Vagus Nerve Stimulation Therapy in Epilepsy: An Overview of Technical and Surgical Method, Patient Selection, and Treatment Outcomes
Abstract
:1. Introduction
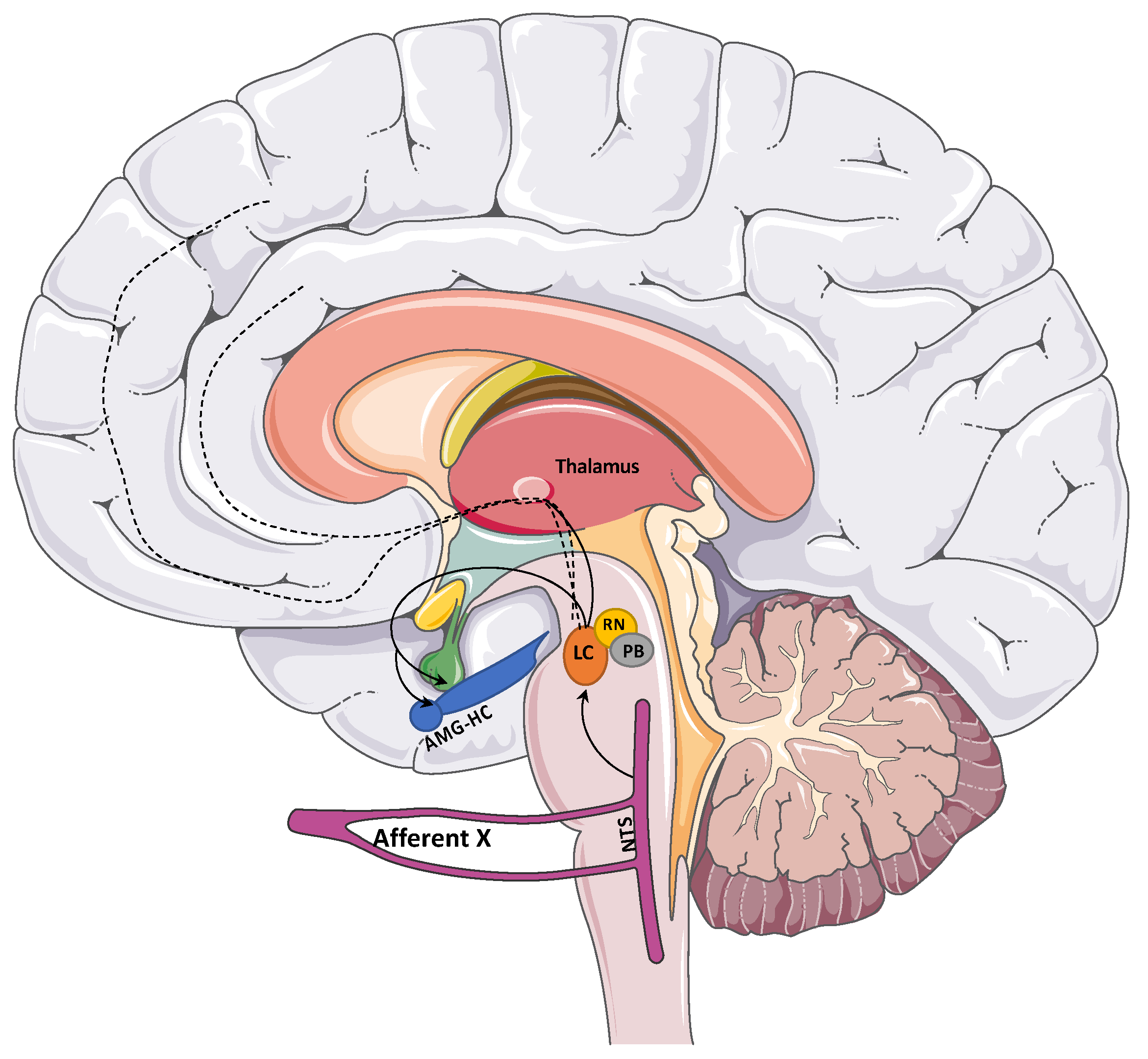
2. Background on VNS Physiology and Evidence
3. Overview of VNS Safety and Efficacy
4. Patient Selection
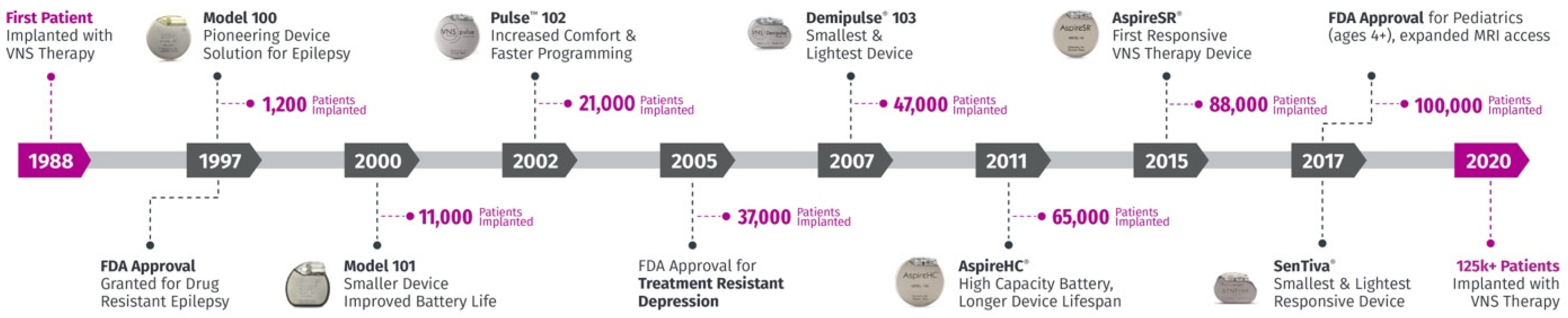
5. Technology Overview
5.1. Equipment
5.2. Technical Upgrades
6. Neuromodulation Programming
7. Surgical Implantation and Complications
7.1. Implantation
7.2. Revision/Removal
7.3. Expected Side Effects of Therapy
8. Complications
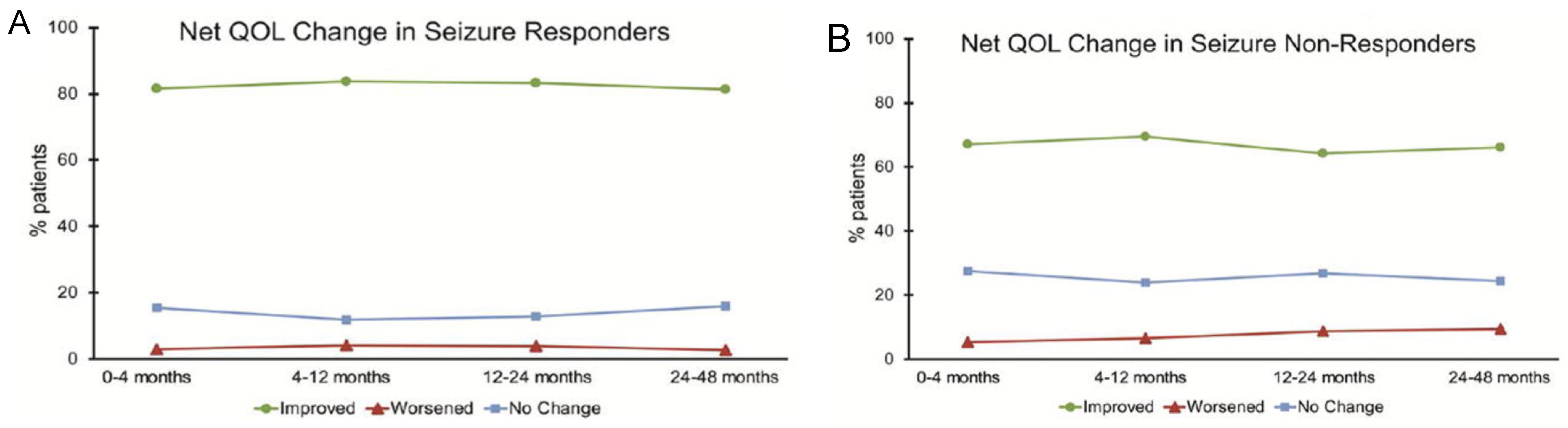
9. Other Considerations
9.1. VNS and Neurological Disorders
9.1.1. Depression
9.1.2. Motor Rehabilitation
10. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krahl, S. Vagus nerve stimulation for epilepsy: A review of the peripheral mechanisms. Surg. Neurol. Int. 2012, 3, 47–52. [Google Scholar] [CrossRef]
- Krahl, S.E.; Clark, K.B. Vagus nerve stimulation for epilepsy: A review of central mechanisms. Surg. Neurol. Int. 2012, 3 (Suppl. S4), 255–259. [Google Scholar] [CrossRef] [PubMed]
- Zabara, J. Peripheral control of hypersynchronous discharge in epilepsy. Electroencephalogr. Clin. Neurophysiol. 1985, 61, S162. [Google Scholar] [CrossRef]
- Giordano, F.; Zicca, A.; Barba, C.; Guerrini, R.; Genitori, L. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia 2017, 58 (Suppl. S1), 85–90. [Google Scholar] [CrossRef]
- Groves, D.A.; Brown, V.J. Vagal nerve stimulation: A review of its applications and potential mechanisms that mediate its clinical effects. Neurosci. Biobehav. Rev. 2005, 29, 493–500. [Google Scholar] [CrossRef]
- George, M.S.; Nahas, Z.; Bohning, D.E.; Mu, Q.; Kozel, F.A.; Borckhardt, J.; Denslow, S. Mechanisms of action of vagus nerve stimulation (VNS). Clin. Neurosci. Res. 2004, 4, 71–79. [Google Scholar] [CrossRef]
- Ricardo, J.A.; Tongju Koh, E. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978, 153, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dorr, A.E.; Debonnel, G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J. Pharmacol. Exp. Ther. 2006, 318, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Hassert, D.L.; Miyashita, T.; Williams, C.L. The Effects of Peripheral Vagal Nerve Stimulation at a Memory-Modulating Intensity on Norepinephrine Output in the Basolateral Amygdala. Behav. Neurosci. 2004, 118, 79–88. [Google Scholar] [CrossRef]
- Roosevelt, R.W.; Smith, D.C.; Clough, R.W.; Jensen, R.A.; Browning, R.A. Increased extracellular concentrations of norepinephrine in cortex and hippocampus following vagus nerve stimulation in the rat. Brain Res. 2006, 1119, 124–132. [Google Scholar] [CrossRef]
- Jin, Z.; Dong, J.; Wang, Y.; Liu, Y. Exploring the potential of vagus nerve stimulation in treating brain diseases: A review of immunologic benefits and neuroprotective efficacy. Eur. J. Med. Res. 2023, 28, 444. [Google Scholar] [CrossRef] [PubMed]
- Broncel, A.; Bocian, R.; Kłos-Wojtczak, P.; Konopacki, J. GABAergic mediation of hippocampal theta rhythm induced by stimulation of the vagal nerve. Brain Res. Bull. 2019, 147, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Broncel, A.; Bocian, R.; Kłos-Wojtczak, P.; Konopacki, J. Vagus nerve stimulation produces a hippocampal formation theta rhythm in anesthetized rats. Brain Res. 2017, 1675, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Volnova, A.; Tsytsarev, V.; Ganina, O.; Velez-Crespo, G.E.; Alves, J.M.; Ingnashchenkova, A.; Inyushin, M. The Anti-Epileptic Effects of Carbenoxolone In Vitro and In Vivo. Int. J. Mol. Sci. 2022, 2022, 663. [Google Scholar] [CrossRef] [PubMed]
- Rx Only VNS Therapy® System Epilepsy Physician’s Manual PulseTM Generator-Model 102 Pulse DuoTM Genera-tor-Model 102R Demipulse® Generator-Model 103 Demipulse Duo® Generator-Model 104 AspireHC ® Genera-tor-Model 105 AspireSR ® Generator-Model 106 SenTiva ® Generator-Model 1000 Lead-Model 302 PerenniaFLEX ® Lead-Model 304 PerenniaDURA® Lead-Model 303. 2019. Available online: www.livanova.com (accessed on 9 May 2024).
- Ben-Menachem, E.; Maiion-Espaillat, R.; Ristanovic, R.R.; Wilder, B.J.; Stefan, H.; Mirza, W.; Tarver, W.B.; Wenicke, J.F. Vagus Nerve Stimulation for Treatment of Partial Seizures: 1. A Controlled Study of Effect on Seizures. Epilepsia 1994, 35, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.E.; Uthman, B.M.; Augustinsson, L.E.; Upton, A.R.; Naritoku, D.; Willis, J.; Trig, T.; Barolat, G.; Wernicke, J.F. Vagus Nerve Stimulation for Treatment of Partial Seizures: 2. Safety, Side Effects, and Tolerability. Epilepsia 1994, 35, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Handforth, A.; DeGiorgio, C.M.; Schachter, S.C.; Uthman, B.M.; Naritoku, D.K.; Tecoma, E.S.; Henry, T.R.; Collins, S.D.; Vaughn, B.V.; Gilmartin, R.C.; et al. Vagus nerve stimulation therapy for partial-onset seizures: A randomized active-control trial. Neurology 1998, 51, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Morris III, G.L.; Mueller, W.M. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01–E05. Neurology 1999, 53, 1731. [Google Scholar] [CrossRef] [PubMed]
- George, R.; Salinsky, M.; Kuzniecky, R.; Rosenfeld, W.; Bergen, D.; Tarver, W.B.; Wernicke, J.F.; First International Vagus Nerve Stimulation Study Group. Vagus Nerve Stimulation for Treatment of Partial Seizures: 3. Long-Term Follow-Up on First 67 Patients Exiting a Controlled Study. Epilepsia 1994, 35, 637–643. [Google Scholar] [CrossRef]
- Ben-Menachem, E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002, 1, 477–482. [Google Scholar] [CrossRef]
- Klinkenberg, S.; Aalbers, M.W.; Vles, J.S.H.; Cornips, E.M.J.; Rijkers, K.; Leenen, L.; Kessels, F.G.H.; Aldenkamp, A.P.; Majoie, M. Vagus nerve stimulation in children with intractable epilepsy: A randomized controlled trial. Dev. Med. Child Neurol. 2012, 54, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Kawai, K.; Tanaka, T.; Baba, H.; Bunker, M.; Ikeda, A.; Inoue, Y.; Kameyama, S.; Kaneko, S.; Kato, A.; Nozawa, T.; et al. Outcome of vagus nerve stimulation for drug-resistant epilepsy: The first three years of a prospective Japanese registry. Epileptic Disord. 2017, 19, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Ryvlin, P.; Gilliam, F.G.; Nguyen, D.K.; Colicchio, G.; Ludice, A.; Tinuper, P.; Zampoi, N.; Aguglia, U.; Wagner, L.; Minotti, L.; et al. The long-term effect of vagus nerve stimulation on quality of life in patients with pharmacoresistant focal epilepsy: The PuLsE (Open Prospective Randomized Long-term Effectiveness) trial. Epilepsia 2014, 55, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Rolston, J.D.; Wright, C.W.; Hassnain, K.H.; Chang, E.F. Rates and Predictors of Seizure Freedom with Vagus Nerve Stimulation for Intractable Epilepsy. Neurosurgery 2016, 79, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; O’Connor, M.; Liporace, J.; Sperling, M.R. Refractory generalized seizures: Response to corpus callosotomy and vagal nerve stimulation. Epilepsia 2006, 47, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Devinsky, O. Vagus nerve stimulation for refractory idiopathic generalised epilepsy. Seizure 2004, 13, 176–178. [Google Scholar] [CrossRef]
- Welch, W.P.; Sitwat, B.; Sogawa, Y. Use of Vagus Nerve Stimulator on Children With Primary Generalized Epilepsy. J. Child Neurol. 2018, 33, 449–452. [Google Scholar] [CrossRef]
- Dibué, M.; Greco, T.; Spoor, J.K.H.; Tahir, Z.; Specchio, N.; Hanggi, D.; Steiger, H.J.; Kamp, M.A. Vagus nerve stimulation in patients with Lennox-Gastaut syndrome: A meta-analysis. Acta Neurol. Scand. 2021, 143, 497–508. [Google Scholar] [CrossRef]
- Majoie, H.J.M.; Berfelo, M.W.; Aldenkamp, A.P.; Renier, W.O.; Kessels, A.G.H. Vagus nerve stimulation in patients with catastrophic childhood epilepsy, a 2-year follow-up study. Seizure 2005, 14, 10–18. [Google Scholar] [CrossRef]
- Rolston, J.D.; Englot, D.J.; Wang, D.D.; Garcia, P.A.; Chang, E.F. Corpus callosotomy versus vagus nerve stimulation for atonic seizures and drop attacks: A systematic review. Epilepsy Behav. 2015, 51, 13–17. [Google Scholar] [CrossRef]
- Lancman, G.; Virk, M.; Shao, H.; Mazumdar, M.; Greenfield, J.P.; Weinstein, S.; Schwartz, T.H. Vagus nerve stimulation vs. corpus callosotomy in the treatment of Lennox-Gastaut syndrome: A meta-Analysis. Seizure 2013, 22, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Montouris, G.; Aboumatar, S.; Burdette, D.; Kothare, S.; Kuzniecky, R.; Rosenfeld, W.; Chung, S. Expert opinion: Proposed diagnostic and treatment algorithms for Lennox–Gastaut syndrome in adult patients. Epilepsy Behav. 2020, 110, 107146. [Google Scholar] [CrossRef]
- Toffa, D.H.; Touma, L.; El Meskine, T.; Bouthillier, A.; Nguyen, D.K. Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: A critical review. Seizure 2020, 83, 104–123. [Google Scholar] [CrossRef]
- Ryvlin, P.; Jehi, L.E. Neuromodulation for Refractory Epilepsy. Epilepsy Curr. 2022, 22, 11–17. [Google Scholar] [CrossRef]
- Benbadis, S.R.; Geller, E.; Ryvlin, P.; Schachter, S.; Wheless, J.; Doyle, W.; Vale, F.L. Putting it all together: Options for intractable epilepsy: An updated algorithm on the use of epilepsy surgery and neurostimulation. Epilepsy Behav. 2018, 88, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Spindler, P.; Bohlmann, K.; Straub, H.B.; Vajkoczy, P.; Schneider, U.C. Effects of vagus nerve stimulation on symptoms of depression in patients with difficult-to-treat epilepsy. Seizure 2019, 69, 77–79. [Google Scholar] [CrossRef]
- Fahoum, F.; Boffini, M.; Kann, L.; Faini, S.; Gordon, C.; Tzadok, M.; el Tahry, R. VNS parameters for clinical response in Epilepsy. Brain Stimul. 2022, 15, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Vonck, K.; Dedeurwaerdere, S.; de Groote, L.; Thadani, V.; Claeys, P.; Gossiaux, F.; van Roost, D.; Boon, P. Generator replacement in epilepsy patients treated with vagus nerve stimulation. Seizure 2005, 14, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Afra, P.; Adamolekun, B.; Aydemir, S.; Watson, G.D.R. Evolution of the Vagus Nerve Stimulation (VNS) Therapy System Technology for Drug-Resistant Epilepsy. Front. Med. Technol. 2021, 3, 696543. [Google Scholar] [CrossRef] [PubMed]
- Boon, P.; Vonck, K.; van Rijckevorsel, K.; el Tahry, R.; Elger, C.E.; Mullatti, N.; Schulze-Bonhage, A.; Wagner, L.; Diehl, B.; Hamer, H.; et al. A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure 2015, 32, 52–61. [Google Scholar] [CrossRef]
- Salanova, V.; Witt, T.; Worth, R.; Henry, T.R.; Gross, R.E.; Nazzaro, J.M.; Labar, D.; Sperling, M.R.; Sharan, A.; Sandok, E.; et al. Long-Term Efficacy and Safety of Thalamic Stimulation for Drug-Resistant Partial Epilepsy. Marshfield Clinic. Neurology 2015, 84, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.; Soryal, I.; Dhahri, P.; Wimalachandra, W.; Leat, A.; Hughes, D.; Toghill, N.; Hodson, J.; Sawlani, V.; Hayton, T.; et al. Clinical outcomes of VNS therapy with AspireSR® (including cardiac-based seizure detection) at a large complex epilepsy and surgery centre. Seizure 2018, 58, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.; Galla, K.M.; Sajja, K.; Wichman, C.; Wang, H.; Madhavan, D. Vagus nerve stimulation with tachycardia detection provides additional seizure reduction compared to traditional vagus nerve stimulation. Epilepsy Behav. 2020, 111, 107280. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.B.; Chevill, B.; Philip, S.; Agrawal, S.; Walsh, A.R. Seizure improvement following vagus nerve stimulator (VNS) battery change with cardiac-based seizure detection automatic stimulation (AutoStim): Early experience in a regional paediatric unit. Child’s Nerv. Syst. 2021, 37, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Osharina, V.; Bagaev, V.; Wallois, F.; Larnicol, N. Autonomic response and Fos expression in the NTS following intermittent vagal stimulation: Importance of pulse frequency. Auton. Neurosci. 2006, 126–127, 72–80. [Google Scholar] [CrossRef]
- Liporace, J.; Hucko, D.; Morrow, R.; Barolat, G.; Nei, M.; Schnur, J.; Sperling, M. Vagal nerve stimulation: Adjustments to reduce painful side effects. Neurology 2001, 57, 885–886. [Google Scholar] [CrossRef] [PubMed]
- Bagić, A.I.; Verner, R.; Afra, P.; Benbadis, S.; Bagić, A.; Nune, G.; Fakhoury, T.; Helmers, S.; Winkel, D.; Kirmani, B.; et al. ASCEND: A randomized controlled trial of titration strategies for vagus nerve stimulation in drug-resistant epilepsy. Epilepsy Behav. 2023, 145, 109333. [Google Scholar] [CrossRef] [PubMed]
- Kayyali, H.; Abdelmoity, S.; Bansal, L.; Kaufman, C.; Smith, K.; Fecske, E.; Pawar, K.; Hall, A.; Gustafson, M.; Abdelmoity, A.; et al. The Efficacy and Safety of Rapid Cycling Vagus Nerve Stimulation in Children With Intractable Epilepsy. Pediatr. Neurol. 2020, 109, 35–38. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, K.; Wang, X.; Chen, M.; Li, X.; Wu, Y. Brain functional connectivity and network characteristics changes after vagus nerve stimulation in patients with refractory epilepsy. Transl. Neurosci. 2023, 14, 20220308. [Google Scholar] [CrossRef]
- Sangare, A.; Marchi, A.; Pruvost-Robieux, E.; Soufflet, C.; Crepon, B.; Ramdani, C.; Chassoux, F.; Turak, B.; Landre, E.; Gavaret, M. The effectiveness of vagus nerve stimulation in drug-resistant epilepsy correlates with vagus nerve stimulation-induced electroencephalography desynchronization. Brain Connect. 2020, 10, 566–577. [Google Scholar] [CrossRef]
- Morrison, E.G.; Danthine, V.; Santalucia, R.; Torres, A.; Cakiroglu, I.; Nonclercq, A.; Tahry, R.E. Characterization of Vagus Nerve Stimulation (VNS) Dose-Dependent Effects on EEG Power Spectrum and Synchronization. Biomedicines 2024, 12, 557. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.G.; Sillau, S.; McDermott, D.; Ernst, L.D.; Spencer, D.C.; Englot, D.J.; González, H.F.J.; Datta, P.; Karakis, I.; Becker, D.; et al. Concurrent brain-responsive and vagus nerve stimulation for treatment of drug-resistant focal epilepsy. Epilepsy Behav. 2022, 129, 108653. [Google Scholar] [CrossRef] [PubMed]
- Freund, B.; Grewal, S.S.; Middlebrooks, E.H.; Moniz-Garcia, D.; Feyissa, A.M.; Tatum, W.O. Dual-Device Neuromodulation in Epilepsy. World Neurosurg. 2022, 161, e596–e601. [Google Scholar] [CrossRef] [PubMed]
- Khankhanian, P.; Lee, A.M.; Drees, C.N.; Decker, B.M.; Becker, D.A. Combined VNS-RNS Neuromodulation for Epilepsy. J. Clin. Neurophysiol. 2022, 39, e5–e9. [Google Scholar] [CrossRef] [PubMed]
- Kulju, T.; Haapasalo, J.; Lehtimäki, K.; Rainesalo, S.; Peltola, J. Similarities between the responses to ANT-DBS and prior VNS in refractory epilepsy. Brain Behav. 2018, 8, e00983. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.A. Surgical Technique for Implantation of the Neurocybernetic Prosthesis. Epilepsia 1990, 31, S38–S39. [Google Scholar] [CrossRef] [PubMed]
- Baltuch, G.H.; Villemure, J.G. Operative Techniques in Epilepsy Surgery. Am. J. Neuroradiol. 2009, 30, E133. [Google Scholar] [CrossRef]
- Couch, J.D.; Gilman, A.M.; Doyle, W.K. Long-term expectations of vagus nerve stimulation: A look at battery replacement and revision surgery. Neurosurgery 2015, 78, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Révész, D.; Rydenhag, B.; Ben-Menachem, E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J. Neurosurg. Pediatr. 2016, 18, 97–104. [Google Scholar] [CrossRef]
- Ng, W.H.; Donner, E.; Go, C.; Abou-Hamden, A.; Rutka, J.T. Revision of vagal nerve stimulation (VNS) electrodes: Review and report on use of ultra-sharp monopolar tip. Child’s Nerv. Syst. 2010, 26, 1081–1084. [Google Scholar] [CrossRef]
- Zalvan, C.; Sulica, L.; Wolf, S.; Cohen, J.; Gonzalez-Yanes, O.; Blitzer, A. Laryngopharyngeal dysfunction from the implant vagal nerve stimulator. Laryngoscope 2003, 113, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Helmers, S.L.; Wheless, J.W.; Frost, M.; Gates, J.; Levisohn, P.; Tardo, C.; Conry, J.A.; Yalnizoglu, D.; Madsen, J.R. Vagus nerve stimulation therapy in pediatric patients with refractory epilepsy: Retrospective study. J. Child Neurol. 2001, 16, 843–848. [Google Scholar] [CrossRef]
- Tatum, I.V.W.O.; Moore, D.B.; Stecker, M.M.; Baltuch, G.H.; French, J.A.; Ferreira, J.A.; Carney, P.M.; Labar, D.R.; Vale, F.L. Ventricular asystole during vagus nerve stimulation for epilepsy in humans. Neurology 1999, 52, 1267. [Google Scholar] [CrossRef] [PubMed]
- Fahy, B.G. Intraoperative and perioperative complications with a vagus nerve stimulation device. J. Clin. Anesth. 2010, 22, 213–222. [Google Scholar] [CrossRef]
- Dlouhy, B.J.; Viljoen S v Kung, D.K.; Vogel, T.W.; Granner, M.A.; Howard, M.A.; Kawasaki, H. Vagus nerve stimulation after lead revision. Neurosurg. Focus 2012, 32, E11. [Google Scholar] [CrossRef] [PubMed]
- Ficker, D.M.; So, E.L.; Shen, W.K.; Annegers, J.F.; O’Brien, P.C.; Cascino, G.D.; Belau, P.O. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology 1998, 51, 1270–1274. [Google Scholar] [CrossRef]
- DeGiorgio, C.M.; Markovic, D.; Mazumder, R.; Moseley, B.D. Ranking the leading risk factors for sudden unexpected death in epilepsy. Front. Neurol. 2017, 8, 473. [Google Scholar] [CrossRef] [PubMed]
- Annegers, J.F.; Coan, S.P.; Hauser, W.A.; Leestma, J. Epilepsy, vagal nerve stimulation by the NCP system, all-cause mortality, and sudden, unexpected, unexplained death. Epilepsia 2000, 41, 549–553. [Google Scholar] [CrossRef]
- Ryvlin, P.; So, E.L.; Gordon, C.M.; Hesdorffer, D.C.; Sperling, M.R.; Devinsky, O.; Bunker, M.T.; Olin, B.; Friedman, D. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia 2018, 59, 562–572. [Google Scholar] [CrossRef]
- Verrier, R.L.; Nearing, B.D.; Olin, B.; Boon, P.; Schachter, S.C. Baseline elevation and reduction in cardiac electrical instability assessed by quantitative T-wave alternans in patients with drug-resistant epilepsy treated with vagus nerve stimulation in the AspireSR E-36 trial. Epilepsy Behav. 2016, 62, 85–89. [Google Scholar] [CrossRef]
- Mikati, M.A.; Ataya, N.F.; El-Ferezli, J.C.; Baghdadi, T.S.; Turkmani, A.H.; Comair, Y.G.; Kansagra, S.; Najjar, M.W. Quality of life after vagal nerve stimulator insertion. Epileptic Disord. 2009, 11, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Sander, J.W.; Taylor, R.J.; Baker, G.A. Predictors of health-related quality of life and costs in adults with epilepsy: A systematic review. Epilepsia 2011, 52, 2168–2180. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Hassnain, K.H.; Rolston, J.D.; Harward, S.C.; Sinha, S.R.; Haglund, M.M. Quality-of-life metrics with vagus nerve stimulation for epilepsy from provider survey data. Epilepsy Behav. 2017, 66, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.S.; Elmaadawi, A.; Mansuri, Z.; Kaur, M.; Shah, K.; Nasr, S. Psychiatric Comorbidities and Outcomes in Epilepsy Patients: An Insight from a Nationwide Inpatient Analysis in the United States. Cureus 2017, 9, e1686. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.M. Depression and Epilepsy: A New Perspective on Two Closely Related Disorders. Epilepsy Curr. 2006, 6, 141–146. [Google Scholar] [CrossRef]
- Harden, C.L.; Pulver, M.C.; Ravdin, L.D.; Nikolov, B.; Halper, J.P.; Labar, D.R. A Pilot Study of Mood in Epilepsy Patients Treated with Vagus Nerve Stimulation. Epilepsy Behav. 2000, 1, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Elger, G.; Hoppe, C.; Falkai, P.; Rush, A.J.; Elger, C.E. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000, 42, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Assenza, G.; Tombini, M.; Lanzone, J.; Ricci, L.; di Lazzaro, V.; Casciato, S.; Morano, A.; Giallonardo, A.T.; di Bonaventura, C.; Beghi, E.; et al. Antidepressant effect of vagal nerve stimulation in epilepsy patients: A systematic review. Neurol. Sci. 2020, 41, 3075–3084. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.R.; Bakay, R.A.E.; Pennell, P.B.; Epstein, C.M.; Votaw, J.R. Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: II. Prolonged effects at high and low levels of stimulation. Epilepsia 2004, 45, 1064–1070. [Google Scholar] [CrossRef]
- Nahas, Z.; Teneback, C.; Chae, J.H.; Mu, Q.; Molnar, C.; Kozel, F.A.; Walker, J.; Anderson, B.; Koola, J.; Kose, S.; et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology 2007, 32, 1649–1660. [Google Scholar] [CrossRef]
- Chae, J.H.; Nahas, Z.; Lomarev, M.; Denslow, S.; Lorberbaum, J.P.; Bohning, D.E.; George, M.S. A review of functional neuroimaging studies of vagus nerve stimulation (VNS). J. Psychiatr. Res. 2003, 37, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Bohning, D.E.; Nahas, Z.; Walker, J.; Anderson, B.; Johnson, K.A.; Denslow, S.; Lomarev, M.; Moghadam, P.; Chae, J.H.; et al. Acute vagus nerve stimulation using different pulse widths produces varying brain effects. Biol. Psychiatry 2004, 55, 816–825. [Google Scholar] [CrossRef]
- Thompson, S.L.; O’Leary, G.H.; Austelle, C.W.; Gruber, E.; Kahn, A.T.; Manett AJShort, B.; Badran, B.W. A Review of Parameter Settings for Invasive and Non-invasive Vagus Nerve Stimulation (VNS) Applied in Neurological and Psychiatric Disorders. Front. Neurosci. 2021, 15, 709436. [Google Scholar] [CrossRef] [PubMed]
- Kilgard, M.P.; Rennaker, R.L.; Alexander, J.; Dawson, J. Vagus nerve stimulation paired with tactile training improved sensory function in a chronic stroke patient. NeuroRehabilitation 2018, 42, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Pierce, D.; Dixit, A.; Kimberley, T.J.; Robertson, M.; Tarver, B.; Hilmi, O.; Mclean, J.; Forbes, K.; Kilgard, M.P.; et al. Safety, feasibility, and efficacy of vagus nerve stimulation paired with upper-limb rehabilitation after ischemic stroke. Stroke 2016, 47, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Kimberley, T.J.; Pierce, D.; Prudente, C.N.; Francisco, G.E.; Yozbatiran, N.; Smith, P.; Tarver, B.; Engineer, N.D.; Dickie, D.A.; Kline, D.K.; et al. Vagus nerve stimulation paired with upper limb rehabilitation after chronic stroke: A blinded randomized pilot study. Stroke 2018, 49, 2789–2792. [Google Scholar] [CrossRef]
- Dawson, J.; Liu, C.Y.; Francisco, G.E.; Cramer, S.C.; Wolf, S.L.; Dixit, A.; Alexander, J.; Ali, R.; Brown, B.L.; Feng, W.; et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): A randomised, blinded, pivotal, device trial. Lancet 2021, 397, 1545–1553. [Google Scholar] [CrossRef]
- McGlone, J.; Valdivia, I.; Penner, M.; Williams, J.; Sadler, R.M.; Clarke, D.B. Quality of life and memory after vagus nerve stimulator implantation for epilepsy. Can. J. Neurol. Sci. 2008, 35, 287–296. [Google Scholar] [CrossRef]
- Dodrill, C.B.; Morris, G.L. Effects of Vagal Nerve Stimulation on Cognition and Quality of Life in Epilepsy. Epilepsy Behav. 2001, 2, 46–53. [Google Scholar] [CrossRef]
- Boon, P.; Moors, I.; de Herdt, V.; Vonck, K. Vagus nerve stimulation and cognition. Seizure 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Vonck, K.; Raedt, R.; Naulaerts, J.; De Vogelaere, F.; Thiery, E.; Van Roost, D.; Aldenkamp, B.; Miatton, M.; Boon, P. Vagus nerve stimulation. 25 years later! What do we know about the effects on cognition? Neurosci. Biobehav. Rev. 2014, 45, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Knorr, C.; Greuter, L.; Constantini, S.; Fried, I.; Kremer, U.; Datta, A.N.; Guzman, R.; Soleman, J. Subgroup analysis of seizure and cognitive outcome after vagal nerve stimulator implantation in children. Child’s Nerv. Syst. 2021, 37, 243–252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdennadher, M.; Rohatgi, P.; Saxena, A. Vagus Nerve Stimulation Therapy in Epilepsy: An Overview of Technical and Surgical Method, Patient Selection, and Treatment Outcomes. Brain Sci. 2024, 14, 675. https://doi.org/10.3390/brainsci14070675
Abdennadher M, Rohatgi P, Saxena A. Vagus Nerve Stimulation Therapy in Epilepsy: An Overview of Technical and Surgical Method, Patient Selection, and Treatment Outcomes. Brain Sciences. 2024; 14(7):675. https://doi.org/10.3390/brainsci14070675
Chicago/Turabian StyleAbdennadher, Myriam, Pratik Rohatgi, and Aneeta Saxena. 2024. "Vagus Nerve Stimulation Therapy in Epilepsy: An Overview of Technical and Surgical Method, Patient Selection, and Treatment Outcomes" Brain Sciences 14, no. 7: 675. https://doi.org/10.3390/brainsci14070675




