Centralizing the Knowledge and Interpretation of Pain in Chemotherapy-Induced Peripheral Neuropathy: A Paradigm Shift towards Brain-Centric Approaches
Abstract
:1. Introduction
2. Clinical Syndrome
3. Clinical Assessment
4. Management of CIPN
5. Mechanisms of CIPN
5.1. The Brain of CIPN Patients
5.2. Contribution of Preclinical Research
5.2.1. New Approaches in Preclinical Studies of CIPN: Imaging Studies Using MRI and Spectroscopy Analysis
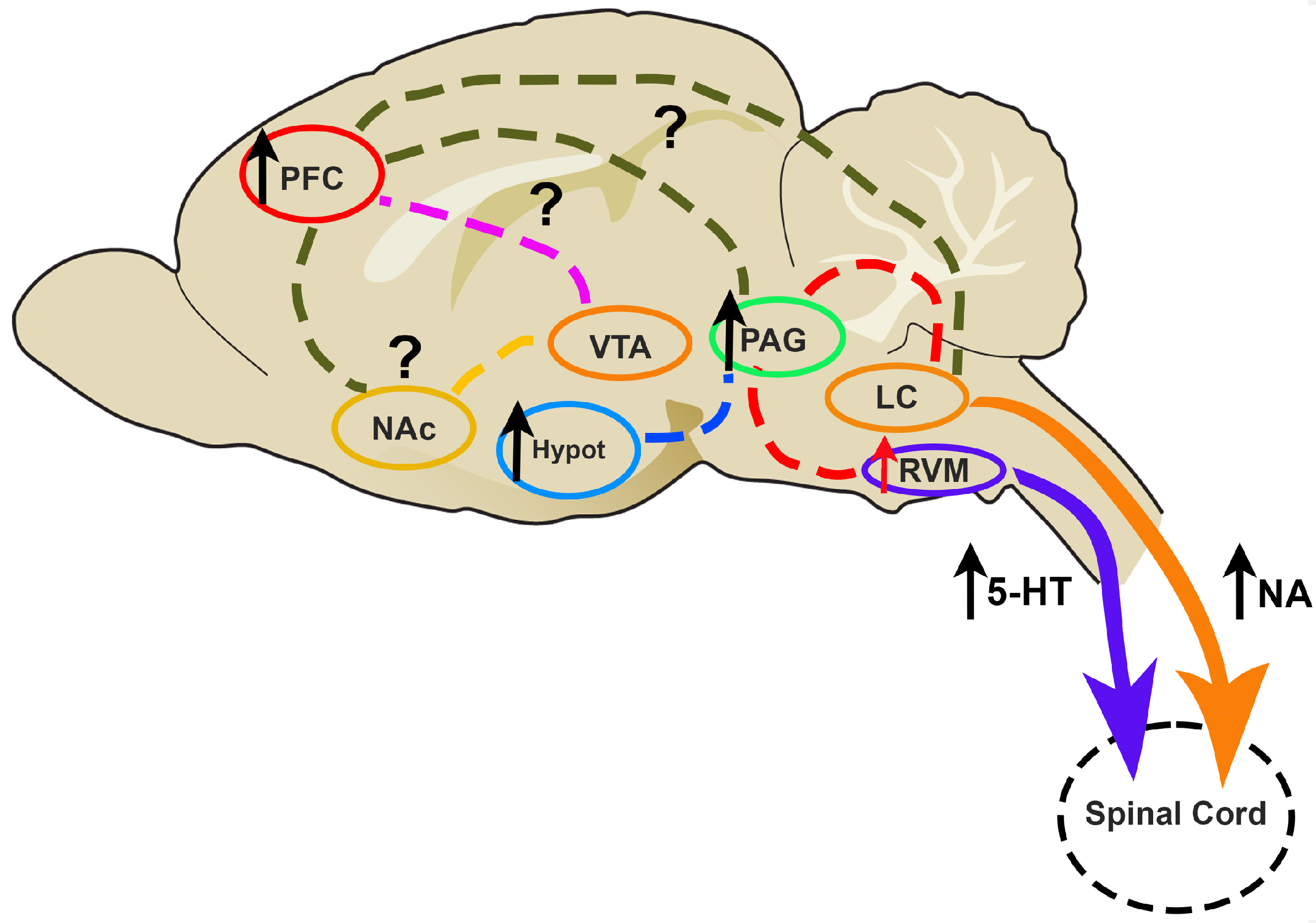
5.2.2. The Study of the Descending Pain Modulation during CIPN: Neurochemical Studies of the Serotoninergic and Noradrenergic Systems
Involvement of 5-HT in Descending Pain Modulation
Involvement of NA in Descending Pain Modulation
Other Neurochemical Systems
Pharmacological Interventions
6. Concluding Remarks and Future Perspectives
- Targeted Therapies: Understanding how the brain processes pain and responds to chemotherapy allows for developing treatments targeting these specific mechanisms. This means that medications and interventions can be tailored to the individual, addressing their unique pain processing and tolerance;
- Reducing Side Effects: A personalized approach based on a patient’s brain responses can reduce the risk of CIPN. By selecting prophylactic treatments, it is possible to mitigate or prevent painful CIPN;
- Improved Treatment Outcomes: The ability to reduce side effects based on an individual’s brain profile can improve treatment outcomes. By avoiding or minimizing CIPN, patients may be more likely to complete their prescribed chemotherapy regimens, leading to improved cancer treatment success;
- Enhanced Quality of Life: CIPN can have a profound impact on a patient’s quality of life, as it often leads to chronic pain and limitations in daily activities. Personalized treatment that minimizes the risk of CIPN can contribute to a better quality of life during and after cancer treatment;
- Reducing Healthcare Costs: Effective personalized treatment of CIPN can potentially reduce healthcare costs associated with treating CIPN-related complications, including pain management and rehabilitative care.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NCI. National Cancer Institute—Side Effects of Cancer Treatment. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 31 October 2023).
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Pike, C.T.; Birnbaum, H.G.; Muehlenbein, C.E.; Pohl, G.M.; Natale, R.B. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother. Res. Pract. 2012, 2012, 913848. [Google Scholar] [CrossRef] [PubMed]
- Colvin, L.A. Chemotherapy-induced peripheral neuropathy: Where are we now? Pain 2019, 160 (Suppl. S1), S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.T. Neuropathic pain in cancer. Br. J. Anaesth. 2013, 111, 105–111. [Google Scholar] [CrossRef]
- Cavaletti, G.; Marmiroli, P. Chemotherapy-induced peripheral neurotoxicity. Curr. Opin. Neurol. 2015, 28, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Sisignano, M.; Baron, R.; Scholich, K.; Geisslinger, G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat. Rev. Neurol. 2014, 10, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Boyette-Davis, J.A.; Hou, S.; Abdi, S.; Dougherty, P.M. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag. 2018, 8, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Kavelaars, A.; Dougherty, P.M.; Heijnen, C.J. Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source. Cancer 2018, 124, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Heinricher, M.M.; Tavares, I.; Leith, J.L.; Lumb, B.M. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res. Rev. 2009, 60, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef] [PubMed]
- Bannister, K.; Dickenson, A.H. What do monoamines do in pain modulation? Curr. Opin. Support. Palliat. Care 2016, 10, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Tavares, I.; Costa-Pereira, J.T.; Martins, I. Monoaminergic and Opioidergic Modulation of Brainstem Circuits: New Insights Into the Clinical Challenges of Pain Treatment? Front. Pain Res. 2021, 2, 696515. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending pain modulation and chronification of pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I.; Mantyh, P.W. The cerebral signature for pain perception and its modulation. Neuron 2007, 55, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Mezzanotte, J.N.; Grimm, M.; Shinde, N.V.; Nolan, T.; Worthen-Chaudhari, L.; Williams, N.O.; Lustberg, M.B. Updates in the Treatment of Chemotherapy-Induced Peripheral Neuropathy. Curr. Treat. Options Oncol. 2022, 23, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I. Neuroimaging mechanisms in pain: From discovery to translation. Pain 2017, 158 (Suppl. S1), S115–S122. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Cioroiu, C.; Weimer, L.H. Update on Chemotherapy-Induced Peripheral Neuropathy. Curr. Neurol. Neurosci. Rep. 2017, 17, 47. [Google Scholar] [CrossRef] [PubMed]
- Maihöfner, C.; Diel, I.; Tesch, H.; Quandel, T.; Baron, R. Chemotherapy-induced peripheral neuropathy (CIPN): Current therapies and topical treatment option with high-concentration capsaicin. Support. Care Cancer 2021, 29, 4223–4238. [Google Scholar] [CrossRef]
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Sprauten, M.; Darrah, T.H.; Peterson, D.R.; Campbell, M.E.; Hannigan, R.E.; Cvancarova, M.; Beard, C.; Haugnes, H.S.; Fosså, S.D.; Oldenburg, J.; et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in Cisplatin-treated survivors of testicular cancer. J. Clin. Oncol. 2012, 30, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on Chemotherapy-Induced Peripheral Neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-induced peripheral neurotoxicity: A critical analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Chemotherapy-induced peripheral neuropathy and rehabilitation: A review. Semin. Oncol. 2021, 48, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Mi, W.L.; Wu, G.C.; Wang, Y.Q.; Mao-Ying, Q.L. Prevention and Treatment for Chemotherapy-Induced Peripheral Neuropathy: Therapies Based on CIPN Mechanisms. Curr. Neuropharmacol. 2019, 17, 184–196. [Google Scholar] [CrossRef]
- Grisold, W.; Cavaletti, G.; Windebank, A.J. Peripheral neuropathies from chemotherapeutics and targeted agents: Diagnosis, treatment, and prevention. Neuro Oncol. 2012, 14 (Suppl. S4), iv45–iv54. [Google Scholar] [CrossRef] [PubMed]
- Bernhardson, B.M.; Tishelman, C.; Rutqvist, L.E. Chemosensory changes experienced by patients undergoing cancer chemotherapy: A qualitative interview study. J. Pain Symptom Manag. 2007, 34, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Kolb, N.A.; Smith, A.G.; Singleton, J.R.; Beck, S.L.; Stoddard, G.J.; Brown, S.; Mooney, K. The Association of Chemotherapy-Induced Peripheral Neuropathy Symptoms and the Risk of Falling. JAMA Neurol. 2016, 73, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Mols, F.; van de Poll-Franse, L.V.; Vreugdenhil, G.; Beijers, A.J.; Kieffer, J.M.; Aaronson, N.K.; Husson, O. Reference data of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-CIPN20 Questionnaire in the general Dutch population. Eur. J. Cancer 2016, 69, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Gewandter, J.S.; Brell, J.; Cavaletti, G.; Dougherty, P.M.; Evans, S.; Howie, L.; McDermott, M.P.; O’Mara, A.; Smith, A.G.; Dastros-Pitei, D.; et al. Trial designs for chemotherapy-induced peripheral neuropathy prevention: ACTTION recommendations. Neurology 2018, 91, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Cavaletti, G.; Cornblath, D.R.; Merkies, I.S.J.; Postma, T.J.; Rossi, E.; Frigeni, B.; Alberti, P.; Bruna, J.; Velasco, R.; Argyriou, A.A.; et al. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: From consensus to the first validity and reliability findings. Ann. Oncol. 2013, 24, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.C., 3rd; Backonja, M.M. Review of neuropathic pain screening and assessment tools. Curr. Pain Headache Rep. 2013, 17, 363. [Google Scholar] [CrossRef]
- Haanpää, M.; Attal, N.; Backonja, M.; Baron, R.; Bennett, M.; Bouhassira, D.; Cruccu, G.; Hansson, P.; Haythornthwaite, J.A.; Iannetti, G.D.; et al. NeuPSIG guidelines on neuropathic pain assessment. Pain 2011, 152, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Park, S.B.; Islam, B.; Tamburin, S.; Velasco, R.; Alberti, P.; Bruna, J.; Psimaras, D.; Cavaletti, G.; Cornblath, D.R. Neurophysiological, nerve imaging and other techniques to assess chemotherapy-induced peripheral neurotoxicity in the clinical and research settings. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Fuglsang-Frederiksen, A.; Pugdahl, K. Current status on electrodiagnostic standards and guidelines in neuromuscular disorders. Clin. Neurophysiol. 2011, 122, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.Y.; Ehrlich, B.E. Prevention of chemotherapy-induced peripheral neuropathy: A review of recent findings. Crit. Rev. Oncol. Hematol. 2020, 145, 102831. [Google Scholar] [CrossRef]
- McCrary, J.M.; Goldstein, D.; Boyle, F.; Cox, K.; Grimison, P.; Kiernan, M.C.; Krishnan, A.V.; Lewis, C.R.; Webber, K.; Baron-Hay, S.; et al. Optimal clinical assessment strategies for chemotherapy-induced peripheral neuropathy (CIPN): A systematic review and Delphi survey. Support. Care Cancer 2017, 25, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, S.G.; Kleckner, I.R.; Barton, D.; Mustian, K.; O’Mara, A.; St Germain, D.; Cavaletti, G.; Danhauer, S.C.; Hershman, D.L.; Hohmann, A.G.; et al. The National Cancer Institute Clinical Trials Planning Meeting for Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. J. Natl. Cancer Inst. 2019, 111, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Pang, H.; Cirrincione, C.; Fleishman, S.; Paskett, E.D.; Ahles, T.; Bressler, L.R.; Fadul, C.E.; Knox, C.; Le-Lindqwister, N.; et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: A randomized clinical trial. JAMA 2013, 309, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef]
- Stillman, M.; Cata, J.P. Management of chemotherapy-induced peripheral neuropathy. Curr. Pain Headache Rep. 2006, 10, 279–287. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Alvarez, G.A.M.; Dombovy-Johnson, M.; Eller, J.; Abd-Elsayed, A. Evidence-Based Treatment of Pain in Chemotherapy-Induced Peripheral Neuropathy. Curr. Pain Headache Rep. 2023, 27, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Farshchian, N.; Alavi, A.; Heydarheydari, S.; Moradian, N. Comparative study of the effects of venlafaxine and duloxetine on chemotherapy-induced peripheral neuropathy. Cancer Chemother. Pharmacol. 2018, 82, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Klafke, N.; Bossert, J.; Kröger, B.; Neuberger, P.; Heyder, U.; Layer, M.; Winkler, M.; Idler, C.; Kaschdailewitsch, E.; Heine, R.; et al. Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy (CIPN) with Non-Pharmacological Interventions: Clinical Recommendations from a Systematic Scoping Review and an Expert Consensus Process. Med. Sci. 2023, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.; Stamou, M.; Bakalidou, D.; Moschovos, C.; Zouvelou, V.; Zis, P.; Tzartos, J.; Chroni, E.; Michopoulos, I.; Tsivgoulis, G. Non-pharmacological Interventions on Pain and Quality of Life in Chemotherapy Induced Polyneuropathy: Systematic Review and Meta-Analysis. In Vivo 2023, 37, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 1, Cd011279. [Google Scholar] [CrossRef]
- Huang, Y.; Tan, T.; Liu, L.; Yan, Z.; Deng, Y.; Li, G.; Li, M.; Xiong, J. Exercise for reducing chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis of randomized controlled trials. Front. Neurol. 2023, 14, 1252259. [Google Scholar] [CrossRef]
- Cao, A.; Cartmel, B.; Li, F.Y.; Gottlieb, L.T.; Harrigan, M.; Ligibel, J.A.; Gogoi, R.; Schwartz, P.E.; Esserman, D.A.; Irwin, M.L.; et al. Effect of Exercise on Chemotherapy-Induced Peripheral Neuropathy Among Patients Treated for Ovarian Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2326463. [Google Scholar] [CrossRef]
- Prinsloo, S.; Novy, D.; Driver, L.; Lyle, R.; Ramondetta, L.; Eng, C.; Lopez, G.; Li, Y.; Cohen, L. The Long-Term Impact of Neurofeedback on Symptom Burden and Interference in Patients With Chronic Chemotherapy-Induced Neuropathy: Analysis of a Randomized Controlled Trial. J. Pain Symptom Manag. 2018, 55, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Prinsloo, S.; Novy, D.; Driver, L.; Lyle, R.; Ramondetta, L.; Eng, C.; McQuade, J.; Lopez, G.; Cohen, L. Randomized controlled trial of neurofeedback on chemotherapy-induced peripheral neuropathy: A pilot study. Cancer 2017, 123, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Tian, Y.; Xu, S.; Chen, H. Oxaliplatin-induced peripheral neuropathy: Clinical features, mechanisms, prevention and treatment. J. Neurol. 2021, 268, 3269–3282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Trendowski, M.R.; Wilkinson, E.; Shahbazi, M.; Dinh, P.C.; Shuey, M.M.; Feldman, D.R.; Hamilton, R.J.; Vaughn, D.J.; Fung, C.; et al. Pharmacogenomics of cisplatin-induced neurotoxicities: Hearing loss, tinnitus, and peripheral sensory neuropathy. Cancer Med. 2022, 11, 2801–2816. [Google Scholar] [CrossRef] [PubMed]
- Triarico, S.; Romano, A.; Attinà, G.; Capozza, M.A.; Maurizi, P.; Mastrangelo, S.; Ruggiero, A. Vincristine-Induced Peripheral Neuropathy (VIPN) in Pediatric Tumors: Mechanisms, Risk Factors, Strategies of Prevention and Treatment. Int. J. Mol. Sci. 2021, 22, 4112. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, L.T.; Thomas, E.S.; Roché, H.H.; Hortobagyi, G.N.; Sparano, J.A.; Yelle, L.; Fornier, M.N.; Martín, M.; Bunnell, C.A.; Mukhopadhyay, P.; et al. Ixabepilone-associated peripheral neuropathy: Data from across the phase II and III clinical trials. Support. Care Cancer 2012, 20, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Tamburin, S.; Park, S.B.; Alberti, P.; Demichelis, C.; Schenone, A.; Argyriou, A.A. Taxane and epothilone-induced peripheral neurotoxicity: From pathogenesis to treatment. J. Peripher. Nerv. Syst. 2019, 24 (Suppl. S2), S40–S51. [Google Scholar] [CrossRef] [PubMed]
- Staff, N.P.; Fehrenbacher, J.C.; Caillaud, M.; Damaj, M.I.; Segal, R.A.; Rieger, S. Pathogenesis of paclitaxel-induced peripheral neuropathy: A current review of in vitro and in vivo findings using rodent and human model systems. Exp. Neurol. 2020, 324, 113121. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.L.; Molassiotis, A.; Leung, A.K.T.; Wong, K.H. Docetaxel-Induced Peripheral Neuropathy in Breast Cancer Patients Treated with Adjuvant or Neo-Adjuvant Chemotherapy. Breast Care 2021, 16, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Egashira, N. Pathological Mechanisms of Bortezomib-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2021, 22, 888. [Google Scholar] [CrossRef] [PubMed]
- Cundari, S.; Cavaletti, G. Thalidomide chemotherapy-induced peripheral neuropathy: Actual status and new perspectives with thalidomide analogues derivatives. Mini Rev. Med. Chem. 2009, 9, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Condé, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Ollodart, J.; Steele, L.R.; Romero-Sandoval, E.A.; Strowd, R.E.; Shiozawa, Y. Contributions of neuroimmune interactions to chemotherapy-induced peripheral neuropathy development and its prevention/therapy. Biochem. Pharmacol. 2024, 222, 116070. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gan, Y.; Au, N.P.B.; Ma, C.H.E. Current understanding of the molecular mechanisms of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2024, 17, 1345811. [Google Scholar] [CrossRef] [PubMed]
- Kacem, H.; Cimini, A.; d’Angelo, M.; Castelli, V. Molecular and Cellular Involvement in CIPN. Biomedicines 2024, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xiao, W.H.; Bennett, G.J. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp. Neurol. 2012, 238, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xiao, W.H.; Bennett, G.J. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp. Neurol. 2011, 232, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.H.; Bennett, G.J. Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain 2012, 153, 704–709. [Google Scholar] [CrossRef]
- Xiao, W.H.; Zheng, H.; Bennett, G.J. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience 2012, 203, 194–206. [Google Scholar] [CrossRef]
- De Grandis, D. Acetyl-L-carnitine for the treatment of chemotherapy-induced peripheral neuropathy: A short review. CNS Drugs 2007, 21 (Suppl. S1), 39–43; discussion 36–45. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Unger, J.M.; Crew, K.D.; Minasian, L.M.; Awad, D.; Moinpour, C.M.; Hansen, L.; Lew, D.L.; Greenlee, H.; Fehrenbacher, L.; et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J. Clin. Oncol. 2013, 31, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, M.; Otake, S.; Murakami, T.; Yamamoto, S.; Makino, T.; Ono, H. Gabapentin prevents oxaliplatin-induced mechanical hyperalgesia in mice. J. Pharmacol. Sci. 2014, 125, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Tateishi, K.; Tsubaki, M.; Takeda, T.; Matsumoto, M.; Tsurushima, K.; Ishizaka, T.; Nishida, S. Gabapentin and Duloxetine Prevent Oxaliplatin- and Paclitaxel-Induced Peripheral Neuropathy by Inhibiting Extracellular Signal-Regulated Kinase 1/2 (ERK1/2) Phosphorylation in Spinal Cords of Mice. Pharmaceuticals 2020, 14, 30. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Chiba, T.; Katagiri, N.; Saduka, M.; Abe, K.; Utsunomiya, I.; Hama, T.; Taguchi, K. Paclitaxel increases high voltage-dependent calcium channel current in dorsal root ganglion neurons of the rat. J. Pharmacol. Sci. 2012, 120, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Tsuboi, M.; Kambe, T.; Abe, K.; Nakatani, Y.; Kawakami, K.; Utsunomiya, I.; Taguchi, K. Oxaliplatin administration increases expression of the voltage-dependent calcium channel α2δ-1 subunit in the rat spinal cord. J. Pharmacol. Sci. 2016, 130, 117–122. [Google Scholar] [CrossRef]
- Pandey, P.; Kumar, A.; Pushpam, D.; Khurana, S.; Malik, P.S.; Gogia, A.; Arunmozhimaran, E.; Singh, M.B.; Chandran, D.S.; Batra, A. Randomized double-blind, placebo-controlled study of oral gabapentin for prevention of neuropathy in patients receiving paclitaxel. Trials 2023, 24, 79. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy. Front. Immunol. 2020, 11, 626687. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pereira, J.T.; Oliveira, R.; Guadilla, I.; Guillen, M.J.; Tavares, I.; Lopez-Larrubia, P. Neuroimaging uncovers neuronal and metabolic changes in pain modulatory brain areas in a rat model of chemotherapy-induced neuropathy—MEMRI and ex vivo spectroscopy studies. Brain Res. Bull. 2023, 192, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Stein, C.; Gaveriaux-Ruff, C. The Oxford Handbook of the Neurobiology of Pain; Oxford University Press: Oxford, UK, 2020. [Google Scholar] [CrossRef]
- Odling-Smee, L. Chronic pain can be treated—So why are millions still suffering? Nature 2023, 615, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.; Branco, P.; Pinto-Ramos, J.; Vigotsky, A.D.; Reis, A.M.; Schnitzer, T.J.; Galhardo, V.; Apkarian, A.V. Subcortical brain anatomy as a potential biomarker of persistent pain after total knee replacement in osteoarthritis. Pain 2023, 164, 2306–2315. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.; Wakaizumi, K.; Reis, A.M.; Baliki, M.; Schnitzer, T.J.; Galhardo, V.; Apkarian, A.V. Reorganization of functional brain network architecture in chronic osteoarthritis pain. Hum. Brain Mapp. 2021, 42, 1206–1222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hu, H.; Das, S.K.; Yang, M.J.; Li, B.; Li, Y.; Xu, X.X.; Yang, H.F. Structural and Functional Brain Abnormalities in Trigeminal Neuralgia: A Systematic Review. J. Oral. Facial Pain Headache 2020, 34, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, M.; Zheng, T.; Xiao, Y.; Wang, S.; Han, F.; Chen, G. Structural and functional brain abnormalities in postherpetic neuralgia: A systematic review of neuroimaging studies. Brain Res. 2021, 1752, 147219. [Google Scholar] [CrossRef]
- Medrano-Escalada, Y.; Plaza-Manzano, G.; Fernández-de-Las-Peñas, C.; Valera-Calero, J.A. Structural, Functional and Neurochemical Cortical Brain Changes Associated with Chronic Low Back Pain. Tomography 2022, 8, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Tseng, M.T.; Hsieh, P.C.; Lin, C.J.; Huang, S.L.; Hsieh, S.T.; Chiang, M.C. Brain Mechanisms of Pain and Dysautonomia in Diabetic Neuropathy: Connectivity Changes in Thalamus and Hypothalamus. J. Clin. Endocrinol. Metab. 2022, 107, e1167–e1180. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.Z.; Waxman, S.G. Neuropathic pain in diabetes--evidence for a central mechanism. Nat. Rev. Neurol. 2010, 6, 462–466. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, K.N.; McDonald, B.C.; Wang, Y.; Smith, D.J.; West, J.D.; O’Neill, D.P.; Zanville, N.R.; Champion, V.L.; Schneider, B.P.; Saykin, A.J. Cerebral Perfusion and Gray Matter Changes Associated With Chemotherapy-Induced Peripheral Neuropathy. J. Clin. Oncol. 2016, 34, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Boland, E.G.; Selvarajah, D.; Hunter, M.; Ezaydi, Y.; Tesfaye, S.; Ahmedzai, S.H.; Snowden, J.A.; Wilkinson, I.D. Central pain processing in chronic chemotherapy-induced peripheral neuropathy: A functional magnetic resonance imaging study. PLoS ONE 2014, 9, e96474. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Romaniuk, L.; Whalley, H.; Sladdin, K.; Lawrie, S.; Warnaby, C.E.; Roberts, N.; Colvin, L.; Tracey, I.; Fallon, M. Neuroimaging reveals a potential brain-based pre-existing mechanism that confers vulnerability to development of chronic painful chemotherapy-induced peripheral neuropathy. Br. J. Anaesth. 2023, 130, 83–93. [Google Scholar] [CrossRef]
- Bacalhau, C.; Costa-Pereira, J.T.; Tavares, I. Preclinical research in paclitaxel-induced neuropathic pain: A systematic review. Front. Vet. Sci. 2023, 10, 1264668. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.F.; Nodine, S.; Pottala, T.; Cai, X.; Knox, T.M.; Fofana, F.H.; Kim, S.; Kulkarni, P.; Crystal, J.D.; Hohmann, A.G. Alterations in brain neurocircuitry following treatment with the chemotherapeutic agent paclitaxel in rats. Neurobiol. Pain 2019, 6, 100034. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, K.; Yamanaka, K.; Ogawa, S.; Takamatsu, H.; Higo, N. Brain activity changes in a macaque model of oxaliplatin-induced neuropathic cold hypersensitivity. Sci. Rep. 2017, 7, 4305. [Google Scholar] [CrossRef] [PubMed]
- Shidahara, Y.; Natsume, T.; Awaga, Y.; Ogawa, S.; Yamoto, K.; Okamoto, S.; Hama, A.; Hayashi, I.; Takamatsu, H.; Magata, Y. Distinguishing analgesic drugs from non-analgesic drugs based on brain activation in macaques with oxaliplatin-induced neuropathic pain. Neuropharmacology 2019, 149, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, T.A.; Ennis, M.; Behbehani, M.M.; Shipley, M.T. Connections between the central nucleus of the amygdala and the midbrain periaqueductal gray: Topography and reciprocity. J. Comp. Neurol. 1991, 303, 121–131. [Google Scholar] [CrossRef]
- Bandler, R.; Keay, K.A. Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog. Brain Res. 1996, 107, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Floyd, N.S.; Price, J.L.; Ferry, A.T.; Keay, K.A.; Bandler, R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J. Comp. Neurol. 2000, 422, 556–578. [Google Scholar] [CrossRef]
- Ennis, M.; Behbehani, M.; Shipley, M.T.; Van Bockstaele, E.J.; Aston-Jones, G. Projections from the periaqueductal gray to the rostromedial pericoerulear region and nucleus locus coeruleus: Anatomic and physiologic studies. J. Comp. Neurol. 1991, 306, 480–494. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Fields, H.L. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984, 7, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Cobos, A.; Tavares, I.; Lima, D. Brain afferents to the medullary dorsal reticular nucleus: A retrograde and anterograde tracing study in the rat. Eur. J. Neurosci. 2002, 16, 81–95. [Google Scholar] [CrossRef]
- Cobos, A.; Lima, D.; Almeida, A.; Tavares, I. Brain afferents to the lateral caudal ventrolateral medulla: A retrograde and anterograde tracing study in the rat. Neuroscience 2003, 120, 485–498. [Google Scholar] [CrossRef]
- Behbehani, M.M.; Fields, H.L. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979, 170, 85–93. [Google Scholar] [CrossRef]
- Porreca, F.; Ossipov, M.H.; Gebhart, G.F. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002, 25, 319–325. [Google Scholar] [CrossRef]
- Bajic, D.; Proudfit, H.K. Projections of neurons in the periaqueductal gray to pontine and medullary catecholamine cell groups involved in the modulation of nociception. J. Comp. Neurol. 1999, 405, 359–379. [Google Scholar] [CrossRef]
- Porreca, F.; Navratilova, E. Reward, motivation, and emotion of pain and its relief. Pain 2017, 158 (Suppl. S1), S43–S49. [Google Scholar] [CrossRef]
- Song, Q.; Wei, A.; Xu, H.; Gu, Y.; Jiang, Y.; Dong, N.; Zheng, C.; Wang, Q.; Gao, M.; Sun, S.; et al. An ACC-VTA-ACC positive-feedback loop mediates the persistence of neuropathic pain and emotional consequences. Nat. Neurosci. 2024, 27, 272–285. [Google Scholar] [CrossRef]
- Juarez-Salinas, D.L.; Braz, J.M.; Etlin, A.; Gee, S.; Sohal, V.; Basbaum, A.I. GABAergic cell transplants in the anterior cingulate cortex reduce neuropathic pain aversiveness. Brain 2019, 142, 2655–2669. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpaa, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Da Silva, J.T.; Seminowicz, D.A. Neuroimaging of pain in animal models: A review of recent literature. Pain Rep. 2019, 4, e732. [Google Scholar] [CrossRef]
- Thompson, S.J.; Bushnell, M.C. Rodent functional and anatomical imaging of pain. Neurosci. Lett. 2012, 520, 131–139. [Google Scholar] [CrossRef]
- Morris, L.S.; Sprenger, C.; Koda, K.; de la Mora, D.M.; Yamada, T.; Mano, H.; Kashiwagi, Y.; Yoshioka, Y.; Morioka, Y.; Seymour, B. Anterior cingulate cortex connectivity is associated with suppression of behaviour in a rat model of chronic pain. Brain Neurosci. Adv. 2018, 2, 2398212818779646. [Google Scholar] [CrossRef]
- Chang, P.C.; Centeno, M.V.; Procissi, D.; Baria, A.; Apkarian, A.V. Brain activity for tactile allodynia: A longitudinal awake rat functional magnetic resonance imaging study tracking emergence of neuropathic pain. Pain 2017, 158, 488–497. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Kang, J.H. Investigation of spinal nerve ligation-mediated functional activation of the rat brain using manganese-enhanced MRI. Exp. Anim. 2018, 67, 23–29. [Google Scholar] [CrossRef]
- Onishi, O.; Ikoma, K.; Oda, R.; Yamazaki, T.; Fujiwara, H.; Yamada, S.; Tanaka, M.; Kubo, T. Sequential variation in brain functional magnetic resonance imaging after peripheral nerve injury: A rat study. Neurosci. Lett. 2018, 673, 150–156. [Google Scholar] [CrossRef]
- Yang, Y.H.; Lin, J.K.; Chen, W.S.; Lin, T.C.; Yang, S.H.; Jiang, J.K.; Chang, S.C.; Lan, Y.T.; Lin, C.C.; Yen, C.C.; et al. Duloxetine improves oxaliplatin-induced neuropathy in patients with colorectal cancer: An open-label pilot study. Support. Care Cancer 2012, 20, 1491–1497. [Google Scholar] [CrossRef]
- Costa-Pereira, J.T.; Serrao, P.; Martins, I.; Tavares, I. Serotoninergic pain modulation from the rostral ventromedial medulla (RVM) in chemotherapy-induced neuropathy: The role of spinal 5-HT3 receptors. Eur. J. Neurosci. 2020, 51, 1756–1769. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Ai, G.; Xu, X.; Niu, X.; Zhang, M. Selective Ablation of Descending Serotonin from the Rostral Ventromedial Medulla Unmasks Its Pro-Nociceptive Role in Chemotherapy-Induced Painful Neuropathy. J. Pain Res. 2020, 13, 3081–3094. [Google Scholar] [CrossRef]
- Gang, J.; Park, K.T.; Kim, S.; Kim, W. Involvement of the Spinal Serotonergic System in the Analgesic Effect of [6]-Shogaol in Oxaliplatin-Induced Neuropathic Pain in Mice. Pharmaceuticals 2023, 16, 1465. [Google Scholar] [CrossRef]
- Kim, Y.O.; Song, J.A.; Kim, W.M.; Yoon, M.H. Antiallodynic Effect of Intrathecal Korean Red Ginseng in Cisplatin-Induced Neuropathic Pain Rats. Pharmacology 2020, 105, 173–180. [Google Scholar] [CrossRef]
- Lee, J.H.; Min, D.; Lee, D.; Kim, W. Zingiber officinale Roscoe Rhizomes Attenuate Oxaliplatin-Induced Neuropathic Pain in Mice. Molecules 2021, 26, 548. [Google Scholar] [CrossRef]
- Andoh, T.; Sakamoto, A.; Kuraishi, Y. Effects of xaliproden, a 5-HT(1)A agonist, on mechanical allodynia caused by chemotherapeutic agents in mice. Eur. J. Pharmacol. 2013, 721, 231–236. [Google Scholar] [CrossRef]
- Thibault, K.; Van Steenwinckel, J.; Brisorgueil, M.J.; Fischer, J.; Hamon, M.; Calvino, B.; Conrath, M. Serotonin 5-HT2A receptor involvement and Fos expression at the spinal level in vincristine-induced neuropathy in the rat. Pain 2008, 140, 305–322. [Google Scholar] [CrossRef]
- Chenaf, C.; Chapuy, E.; Libert, F.; Marchand, F.; Courteix, C.; Bertrand, M.; Gabriel, C.; Mocaer, E.; Eschalier, A.; Authier, N. Agomelatine: A new opportunity to reduce neuropathic pain-preclinical evidence. Pain 2017, 158, 149–160. [Google Scholar] [CrossRef]
- Usman, M.; Malik, H.; Tokhi, A.; Arif, M.; Huma, Z.; Rauf, K.; Sewell, R.D.E. 5,7-Dimethoxycoumarin ameliorates vincristine induced neuropathic pain: Potential role of 5HT(3) receptors and monoamines. Front. Pharmacol. 2023, 14, 1213763. [Google Scholar] [CrossRef]
- Park, K.T.; Kim, S.; Choi, I.; Han, I.H.; Bae, H.; Kim, W. The involvement of the noradrenergic system in the antinociceptive effect of cucurbitacin D on mice with paclitaxel-induced neuropathic pain. Front. Pharmacol. 2022, 13, 1055264. [Google Scholar] [CrossRef]
- Costa-Pereira, J.T.; Ribeiro, J.; Martins, I.; Tavares, I. Role of Spinal Cord alpha(2)-Adrenoreceptors in Noradrenergic Inhibition of Nociceptive Transmission during Chemotherapy-Induced Peripheral Neuropathy. Front. Neurosci. 2019, 13, 1413. [Google Scholar] [CrossRef]
- Messing, R.B.; Lytle, L.D. Serotonin-containing neurons: Their possible role in pain and analgesia. Pain 1977, 4, 1–21. [Google Scholar] [CrossRef]
- Dogrul, A.; Ossipov, M.H.; Porreca, F. Differential mediation of descending pain facilitation and inhibition by spinal 5HT-3 and 5HT-7 receptors. Brain Res. 2009, 1280, 52–59. [Google Scholar] [CrossRef]
- Viisanen, H.; Pertovaara, A. Roles of the rostroventromedial medulla and the spinal 5-HT(1A) receptor in descending antinociception induced by motor cortex stimulation in the neuropathic rat. Neurosci. Lett. 2010, 476, 133–137. [Google Scholar] [CrossRef]
- Rahman, W.; Bannister, K.; Bee, L.A.; Dickenson, A.H. A pronociceptive role for the 5-HT2 receptor on spinal nociceptive transmission: An in vivo electrophysiological study in the rat. Brain Res. 2011, 1382, 29–36. [Google Scholar] [CrossRef]
- Bardoni, R. Serotonergic Modulation of Nociceptive Circuits in Spinal Cord Dorsal Horn. Curr. Neuropharmacol. 2019, 17, 1133–1145. [Google Scholar] [CrossRef]
- Wei, F.; Dubner, R.; Zou, S.; Ren, K.; Bai, G.; Wei, D.; Guo, W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J. Neurosci. 2010, 30, 8624–8636. [Google Scholar] [CrossRef]
- Rahman, W.; Suzuki, R.; Webber, M.; Hunt, S.P.; Dickenson, A.H. Depletion of endogenous spinal 5-HT attenuates the behavioural hypersensitivity to mechanical and cooling stimuli induced by spinal nerve ligation. Pain 2006, 123, 264–274. [Google Scholar] [CrossRef]
- Satoh, O.; Omote, K. Roles of monoaminergic, glycinergic and GABAergic inhibitory systems in the spinal cord in rats with peripheral mononeuropathy. Brain Res. 1996, 728, 27–36. [Google Scholar] [CrossRef]
- Morgado, C.; Silva, L.; Pereira-Terra, P.; Tavares, I. Changes in serotoninergic and noradrenergic descending pain pathways during painful diabetic neuropathy: The preventive action of IGF1. Neurobiol. Dis. 2011, 43, 275–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Xin, W.J.; He, S.; Deng, J.; Ruan, X. Somatostatin Neurons from Periaqueductal Gray to Medulla Facilitate Neuropathic Pain in Male Mice. J. Pain 2023, 24, 1020–1029. [Google Scholar] [CrossRef]
- White, D.; Abdulla, M.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G.; Lees, J.G. Targeting translation: A review of preclinical animal models in the development of treatments for chemotherapy-induced peripheral neuropathy. J. Peripher. Nerv. Syst. 2023, 28, 179–190. [Google Scholar] [CrossRef]
- Andoh, T.; Sakamoto, A.; Kuraishi, Y. 5-HT1A receptor agonists, xaliproden and tandospirone, inhibit the increase in the number of cutaneous mast cells involved in the exacerbation of mechanical allodynia in oxaliplatin-treated mice. J. Pharmacol. Sci. 2016, 131, 284–287. [Google Scholar] [CrossRef]
- Salat, K.; Kolaczkowski, M.; Furgala, A.; Rojek, A.; Sniecikowska, J.; Varney, M.A.; Newman-Tancredi, A. Antinociceptive, antiallodynic and antihyperalgesic effects of the 5-HT(1A) receptor selective agonist, NLX-112 in mouse models of pain. Neuropharmacology 2017, 125, 181–188. [Google Scholar] [CrossRef]
- Rapacz, A.; Obniska, J.; Koczurkiewicz, P.; Wojcik-Pszczola, K.; Siwek, A.; Grybos, A.; Rybka, S.; Karcz, A.; Pekala, E.; Filipek, B. Antiallodynic and antihyperalgesic activity of new 3,3-diphenyl-propionamides with anticonvulsant activity in models of pain in mice. Eur. J. Pharmacol. 2018, 821, 39–48. [Google Scholar] [CrossRef]
- Masuguchi, K.; Watanabe, H.; Kawashiri, T.; Ushio, S.; Ozawa, N.; Morita, H.; Oishi, R.; Egashira, N. Neurotropin(R) relieves oxaliplatin-induced neuropathy via Gi protein-coupled receptors in the monoaminergic descending pain inhibitory system. Life Sci. 2014, 98, 49–54. [Google Scholar] [CrossRef]
- Zeitz, K.P.; Guy, N.; Malmberg, A.B.; Dirajlal, S.; Martin, W.J.; Sun, L.; Bonhaus, D.W.; Stucky, C.L.; Julius, D.; Basbaum, A.I. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J. Neurosci. 2002, 22, 1010–1019. [Google Scholar] [CrossRef]
- Guo, W.; Miyoshi, K.; Dubner, R.; Gu, M.; Li, M.; Liu, J.; Yang, J.; Zou, S.; Ren, K.; Noguchi, K.; et al. Spinal 5-HT3 receptors mediate descending facilitation and contribute to behavioral hypersensitivity via a reciprocal neuron-glial signaling cascade. Mol. Pain 2014, 10, 35. [Google Scholar] [CrossRef]
- Pertovaara, A. Noradrenergic pain modulation. Prog. Neurobiol. 2006, 80, 53–83. [Google Scholar] [CrossRef]
- Clark, F.M.; Proudfit, H.K. The projection of noradrenergic neurons in the A7 catecholamine cell group to the spinal cord in the rat demonstrated by anterograde tracing combined with immunocytochemistry. Brain Res. 1991, 547, 279–288. [Google Scholar] [CrossRef]
- Clark, F.M.; Proudfit, H.K. The projections of noradrenergic neurons in the A5 catecholamine cell group to the spinal cord in the rat: Anatomical evidence that A5 neurons modulate nociception. Brain Res. 1993, 616, 200–210. [Google Scholar] [CrossRef]
- Fritschy, J.M.; Lyons, W.E.; Mullen, C.A.; Kosofsky, B.E.; Molliver, M.E.; Grzanna, R. Distribution of locus coeruleus axons in the rat spinal cord: A combined anterograde transport and immunohistochemical study. Brain Res. 1987, 437, 176–180. [Google Scholar] [CrossRef]
- Tavares, I.; Lima, D.; Coimbra, A. The ventrolateral medulla of the rat is connected with the spinal cord dorsal horn by an indirect descending pathway relayed in the A5 noradrenergic cell group. J. Comp. Neurol. 1996, 374, 84–95. [Google Scholar] [CrossRef]
- Westlund, K.N.; Bowker, R.M.; Ziegler, M.G.; Coulter, J.D. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983, 263, 15–31. [Google Scholar] [CrossRef]
- Kwiat, G.C.; Basbaum, A.I. The origin of brainstem noradrenergic and serotonergic projections to the spinal cord dorsal horn in the rat. Somatosens. Mot. Res. 1992, 9, 157–173. [Google Scholar] [CrossRef]
- Fairbanks, C.A.; Stone, L.S.; Wilcox, G.L. Pharmacological profiles of alpha 2 adrenergic receptor agonists identified using genetically altered mice and isobolographic analysis. Pharmacol. Ther. 2009, 123, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.J.; McMartin, L.R. Adrenoceptors and their second messenger systems. J. Neurochem. 1993, 60, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Llorca-Torralba, M.; Borges, G.; Neto, F.; Mico, J.A.; Berrocoso, E. Noradrenergic Locus Coeruleus pathways in pain modulation. Neuroscience 2016, 338, 93–113. [Google Scholar] [CrossRef] [PubMed]
- Stone, L.S.; Broberger, C.; Vulchanova, L.; Wilcox, G.L.; Hokfelt, T.; Riedl, M.S.; Elde, R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J. Neurosci. 1998, 18, 5928–5937. [Google Scholar] [CrossRef] [PubMed]
- Olave, M.J.; Maxwell, D.J. Axon terminals possessing the alpha 2c-adrenergic receptor in the rat dorsal horn are predominantly excitatory. Brain Res. 2003, 965, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Viisanen, H.; Pertovaara, A. Influence of peripheral nerve injury on response properties of locus coeruleus neurons and coeruleospinal antinociception in the rat. Neuroscience 2007, 146, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Pertovaara, A. Regulation of neuropathic hypersensitivity by alpha(2)-adrenoceptors in the pontine A7 cell group. Basic. Clin. Pharmacol. Toxicol. 2013, 112, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, A. The noradrenergic pain regulation system: A potential target for pain therapy. Eur. J. Pharmacol. 2013, 716, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.; D’Mello, R.; Dickenson, A.H. Peripheral nerve injury-induced changes in spinal alpha(2)-adrenoceptor-mediated modulation of mechanically evoked dorsal horn neuronal responses. J. Pain 2008, 9, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Bantel, C.; Eisenach, J.C.; Duflo, F.; Tobin, J.R.; Childers, S.R. Spinal nerve ligation increases alpha2-adrenergic receptor G-protein coupling in the spinal cord. Brain Res. 2005, 1038, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Eisenach, J.C. Chronic constriction injury of sciatic nerve induces the up-regulation of descending inhibitory noradrenergic innervation to the lumbar dorsal horn of mice. Brain Res. 2003, 970, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Kumamoto, E.; Furue, H.; Yoshimura, M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology 2003, 98, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Yamada, A.; Kim, W.; Kim, S.K.; Furue, H. Noradrenergic inhibition of spinal hyperexcitation elicited by cutaneous cold stimuli in rats with oxaliplatin-induced allodynia: Electrophysiological and behavioral assessments. J. Physiol. Sci. 2017, 67, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Salinas, D.L.; Braz, J.M.; Hamel, K.A.; Basbaum, A.I. Pain relief by supraspinal gabapentin requires descending noradrenergic inhibitory controls. Pain Rep. 2018, 3, e659. [Google Scholar] [CrossRef] [PubMed]
- de Novellis, V.; Mariani, L.; Palazzo, E.; Vita, D.; Marabese, I.; Scafuro, M.; Rossi, F.; Maione, S. Periaqueductal grey CB1 cannabinoid and metabotropic glutamate subtype 5 receptors modulate changes in rostral ventromedial medulla neuronal activities induced by subcutaneous formalin in the rat. Neuroscience 2005, 134, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Blanton, H.L.; Brelsfoard, J.; DeTurk, N.; Pruitt, K.; Narasimhan, M.; Morgan, D.J.; Guindon, J. Cannabinoids: Current and Future Options to Treat Chronic and Chemotherapy-Induced Neuropathic Pain. Drugs 2019, 79, 969–995. [Google Scholar] [CrossRef] [PubMed]
- Burston, J.J.; Woodhams, S.G. Endocannabinoid system and pain: An introduction. Proc. Nutr. Soc. 2014, 73, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002, 68–69, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, C.; Lewis, D.L. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. 1999, 19, 9271–9280. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, R.; Bushlin, I.; Gomes, I.; Tzavaras, N.; Gupta, A.; Neves, S.; Battini, L.; Gusella, G.L.; Lachmann, A.; Ma’ayan, A.; et al. Receptor heteromerization expands the repertoire of cannabinoid signaling in rodent neurons. PLoS ONE 2012, 7, e29239. [Google Scholar] [CrossRef] [PubMed]
- Vinals, X.; Moreno, E.; Lanfumey, L.; Cordomi, A.; Pastor, A.; de La Torre, R.; Gasperini, P.; Navarro, G.; Howell, L.A.; Pardo, L.; et al. Cognitive Impairment Induced by Delta9-tetrahydrocannabinol Occurs through Heteromers between Cannabinoid CB1 and Serotonin 5-HT2A Receptors. PLoS Biol. 2015, 13, e1002194. [Google Scholar] [CrossRef] [PubMed]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, M.; Araque, A. Endocannabinoids mediate neuron-astrocyte communication. Neuron 2008, 57, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Morales, P.; Rodriguez-Cueto, C.; Fernandez-Ruiz, J.; Jagerovic, N.; Franco, R. Targeting Cannabinoid CB2 Receptors in the Central Nervous System. Medicinal Chemistry Approaches with Focus on Neurodegenerative Disorders. Front. Neurosci. 2016, 10, 406. [Google Scholar] [CrossRef]
- Stella, N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 2010, 58, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Ferreira, G.A.; Jamerson, M.J. Endocannabinoids and the Immune System in Health and Disease. Handb. Exp. Pharmacol. 2015, 231, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Riquelme-Sandoval, A.; de Sa-Ferreira, C.O.; Miyakoshi, L.M.; Hedin-Pereira, C. New Insights Into Peptide Cannabinoids: Structure, Biosynthesis and Signaling. Front. Pharmacol. 2020, 11, 596572. [Google Scholar] [CrossRef] [PubMed]
- Guindon, J.; Hohmann, A.G. Cannabinoid CB2 receptors: A therapeutic target for the treatment of inflammatory and neuropathic pain. Br. J. Pharmacol. 2008, 153, 319–334. [Google Scholar] [CrossRef]
- Ghosh, K.; Zhang, G.F.; Chen, H.; Chen, S.R.; Pan, H.L. Cannabinoid CB2 receptors are upregulated via bivalent histone modifications and control primary afferent input to the spinal cord in neuropathic pain. J. Biol. Chem. 2022, 298, 101999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hoffert, C.; Vu, H.K.; Groblewski, T.; Ahmad, S.; O’Donnell, D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003, 17, 2750–2754. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Z.; Carey, L.; Romero, J.; Makriyannis, A.; Hillard, C.J.; Ruggiero, E.; Dockum, M.; Houk, G.; Mackie, K.; et al. A peripheral CB2 cannabinoid receptor mechanism suppresses chemotherapy-induced peripheral neuropathy: Evidence from a CB2 reporter mouse. Pain 2022, 163, 834–851. [Google Scholar] [CrossRef] [PubMed]
- King, K.M.; Myers, A.M.; Soroka-Monzo, A.J.; Tuma, R.F.; Tallarida, R.J.; Walker, E.A.; Ward, S.J. Single and combined effects of Delta(9)-tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy-induced neuropathic pain. Br. J. Pharmacol. 2017, 174, 2832–2841. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Guindon, J.; Vemuri, V.K.; Thakur, G.A.; White, F.A.; Makriyannis, A.; Hohmann, A.G. The maintenance of cisplatin- and paclitaxel-induced mechanical and cold allodynia is suppressed by cannabinoid CB(2) receptor activation and independent of CXCR4 signaling in models of chemotherapy-induced peripheral neuropathy. Mol. Pain 2012, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Pascual, D.; Goicoechea, C.; Suardiaz, M.; Martin, M.I. A cannabinoid agonist, WIN 55,212-2, reduces neuropathic nociception induced by paclitaxel in rats. Pain 2005, 118, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Rahn, E.J.; Makriyannis, A.; Hohmann, A.G. Activation of cannabinoid CB1 and CB2 receptors suppresses neuropathic nociception evoked by the chemotherapeutic agent vincristine in rats. Br. J. Pharmacol. 2007, 152, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Hocevar, M.; Bie, B.; Foss, J.F.; Naguib, M. Cannabinoid Type 2 Receptor System Modulates Paclitaxel-Induced Microglial Dysregulation and Central Sensitization in Rats. J. Pain 2019, 20, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Gupta, A.; Gomes, I.; Fowkes, M.; Ram, A.; Bobeck, E.N.; Devi, L.A. Targeting Cannabinoid 1 and Delta Opioid Receptor Heteromers Alleviates Chemotherapy-Induced Neuropathic Pain. ACS Pharmacol. Transl. Sci. 2019, 2, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Xie, W.; Inoue, M.; Ueda, H. Evidence for the tonic inhibition of spinal pain by nicotinic cholinergic transmission through primary afferents. Mol. Pain 2007, 3, 41. [Google Scholar] [CrossRef] [PubMed]
- Naser, P.V.; Kuner, R. Molecular, Cellular and Circuit Basis of Cholinergic Modulation of Pain. Neuroscience 2018, 387, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors: Therapeutic targets for novel ligands to treat pain and inflammation. Pharmacol. Res. 2023, 190, 106715. [Google Scholar] [CrossRef] [PubMed]
- Picciotto, M.R.; Higley, M.J.; Mineur, Y.S. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron 2012, 76, 116–129. [Google Scholar] [CrossRef]
- Iwamoto, E.T.; Marion, L. Adrenergic, serotonergic and cholinergic components of nicotinic antinociception in rats. J. Pharmacol. Exp. Ther. 1993, 265, 777–789. [Google Scholar] [PubMed]
- Ferrier, J.; Bayet-Robert, M.; Dalmann, R.; El Guerrab, A.; Aissouni, Y.; Graveron-Demilly, D.; Chalus, M.; Pinguet, J.; Eschalier, A.; Richard, D.; et al. Cholinergic Neurotransmission in the Posterior Insular Cortex Is Altered in Preclinical Models of Neuropathic Pain: Key Role of Muscarinic M2 Receptors in Donepezil-Induced Antinociception. J. Neurosci. 2015, 35, 16418–16430. [Google Scholar] [CrossRef] [PubMed]
- Selvy, M.; Mattevi, C.; Dalbos, C.; Aissouni, Y.; Chapuy, E.; Martin, P.Y.; Collin, A.; Richard, D.; Dumontet, C.; Busserolles, J.; et al. Analgesic and preventive effects of donepezil in animal models of chemotherapy-induced peripheral neuropathy: Involvement of spinal muscarinic acetylcholine M2 receptors. Biomed. Pharmacother. 2022, 149, 112915. [Google Scholar] [CrossRef] [PubMed]
- Kanat, O.; Bagdas, D.; Ozboluk, H.Y.; Gurun, M.S. Preclinical evidence for the antihyperalgesic activity of CDP-choline in oxaliplatin-induced neuropathic pain. J. BUON 2013, 18, 1012–1018. [Google Scholar] [PubMed]
- Favre-Guilmard, C.; Auguet, M.; Chabrier, P.E. Different antinociceptive effects of botulinum toxin type A in inflammatory and peripheral polyneuropathic rat models. Eur. J. Pharmacol. 2009, 617, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Kyte, S.L.; Toma, W.; Bagdas, D.; Meade, J.A.; Schurman, L.D.; Lichtman, A.H.; Chen, Z.J.; Del Fabbro, E.; Fang, X.; Bigbee, J.W.; et al. Nicotine Prevents and Reverses Paclitaxel-Induced Mechanical Allodynia in a Mouse Model of CIPN. J. Pharmacol. Exp. Ther. 2018, 364, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.J., 3rd; Wade, C.L.; Mikusa, J.P.; Decker, M.W.; Honore, P. ABT-594 (a nicotinic acetylcholine agonist): Anti-allodynia in a rat chemotherapy-induced pain model. Eur. J. Pharmacol. 2005, 509, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Kim, M.J.; Yoon, I.; Li, D.X.; Bae, H.; Kim, S.K. Nicotinic Acetylcholine Receptors Mediate the Suppressive Effect of an Injection of Diluted Bee Venom into the GV3 Acupoint on Oxaliplatin-Induced Neuropathic Cold Allodynia in Rats. Biol. Pharm. Bull. 2015, 38, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Arias, H.R.; Ghelardini, C.; Lucarini, E.; Tae, H.S.; Yousuf, A.; Marcovich, I.; Manetti, D.; Romanelli, M.N.; Elgoyhen, A.B.; Adams, D.J.; et al. (E)-3-Furan-2-yl-N-p-tolyl-acrylamide and its Derivative DM489 Decrease Neuropathic Pain in Mice Predominantly by alpha7 Nicotinic Acetylcholine Receptor Potentiation. ACS Chem. Neurosci. 2020, 11, 3603–3614. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Pacini, A.; Matera, C.; Zanardelli, M.; Mello, T.; De Amici, M.; Dallanoce, C.; Ghelardini, C. Involvement of alpha7 nAChR subtype in rat oxaliplatin-induced neuropathy: Effects of selective activation. Neuropharmacology 2014, 79, 37–48. [Google Scholar] [CrossRef]
- Toma, W.; Kyte, S.L.; Bagdas, D.; Jackson, A.; Meade, J.A.; Rahman, F.; Chen, Z.J.; Del Fabbro, E.; Cantwell, L.; Kulkarni, A.; et al. The alpha7 nicotinic receptor silent agonist R-47 prevents and reverses paclitaxel-induced peripheral neuropathy in mice without tolerance or altering nicotine reward and withdrawal. Exp. Neurol. 2019, 320, 113010. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Hone, A.J.; Roux, I.; Kniazeff, J.; Pin, J.P.; Upert, G.; Servent, D.; Glowatzki, E.; McIntosh, J.M. RgIA4 Potently Blocks Mouse alpha9alpha10 nAChRs and Provides Long Lasting Protection against Oxaliplatin-Induced Cold Allodynia. Front. Cell Neurosci. 2017, 11, 219. [Google Scholar] [CrossRef]
- Dyachenko, I.A.; Palikova, Y.A.; Palikov, V.A.; Korolkova, Y.V.; Kazakov, V.A.; Egorova, N.S.; Garifulina, A.I.; Utkin, Y.N.; Tsetlin, V.I.; Kryukova, E.V. alpha-Conotoxin RgIA and oligoarginine R8 in the mice model alleviate long-term oxaliplatin induced neuropathy. Biochimie 2022, 194, 127–136. [Google Scholar] [CrossRef]
- Gajewiak, J.; Christensen, S.B.; Dowell, C.; Hararah, F.; Fisher, F.; Huynh, P.N.; Olivera, B.M.; McIntosh, J.M. Selective Penicillamine Substitution Enables Development of a Potent Analgesic Peptide that Acts through a Non-Opioid-Based Mechanism. J. Med. Chem. 2021, 64, 9271–9278. [Google Scholar] [CrossRef] [PubMed]
- Huynh, P.N.; Giuvelis, D.; Christensen, S.; Tucker, K.L.; McIntosh, J.M. RgIA4 Accelerates Recovery from Paclitaxel-Induced Neuropathic Pain in Rats. Mar. Drugs 2019, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Pacini, A.; Micheli, L.; Maresca, M.; Branca, J.J.; McIntosh, J.M.; Ghelardini, C.; Di Cesare Mannelli, L. The alpha9alpha10 nicotinic receptor antagonist alpha-conotoxin RgIA prevents neuropathic pain induced by oxaliplatin treatment. Exp. Neurol. 2016, 282, 37–48. [Google Scholar] [CrossRef]
- Romero, H.K.; Christensen, S.B.; Di Cesare Mannelli, L.; Gajewiak, J.; Ramachandra, R.; Elmslie, K.S.; Vetter, D.E.; Ghelardini, C.; Iadonato, S.P.; Mercado, J.L.; et al. Inhibition of alpha9alpha10 nicotinic acetylcholine receptors prevents chemotherapy-induced neuropathic pain. Proc. Natl. Acad. Sci. USA 2017, 114, E1825–E1832. [Google Scholar] [CrossRef]
- Li, Z.; Han, X.; Hong, X.; Li, X.; Gao, J.; Zhang, H.; Zheng, A. Lyophilization Serves as an Effective Strategy for Drug Development of the alpha9alpha10 Nicotinic Acetylcholine Receptor Antagonist alpha-Conotoxin GeXIVA[1,2]. Mar. Drugs 2021, 19, 121. [Google Scholar] [CrossRef] [PubMed]
- Wala, E.P.; Crooks, P.A.; McIntosh, J.M.; Holtman, J.R., Jr. Novel small molecule alpha9alpha10 nicotinic receptor antagonist prevents and reverses chemotherapy-evoked neuropathic pain in rats. Anesth. Analg. 2012, 115, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhangsun, D.; Yu, G.; Su, R.; Luo, S. The alpha9alpha10 Nicotinic Acetylcholine Receptor Antagonist alphaO-Conotoxin GeXIVA[1,2] Alleviates and Reverses Chemotherapy-Induced Neuropathic Pain. Mar. Drugs 2019, 17, 265. [Google Scholar] [CrossRef] [PubMed]
- Calcutt, N.A.; Smith, D.R.; Frizzi, K.; Sabbir, M.G.; Chowdhury, S.K.; Mixcoatl-Zecuatl, T.; Saleh, A.; Muttalib, N.; Van der Ploeg, R.; Ochoa, J.; et al. Selective antagonism of muscarinic receptors is neuroprotective in peripheral neuropathy. J. Clin. Investig. 2017, 127, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.W.; Martino, G.; Coupal, M.; Lindberg, M.; Schroeder, P.; Santhakumar, V.; Valiquette, M.; Sandin, J.; Widzowski, D.; Laird, J. Broad analgesic activity of a novel, selective M1 agonist. Neuropharmacology 2017, 123, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Motta, E.M.; Dutra, R.C.; Manjavachi, M.N.; Bento, A.F.; Malinsky, F.R.; Pesquero, J.B.; Calixto, J.B. Anti-nociceptive effect of kinin B(1) and B(2) receptor antagonists on peripheral neuropathy induced by paclitaxel in mice. Br. J. Pharmacol. 2011, 164, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, J.; de Assis, P.M.; Cristina Dalazen Goncalves, E.; Daniele Bobermin, L.; Quincozes-Santos, A.; Raposo, N.R.B.; Gomez, M.V.; Dutra, R.C. Systemic, Intrathecal, and Intracerebroventricular Antihyperalgesic Effects of the Calcium Channel Blocker CTK 01512-2 Toxin in Persistent Pain Models. Mol. Neurobiol. 2022, 59, 4436–4452. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, H.; Gao, H.; Zhao, H.; Liu, D.; Li, J. Participation of pro-inflammatory cytokines in neuropathic pain evoked by chemotherapeutic oxaliplatin via central GABAergic pathway. Mol. Pain 2018, 14, 1744806918783535. [Google Scholar] [CrossRef]
- Hache, G.; Guiard, B.P.; Nguyen, T.H.; Quesseveur, G.; Gardier, A.M.; Peters, D.; Munro, G.; Coudore, F. Antinociceptive activity of the new triple reuptake inhibitor NS18283 in a mouse model of chemotherapy-induced neuropathic pain. Eur. J. Pain 2015, 19, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Kanbara, T.; Nakamura, A.; Shibasaki, M.; Mori, T.; Suzuki, T.; Sakaguchi, G.; Kanemasa, T. Morphine and oxycodone, but not fentanyl, exhibit antinociceptive effects mediated by G-protein inwardly rectifying potassium (GIRK) channels in an oxaliplatin-induced neuropathy rat model. Neurosci. Lett. 2014, 580, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kanbara, T.; Nakamura, A.; Takasu, K.; Ogawa, K.; Shibasaki, M.; Mori, T.; Suzuki, T.; Hasegawa, M.; Sakaguchi, G.; Kanemasa, T. The contribution of Gi/o protein to opioid antinociception in an oxaliplatin-induced neuropathy rat model. J. Pharmacol. Sci. 2014, 126, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Norcini, M.; Vivoli, E.; Galeotti, N.; Bianchi, E.; Bartolini, A.; Ghelardini, C. Supraspinal role of protein kinase C in oxaliplatin-induced neuropathy in rat. Pain 2009, 146, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Toyama, S.; Shimoyama, N.; Shimoyama, M. The analgesic effect of orexin-A in a murine model of chemotherapy-induced neuropathic pain. Neuropeptides 2017, 61, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.; Kim, S.K. Therapeutics for Chemotherapy-Induced Peripheral Neuropathy: Approaches with Natural Compounds from Traditional Eastern Medicine. Pharmaceutics 2022, 14, 1407. [Google Scholar] [CrossRef] [PubMed]
- Balayssac, D.; Durif, J.; Lambert, C.; Dalbos, C.; Chapuy, E.; Etienne, M.; Demiot, C.; Busserolles, J.; Martin, V.; Sapin, V. Exploring Serum Biomarkers for Neuropathic Pain in Rat Models of Chemotherapy-Induced Peripheral Neuropathy: A Comparative Pilot Study with Oxaliplatin, Paclitaxel, Bortezomib, and Vincristine. Toxics 2023, 11, 1004. [Google Scholar] [CrossRef] [PubMed]
- Attal, N.; Bouhassira, D.; Colvin, L. Advances and challenges in neuropathic pain: A narrative review and future directions. Br. J. Anaesth. 2023, 131, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Bouhassira, D.; Attal, N. Personalized treatment of neuropathic pain: Where are we now? Eur. J. Pain 2023, 27, 1084–1098. [Google Scholar] [CrossRef]
| Study Design | Type of Chemotherapy | Effects of CIPN in Brain | Ref. |
|---|---|---|---|
| Longitudinal study: 24 women with chemotherapy, 23 women no chemotherapy | Combinations of paclitaxel, docetaxel, carboplatin, and cisplatin | ↑ perfusion in the CG and SFG | [90] |
| Case-control study: 12 patients CIPN 12 healthy volunteers | Bortezomib, thalidomide, or vincristine | ↑ activation in the precuneus ↓ activation in the SFG Activation in the FO associated with worse CIPN. | [91] |
| Prospective, multicenter cohort study: 20 patients | Bortezomib, oxaliplatin, paclitaxel, docetaxel, cisplatin | Prior chemotherapy (punctate stimuli): ↑ activity in insula, somatosensory cortex, thalamus and cerebellum in CIPNþ. ↑ activity of PAG in CIPNe | [92] |
| Neuroimaging Approach | Species (Sex) | CIPN Model | Main Results | Ref. |
|---|---|---|---|---|
| DW imaging—quantitative anisotropy | Rats (males) | Paclitaxel | Reorganization of gray matter in the PFC, amygdala, hippocampus, hypothalamus and striatum/NAc | [94] |
| Rs functional connectivity | Rats (males) | Paclitaxel | Altered connections to the PAG | [94] |
| MEMRI | Rats (males) | Paclitaxel | ↑ activation of hypothalamus and PAG | [80] |
| Ex vivo spectroscopy | Rats (males) | Paclitaxel | Early CIPN: ↑ NAA levels in PFC ↑ NAA and lactate levels in hypothalamus Late CIPN: ↓ NAA levels in PFC ↑ taurine levels in PFC | [80] |
| fMRI | Non-human primates | Oxaliplatin | ↑ activation of SSC and Insula | [95,96] |
| Neurotransmitter System | CIPN Model | CNS Region | Main Results | Ref. |
|---|---|---|---|---|
| Serotoninergic | Paclitaxel | RVM | ↑ 5-HT neuron activation | [118] |
| SC | ↑ 5-HT levels ↑ 5-HT3 receptors | |||
| Paclitaxel | RVM | ↑ 5-HT neuron activation | [119] | |
| Oxaliplatin | SC | ↓ 5-HT levels | [120] | |
| Cisplatin | SC | ↓ 5-HT levels | [121] | |
| Oxaliplatin | SC | ↓ 5-HT1A receptors | [122,123] | |
| Paclitaxel Vincristine | SC | ↔ 5-HT1A receptors | [123] | |
| Vincristine | SC | ↑ 5-HT2A receptors | [124] | |
| Oxaliplatin | SC | ↑ 5-HT2C receptors | [125] | |
| Vincristine | FC Striatum Hippocampus | ↑ 5-HT levels | [126] | |
| Noradrenergic | Paclitaxel | LC | ↑ TH expression | [127] |
| SC | ↑ NA levels ↑ α1-AR receptors ↑ α2-AR receptors | |||
| SC | ↑ DBH expression ↑ α2-AR receptor potency | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, M.; Tavares, I.; Costa-Pereira, J.T. Centralizing the Knowledge and Interpretation of Pain in Chemotherapy-Induced Peripheral Neuropathy: A Paradigm Shift towards Brain-Centric Approaches. Brain Sci. 2024, 14, 659. https://doi.org/10.3390/brainsci14070659
Cunha M, Tavares I, Costa-Pereira JT. Centralizing the Knowledge and Interpretation of Pain in Chemotherapy-Induced Peripheral Neuropathy: A Paradigm Shift towards Brain-Centric Approaches. Brain Sciences. 2024; 14(7):659. https://doi.org/10.3390/brainsci14070659
Chicago/Turabian StyleCunha, Mário, Isaura Tavares, and José Tiago Costa-Pereira. 2024. "Centralizing the Knowledge and Interpretation of Pain in Chemotherapy-Induced Peripheral Neuropathy: A Paradigm Shift towards Brain-Centric Approaches" Brain Sciences 14, no. 7: 659. https://doi.org/10.3390/brainsci14070659






