New Perspectives of Deep Brain Stimulation Indications for Parkinson’s Disease: A Critical Review
Abstract
1. Introduction
2. Methods
3. Choosing the Puzzle
4. Sorting and Grouping Pieces
4.1. Genetics
4.2. PD Phenotypes: The Role of Heterogeneity for DBS Selection
4.3. Early-Stage Indications: Is There a Haste Enemy of Perfection?
4.4. Non-Motor Symptoms Matter
4.5. Neuroimaging: How Can Neuroimaging Contribute to DBS Indications?
4.6. Comorbidities
4.7. Deep Brain Stimulation in Elderly
5. Be Patient: Is It Worth It?
5.1. Align Expectations: Patient’s and Physician’s
5.2. Patient Autonomy and Social Support
6. Conclusions: The Big Picture Perspective
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Lozano, A.M.; Lipsman, N.; Bergman, H.; Brown, P.; Chabardes, S.; Chang, J.W.; Matthews, K.; McIntyre, C.C.; Schlaepfer, T.E.; Schulder, M.; et al. Deep brain stimulation: Current challenges and future directions. Nat. Rev. Neurol. 2019, 15, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Mahlknecht, P.; Foltynie, T.; Limousin, P.; Poewe, W. How Does Deep Brain Stimulation Change the Course of Parkinson’s Disease? Mov. Disord. 2022, 37, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.J.; Fliegen, S.; Groiss, S.J.; Wojtecki, L.; Schnitzler, A. An update on best practice of deep brain stimulation in Parkinson’s disease. Ther. Adv. Neurol. Disord. 2019, 12, 175628641983809. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Butala, A.; Okun, M.S.; Mari, Z.; Mills, K.A. Global Variability in Deep Brain Stimulation Practices for Parkinson’s Disease. Front. Hum. Neurosci. 2021, 15, 667035. [Google Scholar] [CrossRef]
- Tanner, C.M. A Second Honeymoon for Parkinson’s Disease? N. Engl. J. Med. 2013, 368, 675–676. [Google Scholar] [CrossRef]
- Weaver, F.M.; Follett, K.A.; Stern, M.; Luo, P.; Harris, C.L.; Hur, K.; Marks, W.J., Jr.; Rothlind, J.; Sagher, O.; Moy, C.; et al. Randomized trial of deep brain stimulation for Parkinson disease: Thirty-six-month outcomes. Neurology 2012, 79, 55–65. [Google Scholar] [CrossRef]
- Becerra, J.E.; Zorro, O.; Ruiz-Gaviria, R.; Castañeda-Cardona, C.; Otálora-Esteban, M.; Henao, S.; Navarrete, S.; Acevedo, J.C.; Rosselli, D. Economic Analysis of Deep Brain Stimulation in Parkinson Disease: Systematic Review of the Literature. World Neurosurg. 2016, 93, 44–49. [Google Scholar] [CrossRef]
- Pietzsch, J.B.; Garner, A.M.; Marks, J.W.J. Cost-Effectiveness of Deep Brain Stimulation for Advanced Parkinson’s Disease in the United States. Neuromodulation Technol. Neural Interface 2016, 19, 689–697. [Google Scholar] [CrossRef]
- Wolke, R.; Becktepe, J.S.; Paschen, S.; Helmers, A.; Kübler-Weller, D.; Youn, J.; Brinker, D.; Bergman, H.; Kühn, A.A.; Fasano, A.; et al. The Role of Levodopa Challenge in Predicting the Outcome of Subthalamic Deep Brain Stimulation. Mov. Disord. Clin. Pract. 2023, 10, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Artusi, C.A.; Lopiano, L.; Morgante, F. Deep Brain Stimulation Selection Criteria for Parkinson’s Disease: Time to Go beyond CAPSIT-PD. J. Clin. Med. 2020, 9, 3931. [Google Scholar] [CrossRef]
- Antonini, A.; Stoessl, A.J.; Kleinman, L.S.; Skalicky, A.M.; Marshall, T.S.; Sail, K.R.; Onuk, K.; Odin, P.L.A. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multi-country Delphi-panel approach. Curr. Med. Res. Opin. 2018, 34, 2063–2073. [Google Scholar] [CrossRef] [PubMed]
- Santos-Garcia, D.; Fonticoba, T.d.D.; Castro, E.S.; Diaz, A.A.; McAfee, D. 5-2-1 Criteria: A Simple Screening Tool for Identifying Advanced PD Patients Who Need an Optimization of Parkinson’s Treatment. Park. Dis. 2020, 2020, 7537924. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.E.; Shah, B.B.; Huss, D.S.; Dallapiazza, R.F.; Warren, A.; Harrison, M.B.; Sperling, S.A.; Wang, X.-Q.; Gwinn, R.; Witt, J.; et al. Safety and Efficacy of Focused Ultrasound Thalamotomy for Patients with Medication-Refractory, Tremor-Dominant Parkinson Disease. JAMA Neurol. 2017, 74, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, W.A.; Pusso, A.; Sperling, S.A.; Flanigan, J.L.; Huss, D.S.; Harrison, M.B.; Elias, W.J.; Shah, B.B.; Barrett, M.J. Comparison of Parkinson’s Disease Patients’ Characteristics by Indication for Deep Brain Stimulation: Men Are More Likely to Have DBS for Tremor. Tremor and Other Hyperkinetic Movements. Available online: https://tremorjournal.org/article/10.5334/tohm.468/ (accessed on 21 September 2023).
- Artusi, C.A.; Rinaldi, D.; Balestrino, R.; Lopiano, L. Deep brain stimulation for atypical parkinsonism: A systematic review on efficacy and safety. Park. Relat. Disord. 2022, 96, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Vitek, J.L.; Jain, R.; Chen, L.; Tröster, A.I.; Schrock, L.E.; House, P.A.; Giroux, M.L.; Hebb, A.O.; Farris, S.M.; Whiting, D.M.; et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson’s disease (INTREPID): A multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol. 2020, 19, 491–501. [Google Scholar] [CrossRef] [PubMed]
- França, C.; Carra, R.B.; Diniz, J.M.; Munhoz, R.P.; Cury, R.G. Deep brain stimulation in Parkinson’s disease: State of the art and future perspectives. Arq. Neuropsiquiatr. 2022, 80 (Suppl. S1), 105–115. [Google Scholar] [CrossRef]
- Virameteekul, S.; Revesz, T.; Jaunmuktane, Z.; Warner, T.T.; De Pablo-Fernández, E. Clinical Diagnostic Accuracy of Parkinson’s Disease: Where Do We Stand? Mov. Disord. 2023, 38, 558–566. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.M.; Barbosa, E.R.; Aquino, C.C.; Munhoz, R.P.; Fasano, A.; Cury, R.G. Deep Brain Stimulation in Patients With Mutations in Parkinson’s Disease–Related Genes: A Systematic Review. Mov. Disord. Clin. Pract. 2019, 6, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.H.-F. The Role of Genetic Data in Selecting Device-Aided Therapies in Patients with Advanced Parkinson’s Disease: A Mini-Review. Front. Aging Neurosci. 2022, 14, 895430. [Google Scholar] [CrossRef]
- Rizzone, M.G.; Martone, T.; Balestrino, R.; Lopiano, L. Genetic background and outcome of Deep Brain Stimulation in Parkinson’s disease. Park. Relat. Disord. 2018, 64, 8–19. [Google Scholar] [CrossRef]
- Kuusimäki, T.; Korpela, J.; Pekkonen, E.; Martikainen, M.H.; Antonini, A.; Kaasinen, V. Deep brain stimulation for monogenic Parkinson’s disease: A systematic review. J. Neurol. 2020, 267, 883–897. [Google Scholar] [CrossRef]
- Pal, G.D.; Corcos, D.M.; Metman, L.V.; Israel, Z.; Bergman, H.; Arkadir, D. Cognitive Effects of Subthalamic Nucleus Deep Brain Stimulation in Parkinson’s Disease with GBA1 Pathogenic Variants. Mov. Disord. 2023, 38, 2155–2162. [Google Scholar] [CrossRef]
- Pal, G.; Mangone, G.; Hill, E.J.; Ouyang, B.; Liu, Y.; Lythe, V.; Ehrlich, D.; Saunders-Pullman, R.; Shanker, V.; Bressman, S.; et al. Parkinson Disease and Subthalamic Nucleus Deep Brain Stimulation: Cognitive Effects in GBA Mutation Carriers. Ann. Neurol. 2022, 91, 424–435. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.-M.; Zeighami, Y.; Dagher, A.; Postuma, R.B. Clinical criteria for subtyping Parkinson’s disease: Biomarkers and longitudinal progression. Brain 2017, 140, 1959–1976. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Fernández, E.; Lees, A.J.; Holton, J.L.; Warner, T.T. Prognosis and Neuropathologic Correlation of Clinical Subtypes of Parkinson Disease. JAMA Neurol. 2019, 76, 470–479. [Google Scholar] [CrossRef]
- Cavallieri, F.; Fraix, V.; Bove, F.; Mulas, D.; Tondelli, M.; Castrioto, A.; Krack, P.; Meoni, S.; Schmitt, E.; Lhommée, E.; et al. Predictors of Long-Term Outcome of Subthalamic Stimulation in Parkinson Disease. Ann. Neurol. 2020, 89, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Cubo, E.; Martínez-Martín, P.; González-Bernal, J.; Casas, E.; Arnaiz, S.; Miranda, J.; Gámez, P.; Santos-García, D. Effects of Motor Symptom Laterality on Clinical Manifestations and Quality of Life in Parkinson’s Disease. J. Park. Dis. 2020, 10, 1611–1620. [Google Scholar] [CrossRef]
- Stoehr, K.; Pazira, K.; Bonnet, K.; Schlundt, D.; Charles, D.; Hacker, M. Deep Brain Stimulation in Early-Stage Parkinson’s Disease: Patient Experience after 11 Years. Brain Sci. 2022, 12, 766. [Google Scholar] [CrossRef] [PubMed]
- Schuepbach, W.M.M.; Rau, J.; Knudsen, K.; Volkmann, J.; Krack, P.; Timmermann, L.; Hälbig, T.D.; Hesekamp, H.; Navarro, S.M.; Meier, N.; et al. Neurostimulation for Parkinson’s Disease with Early Motor Complications. N. Engl. J. Med. 2013, 368, 610–622. [Google Scholar] [CrossRef]
- Cabrera, L.Y.; Goudreau, J.; Sidiropoulos, C. Critical appraisal of the recent US FDA approval for earlier DBS intervention. Neurology 2018, 91, 133–136. [Google Scholar] [CrossRef]
- Deuschl, G.; Antonini, A.; Costa, J.; Śmiłowska, K.; Berg, D.; Corvol, J.C.; Fabbrini, G.; Ferreira, J.; Foltynie, T.; Mir, P.; et al. European Academy of Neurology/Movement Disorder Society-European Section Guideline on the Treatment of Parkinson’s Disease: I. Invasive Therapies. Mov. Disord. 2022, 37, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Hacker, M.L.; Turchan, M.; Heusinkveld, L.E.; Currie, A.D.; Millan, S.H.; Molinari, A.L.; Konrad, P.E.; Davis, T.L.; Phibbs, F.T.; Hedera, P.; et al. Deep brain stimulation in early-stage Parkinson disease Five-year outcomes. Neurology 2020, 95, E393–E401. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Volkmann, J.; Fasano, A.; Kühn, A.; Krack, P.; Deuschl, G. Changing Gears—DBS For Dopaminergic Desensitization in Parkinson’s Disease? Ann. Neurol. 2021, 90, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Dafsari, H.S.; Silverdale, M.; Strack, M.; Rizos, A.; Ashkan, K.; Mahlstedt, P.; Sachse, L.; Steffen, J.; Dembek, T.A.; Visser-Vandewalle, V.; et al. Nonmotor symptoms evolution during 24 months of bilateral subthalamic stimulation in Parkinson’s disease: 24 months nonmotor effects of STN-DBS in PD. Mov. Disord. 2018, 33, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Dafsari, H.S.; Reddy, P.; Herchenbach, C.; Wawro, S.; Petry-Schmelzer, J.N.; Visser-Vandewalle, V.; Rizos, A.; Silverdale, M.; Ashkan, K.; Samuel, M.; et al. Beneficial Effects of Bilateral Subthalamic Stimulation on Non-Motor Symptoms in Parkinson’s Disease. Brain Stimul. 2015, 9, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Gronich, N.; Auriel, E.; Lavi, I.; Rennert, G.; Saliba, W. Reply to: From beta-blockers to Parkinson’s disease in respect of essential tremor. Mov. Disord. 2019, 34, 154. [Google Scholar] [CrossRef] [PubMed]
- Jost, S.T.; Konitsioti, A.; Loehrer, P.A.; Ashkan, K.; Rizos, A.; Sauerbier, A.; Ghilardi, M.G.d.S.; Rosenkranz, F.; Strobel, L.; Gronostay, A.; et al. Non-motor effects of deep brain stimulation in Parkinson’s disease motor subtypes. Park. Relat. Disord. 2023, 109, 105318. [Google Scholar] [CrossRef] [PubMed]
- Chou, K.L.; Taylor, J.L.; Patil, P.G. The MDS−UPDRS tracks motor and non-motor improvement due to subthalamic nucleus deep brain stimulation in Parkinson disease. Park. Relat. Disord. 2013, 19, 966–969. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.E.; Pahwa, R. Effects of bilateral subthalamic nucleus stimulation on sleep, daytime sleepiness, and early morning dystonia in patients with Parkinson disease. J. Neurosurg. 2006, 104, 502–505. [Google Scholar] [CrossRef]
- Zibetti, M.; Torre, E.; Cinquepalmi, A.; Rosso, M.; Ducati, A.; Bergamasco, B.; Lanotte, M.; Lopiano, L. Motor and Nonmotor Symptom Follow-Up in Parkinsonian Patients after Deep Brain Stimulation of the Subthalamic Nucleus. Eur. Neurol. 2007, 58, 218–223. [Google Scholar] [CrossRef]
- Witjas, T.; Kaphan, E.; Régis, J.; Jouve, E.; Chérif, A.A.; Péragut, J.; Azulay, J.P. Effects of chronic subthalamic stimulation on nonmotor fluctuations in Parkinson’s disease. Mov. Disord. 2007, 22, 1729–1734. [Google Scholar] [CrossRef] [PubMed]
- Mostofi, A.; Morgante, F.; Edwards, M.J.; Brown, P.; Pereira, E.A.C. Pain in Parkinson’s disease and the role of the subthalamic nucleus. Brain 2021, 144, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Smeding, H.M.M.; Speelman, J.D.; Huizenga, H.M.; Schuurman, P.R.; Schmand, B. Predictors of cognitive and psychosocial outcome after STN DBS in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2009, 82, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Kishore, A.; Krishnan, S.; Pisharady, K.K.; Rajan, R.; Sarma, S.G.; Sarma, P.S. Predictors of dementia-free survival after bilateral subthalamic deep brain stimulation for Parkinson’s disease. Neurol. India 2019, 67, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Andersen, K.; Larsen, J.P.; Lolk, A. Prevalence and Characteristics of Dementia in Parkinson Disease: An 8-Year Prospective Study. Arch Neurol. 2003, 60, 387. [Google Scholar] [CrossRef] [PubMed]
- Rački, V.; Hero, M.; Rožmarić, G.; Papić, E.; Raguž, M.; Chudy, D.; Vuletić, V. Cognitive Impact of Deep Brain Stimulation in Parkinson’s Disease Patients: A Systematic Review. Front. Hum. Neurosci. 2022, 16, 867055. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.D.N.; Voulleminot, P.; Vrillon, A.; Hainque, E.; Béreau, M.; Lagha-Boukbiza, O.; Wirth, T.; Montaut, S.; Bardinet, E.; Kyheng, M.; et al. Impact of Subthalamic Deep Brain Stimulation on Impulse Control Disorders in Parkinson’s Disease: A Prospective Study. Mov. Disord. 2021, 36, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Rodriguez-Oroz, M.; Antonini, A.; Brotchie, J.M.; Ray Chaudhuri, K.; Brown, R.G.; Galpern, W.R.; Nirenberg, M.J.; Okun, M.S.; Lang, A.E. Management of impulse control disorders in Parkinson’s disease: Controversies and future approaches: ICDs IN PD. Mov. Disord. 2015, 30, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Lhommée, E.; Wojtecki, L.; Czernecki, V.; Witt, K.; Maier, F.; Tonder, L.; Timmermann, L.; Hälbig, T.D.; Pineau, F.; Durif, F.; et al. Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson’s disease with early motor complications (EARLYSTIM trial): Secondary analysis of an open-label randomised trial. Lancet Neurol. 2018, 17, 223–231. [Google Scholar] [CrossRef]
- Pollak, P. Deep brain stimulation for Parkinson’s disease—Patient selection. In Handbook of Clinical Neurology [Internet]; Elsevier: Amsterdam, The Netherlands, 2013; pp. 97–105. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780444534972000097 (accessed on 25 January 2023).
- Younce, J.R.; Campbell, M.C.; Perlmutter, J.S.; Norris, S.A. Thalamic and ventricular volumes predict motor response to deep brain stimulation for Parkinson’s disease. Park. Relat. Disord. 2019, 61, 64–69. [Google Scholar] [CrossRef]
- Muthuraman, M.; Deuschl, G.; Koirala, N.; Riedel, C.; Volkmann, J.; Groppa, S. Effects of DBS in parkinsonian patients depend on the structural integrity of frontal cortex. Sci. Rep. 2017, 7, 43571. [Google Scholar] [CrossRef] [PubMed]
- Albano, L.; Agosta, F.; Basaia, S.; Cividini, C.; Stojkovic, T.; Sarasso, E.; Stankovic, I.; Tomic, A.; Markovic, V.; Canu, E.; et al. Altered Functional Connectivity of the Subthalamic Nucleus in Parkinson’s Disease: Focus on Candidates for Deep Brain Stimulation. J. Park. Dis. 2023, 13, 797–809. [Google Scholar] [CrossRef]
- Younce, J.R.; Campbell, M.C.; Hershey, T.; Tanenbaum, A.B.; Milchenko, M.; Ushe, M.; Karimi, M.; Tabbal, S.D.; Kim, A.E.; Snyder, A.Z.; et al. Resting-State Functional Connectivity Predicts STN DBS Clinical Response. Mov. Disord. 2021, 36, 662–671. [Google Scholar] [CrossRef]
- Brown, G.; Du, G.; Farace, E.; Lewis, M.M.; Eslinger, P.J.; McInerney, J.; Kong, L.; Li, R.; Huang, X.; De Jesus, S. Subcortical Iron Accumulation Pattern May Predict Neuropsychological Outcomes After Subthalamic Nucleus Deep Brain Stimulation: A Pilot Study. J. Park. Dis. 2022, 12, 851. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Mathkour, M.; Scullen, T.; Houghton, D.; Lea, G.; Dallapiazza, R.F.; Kahn, L.; Smith, R.D. Effects of Medical Comorbidities on the Surgical Outcomes of Deep Brain Stimulation for Parkinson Disease: A Retrospective, Single-Institution Study. World Neurosurg. 2020, 144, e347–e352. [Google Scholar] [CrossRef]
- Ramayya, A.G.; Abdullah, K.G.; Mallela, A.N.; Pierce, J.T.; Thawani, J.; Petrov, D.; Baltuch, G.H. Thirty-Day Readmission Rates Following Deep Brain Stimulation Surgery. Neurosurgery 2017, 81, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Rughani, A.I.; Hodaie, M.; Lozano, A.M. Acute complications of movement disorders surgery: Effects of age and comorbidities: Complications of Movement Disorders Surgery. Mov. Disord. 2013, 28, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.B.; Jimenez-Shahed, J.; Abraham, D.S.; Thibault, D.P.; Mantri, S.; Fullard, M.; Burack, M.A.; Chou, K.L.; Spindler, M.; Jermakowicz, W.J.; et al. Acute readmission following deep brain stimulation surgery for Parkinson’s disease: A nationwide analysis. Park. Relat. Disord. 2020, 70, 96–102. [Google Scholar] [CrossRef]
- Rumalla, K.; Smith, K.A.; Follett, K.A.; Nazzaro, J.M.; Arnold, P.M. Rates, causes, risk factors, and outcomes of readmission following deep brain stimulation for movement disorders: Analysis of the U.S. Nationwide Readmissions Database. Clin. Neurol. Neurosurg. 2018, 171, 129–134. [Google Scholar] [CrossRef]
- Moghavem, N.; Morrison, D.; Ratliff, J.K.; Hernandez-Boussard, T. Cranial neurosurgical 30-day readmissions by clinical indication. J. Neurosurg. 2015, 123, 189–197. [Google Scholar] [CrossRef]
- Heard, T.; Coyne, T.; Silburn, P. Deep Brain Stimulation in Patients with Concomitant Cardiac Pacemakers: A Case Series. Oper. Neurosurg. 2019, 17, 549–553. [Google Scholar] [CrossRef]
- Garcia, J.; Hubsch, C.; Marques, A.; Gurruchaga, J.; Lamirel, C.; Roze, E.; Moulignier, A. Impact of HIV impact on outcomes of deep-brain stimulation of the subthalamic nucleus for Parkinson’s disease. Eur. J. Neurol. 2021, 29, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, P.; Aquino, C.C.; Fasano, A. Surgical Management of Parkinson’s Disease in the Elderly. Mov. Disord. Clin. Pract. 2021, 8, 500–509. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, J.; Chen, T.; Li, X.; Cui, Z. Short- and Long-Term Efficacy and Safety of Deep-Brain Stimulation in Parkinson’s Disease Patients aged 75 Years and Older. Brain Sci. 2022, 12, 1588. [Google Scholar] [CrossRef]
- Vats, A.; Amit, A.; Doshi, P. A comparative study of bilateral subthalamic nucleus DBS in Parkinson’s disease in young versus old: A single institutional study. J. Clin. Neurosci. 2019, 70, 85–91. [Google Scholar] [CrossRef]
- Dafsari, H.S.; Reker, P.; Stalinski, L.; Silverdale, M.; Rizos, A.; Ashkan, K.; Barbe, M.T.; Fink, G.R.; Evans, J.; Steffen, J.; et al. Quality of life outcome after subthalamic stimulation in Parkinson’s disease depends on age: QOL Outcome after STN-DBS in PD Depends on Age. Mov. Disord. 2018, 33, 99–107. [Google Scholar] [CrossRef]
- Vesper, J.; Haak, S.; Ostertag, C.; Nikkhah, G. Subthalamic nucleus deep brain stimulation in elderly patients—Analysis of outcome and complications. BMC Neurol. 2007, 7, 7. [Google Scholar] [CrossRef]
- Hanna, J.A.; Scullen, T.; Kahn, L.; Mathkour, M.; Gouveia, E.E.; Garces, J.; Evans, L.M.; Lea, G.; Houghton, D.J.; Biro, E.; et al. Comparison of elderly and young patient populations treated with deep brain stimulation for Parkinson’s disease: Long-term outcomes with up to 7 years of follow-up. J. Neurosurg. 2019, 131, 807–812. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Vora, N.M.; Tariq, I.; Mujtaba, A.; Caprara, A.L.F. Deep Brain Stimulation for the Management of Refractory Neurological Disorders: A Comprehensive Review. Medicina 2023, 59, 1991. [Google Scholar] [CrossRef]
- Yen, K.; Miyasaki, J.M.; Waldron, M.; Yu, L.; Sankar, T.; Ba, F. DBS-Edmonton App, a Tool to Manage Patient Expectations of DBS in Parkinson Disease. Neurol. Clin. Pract. 2020, 11, E308–E316. [Google Scholar] [CrossRef] [PubMed]
- Radomska, M.; Flores Alves dos Santos, J.; Weber, K.; Baertschi, M.; Burkhard, P.R.; Herrmann, F.; Belayachi, S.; Favez, N.; Canuto, A. Assessing preoperative hope and expectations related to functional neurosurgery: A new questionnaire. BMC Psychol. 2022, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Geraedts, V.; Kuijf, M.; van Hilten, J.; Marinus, J.; Oosterloo, M.; Contarino, M. Selecting candidates for Deep Brain Stimulation in Parkinson’s disease: The role of patients’ expectations. Park. Relat. Disord. 2019, 66, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Agid, Y.; Schupbach, M.; Gargiulo, M.; Mallet, L.; Houeto, J.L.; Behar, C.; Maltête, D.; Mesnage, V.; Welter, M.L. Neurosurgery in Parkinson’s disease: The doctor is happy, the patient less so? In Parkinson’s Disease and Related Disorders [Internet]; Riederer, P., Reichmann, H., Youdim, M.B.H., Gerlach, M., Eds.; Springer: Vienna, Austria, 2006; pp. 409–414. Available online: http://link.springer.com/10.1007/978-3-211-45295-0_61 (accessed on 20 January 2023).
- Maier, F.; Lewis, C.J.; Horstkoetter, N.; Eggers, C.; Kalbe, E.; Maarouf, M.; Kuhn, J.; Zurowski, M.; Moro, E.; Woopen, C. Patients’ expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson’s disease: A mixed-method approach. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Schuepbach, W.M.; Tonder, L.; Schnitzler, A.; Krack, P.; Rau, J.; Hartmann, A.; Hälbig, T.D.; Pineau, F.; Falk, A.; Paschen, L.; et al. Quality of life predicts outcome of deep brain stimulation in early Parkinson disease. Neurology 2019, 92, e1109–e1120. [Google Scholar] [CrossRef]
- Gámez, Y.M.C.; Brugger, F.; Biller-Andorno, N. Parkinson’s Disease and Deep Brain Stimulation Have an Impact on My Life: A Multimodal Study on the Experiences of Patients and Family Caregivers. Int. J. Environ. Res. Public Health 2021, 18, 9516. [Google Scholar] [CrossRef]
- Kubu, C.S.; Ford, P.J. Clinical Ethics in the Context of Deep Brain Stimulation for Movement Disorders. Arch. Clin. Neuropsychol. 2017, 32, 829–839. [Google Scholar] [CrossRef]
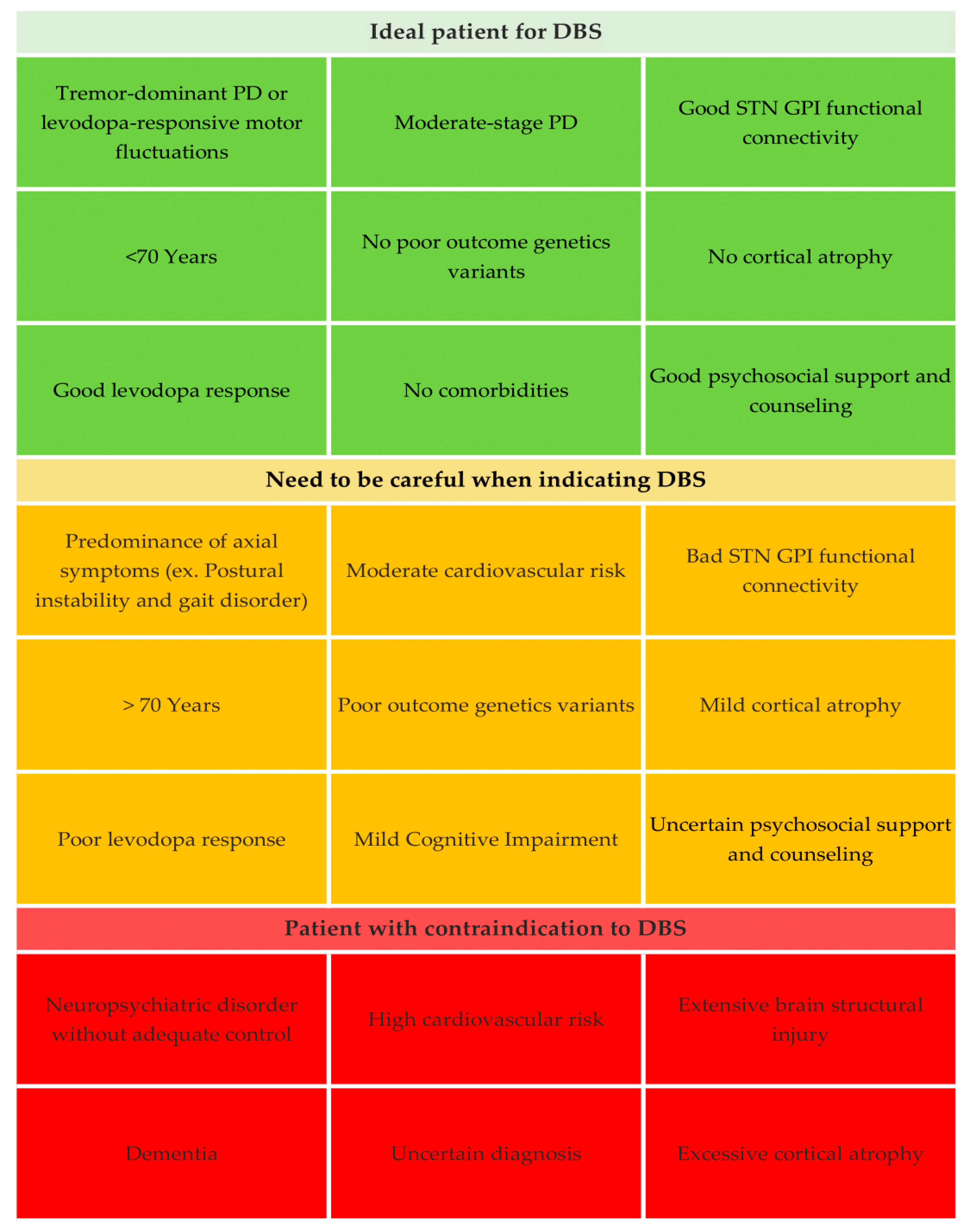
| The Three Indications | Definition | Practical Points |
|---|---|---|
| 1. Motor Complications | Motor fluctuations and dyskinesia | 5-2-1 criteria * |
| 2. Tremor Refractory to Optimized Treatment | Levodopa-resistant tremor [10]. | LEDD of ≥900 mg β |
| 3. Intolerance to Dopaminergic Agents | Patients who have adverse effects that prevent the increase in dose to a level that promotes symptom amelioration | Somnolence, hypotension, nausea, vomiting, impulse control disorders and psychosis secondary to dopaminergic medications |
| The Five Prerequisites | Why Is It Important? |
|---|---|
| 1. The patient must have Parkinson’s Disease | Atypical parkinsonism does not benefit from DBS [15]. |
| 2. More than 4 years after disease onset | This is a measure aimed at avoiding operate atypical parkinsonism * [10,16]. |
| 3. Cut-off of 33% in the levodopa challenge test ** | The need for the test is supported by the good correlation observed between the percentage of amelioration in the test and after DBS surgery [17]. |
| 4. Absence of significant cognitive deficits or uncontrolled neuropsychiatric diseases | Patients with dementia do not benefit, and those with uncontrolled neuropsychiatric diseases have higher risk of complications. |
| 5. Patients must be able to attend frequent medical appointments after surgery | It is paramount that, after the procedure, good programming, medication adjustments when needed, and rehabilitation are performed and prescribed [17]. |
| Gene | Motor Symptom | Non-Motor Symptom | Good DBS Outcome Variants | Bad Outcome Variants |
|---|---|---|---|---|
| LRRK2-AD | Late-onset PD | Mild or absent | p.G2019S p.G2385R p.T2031S, p.Y1699C p.R793M [19]. * | p. R1441G * |
| SNCA-AD | May have atypical features ** | Cognitive decline (70%) | Duplications [19]. *** | p.A53E A |
| VPS35-AD | Similar to tremor-dominant PD | Minimal cognitive, even in the long term | Generally good responses α | - |
| PRKN-AR | Similar to PD. Foot dystonia | Depression | Generally good responses £ | - |
| PINK1-AD | Similar to PRKN | Some may develop dementia at later stages [20]. | Generally good responses € | - |
| GBA-AD | Younger Faster progression More axial symptoms | Dementia, RBD, autonomic dysfunction, and visual hallucination are common and severe | Generally good motor responses ¥ | GPI-DBS led to a lesser motor improvement of around 22% [20]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, R.M.G.; Soares, M.C.; Portela, D.M.M.C.; Guimarães, T.G.; Cury, R.G. New Perspectives of Deep Brain Stimulation Indications for Parkinson’s Disease: A Critical Review. Brain Sci. 2024, 14, 638. https://doi.org/10.3390/brainsci14070638
Barbosa RMG, Soares MC, Portela DMMC, Guimarães TG, Cury RG. New Perspectives of Deep Brain Stimulation Indications for Parkinson’s Disease: A Critical Review. Brain Sciences. 2024; 14(7):638. https://doi.org/10.3390/brainsci14070638
Chicago/Turabian StyleBarbosa, Renata Montes Garcia, Miriam Carvalho Soares, Denise Maria Meneses Cury Portela, Thiago Gonçalves Guimarães, and Rubens Gisbert Cury. 2024. "New Perspectives of Deep Brain Stimulation Indications for Parkinson’s Disease: A Critical Review" Brain Sciences 14, no. 7: 638. https://doi.org/10.3390/brainsci14070638
APA StyleBarbosa, R. M. G., Soares, M. C., Portela, D. M. M. C., Guimarães, T. G., & Cury, R. G. (2024). New Perspectives of Deep Brain Stimulation Indications for Parkinson’s Disease: A Critical Review. Brain Sciences, 14(7), 638. https://doi.org/10.3390/brainsci14070638





