.P. Hippocampal interictal spikes disrupt cognition in rats. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2010, 67, 250–257. [Google Scholar] [CrossRef]
- Khan, O.I.; Zhao, Q.; Miller, F.; Holmes, G.L. Interictal spikes in developing rats cause long-standing cognitive deficits. Neurobiol. Dis. 2010, 39, 362–371. [Google Scholar] [CrossRef]
- Serafini, R.; Dettloff, S.; Loeb, J. Neocortical slices from adult chronic epileptic rats exhibit discharges of higher voltages and broader spread. Neuroscience 2016, 322, 509–524. [Google Scholar] [CrossRef]
- Geraghty, J.R.; Senador, D.; Maharathi, B.; Butler, M.P.; Sudhakar, D.; Smith, R.A.; Wu, Y.; Loeb, J.A. Modulation of locomotor behaviors by location-specific epileptic spiking and seizures. Epilepsy Behav. 2021, 114, 107652. [Google Scholar] [CrossRef]
- Smith, R.A.; Mir, F.; Butler, M.P.; Maharathi, B.; Loeb, J.A. Spike-induced cytoarchitectonic changes in epileptic human cortex are reduced via MAP2K inhibition. Brain Commun. 2024, 6, fcae152. [Google Scholar] [CrossRef]
- Barkmeier, D.; Loeb, J. An animal model to study the clinical significance of interictal spiking. Clin. EEG Neurosci. 2009, 40, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Crisp, D.N.; Cheung, W.; Gliske, S.V.; Lai, A.; Freestone, D.R.; Grayden, D.B.; Cook, M.J.; Stacey, W.C. Quantifying epileptogenesis in rats with spontaneous and responsive brain state dynamics. Brain Commun. 2020, 2, fcaa048. [Google Scholar] [CrossRef]
- Finnerty, G.; Jefferys, J. Investigation of the neuronal aggregate generating seizures in the rat tetanus toxin model of epilepsy. J. Neurophysiol. 2002, 88, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Mellanby, J.; Hawkins, C.; Mellanby, H.; Rawlins, J.; Impey, M. Tetanus toxin as a tool for studying epilepsy. J. Physiol. 1984, 79, 207–215. [Google Scholar]
- van Heyningen, S. Tetanus toxin. Pharmacol. Ther. 1980, 11, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Louis, E.D.; Williamson, P.D.; Darcey, T. Experimental models of chronic focal epilepsy: A critical review of four models. Yale J. Biol. Med. 1987, 60, 255. [Google Scholar]
- Ferecskó, A.S.; Jiruska, P.; Foss, L.; Powell, A.D.; Chang, W.-C.; Sik, A.; Jefferys, J.G. Structural and functional substrates of tetanus toxin in an animal model of temporal lobe epilepsy. Brain Struct. Funct. 2015, 220, 1013–1029. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.C.; Oliván, S.; Manzano, R.; Zaragoza, P.; Aguilera, J.; Osta, R. Fragment C of tetanus toxin: New insights into its neuronal signaling pathway. Int. J. Mol. Sci. 2012, 13, 6883–6901. [Google Scholar] [CrossRef]
- Benke, T.A.; Swann, J. The tetanus toxin model of chronic epilepsy. Recent Adv. Epilepsy Res. 2004, 548, 226–238. [Google Scholar]
- Curtis, D.; Felix, D.; Game, C.; McCulloch, R. Tetanus toxin and the synaptic release of GABA. Brain Res. 1973, 51, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Empson, R.; Amitai, Y.; Jefferys, J.; Gutnick, M. Injection of tetanus toxin into the neocortex elicits persistent epileptiform activity but only transient impairment of GABA release. Neuroscience 1993, 57, 235–239. [Google Scholar] [CrossRef]
- Carrea, R.; Lanari, A. Chronic effect of tetanus toxin applied locally to the cerebral cortex of the dog. Science 1962, 137, 342–343. [Google Scholar] [CrossRef]
- Brooks, V.; Asanuma, H. Action of tetanus toxin in the cerebral cortex. Science 1962, 137, 674–676. [Google Scholar] [CrossRef] [PubMed]
- Brener, K.; Amitai, Y.; Jefferys, J.G.; Gutnick, M.J. Chronic epileptic foci in neocortex: In vivo and in vitro effects of tetanus toxin. Eur. J. Neurosci. 1991, 3, 47–54. [Google Scholar] [CrossRef]
- Vannini, E.; Caleo, M.; Chillemi, S.; Di Garbo, A. Dynamical properties of LFPs from mice with unilateral injection of TeNT. Biosystems 2017, 161, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Mainardi, M.; Pietrasanta, M.; Vannini, E.; Rossetto, O.; Caleo, M. Tetanus neurotoxin–induced epilepsy in mouse visual cortex. Epilepsia 2012, 53, e132–e136. [Google Scholar] [CrossRef] [PubMed]
- Barkmeier, D.T.; Senador, D.; Leclercq, K.; Pai, D.; Hua, J.; Boutros, N.N.; Kaminski, R.M.; Loeb, J.A. Electrical, molecular and behavioral effects of interictal spiking in the rat. Neurobiol. Dis. 2012, 47, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, T.L.; Yao, B.; Shah, A.; Kapatos, G.; Loeb, J.A. Layer-specific CREB target gene induction in human neocortical epilepsy. J. Neurosci. 2012, 32, 14389–14401a. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, A.; Bagla, S.; Dachet, F.; Loeb, J. DUSP4 appears to be a highly localized endogenous inhibitor of epileptic signaling in human neocortex. Neurobiol. Dis. 2020, 145, 105073. [Google Scholar] [CrossRef]
- Kirchner, A.; Dachet, F.; Lipovich, L.; Loeb, J.A. Activity-dependent non-coding RNA MAPK interactome of the human epileptic brain. Non Coding RNA 2023, 9, 3. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eslami, F.; Djedovic, A.; Loeb, J.A. Modeling the Interictal Epileptic State for Therapeutic Development with Tetanus Toxin. Brain Sci. 2024, 14, 634. https://doi.org/10.3390/brainsci14070634
Eslami F, Djedovic A, Loeb JA. Modeling the Interictal Epileptic State for Therapeutic Development with Tetanus Toxin. Brain Sciences. 2024; 14(7):634. https://doi.org/10.3390/brainsci14070634
Chicago/Turabian StyleEslami, Faezeh, Arden Djedovic, and Jeffrey A. Loeb. 2024. "Modeling the Interictal Epileptic State for Therapeutic Development with Tetanus Toxin" Brain Sciences 14, no. 7: 634. https://doi.org/10.3390/brainsci14070634
APA StyleEslami, F., Djedovic, A., & Loeb, J. A. (2024). Modeling the Interictal Epileptic State for Therapeutic Development with Tetanus Toxin. Brain Sciences, 14(7), 634. https://doi.org/10.3390/brainsci14070634





