Translating Molecular Approaches to Oligodendrocyte-Mediated Neurological Circuit Modulation
Abstract
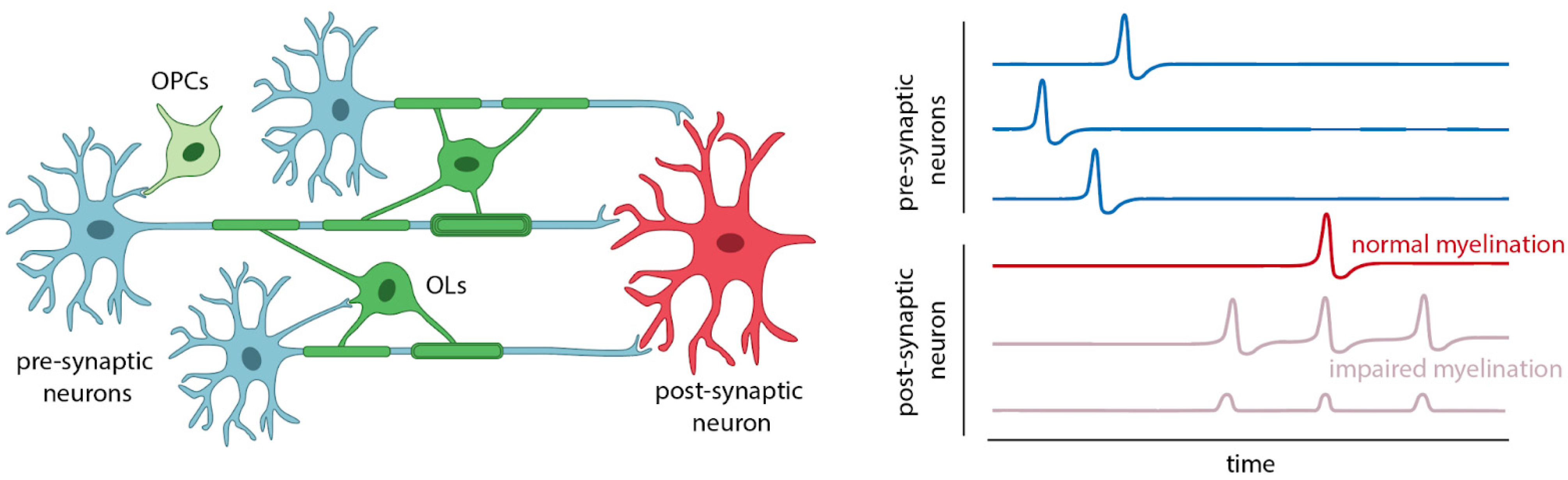
:1. Introduction
2. OLs, OPCs, and Myelin in Neural Circuit Disorders
2.1. Epilepsy
2.2. Glioma
2.3. Psychiatric Disorders
2.4. Neurodevelopment
3. Diagnostics and Therapeutic Approaches
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- LoTurco, J.J.; Owens, D.F.; Heath, M.J.; Davis, M.B.; Kriegstein, A.R. GABA and Glutamate Depolarize Cortical Progenitor Cells and Inhibit DNA Synthesis. Neuron 1995, 15, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Luk, K.C.; Kennedy, T.E.; Sadikot, A.F. Glutamate Promotes Proliferation of Striatal Neuronal Progenitors by an NMDA Receptor-Mediated Mechanism. J. Neurosci. 2003, 23, 2239–2250. [Google Scholar] [CrossRef]
- Luk, K.C.; Sadikot, A.F. Glutamate and Regulation of Proliferation in the Developing Mammalian Telencephalon. Dev. Neurosci. 2004, 26, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.-Y.; Hanumaihgari, P.; Hsu, E.T.; Agarwal, A.; Kawaguchi, R.; Calabresi, P.A.; Bergles, D.E. Norepinephrine Modulates Calcium Dynamics in Cortical Oligodendrocyte Precursor Cells Promoting Proliferation during Arousal in Mice. Nat. Neurosci. 2023, 26, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Huxley, A.F.; Stämpfli, R. Evidence for Saltatory Conduction in Peripheral Myelinated Nerve Fibres. J. Physiol. 1949, 108, 315–339. [Google Scholar] [CrossRef] [PubMed]
- Steadman, P.E.; Xia, F.; Ahmed, M.; Mocle, A.J.; Penning, A.R.A.; Geraghty, A.C.; Steenland, H.W.; Monje, M.; Josselyn, S.A.; Frankland, P.W. Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 2020, 105, 150–164.e6. [Google Scholar] [CrossRef] [PubMed]
- Hartline, D.K.; Colman, D.R. Rapid Conduction and the Evolution of Giant Axons and Myelinated Fibers. Curr. Biol. 2007, 17, R29–R35. [Google Scholar] [CrossRef] [PubMed]
- Makinodan, M.; Rosen, K.M.; Ito, S.; Corfas, G. A Critical Period for Social Experience–Dependent Oligodendrocyte Maturation and Myelination. Science 2012, 337, 1357–1360. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef]
- Osso, L.A.; Rankin, K.A.; Chan, J.R. Experience-Dependent Myelination Following Stress Is Mediated by the Neuropeptide Dynorphin. Neuron 2021, 109, 3619–3632.e5. [Google Scholar] [CrossRef]
- Tomassy, G.S.; Berger, D.R.; Chen, H.-H.; Kasthuri, N.; Hayworth, K.J.; Vercelli, A.; Seung, H.S.; Lichtman, J.W.; Arlotta, P. Distinct Profiles of Myelin Distribution along Single Axons of Pyramidal Neurons in the Neocortex. Science 2014, 344, 319–324.e5. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Orthmann-Murphy, J.L.; Langseth, A.J.; Bergles, D.E. Myelin Remodeling through Experience-Dependent Oligodendrogenesis in the Adult Somatosensory Cortex. Nat. Neurosci. 2018, 21, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.G. The Rules of Attraction in Central Nervous System Myelination. Front. Cell. Neurosci. 2018, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Zuchero, J.B. Anchors Away: Glia-Neuron Adhesion Regulates Myelin Targeting and Growth. Dev. Cell 2019, 51, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Zuchero, J.B.; Fu, M.-M.; Sloan, S.A.; Ibrahim, A.; Olson, A.; Zaremba, A.; Dugas, J.C.; Wienbar, S.; Caprariello, A.V.; Kantor, C.; et al. CNS Myelin Wrapping Is Driven by Actin Disassembly. Dev. Cell 2015, 34, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.; Sánchez, P.; Schmitt, S.; Snaidero, N.; Mitkovski, M.; Velte, C.; Brückner, B.R.; Alexopoulos, I.; Czopka, T.; Jung, S.Y.; et al. Actin Filament Turnover Drives Leading Edge Growth during Myelin Sheath Formation in the Central Nervous System. Dev. Cell 2015, 34, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Takeo, K.; Almeida, R.G.; Cooper, M.H.; Wu, K.; Iyer, M.; Kantarci, H.; Zuchero, J.B. CNS Myelination Requires VAMP2/3-Mediated Membrane Expansion in Oligodendrocytes. Nat. Commun. 2022, 13, 5583. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Trimarco, A.; Zhang, A.J.; Fujimori, K.; Urade, Y.; Sun, L.O.; Taveggia, C.; Zhang, Y. Oligodendrocyte-Lineage Cell Exocytosis and L-Type Prostaglandin D Synthase Promote Oligodendrocyte Development and Myelination. Elife 2023, 12, e77441. [Google Scholar] [CrossRef] [PubMed]
- Snaidero, N.; Möbius, W.; Czopka, T.; Hekking, L.H.P.; Mathisen, C.; Verkleij, D.; Goebbels, S.; Edgar, J.; Merkler, D.; Lyons, D.A.; et al. Myelin Membrane Wrapping of CNS Axons by PI(3,4,5)P3-Dependent Polarized Growth at the Inner Tongue. Cell 2014, 156, 277–290. [Google Scholar] [CrossRef]
- Lundgaard, I.; Luzhynskaya, A.; Stockley, J.H.; Wang, Z.; Evans, K.A.; Swire, M.; Volbracht, K.; Gautier, H.O.B.; Franklin, R.J.M.; Charles, F.-C.; et al. Neuregulin and BDNF Induce a Switch to NMDA Receptor-Dependent Myelination by Oligodendrocytes. PLoS Biol. 2013, 11, e1001743. [Google Scholar] [CrossRef]
- Bechler, M.E.; Swire, M.; Ffrench-Constant, C. Intrinsic and Adaptive Myelination-A Sequential Mechanism for Smart Wiring in the Brain. Dev. Neurobiol. 2018, 78, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Sampaio-Baptista, C.; Khrapitchev, A.A.; Foxley, S.; Schlagheck, T.; Scholz, J.; Jbabdi, S.; DeLuca, G.C.; Miller, K.L.; Taylor, A.; Thomas, N.; et al. Motor Skill Learning Induces Changes in White Matter Microstructure and Myelination. J. Neurosci. 2013, 33, 19499–19503. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; de Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor Skill Learning Requires Active Central Myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Bacmeister, C.M.; Barr, H.J.; McClain, C.R.; Thornton, M.A.; Nettles, D.; Welle, C.G.; Hughes, E.G. Motor Learning Promotes Remyelination via New and Surviving Oligodendrocytes. Nat. Neurosci. 2020, 23, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Bacmeister, C.M.; Huang, R.; Osso, L.A.; Thornton, M.A.; Conant, L.; Chavez, A.R.; Poleg-Polsky, A.; Hughes, E.G. Motor Learning Drives Dynamic Patterns of Intermittent Myelination on Learning-Activated Axons. Nat. Neurosci. 2022, 25, 1300–1313. [Google Scholar] [CrossRef] [PubMed]
- Cullen, C.L.; Pepper, R.E.; Clutterbuck, M.T.; Pitman, K.A.; Oorschot, V.; Auderset, L.; Tang, A.D.; Ramm, G.; Emery, B.; Rodger, J.; et al. Periaxonal and Nodal Plasticities Modulate Action Potential Conduction in the Adult Mouse Brain. Cell Rep. 2021, 34, 108641. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.M.; Michel, K.; Jokhi, V.; Nedivi, E.; Arlotta, P. Neuron Class–specific Responses Govern Adaptive Myelin Remodeling in the Neocortex. Science 2020, 370, eabd2109. [Google Scholar] [CrossRef] [PubMed]
- Kato, D.; Wake, H. Myelin Plasticity Modulates Neural Circuitry Required for Learning and Behavior. Neurosci. Res. 2021, 167, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Buch, V.P.; Bernabei, J.M.; Ng, G.; Richardson, A.G.; Ramayya, A.; Brandon, C.; Stiso, J.; Bassett, D.S.; Lucas, T.H. “Primed to Perform”: Dynamic White Matter Graph Communicability May Drive Metastable Network Representations of Enhanced Preparatory Cognitive Control. bioRxiv 2022. [Google Scholar] [CrossRef]
- Arancibia-Cárcamo, I.L.; Ford, M.C.; Cossell, L.; Ishida, K.; Tohyama, K.; Attwell, D. Node of Ranvier Length as a Potential Regulator of Myelinated Axon Conduction Speed. Elife 2017, 6, e23329. [Google Scholar] [CrossRef]
- Munyeshyaka, M.; Fields, R.D. Oligodendroglia Are Emerging Players in Several Forms of Learning and Memory. Commun. Biol. 2022, 5, 1148. [Google Scholar] [CrossRef]
- Ulloa Severino, F.P.; Lawal, O.O.; Sakers, K.; Wang, S.; Kim, N.; Friedman, A.D.; Johnson, S.A.; Sriworarat, C.; Hughes, R.H.; Soderling, S.H.; et al. Training-Induced Circuit-Specific Excitatory Synaptogenesis in Mice Is Required for Effort Control. Nat. Commun. 2023, 14, 5522. [Google Scholar] [CrossRef] [PubMed]
- Pajevic, S.; Plenz, D.; Basser, P.J.; Fields, R.D. Oligodendrocyte-Mediated Myelin Plasticity and Its Role in Neural Synchronization. Elife 2023, 12, e81982. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, B.; Pomrenze, M.B.; Malacon, K.; Drexler, R.; Rogers, A.E.; Shamardani, K.; Chau, I.J.; Taylor, K.R.; Ni, L.; Contreras-Esquivel, D.; et al. Myelin Plasticity in the Ventral Tegmental Area Is Required for Opioid Reward. Nature 2024, 630, 677–685. [Google Scholar] [CrossRef]
- Hughes, E.G.; Appel, B. The Cell Biology of CNS Myelination. Curr. Opin. Neurobiol. 2016, 39, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, C.; Timmler, S.; Barrantes-Freer, A.; Simons, M. Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol. Rev. 2019, 99, 1381–1431. [Google Scholar] [CrossRef]
- Bedner, P.; Steinhäuser, C. Role of Impaired Astrocyte Gap Junction Coupling in Epileptogenesis. Cells 2023, 12, 1669. [Google Scholar] [CrossRef]
- Purnell, B.S.; Alves, M.; Boison, D. Astrocyte-Neuron Circuits in Epilepsy. Neurobiol. Dis. 2023, 179, 106058. [Google Scholar] [CrossRef]
- Volnova, A.; Tsytsarev, V.; Ganina, O.; Vélez-Crespo, G.E.; Alves, J.M.; Ignashchenkova, A.; Inyushin, M. The Anti-Epileptic Effects of Carbenoxolone In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 23, 663. [Google Scholar] [CrossRef]
- Knowles, J.K.; Xu, H.; Soane, C.; Batra, A.; Saucedo, T.; Frost, E.; Tam, L.T.; Fraga, D.; Ni, L.; Villar, K.; et al. Maladaptive Myelination Promotes Generalized Epilepsy Progression. Nat. Neurosci. 2022, 25, 596–606. [Google Scholar] [CrossRef]
- Larson, V.A.; Mironova, Y.; Vanderpool, K.G.; Waisman, A.; Rash, J.E.; Agarwal, A.; Bergles, D.E. Oligodendrocytes Control Potassium Accumulation in White Matter and Seizure Susceptibility. Elife 2018, 7, e34829. [Google Scholar] [CrossRef]
- Lindquist, B.E.; Timbie, C.; Voskobiynyk, Y.; Paz, J.T. Thalamocortical Circuits in Generalized Epilepsy: Pathophysiologic Mechanisms and Therapeutic Targets. Neurobiol. Dis. 2023, 181, 106094. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, C.J.; Liew, Y.J.; Stanley, G.B. Thalamic State Influences Timing Precision in the Thalamocortical Circuit. J. Neurophysiol. 2021, 125, 1833–1850. [Google Scholar] [CrossRef]
- Scott, G.; Hellyer, P.J.; Ramlackhansingh, A.F.; Brooks, D.J.; Matthews, P.M.; Sharp, D.J. Thalamic Inflammation after Brain Trauma Is Associated with Thalamo-Cortical White Matter Damage. J. Neuroinflamm. 2015, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.N.; Fields, R.D. Regulation of Myelination by Microglia. Sci. Adv. 2021, 7, eabk1131. [Google Scholar] [CrossRef] [PubMed]
- McNamara, N.B.; Munro, D.A.D.; Bestard-Cuche, N.; Uyeda, A.; Bogie, J.F.J.; Hoffmann, A.; Holloway, R.K.; Molina-Gonzalez, I.; Askew, K.E.; Mitchell, S.; et al. Microglia Regulate Central Nervous System Myelin Growth and Integrity. Nature 2023, 613, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.N.; Appel, B. Microglia Phagocytose Myelin Sheaths to Modify Developmental Myelination. Nat. Neurosci. 2020, 23, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Djannatian, M.; Radha, S.; Weikert, U.; Safaiyan, S.; Wrede, C.; Deichsel, C.; Kislinger, G.; Rhomberg, A.; Ruhwedel, T.; Campbell, D.S.; et al. Myelination Generates Aberrant Ultrastructure That Is Resolved by Microglia. J. Cell Biol. 2023, 222, e202204010. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Nagaraja, S.; Ocampo, A.; Tam, L.T.; Wood, L.S.; Pallegar, P.N.; Greene, J.J.; Geraghty, A.C.; Goldstein, A.K.; Ni, L.; et al. Methotrexate Chemotherapy Induces Persistent Tri-Glial Dysregulation That Underlies Chemotherapy-Related Cognitive Impairment. Cell 2019, 176, 43–55.e13. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.-H.; Wood, J.; O’Dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild Respiratory COVID Can Cause Multi-Lineage Neural Cell and Myelin Dysregulation. Cell 2022, 185, 2452–2468.e16. [Google Scholar] [CrossRef]
- Taylor, K.R.; Barron, T.; Hui, A.; Spitzer, A.; Yalçin, B.; Ivec, A.E.; Geraghty, A.C.; Hartmann, G.G.; Arzt, M.; Gillespie, S.M.; et al. Glioma Synapses Recruit Mechanisms of Adaptive Plasticity. Nature 2023, 623, 366–374. [Google Scholar] [CrossRef]
- Yang, C.; Farias, F.H.G.; Ibanez, L.; Suhy, A.; Sadler, B.; Fernandez, M.V.; Wang, F.; Bradley, J.L.; Eiffert, B.; Bahena, J.A.; et al. Genomic Atlas of the Proteome from Brain, CSF and Plasma Prioritizes Proteins Implicated in Neurological Disorders. Nat. Neurosci. 2021, 24, 1302–1312. [Google Scholar] [CrossRef]
- Mayilyan, K.R.; Dodds, A.W.; Boyajyan, A.S.; Soghoyan, A.F.; Sim, R.B. Complement C4B Protein in Schizophrenia. World J. Biol. Psychiatry 2008, 9, 225–230. [Google Scholar] [CrossRef]
- Mostafa, G.A.; Shehab, A.A. The Link of C4B Null Allele to Autism and to a Family History of Autoimmunity in Egyptian Autistic Children. J. Neuroimmunol. 2010, 223, 115–119. [Google Scholar] [CrossRef]
- Zhou, D.; Rudnicki, M.; Chua, G.T.; Lawrance, S.K.; Zhou, B.; Drew, J.L.; Barbar-Smiley, F.; Armstrong, T.K.; Hilt, M.E.; Birmingham, D.J.; et al. Human Complement C4B Allotypes and Deficiencies in Selected Cases with Autoimmune Diseases. Front. Immunol. 2021, 12, 739430. [Google Scholar] [CrossRef]
- Kenigsbuch, M.; Bost, P.; Halevi, S.; Chang, Y.; Chen, S.; Ma, Q.; Hajbi, R.; Schwikowski, B.; Bodenmiller, B.; Fu, H.; et al. A Shared Disease-Associated Oligodendrocyte Signature among Multiple CNS Pathologies. Nat. Neurosci. 2022, 25, 876–886. [Google Scholar] [CrossRef]
- Wang, Y.; Fu, W.-Y.; Cheung, K.; Hung, K.-W.; Chen, C.; Geng, H.; Yung, W.-H.; Qu, J.Y.; Fu, A.K.Y.; Ip, N.Y. Astrocyte-Secreted IL-33 Mediates Homeostatic Synaptic Plasticity in the Adult Hippocampus. Proc. Natl. Acad. Sci. USA 2021, 118, e2020810118. [Google Scholar] [CrossRef]
- Vainchtein, I.D.; Chin, G.; Cho, F.S.; Kelley, K.W.; Miller, J.G.; Chien, E.C.; Liddelow, S.A.; Nguyen, P.T.; Nakao-Inoue, H.; Dorman, L.C.; et al. Astrocyte-Derived Interleukin-33 Promotes Microglial Synapse Engulfment and Neural Circuit Development. Science 2018, 359, 1269–1273. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Dorman, L.C.; Pan, S.; Vainchtein, I.D.; Han, R.T.; Nakao-Inoue, H.; Taloma, S.E.; Barron, J.J.; Molofsky, A.B.; Kheirbek, M.A.; et al. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 2020, 182, 388–403.e15. [Google Scholar] [CrossRef]
- Han, R.T.; Vainchtein, I.D.; Schlachetzki, J.C.M.; Cho, F.S.; Dorman, L.C.; Ahn, E.; Kim, D.K.; Barron, J.J.; Nakao-Inoue, H.; Molofsky, A.B.; et al. Microglial Pattern Recognition via IL-33 Promotes Synaptic Refinement in Developing Corticothalamic Circuits in Mice. J. Exp. Med. 2023, 220, e20220605. [Google Scholar] [CrossRef]
- Han, R.T.; Vainchtein, I.D.; Schlachetzki, J.C.M.; Cho, F.S.; Dorman, L.C.; Johung, T.; Ahn, E.; Barron, J.T.; Nakao-Inoue, H.; Joshi, A.; et al. Interleukin-33 Coordinates a Microglial Phagocytic Response and Limits Corticothalamic Excitability and Seizure Susceptibility. bioRxiv 2021. [Google Scholar] [CrossRef]
- Dohi, E.; Choi, E.Y.; Rose, I.V.L.; Murata, A.S.; Chow, S.; Niwa, M.; Kano, S.-I. Behavioral Changes in Mice Lacking Interleukin-33. eNeuro 2017, 4. [Google Scholar] [CrossRef]
- Sung, H.-Y.; Chen, W.-Y.; Huang, H.-T.; Wang, C.-Y.; Chang, S.-B.; Tzeng, S.-F. Down-Regulation of Interleukin-33 Expression in Oligodendrocyte Precursor Cells Impairs Oligodendrocyte Lineage Progression. J. Neurochem. 2019, 150, 691–708. [Google Scholar] [CrossRef]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain Tumour Cells Interconnect to a Functional and Resistant Network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef]
- Winkler, F.; Wick, W. Harmful Networks in the Brain and beyond. Science 2018, 359, 1100–1101. [Google Scholar] [CrossRef]
- Pan, Y.; Hysinger, J.D.; Barron, T.; Schindler, N.F.; Cobb, O.; Guo, X.; Yalçın, B.; Anastasaki, C.; Mulinyawe, S.B.; Ponnuswami, A.; et al. NF1 Mutation Drives Neuronal Activity-Dependent Initiation of Optic Glioma. Nature 2021, 594, 277–282. [Google Scholar] [CrossRef]
- Chen, P.; Wang, W.; Liu, R.; Lyu, J.; Zhang, L.; Li, B.; Qiu, B.; Tian, A.; Jiang, W.; Ying, H.; et al. Olfactory Sensory Experience Regulates Gliomagenesis via Neuronal IGF1. Nature 2022, 606, 550–556. [Google Scholar] [CrossRef]
- Venkataramani, V.; Yang, Y.; Schubert, M.C.; Reyhan, E.; Tetzlaff, S.K.; Wißmann, N.; Botz, M.; Soyka, S.J.; Beretta, C.A.; Pramatarov, R.L.; et al. Glioblastoma Hijacks Neuronal Mechanisms for Brain Invasion. Cell 2022, 185, 2899–2917.e31. [Google Scholar] [CrossRef]
- Chen, T.-J.; Kula, B.; Nagy, B.; Barzan, R.; Gall, A.; Ehrlich, I.; Kukley, M. In Vivo Regulation of Oligodendrocyte Precursor Cell Proliferation and Differentiation by the AMPA-Receptor Subunit GluA2. Cell Rep. 2018, 25, 852–861.e7. [Google Scholar] [CrossRef]
- Kougioumtzidou, E.; Shimizu, T.; Hamilton, N.B.; Tohyama, K.; Sprengel, R.; Monyer, H.; Attwell, D.; Richardson, W.D. Signalling through AMPA Receptors on Oligodendrocyte Precursors Promotes Myelination by Enhancing Oligodendrocyte Survival. Elife 2017, 6, e28080. [Google Scholar] [CrossRef]
- Cull-Candy, S.G.; Farrant, M. Ca2+-Permeable AMPA Receptors and Their Auxiliary Subunits in Synaptic Plasticity and Disease. J. Physiol. 2021, 599, 2655–2671. [Google Scholar] [CrossRef]
- Taylor, K.R.; Monje, M. Neuron–oligodendroglial Interactions in Health and Malignant Disease. Nat. Rev. Neurosci. 2023, 24, 733–746. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and Synaptic Integration of Glioma into Neural Circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef]
- Hausmann, D.; Hoffmann, D.C.; Venkataramani, V.; Jung, E.; Horschitz, S.; Tetzlaff, S.K.; Jabali, A.; Hai, L.; Kessler, T.; Azoŕin, D.D.; et al. Autonomous Rhythmic Activity in Glioma Networks Drives Brain Tumour Growth. Nature 2023, 613, 179–186. [Google Scholar] [CrossRef]
- Krishna, S.; Choudhury, A.; Keough, M.B.; Seo, K.; Ni, L.; Kakaizada, S.; Lee, A.; Aabedi, A.; Popova, G.; Lipkin, B.; et al. Glioblastoma Remodelling of Human Neural Circuits Decreases Survival. Nature 2023, 617, 599–607. [Google Scholar] [CrossRef]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic Synaptic Input to Glioma Cells Drives Brain Tumour Progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef]
- Rahimian, R.; Perlman, K.; Canonne, C.; Mechawar, N. Targeting Microglia-Oligodendrocyte Crosstalk in Neurodegenerative and Psychiatric Disorders. Drug Discov. Today 2022, 27, 2562–2573. [Google Scholar] [CrossRef]
- Poggi, G.; Albiez, J.; Pryce, C.R. Effects of Chronic Social Stress on Oligodendrocyte Proliferation-Maturation and Myelin Status in Prefrontal Cortex and Amygdala in Adult Mice. Neurobiol. Stress 2022, 18, 100451. [Google Scholar] [CrossRef]
- Tizabi, Y.; Getachew, B.; Hauser, S.R.; Tsytsarev, V.; Manhães, A.C.; da Silva, V.D.A. Role of Glial Cells in Neuronal Function, Mood Disorders, and Drug Addiction. Brain Sci. 2024, 14, 558. [Google Scholar] [CrossRef]
- Nave, K.-A.; Ehrenreich, H. Myelination and Oligodendrocyte Functions in Psychiatric Diseases. JAMA Psychiatry 2014, 71, 582–584. [Google Scholar] [CrossRef]
- Hakak, Y.; Walker, J.R.; Li, C.; Wong, W.H.; Davis, K.L.; Buxbaum, J.D.; Haroutunian, V.; Fienberg, A.A. Genome-Wide Expression Analysis Reveals Dysregulation of Myelination-Related Genes in Chronic Schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 4746–4751. [Google Scholar] [CrossRef]
- Peirce, T.R.; Bray, N.J.; Williams, N.M.; Norton, N.; Moskvina, V.; Preece, A.; Haroutunian, V.; Buxbaum, J.D.; Owen, M.J.; O’Donovan, M.C. Convergent Evidence for 2′,3′-Cyclic Nucleotide 3′-Phosphodiesterase as a Possible Susceptibility Gene for Schizophrenia. Arch. Gen. Psychiatry 2006, 63, 18–24. [Google Scholar] [CrossRef]
- Iwamoto, K.; Bundo, M.; Yamada, K.; Takao, H.; Iwayama-Shigeno, Y.; Yoshikawa, T.; Kato, T. DNA Methylation Status of SOX10 Correlates with Its Downregulation and Oligodendrocyte Dysfunction in Schizophrenia. J. Neurosci. 2005, 25, 5376–5381. [Google Scholar] [CrossRef]
- Roy, K.; Murtie, J.C.; El-Khodor, B.F.; Edgar, N.; Sardi, S.P.; Hooks, B.M.; Benoit-Marand, M.; Chen, C.; Moore, H.; O’Donnell, P.; et al. Loss of erbB Signaling in Oligodendrocytes Alters Myelin and Dopaminergic Function, a Potential Mechanism for Neuropsychiatric Disorders. Proc. Natl. Acad. Sci. USA 2007, 104, 8131–8136. [Google Scholar] [CrossRef]
- Giera, S.; Luo, R.; Ying, Y.; Ackerman, S.D.; Jeong, S.-J.; Stoveken, H.M.; Folts, C.J.; Welsh, C.A.; Tall, G.G.; Stevens, B.; et al. Microglial Transglutaminase-2 Drives Myelination and Myelin Repair via GPR56/ADGRG1 in Oligodendrocyte Precursor Cells. Elife 2018, 7, e33385. [Google Scholar] [CrossRef]
- Nagy, C.; Maitra, M.; Tanti, A.; Suderman, M.; Théroux, J.-F.; Davoli, M.A.; Perlman, K.; Yerko, V.; Wang, Y.C.; Tripathy, S.J.; et al. Single-Nucleus Transcriptomics of the Prefrontal Cortex in Major Depressive Disorder Implicates Oligodendrocyte Precursor Cells and Excitatory Neurons. Nat. Neurosci. 2020, 23, 771–781. [Google Scholar] [CrossRef]
- Barysheva, M.; Jahanshad, N.; Foland-Ross, L.; Altshuler, L.L.; Thompson, P.M. White Matter Microstructural Abnormalities in Bipolar Disorder: A Whole Brain Diffusion Tensor Imaging Study. Neuroimage Clin. 2013, 2, 558–568. [Google Scholar] [CrossRef]
- Kubicki, M.; McCarley, R.W.; Shenton, M.E. Evidence for White Matter Abnormalities in Schizophrenia. Curr. Opin. Psychiatry 2005, 18, 121–134. [Google Scholar] [CrossRef]
- Lener, M.S.; Wong, E.; Tang, C.Y.; Byne, W.; Goldstein, K.E.; Blair, N.J.; Haznedar, M.M.; New, A.S.; Chemerinski, E.; Chu, K.-W.; et al. White Matter Abnormalities in Schizophrenia and Schizotypal Personality Disorder. Schizophr. Bull. 2015, 41, 300–310. [Google Scholar] [CrossRef]
- Stämpfli, P.; Sommer, S.; Manoliu, A.; Burrer, A.; Schmidt, A.; Herdener, M.; Seifritz, E.; Kaiser, S.; Kirschner, M. Subtle White Matter Alterations in Schizophrenia Identified with a New Measure of Fiber Density. Sci. Rep. 2019, 9, 4636. [Google Scholar] [CrossRef]
- Shen, X.; Reus, L.M.; Cox, S.R.; Adams, M.J.; Liewald, D.C.; Bastin, M.E.; Smith, D.J.; Deary, I.J.; Whalley, H.C.; McIntosh, A.M. Subcortical Volume and White Matter Integrity Abnormalities in Major Depressive Disorder: Findings from UK Biobank Imaging Data. Sci. Rep. 2017, 7, 5547. [Google Scholar] [CrossRef] [PubMed]
- He, E.; Liu, M.; Gong, S.; Fu, X.; Han, Y.; Deng, F. White Matter Alterations in Depressive Disorder. Front. Immunol. 2022, 13, 826812. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Mei, B.; Hou, X.; Wang, F.; Zang, C.; Zhang, X.; Zhang, Z. White Matter Microstructure Changes in Adults with Major Depressive Disorder: Evidence from Diffusion Magnetic Resonance Imaging. BJPsych Open 2023, 9, e101. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, L.S.; Kelly, S.; Isaev, D.; Aleman, A.; Aftanas, L.I.; Bauer, J.; Baune, B.T.; Brak, I.V.; Carballedo, A.; Connolly, C.G.; et al. White Matter Disturbances in Major Depressive Disorder: A Coordinated Analysis across 20 International Cohorts in the ENIGMA MDD Working Group. Mol. Psychiatry 2020, 25, 1511–1525. [Google Scholar] [CrossRef]
- Vriend, C.; de Joode, N.T.; Pouwels, P.J.W.; Liu, F.; Otaduy, M.C.G.; Pastorello, B.; Robertson, F.C.; Ipser, J.; Lee, S.; Hezel, D.M.; et al. Age of Onset of Obsessive-Compulsive Disorder Differentially Affects White Matter Microstructure. Mol. Psychiatry 2024, 29, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Zhong, M.; Fan, J.; Liu, W.; Niu, C.; Cai, S.; Zou, L.; Wang, Y.; Wang, Y.; Tan, C.; et al. Abnormal White Matter Structural Connectivity in Adults with Obsessive-Compulsive Disorder. Transl. Psychiatry 2017, 7, e1062. [Google Scholar] [CrossRef]
- Maziero, M.P.; Seitz-Holland, J.; Cho, K.I.K.; Goldenberg, J.E.; Tanamatis, T.W.; Diniz, J.B.; Cappi, C.; Alice de Mathis, M.; Otaduy, M.C.G.; da Graça Morais Martin, M.; et al. Cellular and Extracellular White Matter Abnormalities in Obsessive-Compulsive Disorder: A Diffusion Magnetic Resonance Imaging Study. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 983–991. [Google Scholar] [CrossRef]
- Azarvand Damirichi, M.; Karimi Moridani, M.; Mohammadi, S.E. Relationship between White Matter Alterations and Contamination Subgroup in Obsessive Compulsive Disorder: A Diffusion Tensor Imaging Study. Hum. Brain Mapp. 2023, 44, 3302–3310. [Google Scholar] [CrossRef] [PubMed]
- Byne, W.; Tatusov, A.; Yiannoulos, G.; Vong, G.S.; Marcus, S. Effects of Mental Illness and Aging in Two Thalamic Nuclei. Schizophr. Res. 2008, 106, 172–181. [Google Scholar] [CrossRef]
- Cassoli, J.S.; Guest, P.C.; Malchow, B.; Schmitt, A.; Falkai, P.; Martins-de-Souza, D. Disturbed Macro-Connectivity in Schizophrenia Linked to Oligodendrocyte Dysfunction: From Structural Findings to Molecules. NPJ Schizophr. 2015, 1, 15034. [Google Scholar] [CrossRef]
- Hof, P.R.; Haroutunian, V.; Friedrich, V.L., Jr.; Byne, W.; Buitron, C.; Perl, D.P.; Davis, K.L. Loss and Altered Spatial Distribution of Oligodendrocytes in the Superior Frontal Gyrus in Schizophrenia. Biol. Psychiatry 2003, 53, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhu, Z.; Ransom, B.R.; Tong, X. Oligodendrocyte Lineage Cells and Depression. Mol. Psychiatry 2021, 26, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiao, J.; Zhang, Y.; Wang, H.; Zhu, S.; Zhang, H.; Hartle, K.; Guo, H.; Guo, W.; He, J.; et al. Desvenlafaxine Prevents White Matter Injury and Improves the Decreased Phosphorylation of the Rate-Limiting Enzyme of Cholesterol Synthesis in a Chronic Mouse Model of Depression. J. Neurochem. 2014, 131, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.B.; Cabeen, R.P.; Jann, K.; Tadayonnejad, R.; Strober, M.; Feusner, J.D. White Matter Microstructure in Habit and Reward Circuits in Anorexia Nervosa: Insights from a Neurite Orientation Dispersion and Density Imaging Study. Acta Psychiatr. Scand. 2023, 147, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.W.R. Regional Development of the Brain in Early Life. Arch. Dis. Child. 1968, 43, 388. [Google Scholar]
- Grydeland, H.; Vértes, P.E.; Váša, F.; Romero-Garcia, R.; Whitaker, K.; Alexander-Bloch, A.F.; Bjørnerud, A.; Patel, A.X.; Sederevicius, D.; Tamnes, C.K.; et al. Waves of Maturation and Senescence in Micro-Structural MRI Markers of Human Cortical Myelination over the Lifespan. Cereb. Cortex 2019, 29, 1369–1381. [Google Scholar] [CrossRef]
- de Faria, O., Jr.; Pivonkova, H.; Varga, B.; Timmler, S.; Evans, K.A.; Káradóttir, R.T. Periods of Synchronized Myelin Changes Shape Brain Function and Plasticity. Nat. Neurosci. 2021, 24, 1508–1521. [Google Scholar] [CrossRef]
- Battefeld, A.; Popovic, M.A.; de Vries, S.I.; Kole, M.H.P. High-Frequency Microdomain Ca2+ Transients and Waves during Early Myelin Internode Remodeling. Cell Rep. 2019, 26, 182–191.e5. [Google Scholar] [CrossRef]
- Pan, Y.; Monje, M. Activity Shapes Neural Circuit Form and Function: A Historical Perspective. J. Neurosci. 2020, 40, 944–954. [Google Scholar] [CrossRef]
- Saab, A.S.; Tzvetanova, I.D.; Nave, K.-A. The Role of Myelin and Oligodendrocytes in Axonal Energy Metabolism. Curr. Opin. Neurobiol. 2013, 23, 1065–1072. [Google Scholar] [CrossRef]
- Pajevic, S.; Basser, P.J.; Fields, R.D. Role of Myelin Plasticity in Oscillations and Synchrony of Neuronal Activity. Neuroscience 2014, 276, 135–147. [Google Scholar] [CrossRef]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain Development during Childhood and Adolescence: A Longitudinal MRI Study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Looser, Z.J.; Ravotto, L.; Jung, R.B.; Werner, H.B.; Ruhwedel, T.; Möbius, W.; Bergles, D.E.; Felipe Barros, L.; Nave, K.-A.; Weber, B.; et al. Potassium Regulates Axon-Oligodendrocyte Signaling and Metabolic Coupling in White Matter. bioRxiv 2022. [Google Scholar] [CrossRef]
- Iyer, M.; Kantarci, H.; Ambiel, N.; Novak, S.W.; Andrade, L.R.; Lam, M.; Münch, A.E.; Yu, X.; Khakh, B.S.; Manor, U.; et al. Oligodendrocyte Calcium Signaling Sculpts Myelin Sheath Morphology. bioRxiv 2023. [Google Scholar] [CrossRef]
- Lutz, P.-E.; Tanti, A.; Gasecka, A.; Barnett-Burns, S.; Kim, J.J.; Zhou, Y.; Chen, G.G.; Wakid, M.; Shaw, M.; Almeida, D.; et al. Association of a History of Child Abuse with Impaired Myelination in the Anterior Cingulate Cortex: Convergent Epigenetic, Transcriptional, and Morphological Evidence. Am. J. Psychiatry 2017, 174, 1185–1194. [Google Scholar] [CrossRef]
- Piller, M.; Werkman, I.L.; Brown, R.I.; Latimer, A.J.; Kucenas, S. Glutamate Signaling via the AMPAR Subunit GluR4 Regulates Oligodendrocyte Progenitor Cell Migration in the Developing Spinal Cord. J. Neurosci. 2021, 41, 5353–5371. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Hou, Y.; Huang, W.; Wang, Y.; Jiang, T.; Huang, X.; Wang, Z.; Wu, F.; Zheng, J.; Zhang, J.; et al. Loss of Schizophrenia-Related miR-501-3p in Mice Impairs Sociability and Memory by Enhancing mGluR5-Mediated Glutamatergic Transmission. Sci. Adv. 2022, 8, eabn7357. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dietz, K.; DeLoyht, J.M.; Pedre, X.; Kelkar, D.; Kaur, J.; Vialou, V.; Lobo, M.K.; Dietz, D.M.; Nestler, E.J.; et al. Impaired Adult Myelination in the Prefrontal Cortex of Socially Isolated Mice. Nat. Neurosci. 2012, 15, 1621–1623. [Google Scholar] [CrossRef] [PubMed]
- Paez, P.M.; Lyons, D.A. Calcium Signaling in the Oligodendrocyte Lineage: Regulators and Consequences. Annu. Rev. Neurosci. 2020, 43, 163–186. [Google Scholar] [CrossRef] [PubMed]
- Baraban, M.; Koudelka, S.; Lyons, D.A. Ca2+ Activity Signatures of Myelin Sheath Formation and Growth In Vivo. Nat. Neurosci. 2017, 21, 19–23. [Google Scholar] [CrossRef]
- Bergles, D.E.; Roberts, J.D.; Somogyi, P.; Jahr, C.E. Glutamatergic Synapses on Oligodendrocyte Precursor Cells in the Hippocampus. Nature 2000, 405, 187–191. [Google Scholar] [CrossRef]
- Tabuchi, K.; Blundell, J.; Etherton, M.R.; Hammer, R.E.; Liu, X.; Powell, C.M.; Südhof, T.C. A Neuroligin-3 Mutation Implicated in Autism Increases Inhibitory Synaptic Transmission in Mice. Science 2007, 318, 71–76. [Google Scholar] [CrossRef]
- Deutsch, S.I.; Rosse, R.B.; Schwartz, B.L.; Mastropaolo, J. A Revised Excitotoxic Hypothesis of Schizophrenia: Therapeutic Implications. Clin. Neuropharmacol. 2001, 24, 43–49. [Google Scholar] [CrossRef]
- Kleschevnikov, A.M.; Belichenko, P.V.; Villar, A.J.; Epstein, C.J.; Malenka, R.C.; Mobley, W.C. Hippocampal Long-Term Potentiation Suppressed by Increased Inhibition in the Ts65Dn Mouse, a Genetic Model of Down Syndrome. J. Neurosci. 2004, 24, 8153–8160. [Google Scholar] [CrossRef]
- Gao, Y.; Hong, Y.; Huang, L.; Zheng, S.; Zhang, H.; Wang, S.; Yao, Y.; Zhao, Y.; Zhu, L.; Xu, Q.; et al. β2-Microglobulin Functions as an Endogenous NMDAR Antagonist to Impair Synaptic Function. Cell 2023, 186, 1026–1038.e20. [Google Scholar] [CrossRef]
- Richter, M.M.; Ehlis, A.-C.; Jacob, C.P.; Fallgatter, A.J. Cortical Excitability in Adult Patients with Attention-Deficit/hyperactivity Disorder (ADHD). Neurosci. Lett. 2007, 419, 137–141. [Google Scholar] [CrossRef]
- Pouget, P.; Wattiez, N.; Rivaud-Péchoux, S.; Gaymard, B. A Fragile Balance: Perturbation of GABA Mediated Circuit in Prefrontal Cortex Generates High Intraindividual Performance Variability. PLoS ONE 2009, 4, e5208. [Google Scholar] [CrossRef]
- Sechi, E.; Cacciaguerra, L.; Chen, J.J.; Mariotto, S.; Fadda, G.; Dinoto, A.; Lopez-Chiriboga, A.S.; Pittock, S.J.; Flanagan, E.P. Myelin Oligodendrocyte Glycoprotein Antibody-Associated Disease (MOGAD): A Review of Clinical and MRI Features, Diagnosis, and Management. Front. Neurol. 2022, 13, 885218. [Google Scholar] [CrossRef]
- Parastarfeizabadi, M.; Kouzani, A.Z. Advances in Closed-Loop Deep Brain Stimulation Devices. J. Neuroeng. Rehabil. 2017, 14, 79. [Google Scholar] [CrossRef]
- Gilron, R.; Little, S.; Perrone, R.; Wilt, R.; de Hemptinne, C.; Yaroshinsky, M.S.; Racine, C.A.; Wang, S.S.; Ostrem, J.L.; Larson, P.S.; et al. Long-Term Wireless Streaming of Neural Recordings for Circuit Discovery and Adaptive Stimulation in Individuals with Parkinson’s Disease. Nat. Biotechnol. 2021, 39, 1078–1085. [Google Scholar] [CrossRef]
- Sun, F.T.; Morrell, M.J. The RNS System: Responsive Cortical Stimulation for the Treatment of Refractory Partial Epilepsy. Expert Rev. Med. Devices 2014, 11, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Shirvalkar, P.; Veuthey, T.L.; Dawes, H.E.; Chang, E.F. Closed-Loop Deep Brain Stimulation for Refractory Chronic Pain. Front. Comput. Neurosci. 2018, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Prosky, J.; Cagle, J.; Sellers, K.K.; Gilron, R.; de Hemptinne, C.; Schmitgen, A.; Starr, P.A.; Chang, E.F.; Shirvalkar, P. Practical Closed-Loop Strategies for Deep Brain Stimulation: Lessons From Chronic Pain. Front. Neurosci. 2021, 15, 762097. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.J.; Steinmetz, N.A.; Siegle, J.H.; Denman, D.J.; Bauza, M.; Barbarits, B.; Lee, A.K.; Anastassiou, C.A.; Andrei, A.; Aydın, Ç.; et al. Fully Integrated Silicon Probes for High-Density Recording of Neural Activity. Nature 2017, 551, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Hudry, E.; Vandenberghe, L.H. Therapeutic AAV Gene Transfer to the Nervous System: A Clinical Reality. Neuron 2019, 102, 263. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Patel, R.V.; Sharif, M.; Ashokan, A.; Michaelides, M. Chemogenetics as a Neuromodulatory Approach to Treating Neuropsychiatric Diseases and Disorders. Mol. Ther. 2022, 30, 990–1005. [Google Scholar] [CrossRef]
- Saika, F.; Matsuzaki, S.; Kobayashi, D.; Ideguchi, Y.; Nakamura, T.Y.; Kishioka, S.; Kiguchi, N. Chemogenetic Regulation of CX3CR1-Expressing Microglia Using Gi-DREADD Exerts Sex-Dependent Anti-Allodynic Effects in Mouse Models of Neuropathic Pain. Front. Pharmacol. 2020, 11, 925. [Google Scholar] [CrossRef]
- Grace, P.M.; Wang, X.; Strand, K.A.; Baratta, M.V.; Zhang, Y.; Galer, E.L.; Yin, H.; Maier, S.F.; Watkins, L.R. DREADDed Microglia in Pain: Implications for Spinal Inflammatory Signaling in Male Rats. Exp. Neurol. 2018, 304, 125–131. [Google Scholar] [CrossRef]
- Scofield, M.D.; Boger, H.A.; Smith, R.J.; Li, H.; Haydon, P.G.; Kalivas, P.W. Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-Induced Cocaine Seeking. Biol. Psychiatry 2015, 78, 441–451. [Google Scholar] [CrossRef]
- Lu, J.; Yang, L.; Xu, Y.; Ai, L.; Chen, J.; Xiong, F.; Hu, L.; Chen, H.; Liu, J.; Yan, X.; et al. The Modulatory Effect of Motor Cortex Astrocytes on Diabetic Neuropathic Pain. J. Neurosci. 2021, 41, 5287–5302. [Google Scholar] [CrossRef]
- Nwachukwu, K.N.; Evans, W.A.; Sides, T.R.; Trevisani, C.P.; Davis, A.; Marshall, S.A. Chemogenetic Manipulation of Astrocytic Signaling in the Basolateral Amygdala Reduces Binge-like Alcohol Consumption in Male Mice. J. Neurosci. Res. 2021, 99, 1957–1972. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Saglam, A.; Zuchero, J.B.; Buch, V.P. Translating Molecular Approaches to Oligodendrocyte-Mediated Neurological Circuit Modulation. Brain Sci. 2024, 14, 648. https://doi.org/10.3390/brainsci14070648
Song J, Saglam A, Zuchero JB, Buch VP. Translating Molecular Approaches to Oligodendrocyte-Mediated Neurological Circuit Modulation. Brain Sciences. 2024; 14(7):648. https://doi.org/10.3390/brainsci14070648
Chicago/Turabian StyleSong, Jingwei, Aybike Saglam, J. Bradley Zuchero, and Vivek P. Buch. 2024. "Translating Molecular Approaches to Oligodendrocyte-Mediated Neurological Circuit Modulation" Brain Sciences 14, no. 7: 648. https://doi.org/10.3390/brainsci14070648





