OculoMotor & Vestibular Endurance Screening (MoVES) Normative, Repeatability, and Reliability Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Equity, Diversity, and Inclusion Statement
2.3. Data Collection and Protocol
2.4. Statistical Analysis
3. Results
3.1. Demographic Characteristics of Athletes and Symptomology
3.2. Summary Statistics for all Seven Assessments in Athletes at Pre-Season and Post-Season
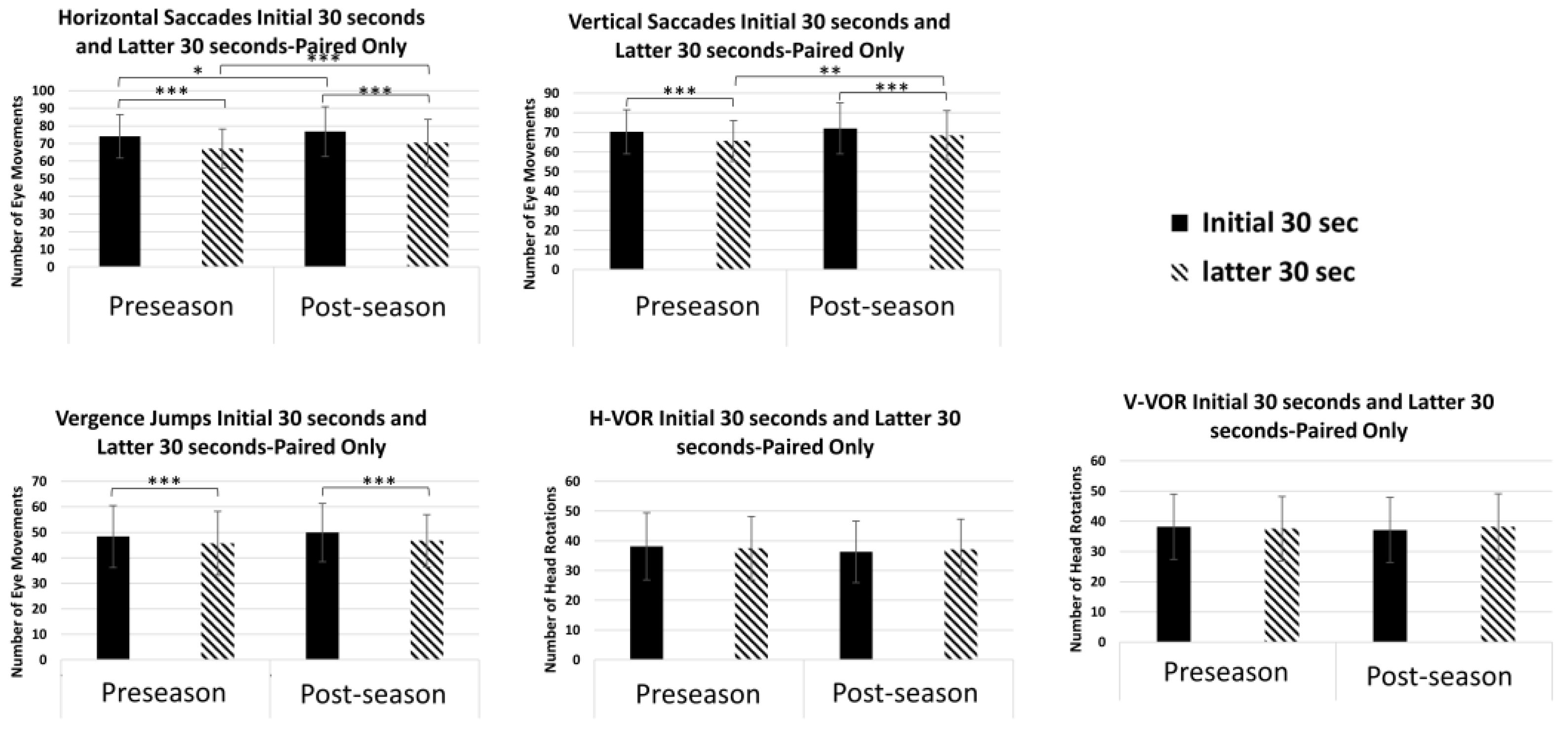
3.3. Performance for Horizontal Saccades
3.4. Performance for Vertical Saccades
3.5. Performance for Vergence Jumps
3.6. Performance for Vertical and Horizontal VOR
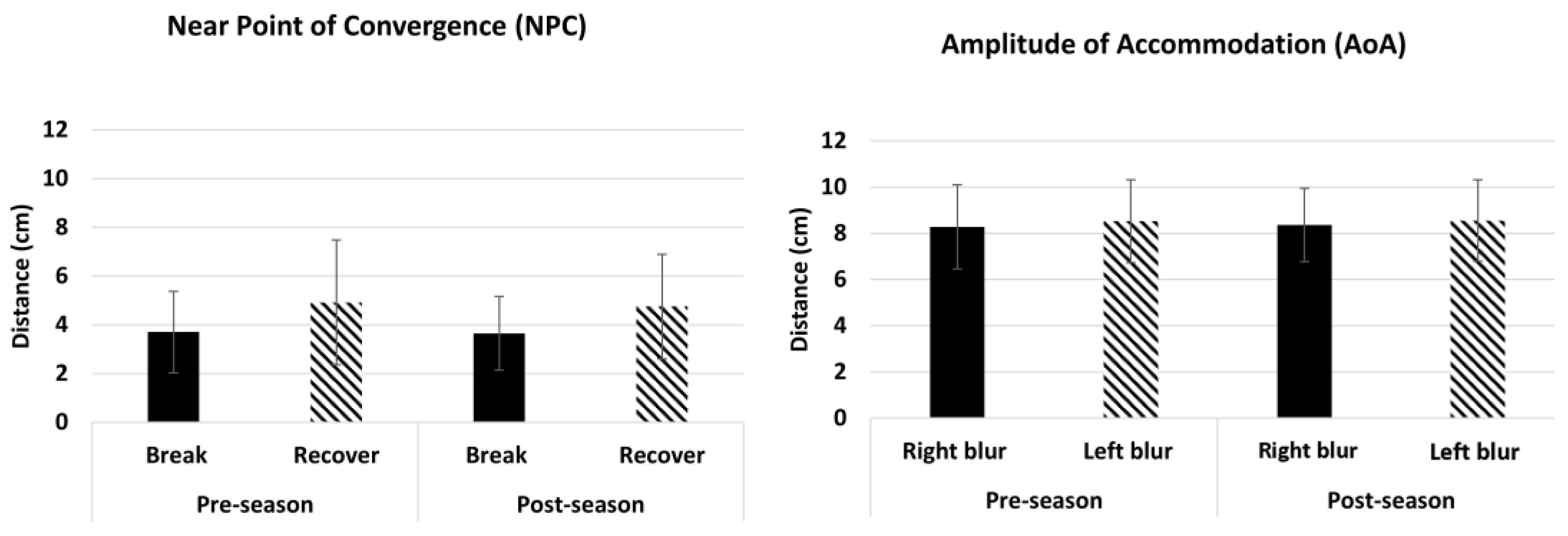
3.7. Performance for Amplitude of Accommodation (AoA) and Near Point of Convergence (NPC)
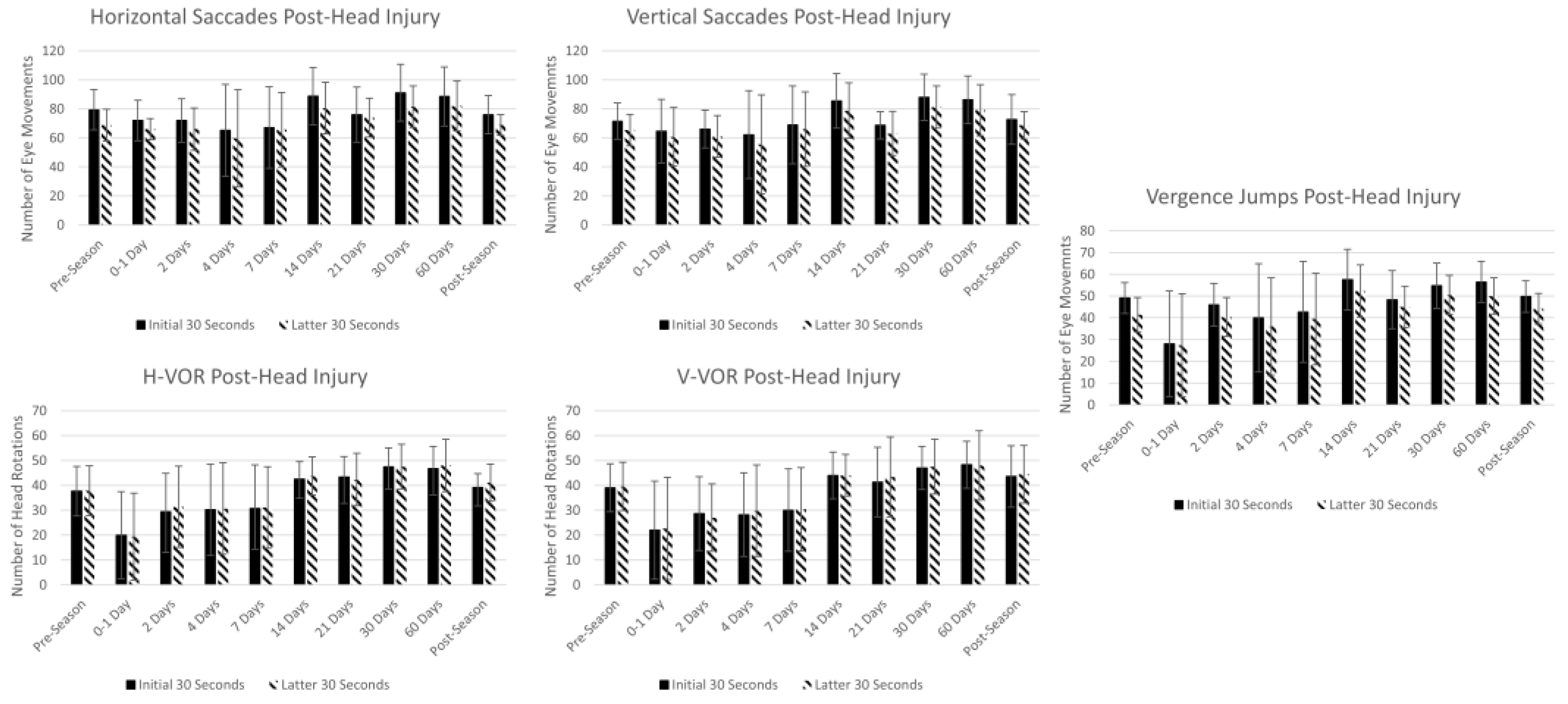
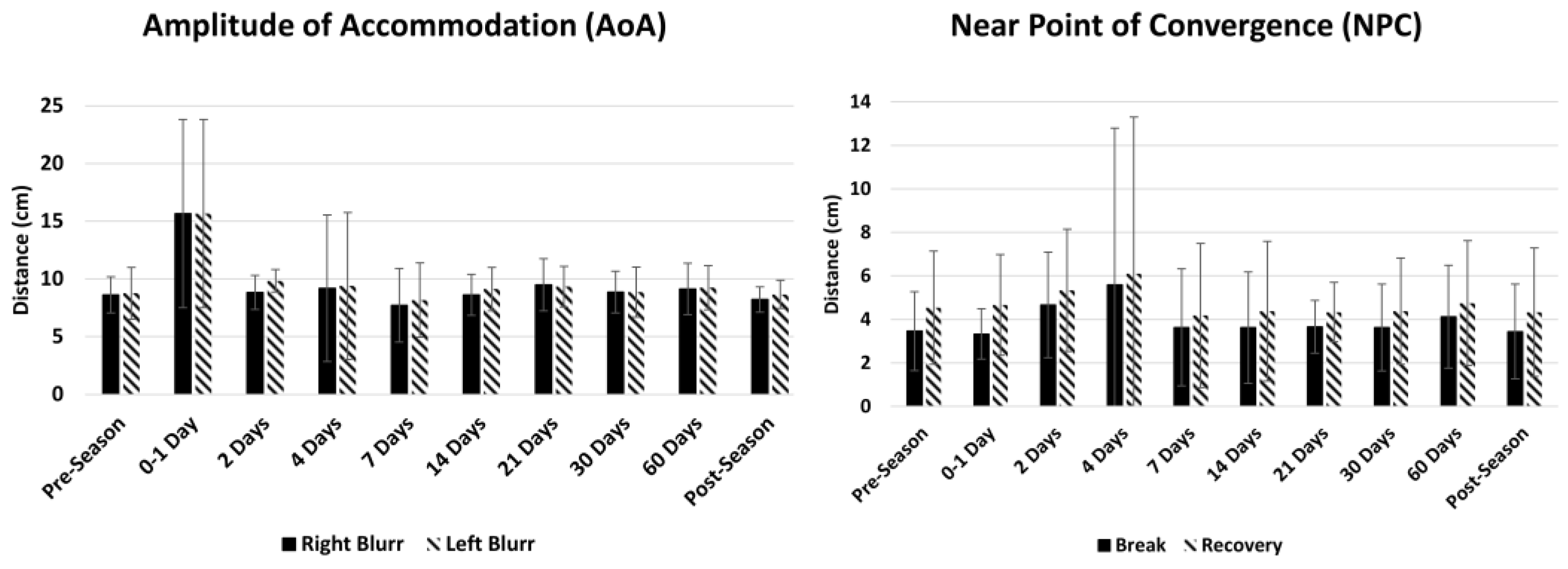
3.8. Summary Statistics in Athletes at Pre-Season, Post-Head Injury and Post-Season
4. Discussion
4.1. Horizontal and Vertical Saccades
4.1.1. Vergence Jumps
4.1.2. Vertical and Horizontal VOR
4.1.3. Amplitude of Accommodation (AoA) and Near Point of Convergence (NPC)
4.1.4. Trends in Athletes at Pre-Season, Post-Head Injury, and Post-Season
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patricios, J.S.; Schneider, K.J.; Dvorak, J.; Ahmed, O.H.; Blauwet, C.; Cantu, R.C.; Davis, G.A.; Echemendia, R.J.; Makdissi, M.; McNamee, M.; et al. Consensus statement on concussion in sport: The 6th International Conference on Concussion in Sport–Amsterdam, October 2022. Br. J. Sports Med. 2023, 57, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Master, C.L.; Bacal, D.; Grady, M.F.; Hertle, R.; Shah, A.S.; Strominger, M.; Whitecross, S.; Bradford, G.E.; Lum, F.; Donahue, S.P.; et al. Vision and Concussion: Symptoms, Signs, Evaluation, and Treatment. Pediatrics 2022, 150, e2021056047. [Google Scholar] [CrossRef] [PubMed]
- Master, C.L.; Scheiman, M.; Gallaway, M.; Goodman, A.; Robinson, R.L.; Master, S.R.; Grady, M.F. Vision Diagnoses Are Common after Concussion in Adolescents. Clin. Pediatr. 2015, 55, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Cordingley, D.; Vis, S.; Reimer, K.; Leiter, J.; Russell, K. Vestibulo-ocular dysfunction in pediatric sports-related concussion. J. Neurosurg. Pediatr. 2015, 16, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Coiner, B.; Pan, H.; Bennett, M.L.; Bodien, Y.G.; Iyer, S.; O’Neil-Pirozzi, T.M.; Leung, L.; Giacino, J.T.; Stern, E. Functional neuroanatomy of the human eye movement network: A review and atlas. Brain Struct. Funct. 2019, 224, 2603–2617. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Hammer, W.; Vleeming, A.; De Caro, R. (Eds.) 3—Deep Fasciae. In Functional Atlas of the Human Fascial System; Churchill Livingstone: London, UK, 2015; pp. 51–102. [Google Scholar]
- den Berg, F.V. 4.1—The physiology of fascia: An introduction. In Fascia: The Tensional Network of the Human Body; Schleip, R., Findley, T.W., Chaitow, L., Huijing, P.A., Eds.; Churchill Livingstone: Oxford, UK, 2012; pp. 149–155. [Google Scholar]
- Mucha, A.; Collins, M.W.; Elbin, R.J.; Furman, J.M.; Troutman-Enseki, C.; DeWolf, R.M.; Marchetti, G.; Kontos, A.P. A Brief Vestibular/Ocular Motor Screening (VOMS) assessment to evaluate concussions: Preliminary findings. Am. J. Sports Med. 2014, 42, 2479–2486. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, M.; Grady, M.F.; Jenewein, E.; Shoge, R.; Podolak, O.E.; Howell, D.H.; Master, C.L. Frequency of oculomotor disorders in adolescents 11 to 17 years of age with concussion, 4 to 12 weeks post injury. Vis. Res. 2021, 183, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.R.; O’Brien, M.J.; Raghuram, A.; Shah, A.S.; Meehan, W.P. Near Point of Convergence and Gait Deficits in Adolescents after Sport-Related Concussion. Clin. J. Sport Med. 2018, 28, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, T.L.; Kim, E.H.; Vicci, V.R.; Dhar, S.K.; Biswal, B.B.; Barrett, A.M. Concurrent vision dysfunctions in convergence insufficiency with traumatic brain injury. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2012, 89, 1740–1751. [Google Scholar] [CrossRef]
- Suleiman, A.; Lithgow, B.J.; Anssari, N.; Ashiri, M.; Moussavi, Z.; Mansouri, B. Correlation between Ocular and Vestibular Abnormalities and Convergence Insufficiency in Post-Concussion Syndrome. Neuro-Ophthalmology 2019, 44, 157–167. [Google Scholar] [CrossRef]
- Debacker, J.; Ventura, R.; Galetta, S.L.; Balcer, L.J.; Rucker, J.C. Neuro-ophthalmologic disorders following concussion. Handb. Clin. Neurol. 2018, 158, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Humphreys, D.; Heath, M. Oculomotor Executive Dysfunction during the Early and Later Stages of Sport-Related Concussion Recovery. J. Neurotrauma 2018, 35, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Kontos, A.P.; Deitrick, J.M.; Collins, M.W.; Mucha, A. Review of Vestibular and Oculomotor Screening and Concussion Rehabilitation. J. Athl. Train. 2017, 52, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Brown, C.; Hirst, R.B.; Thompson, R.C. Commonality in Abnormality: “Abnormal” Neuropsychological Scores Are Common in Baseline Testing of Youth Athletes. Arch. Clin. Neuropsychol. 2020, 35, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Iverson, G.L.; Karr, J.E.; Gardner, A.J.; Silverberg, N.D.; Terry, D.P. Results of scoping review do not support mild traumatic brain injury being associated with a high incidence of chronic cognitive impairment: Commentary on McInnes et al. 2017. PLoS ONE 2019, 14, e0218997. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, M.; Yaramothu, C.; Alvarez, T.L. Changes in the Disparity Vergence Main Sequence after Treatment of Symptomatic Convergence Insufficiency in Children. J. Eye Mov. Res. 2019, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Yaramothu, C.; Morris, C.J.; d’Antonio-Bertagnolli, J.V.; Alvarez, T.L. OculoMotor Assessment Tool Test Procedure and Normative Data. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2021, 98, 636–643. [Google Scholar] [CrossRef]
- Yorke, A.M.; Smith, L.; Babcock, M.; Alsalaheen, B. Validity and Reliability of the Vestibular/Ocular Motor Screening and Associations with Common Concussion Screening Tools. Sports Health 2017, 9, 174–180. [Google Scholar] [CrossRef]
- Murray, N.G.; Szekely, B.; Islas, A.; Munkasy, B.; Gore, R.; Berryhill, M.; Reed-Jones, R.J. Smooth Pursuit and Saccades after Sport-Related Concussion. J. Neurotrauma 2020, 37, 340–346. [Google Scholar] [CrossRef]
- Yaramothu, C.; Greenspan, L.D.; Scheiman, M.; Alvarez, T.L. Vergence Endurance Test: A Pilot Study for a Concussion Biomarker. J. Neurotrauma 2019, 36, 2200–2212. [Google Scholar] [CrossRef]
- Yaramothu, C.; Santos, E.M.; Alvarez, T.L. Effects of visual distractors on vergence eye movements. J. Vis. 2018, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, T.L.; Yaramothu, C.; Scheiman, M.; Goodman, A.; Cotter, S.A.; Huang, K.; Chen, A.M.; Grady, M.; Mozel, A.E.; Podolak, O.E.; et al. Disparity vergence differences between typically occurring and concussion-related convergence insufficiency pediatric patients. Vis. Res. 2021, 185, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Dungan, M.E.; Scheiman, M.; Yaramothu, C. Vision Quality of Life with Time Survey: Normative Data and Repeatability. Clin. Optom. 2023, 15, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.; Nygren de Boussard, C.; Öqvist Seimyr, G.; Pansell, T. The effect of spectacle treatment in patients with mild traumatic brain injury: A pilot study. Clin. Exp. Optom. 2017, 100, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Donker-Cools, B.; Schouten, M.J.E.; Wind, H.; Frings-Dresen, M.H.W. Return to work following acquired brain injury: The views of patients and employers. Disabil. Rehabil. 2018, 40, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Graff, H.J.; Deleu, N.W.; Christiansen, P.; Rytter, H.M. Facilitators of and barriers to return to work after mild traumatic brain injury: A thematic analysis. Neuropsychol. Rehabil. 2021, 31, 1349–1373. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, C.; Kristman, V.L.; Cassidy, J.D.; Hincapié, C.A.; Côté, P.; Boyle, E.; Carroll, L.J.; Stålnacke, B.M.; Nygren-de Boussard, C.; Borg, J. Systematic review of return to work after mild traumatic brain injury: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch. Phys. Med. Rehabil. 2014, 95, S201–S209. [Google Scholar] [CrossRef] [PubMed]
- Zargari Marandi, R.; Madeleine, P.; Omland, Ø.; Vuillerme, N.; Samani, A. Eye movement characteristics reflected fatigue development in both young and elderly individuals. Sci. Rep. 2018, 8, 13148. [Google Scholar] [CrossRef] [PubMed]
- Nij Bijvank, J.A.; van Rijn, L.J.; Kamminga, M.; Tan, H.S.; Uitdehaag, B.M.J.; Petzold, A.; Balk, L.J. Saccadic fatigability in the oculomotor system. J. Neurol. Sci. 2019, 402, 167–174. [Google Scholar] [CrossRef]
- Raghuram, A.; Cotter, S.A.; Gowrisankaran, S.; Kanji, J.; Howell, D.R.; Meehan, W.P.; Shah, A.S. Postconcussion: Receded Near Point of Convergence is not Diagnostic of Convergence Insufficiency. Am. J. Ophthalmol. 2019, 206, 235–244. [Google Scholar] [CrossRef]
- Brahm, K.D.; Wilgenburg, H.M.; Kirby, J.; Ingalla, S.; Chang, C.-Y.; Goodrich, G.L. Visual Impairment and Dysfunction in Combat-Injured Servicemembers with Traumatic Brain Injury. Optom. Vis. Sci. 2009, 86, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Gallaway, M.; Scheiman, M.; Mitchell, G.L. Vision Therapy for Post-Concussion Vision Disorders. Optom. Vis. Sci. 2017, 94, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Semmlow, J.L. The influence of repetitive eye movements on vergence performance. Vis. Res. 2000, 40, 3089–3098. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, K.J.; Ludlam, D.P.; Thiagarajan, P.; Yadav, N.K.; Capo-Aponte, J. Proposed objective visual system biomarkers for mild traumatic brain injury. Mil. Med. 2014, 179, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, M.; Talasan, H.; Alvarez, T.L. Objective Assessment of Disparity Vergence after Treatment of Symptomatic Convergence Insufficiency in Children. Optom. Vis. Sci. Off. Publ. Am. Acad. Optom. 2019, 96, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, P.; Ciuffreda, K.J. Effect of oculomotor rehabilitation on vergence responsivity in mild traumatic brain injury. J. Rehabil. Res. Dev. 2013, 50, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, M.E.; Gottshall, K.R.; Moore, R.; Balough, B.J.; Wester, D. Characterizing and treating dizziness after mild head trauma. Otol. Neurotol. 2004, 25, 135–138. [Google Scholar] [CrossRef]
- Corwin, D.J.; Wiebe, D.J.; Zonfrillo, M.R.; Grady, M.F.; Robinson, R.L.; Goodman, A.M.; Master, C.L. Vestibular Deficits following Youth Concussion. J. Pediatr. 2015, 166, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R. Principles of Neural Science, 5th ed.; McGraw Hill: New York, NY, USA, 2013. [Google Scholar]
- Guskiewicz, K.M. Balance assessment in the management of sport-related concussion. Clin. Sports Med. 2011, 30, 89–102. [Google Scholar] [CrossRef]
- Galetta, K.M.; Barrett, J.; Allen, M.; Madda, F.; Delicata, D.; Tennant, A.T.; Branas, C.C.; Maguire, M.G.; Messner, L.V.; Devick, S.; et al. The King-Devick test as a determinant of head trauma and concussion in boxers and MMA fighters. Neurology 2011, 76, 1456–1462. [Google Scholar] [CrossRef]
- Ventura, R.; Balcer, L.; Galetta, S. The Concussion Toolbox: The Role of Vision in the Assessment of Concussion. Semin. Neurol. 2015, 35, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Mays, L.E.; Gamlin, P.D. Neuronal circuitry controlling the near response. Curr. Opin. Neurobiol. 1995, 5, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.; Starzynski, S.; Mohan, S.; Yaramothu, C. Presence of Accommodative Dysfunction Postconcussion in an Adult Population. J. Head Trauma Rehabil. 2022, 37, E535. [Google Scholar]
- Leonard, B.T.; Kontos, A.P.; Marchetti, G.F.; Zhang, M.; Eagle, S.R.; Reecher, H.M.; Bensinger, E.S.; Snyder, V.C.; Holland, C.L.; Sheehy, C.K.; et al. Fixational eye movements following concussion. J. Vis. 2021, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.J.; Mehmel, B.M.; Rossi, E.A.; Trbovich, A.M.; Eagle, S.R.; Kontos, A.P. Temporal Changes in Fixational Eye Movements after Concussion in Adolescents and Adults: Preliminary Findings. J. Neurotrauma 2023, 41, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Rochefort, C.; Legace, E.; Boulay, C.; Macartney, G.; Goulet, K.; Zemek, R.; Sveistrup, H. Balance Markers and Saccadic Eye-Movement Measures in Adolescents with Postconcussion Syndrome. J. Athl. Train. 2020, 55, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Wiecek, E.K.; Roberts, T.L.; Shah, A.S.; Raghuram, A. Vergence, accommodation, and visual tracking in children and adolescents evaluated in a multidisciplinary concussion clinic. Vis. Res. 2021, 184, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Len, T.K.; Neary, J.P. Cerebrovascular pathophysiology following mild traumatic brain injury. Clin. Physiol. Funct. Imaging 2011, 31, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.D.; Smirl, J.D.; Bryk, K.; Fraser, S.; Jakovac, M.; van Donkelaar, P. Sport-Related Concussion Alters Indices of Dynamic Cerebral Autoregulation. Front. Neurol. 2018, 9, 196. [Google Scholar] [CrossRef]
- Hutchison, M.G.; Schweizer, T.A.; Tam, F.; Graham, S.J.; Comper, P. FMRI and brain activation after sport concussion: A tale of two cases. Front. Neurol. 2014, 5, 46. [Google Scholar] [CrossRef]
- Arshad, Q.; Seemungal, B.M. Age-Related Vestibular Loss: Current Understanding and Future Research Directions. Front. Neurol. 2016, 7, 231. [Google Scholar] [CrossRef]
- Gabriel, G.A.; Harris, L.R.; Gnanasegaram, J.J.; Cushing, S.L.; Gordon, K.A.; Haycock, B.C.; Campos, J.L. Age-related changes to vestibular heave and pitch perception and associations with postural control. Sci. Rep. 2022, 12, 6426. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
| Test | Time Interval | Average (±SD) | Non- Zero Minimum | Maximum | Median (95% Confidence Interval) |
|---|---|---|---|---|---|
| Horizontal Saccades | Initial 30 | 73.5 ± 13.3 | 38 | 140 | 74 (72–75) |
| Latter 30 | 66.6 ± 11.6 | 34 | 102 | 66 (65–68) | |
| Total | 140.0 ± 24.1 | 72 | 242 | 140 (137–143) | |
| Vertical Saccades | Initial 30 | 69.7 ± 11.8 | 40 | 104 | 68 (68–71) |
| Latter 30 | 65.2 ± 11.3 | 22 | 88 | 66 (64–66) | |
| Total | 134.9 ± 22.3 | 68 | 188 | 136 (132–137) | |
| Vergence Jumps | Initial 30 | 45.7 ± 11.9 | 6 | 86 | 44 (44–47) |
| Latter 30 | 43.0 ± 11.7 | 5 | 90 | 42 (42–44) | |
| Total | 88.7 ± 22.9 | 10 | 172 | 86 (86–91) | |
| Horizontal VOR | Initial 30 | 36.6 ± 11.1 | 6 | 84 | 36 (35–38) |
| Latter 30 | 36.7 ± 10.6 | 2 | 74 | 36 (36–38) | |
| Total | 73.3 ± 21.2 | 12 | 158 | 72 (71–76) | |
| Vertical VOR | Initial 30 | 37.3± 10.9 | 8 | 74 | 36 (36–38) |
| Latter 30 | 38.0 ± 10.8 | 10 | 68 | 38 (37–39) | |
| Total | 75.3 ± 21.3 | 20 | 140 | 74 (73–78) | |
| Near Point of Convergence | Break (cm) | 3.3 ± 2 | 2 | 10 | 3 (3–4) |
| Recovery (cm) | 4.4 ± 2 | 2 | 16 | 4 (4–5) | |
| Amplitude of Accommodation | Right (cm) | 8.3 ± 2 | 4.5 | 16 | 8 (8–9) |
| Left (cm) | 8.6 ± 2 | 4.0 | 16 | 8 (8–9) |
| Pre-Season | Post-Season | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Test | Time Interval | Average (±SD) | Non- Zero Minimum | Maximum | Median (95% Confidence Interval) | Average (±SD) | Non- Zero Minimum | Maximum | Median (95% Confidence Interval) |
| Horizontal Saccades | Initial 30 | 74.1 ± 12 | 46 | 102 | 74 (72–76) | 76.8 ± 14 | 40 | 116 | 76 (74–79) |
| Latter 30 | 67.1 ± 11 | 38 | 94 | 68 (65–69) | 70.5 ± 13 | 38 | 112 | 70 (68–72) | |
| Total | 141.2 ± 23 | 90 | 194 | 142 (137–145) | 147.3 ± 27 | 78 | 224 | 144 (142–152) | |
| Vertical Saccades | Initial 30 | 70.3 ± 11 | 44 | 96 | 69 (68–72) | 72.1 ± 13 | 38 | 104 | 72 (70–74) |
| Latter 30 | 65.6 ± 10 | 36 | 88 | 66 (63–67) | 68.5 ± 13 | 38 | 106 | 68 (66–71) | |
| Total | 135.9 ± 21 | 86 | 184 | 136 (132–140) | 140.6 ± 25 | 78 | 206 | 140 (136–145) | |
| Vergence Jumps | Initial 30 | 48.4 ± 12 | 20 | 86 | 48 (46–51) | 49.9 ± 11 | 28 | 82 | 49 (48–52) |
| Latter 30 | 45.7 ± 13 | 24 | 90 | 46 (43–48) | 46.7 ± 10 | 24 | 76 | 44 (45–49) | |
| Total | 94.1 ± 24 | 40 | 172 | 96 (90–98) | 96.6 ± 21 | 54 | 154 | 94 (93–100) | |
| Horizontal VOR | Initial 30 | 38.2 ± 11 | 14 | 74 | 38 (36–40) | 36.3 ± 10 | 12 | 74 | 36 (34–38) |
| Latter 30 | 37.6 ± 11 | 2 | 68 | 36 (36–39) | 37.1 ± 10 | 12 | 74 | 36 (35–39) | |
| Total | 75.7 ± 21 | 32 | 134 | 76 (72–80) | 73.4 ± 20 | 26 | 148 | 72 (70–77) | |
| Vertical VOR | Initial 30 | 38.2 ± 11 | 20 | 66 | 36 (36–40) | 37.1 ± 11 | 18 | 80 | 35 (35–39) |
| Latter 30 | 37.6 ± 11 | 2 | 68 | 36 (36–40) | 38.2 ± 11 | 18 | 80 | 36 (36–40) | |
| Total | 76.2 ± 21 | 36 | 134 | 73 (72–80) | 75.4 ± 21 | 36 | 156 | 71 (71–79) | |
| Near Point of Convergence | Break (cm) | 3.7 ± 2 | 2 | 9.5 | 3 (3–4) | 3.5 ± 2 | 2 | 9.5 | 3 (3–4) |
| Recovery (cm) | 4.9 ± 2 | 2 | 12.5 | 5 (4–5) | 5.0 ± 2 | 2 | 11.5 | 5 (4–5) | |
| Amplitude of Accommodation | Right (cm) | 8.3 ± 2 | 4.5 | 14.0 | 8 (8–9) | 8.5 ± 2 | 4.5 | 16.0 | 9 (8–9) |
| Left (cm) | 8.5 ± 2 | 4.5 | 15.5 | 9 (8–9) | 8.5 ± 2 | 4.5 | 16.0 | 9 (8–9) | |
| Time Point | Horizontal Saccades | Vertical Saccades | Vergence Jumps | Horizontal VOR | Vertical VOR | Amplitude of Accommodation | Near Point of Convergence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial 30 s | Latter 30 s | Initial 30 s | Latter 30 s | Initial 30 s | Latter 30 s | Initial 30 s | Latter 30 s | Initial 30 s | Latter 30 s | Right (cm) | Left (cm) | Break (cm) | Recovery (cm) | |
| Pre-Season (n = 15) | 79.4 ± 14 | 68.8 ± 11 | 71.5 ± 13 | 65.4 ± 11 | 49.1 ± 7 | 41.4 ± 8 | 37.7 ± 10 | 37.8 ± 10 | 39.1 ± 10 | 39.5 ± 10 | 8.6 ± 2 | 8.8 ± 2 | 3.5 ± 2 | 4.5 ± 3 |
| 0–1 Day (n = 3) | 72.0 ± 14 | 66.0 ± 7 | 64.7 ± 22 | 60.7 ± 20 | 28.0 ± 24 | 27.3 ± 24 | 20.0 ± 17 | 19.3 ± 17 | 22.0 ± 20 | 22.7 ± 21 | 15.7 ± 8 | 15.7 ± 8 | 3.3 ± 1 | 4.7 ± 2 |
| 2 Days (n = 7) | 72.0 ± 15 | 66.0 ± 15 | 66.0 ± 13 | 61.0 ± 14 | 46.0 ± 10 | 40.3 ± 9 | 29.3 ± 16 | 31.3 ± 16 | 28.7 ± 15 | 27.0 ± 14 | 8.8 ± 2 | 9.8 ± 1 | 4.7 ± 2 | 5.3 ± 3 |
| 4 Days (n = 11) | 65.2 ± 32 | 59.6 ± 33 | 62.2 ± 30 | 55.6 ± 34 | 40.0 ± 25 | 36.0 ± 22 | 30.2 ± 18 | 30.6 ± 18 | 28.2 ± 17 | 29.8 ± 18 | 9.2 ± 6 | 9.4 ± 6 | 5.6 ± 7 | 6.1 ± 7 |
| 7 Days (n = 12) | 67.1 ± 28 | 65.6 ± 26 | 68.9 ± 27 | 66.0 ± 26 | 42.7 ± 23 | 39.5 ± 21 | 30.7 ± 17 | 31.1 ± 16 | 30.0 ± 17 | 30.4 ± 17 | 7.7 ± 3 | 8.2 ± 3 | 3.6 ± 3 | 4.2 ± 3 |
| 14 Days (n = 9) | 88.8 ± 20 | 80.5 ± 18 | 85.5 ± 19 | 78.8 ± 19 | 57.5 ± 14 | 52.3 ± 12 | 42.5 ± 7 | 43.7 ± 8 | 44.0 ± 9 | 44.0 ± 8 | 8.6 ± 2 | 9.1 ± 2 | 3.6 ± 3 | 4.4 ± 3 |
| 21 Days (n = 7) | 76.0 ± 19 | 74.0 ± 13 | 68.7 ± 9 | 63.0 ± 15 | 48.3 ± 13 | 45.0 ± 10 | 43.3 ± 8 | 42.3 ± 11 | 41.3 ± 14 | 43.3 ± 16 | 9.5 ± 2 | 9.4 ± 2 | 3.7 ± 1 | 4.3 ± 1 |
| 30 Days (n = 9) | 91.0 ± 20 | 81.5 ± 14 | 88.0 ± 16 | 81.3 ± 15 | 54.8 ± 10 | 50.5 ± 9 | 47.5 ± 8 | 47.5 ± 9 | 47.0 ± 9 | 47.5 ± 11 | 8.9 ± 2 | 8.9 ± 2 | 3.6 ± 2 | 4.4 ± 2 |
| 60 Days (n = 9) | 88.5 ± 20 | 82.0 ± 17 | 86.3 ± 16 | 79.0 ± 18 | 56.5 ± 9 | 50.0 ± 8 | 46.8 ± 9 | 48.0 ± 11 | 48.3 ± 9 | 48.0 ± 14 | 9.1 ± 2 | 9.3 ± 2 | 4.1 ± 2 | 4.7 ± 3 |
| Post-Season (n = 15) | 76.0 ± 13 | 68.9 ± 7 | 72.7 ± 17 | 68.7 ± 9 | 49.8 ± 7 | 44.2 ± 7 | 39.1 ± 6 | 41.1 ± 8 | 43.6 ± 12 | 44.4 ± 12 | 8.2 ± 1 | 8.7 ± 1 | 3.4 ± 2 | 4.3 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iring-Sanchez, S.; Dungan, M.E.; Jones, A.; Malakhov, M.; Mohan, S.; Yaramothu, C. OculoMotor & Vestibular Endurance Screening (MoVES) Normative, Repeatability, and Reliability Data. Brain Sci. 2024, 14, 704. https://doi.org/10.3390/brainsci14070704
Iring-Sanchez S, Dungan ME, Jones A, Malakhov M, Mohan S, Yaramothu C. OculoMotor & Vestibular Endurance Screening (MoVES) Normative, Repeatability, and Reliability Data. Brain Sciences. 2024; 14(7):704. https://doi.org/10.3390/brainsci14070704
Chicago/Turabian StyleIring-Sanchez, Stephanie, Michaela E. Dungan, Andrew Jones, Mitchell Malakhov, Stuti Mohan, and Chang Yaramothu. 2024. "OculoMotor & Vestibular Endurance Screening (MoVES) Normative, Repeatability, and Reliability Data" Brain Sciences 14, no. 7: 704. https://doi.org/10.3390/brainsci14070704
APA StyleIring-Sanchez, S., Dungan, M. E., Jones, A., Malakhov, M., Mohan, S., & Yaramothu, C. (2024). OculoMotor & Vestibular Endurance Screening (MoVES) Normative, Repeatability, and Reliability Data. Brain Sciences, 14(7), 704. https://doi.org/10.3390/brainsci14070704






