Neurohabilitation of Cognitive Functions in Pediatric Epilepsy Patients through LEGO®-Based Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Settings, and Ethical Considerations
2.2. Patients
2.3. Instruments
2.3.1. Neuropsychological Attention and Memory Battery (NEUROPSI)
2.3.2. Neuropsychological Battery of Executive Functions (BANFE-2)
2.3.3. Evaluation of the Execution of LEGO®-Based Therapy
2.4. General Procedure
2.5. Sessions of Intervention through LEGO®-Based Therapy
2.6. Statistical Analysis
3. Results
3.1. Descriptive Analysis of the Population
3.2. Cognitive Evaluations of Pediatric Patients with Epilepsy
3.3. Execution of LEGO®-Based Therapy for Pediatric Patients with Epilepsy
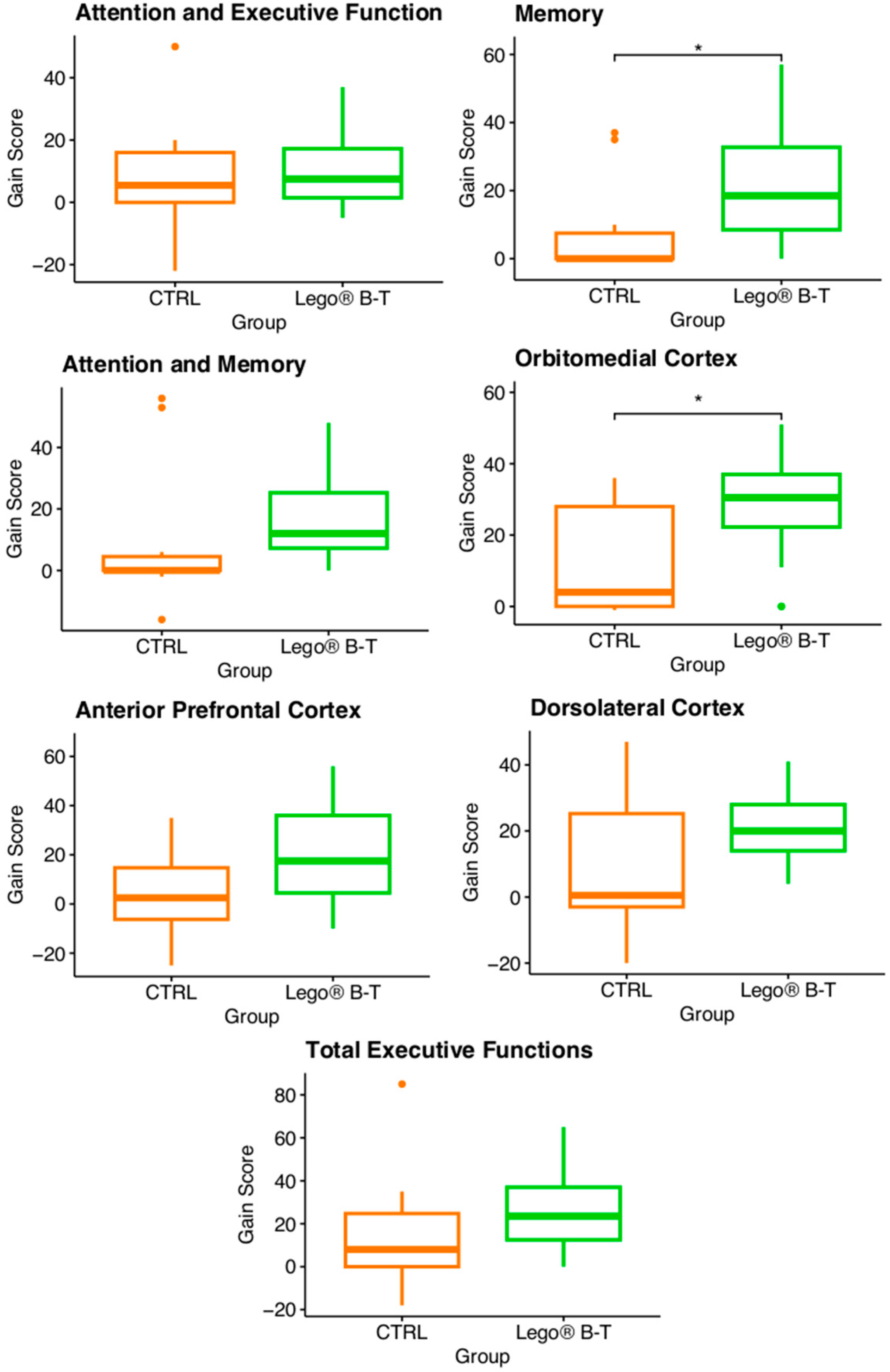
3.4. Effect of LEGO® B-T in Pediatric Patients with Epilepsy
3.5. Analysis of the Gain Score
4. Discussion
5. Limitation of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Camfield, P.; Camfield, C. Incidence, prevalence and etiology of seizures and epilepsy in children. Epileptic Disord. 2015, 17, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cheng, H.; Wang, Y.; Chen, Z. Revealing the Precise Role of Calretinin Neurons in Epilepsy: We Are on the Way. Neurosci Bull. 2022, 38, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Silva-Barragán, M.; Ramos-Galarza, C. Etiología del daño Cerebral: Un aporte neuropsicológico en su construcción teórica (primera parte). Rev. ecuat. Neurol. 2021, 30, 154–165. [Google Scholar] [CrossRef]
- Novak, A.; Vizjak, K.; Rakusa, M. Cognitive Impairment in People with Epilepsy. J. Clin. Med. 2022, 11, 267. [Google Scholar] [CrossRef]
- Elger, C.E.; Helmstaedter, C.; Kurthen, M. Chronic epilepsy and cognition. Lancet Neurol. 2004, 3, 663–672. [Google Scholar] [CrossRef]
- Sutula, T.P.; Hagen, J.; Pitkänen, A. Do epileptic seizures damage the brain? Curr. Opin. Neurol. 2003, 16, 189–195. [Google Scholar] [CrossRef]
- Xi, Y.; Lan, Z.; Chen, Y.; Zhang, Q.; Wu, Z.; Li, G. Patients with epilepsy without cognitive impairment show altered brain networks in multiple frequency bands in an audiovisual integration task. Neurophysiol. Clin. 2023, 53, 102888. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, M.; Deepa, P.; Park, S.J.; Kim, S. Application of the P300 Event-Related Potential in the Diagnosis of Epilepsy Disorder: A Review. Sci. Pharm. 2018, 86, 10. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.A.; Helmstaedter, C. Should cognition be screened in new-onset epilepsies? A study in 247 untreated patients. J. Neurol. 2012, 259, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.M.; Aldin, M.T.K.; Ali, S.E.; Hendi, A.E. Cognitive functions and epilepsy-related characteristics in patients with generalized tonic–clonic epilepsy: A cross-sectional study. Middle East Curr. Psychiatry 2023, 30, 15. [Google Scholar] [CrossRef]
- Miller, L.A.; Galioto, R.; Tremont, G.; Davis, J.; Bryant, K.; Roth, J.; LaFrance, W.C., Jr.; Blum, A.S. Cognitive impairment in older adults with epilepsy: Characterization and risk factor analysis. Epilepsy Behav. 2016, 56, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Vinukonda, G.; Dummula, K.; Malik, S.; Hu, F.; Thompson, C.I.; Csiszar, A.; Ungvari, Z.; Ballabh, P. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke 2010, 41, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Carson, R.; Monaghan-Nichols, A.P.; DeFranco, D.B.; Rudine, A.C. Effects of antenatal glucocorticoids on the developing brain. Steroids 2016, 114, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Crowther, C.A.; Middleton, P.; Marret, S. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst. Rev. 2007, 3, CD004661. [Google Scholar] [CrossRef]
- Crowther, C.A.; Middleton, P.F.; Voysey, M.; Askie, L.; Duley, L.; Pryde, P.G.; Marret, S.; Doyle, L.W.; AMICABLE Group. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis. PLoS Med. 2017, 14, e1002398. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.J.; Paton, M.C.B.; Fahey, M.C.; Jenkin, G.; Miller, S.L.; Finch-Edmondson, M.; McDonald, C.A. Neural stem cell treatment for perinatal brain injury: A systematic review and meta-analysis of preclinical studies. Stem Cells Transl. Med. 2021, 10, 1621–1636. [Google Scholar] [CrossRef]
- Penny, T.R.; Pham, Y.; Sutherland, A.E.; Mihelakis, J.G.; Lee, J.; Jenkin, G.; Fahey, M.C.; Miller, S.L.; McDonald, C.A. Multiple doses of umbilical cord blood cells improve long-term brain injury in the neonatal rat. Brain Res. 2020, 1746, 147001. [Google Scholar] [CrossRef]
- van Velthoven, C.T.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Repeated mesenchymal stem cell treatment after neonatal hypoxia-ischemia has distinct effects on formation and maturation of new neurons and oligodendrocytes leading to restoration of damage, corticospinal motor tract activity, and sensorimotor function. J. Neurosci. 2010, 30, 9603–9611. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Novak, I.; Miller, S.L.; Jenkin, G. Autologous transplantation of umbilical cord blood-derived cells in extreme preterm infants: Protocol for a safety and feasibility study. BMJ Open 2020, 10, e036065. [Google Scholar] [CrossRef]
- Bian, X.X.; Yuan, X.S.; Qi, C.P. Effect of recombinant human erythropoietin on serum S100B protein and interleukin-6 levels after traumatic brain injury in the rat. Neurol. Med. Chir. 2010, 50, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Yatsiv, I.; Grigoriadis, N.; Simeonidou, C.; Stahel, P.F.; Schmidt, O.I.; Alexandrovitch, A.G.; Tsenter, J.; Shohami, E. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005, 19, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- Robertson, N.J.; Tan, S.; Groenendaal, F.; van Bel, F.; Juul, S.E.; Bennet, L.; Derrick, M.; Back, S.A.; Valdez, R.C.; Northington, F.; et al. Which neuroprotective agents are ready for bench to bedside translation in the newborn infant? J. Pediatr. 2012, 160, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Häusler, S.; Robertson, N.J.; Golhen, K.; van den Anker, J.; Tucker, K.; Felder, T.K. Melatonin as a Therapy for Preterm Brain Injury: What Is the Evidence? Antioxidants 2023, 12, 1630. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.J.; Demers, E.J.; Juul, S.E. Safety of high-dose recombinant erythropoietin in a neonatal rat model. Neonatology 2007, 91, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W. Caffeine for Apnea of Prematurity Trial Group. Long-term effects of caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2007, 357, 1893–1902. [Google Scholar] [CrossRef]
- Bruschettini, M.; Brattström, P.; Russo, C.; Onland, W.; Davis, P.G.; Soll, R. Caffeine dosing regimens in preterm infants with or at risk for apnea of prematurity. Cochrane Database Syst. Rev. 2023, 4, CD013873. [Google Scholar] [CrossRef]
- WHO Immediate KMC Study Group. Impact of continuous Kangaroo Mother Care initiated immediately after birth (iKMC) on survival of newborns with birth weight between 1.0 to <1.8 kg: Study protocol for a randomized controlled trial. Trials 2020, 21, 280. [Google Scholar] [CrossRef]
- Cho, E.S.; Kim, S.J.; Kwon, M.S.; Cho, H.; Kim, E.H.; Jun, E.M.; Lee, S. The Effects of Kangaroo Care in the Neonatal Intensive Care Unit on the Physiological Functions of Preterm Infants, Maternal-Infant Attachment, and Maternal Stress. J. Pediatr. Nurs. 2016, 31, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Gonya, J.; Ray, W.C.; Rumpf, R.W.; Brock, G. Investigating skin-to-skin care patterns with extremely preterm infants in the NICU and their effect on early cognitive and communication performance: A retrospective cohort study. BMJ Open 2017, 7, e012985. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional influences on brain development. Acta Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Ottolini, K.M.; Andescavage, N.; Keller, S.; Limperopoulos, C. Nutrition and the developing brain: The road to optimizing early neurodevelopment: A systematic review. Pediatr. Res. 2020, 87, 194–201. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Robson, K.; Bracht, M.; Cruz, M.; Lui, K.; Alvaro, R.; da Silva, O.; Monterrosa, L.; Narvey, M.; Ng, E.; et al. Effectiveness of Family Integrated Care in neonatal intensive care units on infant and parent outcomes: A multicentre, multinational, cluster-randomised controlled trial. Lancet Child Adolesc. Health 2018, 2, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Procianoy, R.S.; Mendes, E.W.; Silveira, R.C. Massage therapy improves neurodevelopment outcome at two years corrected age for very low birth weight infants. Early Hum. Dev. 2010, 86, 7–11. [Google Scholar] [CrossRef]
- Feldman, R.; Rosenthal, Z.; Eidelman, A.I. Maternal-preterm skin-to-skin contact enhances child physiologic organization and cognitive control across the first 10 years of life. Biol. Psychiatry 2014, 75, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Braid, S.; Bernstein, J. Improved Cognitive Development in Preterm Infants with Shared Book Reading. Neonatal. Netw. 2015, 34, 10–17. [Google Scholar] [CrossRef]
- Shellhaas, R.A.; Burns, J.W.; Hassan, F.; Carlson, M.D.; Barks, J.D.E.; Chervin, R.D. Neonatal Sleep-Wake Analyses Predict 18-month Neurodevelopmental Outcomes. Sleep 2017, 40, zsx144. [Google Scholar] [CrossRef]
- Forcada-Guex, M.; Pierrehumbert, B.; Borghini, A.; Moessinger, A.; Muller-Nix, C. Early dyadic patterns of mother-infant interactions and outcomes of prematurity at 18 months. Pediatrics 2006, 118, e107–e114. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.S.; Willment, K.C.; Sarkis, R.A. Non-invasive Cognitive Enhancement in Epilepsy. Front. Neurol. 2019, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- van Walsem, M.R.; Piira, A.; Mikalsen, G.; Fossmo, H.L.; Howe, E.I.; Knutsen, S.F.; Frich, J.C. Cognitive Performance After a One-Year Multidisciplinary Intensive Rehabilitation Program for Huntington’s Disease: An Observational Study. J. Huntingtons. Dis. 2018, 7, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, S.; Hounsell, K.G.; Cassiani, C. A scoping review of the role of LEGO® therapy for improving inclusion and social skills among children and youth with autism. Disabil. Health J. 2017, 10, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Garamendi, E.; Labra-Ruiz, N.A.; Naranjo, L.; Chávez-Mejía, C.A.; Valenzuela-Alarcón, E.; Mendoza-Torreblanca, J.G. Habilitation of Executive Functions in Pediatric Congenital Heart Disease Patients through LEGO®-Based Therapy: A Quasi-Experimental Study. Healthcare 2022, 10, 2348. [Google Scholar] [CrossRef] [PubMed]
- LeGoff, D.B. Use of LEGO as a therapeutic medium for improving social competence. J. Autism. Dev. Disord. 2004, 34, 557–571. [Google Scholar] [CrossRef] [PubMed]
- LeGoff, D.B.; Gómez, G.; Krauss, G.W.; Baron-Cohen, S. LEGO-Based Therapy: Current Theory and Practice, 1st ed.; Universal-Publishers: Irvine, CA, USA; Boca Raton, FL, USA, 2023; pp. 1–155. [Google Scholar]
- Ostrosky, F.; Gómez, M.E.; Matute, E.; Rosselli, M.; Ardila, A.; Pineda, D. Neuropsi Atención y Memoria, 3rd ed.; Manual Moderno: Mexico City, Mexico, 2019; pp. 1–61. [Google Scholar]
- Flores, J.C.; Ostrosky, F.; Lozano, A. Batería Neuropsicológica de las Funciones Ejecutivas y Lóbulos Frontales, 2nd ed.; Manual Moderno: Mexico City, Mexico, 2014; pp. 1–39. [Google Scholar]
- Espinosa-Garamendi, E.; García-Benítez, L.A.; Valenzuela, E.; Mendoza-Torreblanca, J.G. Habilitación de funciones frontales básicas en cardiópatas congénitos a través de LEGO® Education. Rev. Chil. Neuro-Psiquiat. 2012, 16, 11–16. [Google Scholar]
- Dimitrov, D.M.; Rumrill, P.D., Jr. Pretest-posttest designs and measurement of change. Work 2003, 20, 159–165. [Google Scholar] [PubMed]
- Montgomery, D. Design and Analysis of Experiments, 8th ed.; Wiley: Toronto, ON, Canada, 2012. [Google Scholar]
- Gibbons, J.D.; Chakraborti, S. Nonparametric Statistical Inference; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, Series Use R! 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Fine, A.; Wirrell, E.C. Seizures in Children. Pediatr. Rev. 2020, 41, 321–347. [Google Scholar] [CrossRef]
- Zylicz, S.A.; Schippers, H.M.; Tromp, S.C. Lego-induced seizures: From an exceptional case toward the building blocks of generalized epilepsy. Seizure 2013, 22, 326–327. [Google Scholar] [CrossRef]
- Molnár, Z.; Clowry, G.J.; Šestan, N.; Alzu’bi, A.; Bakken, T.; Hevner, R.F.; Hüppi, P.S.; Kostović, I.; Rakic, P.; Anton, E.S.; et al. New insights into the development of the human cerebral cortex. J. Anat. 2019, 235, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Hrvoj-Mihic, B.; Bienvenu, T.; Stefanacci, L.; Muotri, A.R.; Semendeferi, K. Evolution, development, and plasticity of the human brain: From molecules to bones. Front. Hum. Neurosci. 2013, 7, 57752. [Google Scholar] [CrossRef] [PubMed]
- Barakova, E.I.; Bajracharya, P.; Willemsen, M.; Lourens, T.; Huskens, B. Long-term LEGO therapy with humanoid robot for children with ASD. Expert Syst. 2015, 32, 698–709. [Google Scholar] [CrossRef]
- Huskens, B.; Palmen, A.; Van der Werff, M.; Lourens, T.; Barakova, E. Improving Collaborative Play Between Children with Autism Spectrum Disorders and Their Siblings: The Effectiveness of a Robot-Mediated Intervention Based on Lego® Therapy. J. Autism. Dev. Disord. 2015, 45, 3746–3755. [Google Scholar] [CrossRef] [PubMed]
- Sayis, B.; Ramirez, R.; Pares, N. Mixed reality or LEGO game play? Fostering social interaction in children with Autism. Virtual Real. 2022, 26, 771–787. [Google Scholar] [CrossRef]
- Hsieh, H.C.; Liu, C.K.; Chen, P.K.H. Lego Robots in Puppet Play for Children with Cerebral Palsy. In Universal Access in Human-Computer Interaction. Design Approaches and Supporting Technologies. HCII 2020; Antona, M., Stephanidis, C., Eds.; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2020; Volume 12188. [Google Scholar] [CrossRef]
- Bazoolnejad, M.; Vakili, S.; Vahid, L.K.; Yaripour, M. The effectiveness of LEGO therapy on the resiliency of gifted children. JFMH 2021, 23, 359–366. [Google Scholar]
- Shields, M.; Hunnell, W.; Tucker, M.; Price, A. Building blocks and coloring away stress: Utilizing LEGO® and coloring as stress reduction strategies among university students. JHET 2020, 11, 24–31. [Google Scholar]
- Lopez-Samaniego, L.; Garcia-Zapirain, B.; Mendez-Zorrilla, A. Memory and accurate processing brain rehabilitation for elderly individuals: LEGO robot and iPad case study. Biomed. Mater. Eng. 2014, 24, 3549–3556. [Google Scholar] [CrossRef]
- Lopez-Samaniego, L.; Garcia-Zapirain, B. A Robot-Based Tool for Physical and Cognitive Rehabilitation of Elderly People Using Biofeedback. Int. J. Environ. Res. Public Health 2016, 13, 1176. [Google Scholar] [CrossRef]
- Smith, P.S.; Dinse, H.R.; Kalisch, T.; Johnson, M.; Walker-Batson, D. Effects of repetitive electrical stimulation to treat sensory loss in persons poststroke. Arch. Phys. Med. Rehabil. 2009, 90, 2108–2111. [Google Scholar] [CrossRef]
- Taghizadeh, G.; Azad, A.; Kashefi, S.; Fallah, S.; Daneshjoo, F. The effect of sensory-motor training on hand and upper extremity sensory and motor function in patients with idiopathic Parkinson disease. J. Hand Ther. 2018, 31, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Harn, P.A. Preliminary Study of the Empowerment Effects of Strength-Based LEGO® SERIOUS PLAY® on Two Taiwanese Adult Survivors by Earlier Domestic Violence. Psychol. Stud. 2017, 62, 142–151. [Google Scholar] [CrossRef]
- Tseng, W.-C. An Intervention Using LEGO® SERIOUS PLAY® on Fostering Narrative Identity Among Economically Disadvantaged College Students in Taiwan. JCSD 2017, 58, 264–282. [Google Scholar] [CrossRef]
- Camfield, C.S.; Chaplin, S.; Doyle, A.B.; Shapiro, S.H.; Cummings, C.; Camfield, P.R. Side effects of phenobarbital in toddlers; behavioral and cognitive aspects. J. Pediatr. 1979, 95, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Aikiä, M.; Kälviäinen, R.; Sivenius, J.; Halonen, T.; Riekkinen, P.J. Cognitive effects of oxcarbazepine and phenytoin monotherapy in newly diagnosed epilepsy: One year follow-up. Epilepsy Res. 1992, 11, 199–203. [Google Scholar] [CrossRef]
- Donati, F.; Gobbi, G.; Campistol, J.; Rapatz, G.; Daehler, M.; Sturm, Y.; Aldenkamp, A.P.; Oxcarbazepine Cognitive Study Group. The cognitive effects of oxcarbazepine versus carbamazepine or valproate in newly diagnosed children with partial seizures. Seizure 2007, 16, 670–679. [Google Scholar] [CrossRef]
- Burton, L.A.; Harden, C. Effect of topiramate on attention. Epilepsy Res. 1997, 27, 29–32. [Google Scholar] [CrossRef]
- Coppola, G.; Verrotti, A.; Resicato, G.; Ferrarelli, S.; Auricchio, G.; Operto, F.F.; Pascotto, A. Topiramate in children and adolescents with epilepsy and mental retardation: A prospective study on behavior and cognitive effects. Epilepsy Behav. 2008, 12, 253–256. [Google Scholar] [CrossRef]
- Strzelczyk, A.; Schubert-Bast, S. Psychobehavioural and Cognitive Adverse Events of Anti-Seizure Medications for the Treatment of Developmental and Epileptic Encephalopathies. CNS Drugs 2022, 36, 1079–1111. [Google Scholar] [CrossRef]
- Marson, A.G.; Hutton, J.L.; Leach, J.P.; Castillo, S.; Schmidt, D.; White, S.; Chaisewikul, R.; Privitera, M.; Chadwick, D.W. Levetiracetam, oxcarbazepine, remacemide and zonisamide for drug resistant localization-related epilepsy: A systematic review. Epilepsy Res. 2001, 46, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Kuzniecky, R.; Ho, S.; Hetherington, H.; Pan, J.; Sinclair, K.; Gilliam, F.; Faught, E. Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology 1999, 52, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Borm, G.F.; Fransen, J.; Lemmens, W.A. A simple sample size formula for analysis of covariance in randomized clinical trials. J. Clin. Epidemiol. 2007, 60, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Ma, C. A Comment on sample size calculation for analysis of covariance in parallel arm studies. BBIJ 2014, 5, 1. [Google Scholar]
- Bujang, M.A.; Sa’at, N.; Bakar, T.M.I.T.A. Determination of minimum sample size requirement for multiple linear regression and analysis of covariance based on experimental and non-experimental studies. Epidemiol. Biostat. Public Health 2017, 14, e12117-1–e12117-9. [Google Scholar] [CrossRef]




| Sessions | Objectives | Tasks | Sets |
|---|---|---|---|
| (1) Free game with programming | Initial interaction, familiarizing the patient with the material and conducting the therapeutic interaction |
|
|
| (2) and (3) Working and visuospatial memory, inhibitory control | Gradually stimulates areas of the cerebral cortex to improve selective attention, inhibitory control, and short-term memory |
|
|
| (4) to (8) Working memory, inhibitory control, risk selection and planning | Stimulation of cerebral cortex areas to improve the effort investment process of related functions |
|
|
| (9) to (16) Integration of follow-up and executive functions integration | Cognitive habilitation and developmental monitoring |
|
|
| Variable | Characteristic | CTRL Group | LEGO® B-T Group |
|---|---|---|---|
| Sex 1 | Male | 4 (40%) | 7 (58%) |
| Female | 6 (60%) | 5 (42%) | |
| Age 2 | Years | 12 (10–15) | 9.5 (8–12.5) |
| Etiology 1 | Structural | 8 (80%) | 4 (33%) |
| Genetic | 1 (10%) | 2 (16%) | |
| Unknown | 1 (10%) | 6 (50%) | |
| Epilepsy type 1 | Focal | 7 (70%) | 5 (42%) |
| Generalized | 0 (0%) | 2 (16%) | |
| Combined generalized and focal | 3 (30%) | 5 (42%) | |
| EEG 1 | With paroxysmal activity | 5 (50%) | 6 (50%) |
| Without activity | 4 (40%) | 5 (42%) | |
| Not performed | 1 (10%) | 1 (8%) |
| CTRL | LEGO® B-T Group | ||||
|---|---|---|---|---|---|
| Patient | AED | Dose (mg/kg/Day) | Patient | AED | Dose (mg/kg/Day) |
| 1 | LEV | 37 | 1 | VPA | 19 |
| 2 | LEV | 27 | 2 | OXCBZ | 26 |
| 3 | VPA | 17 | 3 | LEV | 47 |
| 4 | OXCBZ | 25 | 4 | LEV | 27 |
| 5 | LTG | 4.2 | 5 | LEV | 36 |
| 6 | LEV | 30 | 6 | LEV | 40 |
| 7 | LEV TPM | 55 5.5 | 7 | OXCBZ LTG | 22 5.6 |
| 8 | LEV VPA | 20 46 | 8 | LEV OXCBZ | 50 30 |
| 9 | VPA LEV | 21.5 40 | 9 | LEV OXCBZ | 65 13 |
| 10 | LEV | 18 | 10 | VPA | 24 |
| LTG | 3.5 | 11 | Suspended | -- | |
| VPA | 31 | 12 | OXCBZ LEV | 30 42 | |
| CTRL Patients with Epilepsy | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NEUROPSI | BANFE-2 | |||||||||||||
| Patient | Attention and Executive Functions | Memory | Attention and Memory | OMC | APC | DLC | Total Executive Functions | |||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 62 | 72 | 60 | 97 | 34 | 87 | 89 | 117 | 64 | 80 | 44 | 91 | 45 | 130 |
| 2 | 45 | 61 | 45 | 45 | 45 | 45 | 64 | 92 | 117 | 110 | 43 | 66 | 45 | 69 |
| 3 | 54 | 74 | 60 | 60 | 85 | 69 | 44 | 80 | 63 | 70 | 50 | 76 | 45 | 70 |
| 4 | 68 | 46 | 45 | 45 | 47 | 45 | 44 | 44 | 132 | 107 | 81 | 72 | 44 | 60 |
| 5 | 45 | 46 | 45 | 45 | 45 | 45 | 43 | 43 | 56 | 48 | 45 | 41 | 45 | 45 |
| 6 | 48 | 98 | 81 | 116 | 57 | 113 | 70 | 103 | 85 | 99 | 57 | 86 | 56 | 91 |
| 7 | 45 | 45 | 45 | 45 | 45 | 45 | 46 | 46 | 82 | 80 | 44 | 44 | 45 | 45 |
| 8 | 46 | 46 | 45 | 45 | 45 | 45 | 43 | 44 | 48 | 44 | 45 | 45 | 45 | 45 |
| 9 | 46 | 46 | 45 | 45 | 45 | 45 | 46 | 45 | 40 | 75 | 44 | 45 | 45 | 45 |
| 10 | 74 | 90 | 72 | 82 | 72 | 78 | 74 | 81 | 92 | 107 | 96 | 76 | 94 | 76 |
| LEGO® B-T patients with epilepsy | ||||||||||||||
| NEUROPSI | BANFE-2 | |||||||||||||
| Patient | Attention Executive Function | Memory | Attention and Memory | OMC | APC | DLC | Total Executive Functions | |||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 84 | 94 | 50 | 72 | 57 | 77 | 46 | 97 | 58 | 94 | 87 | 113 | 68 | 108 |
| 2 | 95 | 104 | 63 | 98 | 69 | 94 | 92 | 120 | 96 | 103 | 90 | 111 | 94 | 109 |
| 3 | 45 | 45 | 48 | 63 | 45 | 45 | 44 | 44 | 97 | 107 | 62 | 66 | 44 | 44 |
| 4 | 74 | 69 | 45 | 45 | 45 | 45 | 46 | 69 | 64 | 70 | 44 | 60 | 45 | 50 |
| 5 | 101 | 105 | 89 | 146 | 90 | 98 | 84 | 117 | 107 | 132 | 98 | 112 | 92 | 120 |
| 6 | 66 | 90 | 45 | 77 | 71 | 79 | 69 | 105 | 89 | 89 | 58 | 95 | 54 | 96 |
| 7 | 45 | 51 | 45 | 45 | 40 | 45 | 46 | 86 | 47 | 103 | 45 | 64 | 66 | 77 |
| 8 | 94 | 96 | 68 | 120 | 67 | 115 | 79 | 90 | 85 | 85 | 96 | 120 | 92 | 105 |
| 9 | 106 | 121 | 78 | 91 | 87 | 101 | 44 | 86 | 117 | 107 | 108 | 114 | 44 | 109 |
| 10 | 85 | 111 | 107 | 111 | 98 | 108 | 46 | 81 | 82 | 118 | 106 | 120 | 90 | 114 |
| 11 | 90 | 90 | 58 | 86 | 55 | 81 | 85 | 108 | 63 | 107 | 79 | 120 | 85 | 108 |
| 12 | 46 | 83 | 57 | 67 | 45 | 73 | 71 | 91 | 78 | 107 | 66 | 100 | 63 | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaldumbide-Alcocer, F.L.; Labra-Ruiz, N.A.; Carbó-Godinez, A.A.; Ruíz-García, M.; Mendoza-Torreblanca, J.G.; Naranjo-Albarrán, L.; Cárdenas-Rodríguez, N.; Valenzuela-Alarcón, E.; Espinosa-Garamendi, E. Neurohabilitation of Cognitive Functions in Pediatric Epilepsy Patients through LEGO®-Based Therapy. Brain Sci. 2024, 14, 702. https://doi.org/10.3390/brainsci14070702
Zaldumbide-Alcocer FL, Labra-Ruiz NA, Carbó-Godinez AA, Ruíz-García M, Mendoza-Torreblanca JG, Naranjo-Albarrán L, Cárdenas-Rodríguez N, Valenzuela-Alarcón E, Espinosa-Garamendi E. Neurohabilitation of Cognitive Functions in Pediatric Epilepsy Patients through LEGO®-Based Therapy. Brain Sciences. 2024; 14(7):702. https://doi.org/10.3390/brainsci14070702
Chicago/Turabian StyleZaldumbide-Alcocer, Flor Lorena, Norma Angélica Labra-Ruiz, Abril Astrid Carbó-Godinez, Matilde Ruíz-García, Julieta Griselda Mendoza-Torreblanca, Lizbeth Naranjo-Albarrán, Noemí Cárdenas-Rodríguez, Erika Valenzuela-Alarcón, and Eduardo Espinosa-Garamendi. 2024. "Neurohabilitation of Cognitive Functions in Pediatric Epilepsy Patients through LEGO®-Based Therapy" Brain Sciences 14, no. 7: 702. https://doi.org/10.3390/brainsci14070702
APA StyleZaldumbide-Alcocer, F. L., Labra-Ruiz, N. A., Carbó-Godinez, A. A., Ruíz-García, M., Mendoza-Torreblanca, J. G., Naranjo-Albarrán, L., Cárdenas-Rodríguez, N., Valenzuela-Alarcón, E., & Espinosa-Garamendi, E. (2024). Neurohabilitation of Cognitive Functions in Pediatric Epilepsy Patients through LEGO®-Based Therapy. Brain Sciences, 14(7), 702. https://doi.org/10.3390/brainsci14070702









