Effects of Adapted Physical Activity on White Matter Integrity in Patients with Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Participants
2.2. e-APA Intervention
2.3. Cardiorespiratory Fitness
2.4. Clinical Symptoms
2.5. Neuroimaging
2.5.1. Data Acquisition
2.5.2. Data Processing
2.6. Statistical Analyses
2.6.1. TBSS Analyses
2.6.2. Correlation Analyses
3. Results
3.1. Per Protocol Population Characteristics
3.2. Diffusion Tensor Imaging
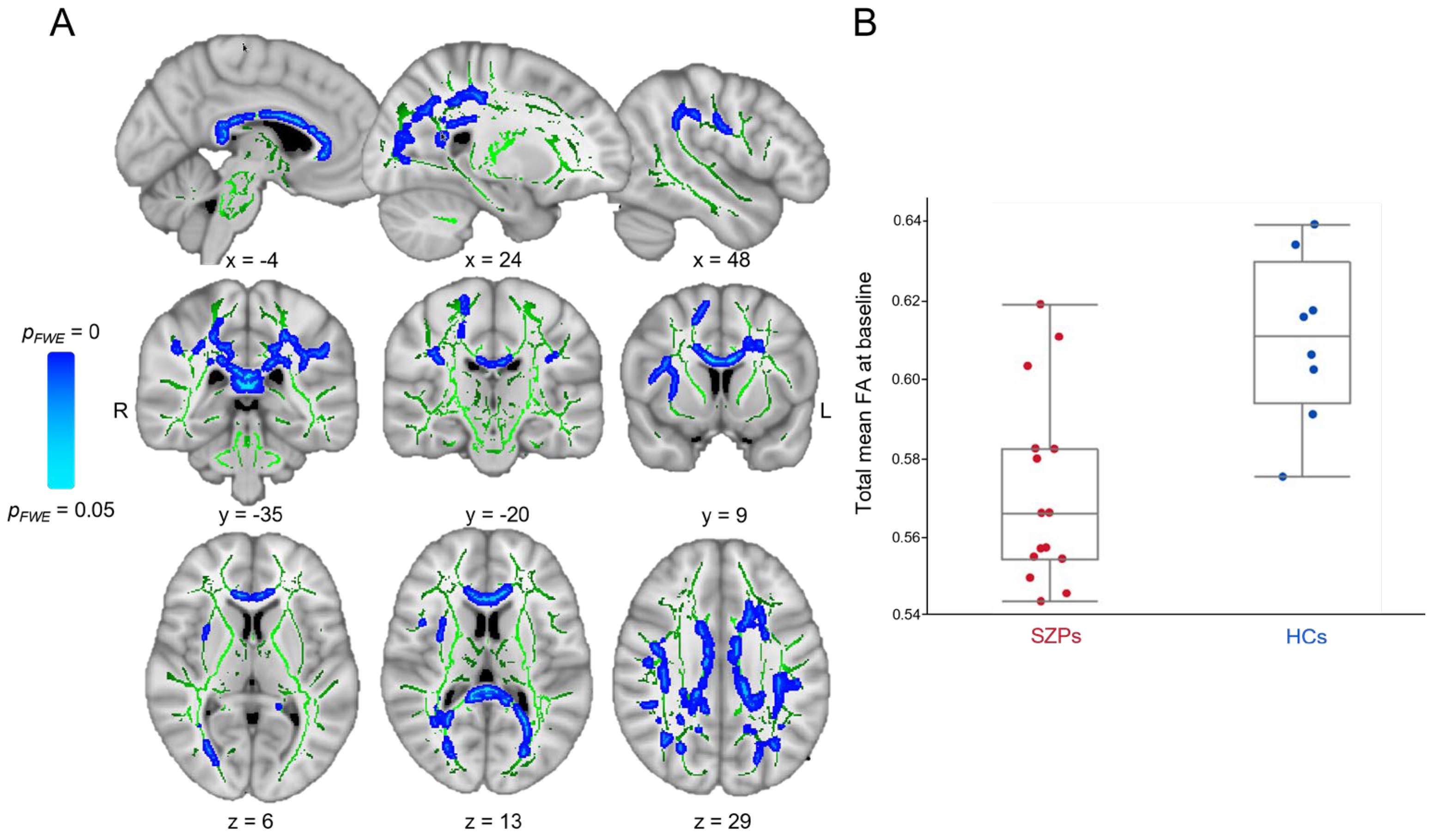
3.2.1. Baseline
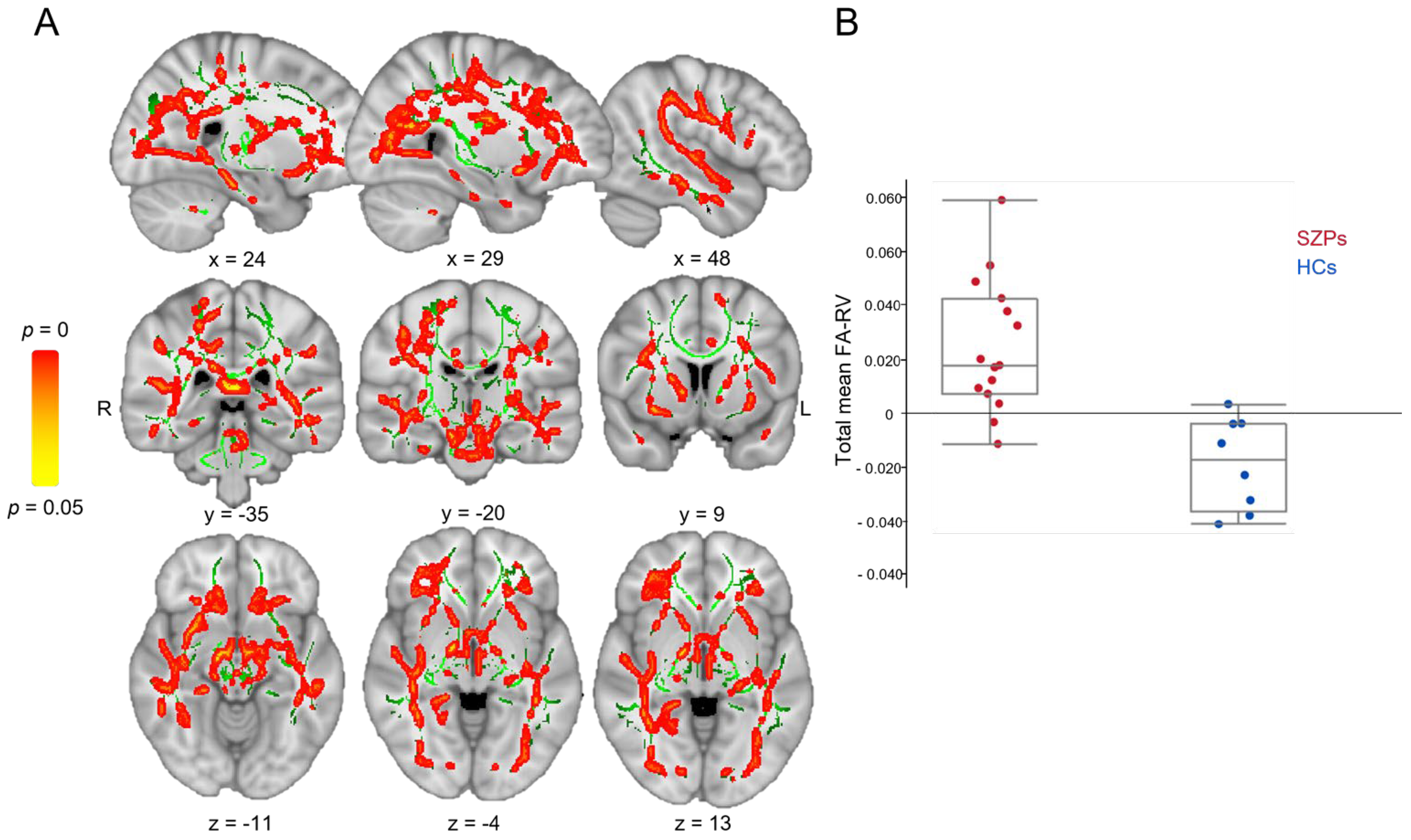
3.2.2. Longitudinal
3.3. Relationships between White Matter Integrity and Cardiorespiratory Fitness/Clinical Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietsche, B.; Kircher, T.; Falkenberg, I. Structural Brain Changes in Schizophrenia at Different Stages of the Illness: A Selective Review of Longitudinal Magnetic Resonance Imaging Studies. Aust. N. Z. J. Psychiatry 2017, 51, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Olabi, B.; Ellison-Wright, I.; McIntosh, A.M.; Wood, S.J.; Bullmore, E.; Lawrie, S.M. Are There Progressive Brain Changes in Schizophrenia? A Meta-Analysis of Structural Magnetic Resonance Imaging Studies. Biol. Psychiatry 2011, 70, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.; De, P.L.; Deste, G.; Sacchetti, E. Progressive Loss of Cortical Gray Matter in Schizophrenia: A Meta-Analysis and Meta-Regression of Longitudinal MRI Studies. Transl. Psychiatry 2012, 2, e190. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.A.; White, D.M.; Kraguljac, N.V.; Lahti, A.C. A Combined Diffusion Tensor Imaging and Magnetic Resonance Spectroscopy Study of Patients with Schizophrenia. Schizophr. Res. 2016, 170, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Ellison-Wright, I.; Nathan, P.J.; Bullmore, E.T.; Zaman, R.; Dudas, R.B.; Agius, M.; Fernandez-Egea, E.; Müller, U.; Dodds, C.M.; Forde, N.J.; et al. Distribution of Tract Deficits in Schizophrenia. BMC Psychiatry 2014, 14, 99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, X.; Lai, Y.; Wang, X.; Hao, C.; Chen, L.; Zhou, Z.; Yu, X.; Hong, N. A Combined DTI and Structural MRI Study in Medicated-Naïve Chronic Schizophrenia. Magn. Reson. Imaging 2014, 32, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, E.; Tatu, M.K.; Pignolo, C.; Cauda, F.; Costa, T.; Ando’, A.; Zennaro, A. White Matter and Schizophrenia: A Meta-Analysis of Voxel-Based Morphometry and Diffusion Tensor Imaging Studies. Psychiatry Res. Neuroimaging 2017, 270, 8–21. [Google Scholar] [CrossRef]
- Bora, E.; Fornito, A.; Radua, J.; Walterfang, M.; Seal, M.; Wood, S.J.; Yucel, M.; Velakoulis, D.; Pantelis, C. Neuroanatomical Abnormalities in Schizophrenia: A Multimodal Voxelwise Meta-Analysis and Meta-Regression Analysis. Schizophr. Res. 2011, 127, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, M.; Dehghani Firouzabadi, F.; Yang, K.; Barker, P.B.; Lee, R.R.; Yousem, D.M. Neuroimaging in Schizophrenia: A Review Article. Front. Neurosci. 2022, 16, 1042814. [Google Scholar] [CrossRef]
- Heilbronner, U.; Samara, M.; Leucht, S.; Falkai, P.; Schulze, T.G. The Longitudinal Course of Schizophrenia Across the Lifespan: Clinical, Cognitive, and Neurobiological Aspects. Harv. Rev. Psychiatry 2016, 24, 118–128. [Google Scholar] [CrossRef]
- Kaur, A.; Basavanagowda, D.M.; Rathod, B.; Mishra, N.; Fuad, S.; Nosher, S.; Alrashid, Z.A.; Mohan, D.; Heindl, S.E. Structural and Functional Alterations of the Temporal Lobe in Schizophrenia: A Literature Review. Cureus 2020, 12, e11177. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G.; Lu, P.H.; Nuechterlein, K.H.; Gitlin, M.; Doi, C.; Edwards, N.; Lieu, C.; Altshuler, L.L.; Mintz, J. Differential Effects of Typical and Atypical Antipsychotics on Brain Myelination in Schizophrenia. Schizophr. Res. 2007, 93, 13–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Leroux, E.; Vandevelde, A.; Trehout, M.; Dollfus, S. Abnormalities of Fronto-Subcortical Pathways in Schizophrenia and the Differential Impacts of Antipsychotic Treatment: A DTI-Based Tractography Study. Psychiatry Res. Neuroimaging 2018, 280, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Correll, C.U.; Detraux, J.; De, L.J.; De, H.M. Effects of Antipsychotics, Antidepressants and Mood Stabilizers on Risk for Physical Diseases in People with Schizophrenia, Depression and Bipolar Disorder. World Psychiatry 2015, 14, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, S.; Kaiser, S.; Bitter, I.; Nordentoft, M.; Mucci, A.; Sabé, M.; Giordano, G.M.; Nielsen, M.Ø.; Glenthøj, L.B.; Pezzella, P.; et al. EPA Guidance on Treatment of Negative Symptoms in Schizophrenia. Eur. Psychiatry 2021, 64, e21. [Google Scholar] [CrossRef] [PubMed]
- Keefe, R.S.E.; Bilder, R.M.; Davis, S.M.; Harvey, P.D.; Palmer, B.W.; Gold, J.M.; Meltzer, H.Y.; Green, M.F.; Capuano, G.; Stroup, T.S.; et al. Neurocognitive Effects of Antipsychotic Medications in Patients with Chronic Schizophrenia in the CATIE Trial. Arch. Gen. Psychiatry 2007, 64, 633–647. [Google Scholar] [CrossRef]
- Carli, M.; Kolachalam, S.; Longoni, B.; Pintaudi, A.; Baldini, M.; Aringhieri, S.; Fasciani, I.; Annibale, P.; Maggio, R.; Scarselli, M. Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences. Pharmaceuticals 2021, 14, 238. [Google Scholar] [CrossRef]
- Firth, J.; Solmi, M.; Wootton, R.E.; Vancampfort, D.; Schuch, F.B.; Hoare, E.; Gilbody, S.; Torous, J.; Teasdale, S.B.; Jackson, S.E.; et al. A Meta-Review of “Lifestyle Psychiatry”: The Role of Exercise, Smoking, Diet and Sleep in the Prevention and Treatment of Mental Disorders. World Psychiatry 2020, 19, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, J.; Gandhi, S.; Damodharan, D.; Ganesan, V.; Palaniappan, M. Exercise, Diet and Educational Interventions for Metabolic Syndrome in Persons with Schizophrenia: A Systematic Review. Asian J. Psychiatry 2018, 36, 73–85. [Google Scholar] [CrossRef]
- Singh, R.; Bansal, Y.; Medhi, B.; Kuhad, A. Antipsychotics-Induced Metabolic Alterations: Recounting the Mechanistic Insights, Therapeutic Targets and Pharmacological Alternatives. Eur. J. Pharmacol. 2019, 844, 231–240. [Google Scholar] [CrossRef]
- Falkai, P.; Schmitt, A.; Rosenbeiger, C.P.; Maurus, I.; Hattenkofer, L.; Hasan, A.; Malchow, B.; Heim-Ohmayer, P.; Halle, M.; Heitkamp, M. Aerobic Exercise in Severe Mental Illness: Requirements from the Perspective of Sports Medicine. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 643–677. [Google Scholar] [CrossRef]
- Hjorth, P.; Davidsen, A.S.; Kilian, R.; Skrubbeltrang, C. A Systematic Review of Controlled Interventions to Reduce Overweight and Obesity in People with Schizophrenia. Acta Psychiatr. Scand. 2014, 130, 279–289. [Google Scholar] [CrossRef]
- Holt, R.I.G. The Prevention of Diabetes and Cardiovascular Disease in People with Schizophrenia. Acta Psychiatr. Scand. 2015, 132, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.D.; Lang, D.J.; Warburton, D.E.R.; Barr, A.M.; Smith, G.N.; Thornton, A.E.; White, R.F.; Honer, W.G.; Procyshyn, R.M. Effects of Exercise on Serum Triglycerides and Symptoms of Schizophrenia. J. Clin. Psychopharmacol. 2017, 37, 273–274. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Maurus, I.; Rossner, M.J.; Röh, A.; Lembeck, M.; von Wilmsdorff, M.; Takahashi, S.; Rauchmann, B.; Keeser, D.; Hasan, A.; et al. Effects of Aerobic Exercise on Metabolic Syndrome, Cardiorespiratory Fitness, and Symptoms in Schizophrenia Include Decreased Mortality. Front. Psychiatry 2018, 9, 690. [Google Scholar] [CrossRef]
- Svatkova, A.; Mandl, R.C.W.; Scheewe, T.W.; Cahn, W.; Kahn, R.S.; Hulshoff Pol, H.E. Physical Exercise Keeps the Brain Connected: Biking Increases White Matter Integrity in Patients with Schizophrenia and Healthy Controls. Schizophr. Bull. 2015, 41, 869–878. [Google Scholar] [CrossRef]
- Maurus, I.; Röll, L.; Keeser, D.; Karali, T.; Papazov, B.; Hasan, A.; Schmitt, A.; Papazova, I.; Lembeck, M.; Hirjak, D.; et al. Associations between Aerobic Fitness, Negative Symptoms, Cognitive Deficits and Brain Structure in Schizophrenia—A Cross-Sectional Study. Schizophrenia 2022, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Tréhout, M.; Leroux, E.; Bigot, L.; Jego, S.; Leconte, P.; Reboursière, E.; Morello, R.; Chapon, P.-A.; Herbinet, A.; Quarck, G.; et al. A Web-Based Adapted Physical Activity Program (e-APA) versus Health Education Program (e-HE) in Patients with Schizophrenia and Healthy Volunteers: Study Protocol for a Randomized Controlled Trial (PEPSY V@Si). Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Leroux, E.; Tréhout, M.; Reboursiere, E.; de Flores, R.; Morello, R.; Guillin, O.; Quarck, G.; Dollfus, S. Effects of Web-Based Adapted Physical Activity on Hippocampal Plasticity, Cardiorespiratory Fitness, Symptoms, and Cardiometabolic Markers in Patients with Schizophrenia: A Randomized, Controlled Study. Eur. Arch. Psychiatry Clin. Neurosci. 2024. [Google Scholar] [CrossRef]
- Maurus, I.; Roell, L.; Lembeck, M.; Papazova, I.; Greska, D.; Muenz, S.; Wagner, E.; Campana, M.; Schwaiger, R.; Schneider-Axmann, T.; et al. Exercise as an Add-on Treatment in Individuals with Schizophrenia: Results from a Large Multicenter Randomized Controlled Trial. Psychiatry Res. 2023, 328, 115480. [Google Scholar] [CrossRef]
- Stubbs, B.; Vancampfort, D.; Hallgren, M.; Firth, J.; Veronese, N.; Solmi, M.; Brand, S.; Cordes, J.; Malchow, B.; Gerber, M.; et al. EPA Guidance on Physical Activity as a Treatment for Severe Mental Illness: A Meta-Review of the Evidence and Position Statement from the European Psychiatric Association (EPA), Supported by the International Organization of Physical Therapists in Mental Health (IOPTMH). Eur. Psychiatry 2018, 54, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine American College of Sports Medicine Position Stand. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Dollfus, S.; Delouche, C.; Hervochon, C.; Mach, C.; Bourgeois, V.; Rotharmel, M.; Tréhout, M.; Vandevelde, A.; Guillin, O.; Morello, R. Specificity and Sensitivity of the Self-Assessment of Negative Symptoms (SNS) in Patients with Schizophrenia. Schizophr. Res. 2019, 211, 51–55. [Google Scholar] [CrossRef]
- Kirkpatrick, B.; Saoud, J.B.; Strauss, G.P.; Ahmed, A.O.; Tatsumi, K.; Opler, M.; Luthringer, R.; Davidson, M. The Brief Negative Symptom Scale (BNSS): Sensitivity to Treatment Effects. Schizophr. Res. 2018, 197, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. FSL. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.L.R.; Skare, S.; Ashburner, J. How to Correct Susceptibility Distortions in Spin-Echo Echo-Planar Images: Application to Diffusion Tensor Imaging. Neuroimage 2003, 20, 870–888. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Graham, M.S.; Drobnjak, I.; Zhang, H.; Filippini, N.; Bastiani, M. Towards a Comprehensive Framework for Movement and Distortion Correction of Diffusion MR Images: Within Volume Movement. Neuroimage 2017, 152, 450–466. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Graham, M.S.; Zsoldos, E.; Sotiropoulos, S.N. Incorporating Outlier Detection and Replacement into a Non-Parametric Framework for Movement and Distortion Correction of Diffusion MR Images. Neuroimage 2016, 141, 556–572. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Sotiropoulos, S.N. An Integrated Approach to Correction for Off-Resonance Effects and Subject Movement in Diffusion MR Imaging. Neuroimage 2016, 125, 1063–1078. [Google Scholar] [CrossRef]
- Smith, S.M.; Jenkinson, M.; Johansen-Berg, H.; Rueckert, D.; Nichols, T.E.; Mackay, C.E.; Watkins, K.E.; Ciccarelli, O.; Cader, M.Z.; Matthews, P.M.; et al. Tract-Based Spatial Statistics: Voxelwise Analysis of Multi-Subject Diffusion Data. Neuroimage 2006, 31, 1487–1505. [Google Scholar] [CrossRef]
- Winkler, A.M.; Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation Inference for the General Linear Model. Neuroimage 2014, 92, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Nichols, T.E. Threshold-Free Cluster Enhancement: Addressing Problems of Smoothing, Threshold Dependence and Localisation in Cluster Inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Wakana, S.; Caprihan, A.; Panzenboeck, M.M.; Fallon, J.H.; Perry, M.; Gollub, R.L.; Hua, K.; Zhang, J.; Jiang, H.; Dubey, P.; et al. Reproducibility of Quantitative Tractography Methods Applied to Cerebral White Matter. Neuroimage. 2007, 36, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion Tensor Imaging of the Brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, C. The Basis of Anisotropic Water Diffusion in the Nervous System—A Technical Review. NMR Biomed. 2002, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Leroux, E.; Delcroix, N.; Alary, M.; Razafimandimby, A.; Brazo, P.; Delamillieure, P.; Dollfus, S. Functional and White Matter Abnormalities in the Language Network in Patients with Schizophrenia: A Combined Study with Diffusion Tensor Imaging and Functional Magnetic Resonance Imaging. Schizophr. Res. 2013, 150, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H. The Corticospinal System: From Development to Motor Control. Neuroscientist 2005, 11, 161–173. [Google Scholar] [CrossRef]
- Morales, H.; Tomsick, T. Middle Cerebellar Peduncles: Magnetic Resonance Imaging and Pathophysiologic Correlate. World J. Radiol. 2015, 7, 438–447. [Google Scholar] [CrossRef]
- Ikuta, T.; Loprinzi, P.D. Integrity of the Cortico-Spinal Tract Is Associated with Physical Activity. Int. J. Neurosci. 2020, 130, 413–416. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Harper, J.; Ikuta, T. The Effects of Aerobic Exercise on Corpus Callosum Integrity: Systematic Review. Physician Sportsmed. 2020, 48, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.; Hendrikse, J.; Chye, Y.; Caeyenberghs, K.; Coxon, J.P.; Oldham, S.; Suo, C.; Yücel, M. Associations of Cardiorespiratory Fitness and Exercise with Brain White Matter in Healthy Adults: A Systematic Review and Meta-Analysis. Brain Imaging Behav. 2022, 16, 2402–2425. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.; Kennedy, D.N.; McInerney, S.; Sorensen, A.G.; Wang, R.; Caviness, V.S.; Pandya, D.N. Segmentation of Subcomponents within the Superior Longitudinal Fascicle in Humans: A Quantitative, In Vivo, DT-MRI Study. Cereb. Cortex 2005, 15, 854–869. [Google Scholar] [CrossRef]
- Wang, X.; Pathak, S.; Stefaneanu, L.; Yeh, F.-C.; Li, S.; Fernandez-Miranda, J.C. Subcomponents and Connectivity of the Superior Longitudinal Fasciculus in the Human Brain. Brain Struct. Funct. 2016, 221, 2075–2092. [Google Scholar] [CrossRef] [PubMed]
- Leroux, E.; Poirel, N.; Dollfus, S. Anatomical Connectivity of the Visuospatial Attentional Network in Schizophrenia: A Diffusion Tensor Imaging Tractography Study. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Leroux, E.; Delcroix, N.; Dollfus, S. Abnormalities of Language Pathways in Schizophrenia Patients with and without a Lifetime History of Auditory Verbal Hallucinations: A DTI-Based Tractography Study. World J. Biol. Psychiatry 2017, 18, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Leroux, E.; Delcroix, N.; Dollfus, S. Left Fronto-Temporal Dysconnectivity within the Language Network in Schizophrenia: An fMRI and DTI Study. Psychiatry Res. 2014, 223, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, K.; Trivedi, R.; Goyal, S.; Kaur, P.; Singh, N.; Bhatia, T.; Deshpande, S.N.; Khushu, S. Microstructural Abnormalities of Uncinate Fasciculus as a Function of Impaired Cognition in Schizophrenia: A DTI Study. J. Biosci. 2016, 41, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Whitford, T.J.; Lee, S.W.; Oh, J.S.; de Luis-Garcia, R.; Savadjiev, P.; Alvarado, J.L.; Westin, C.-F.; Niznikiewicz, M.; Nestor, P.G.; McCarley, R.W.; et al. Localized Abnormalities in the Cingulum Bundle in Patients with Schizophrenia: A Diffusion Tensor Tractography Study. Neuroimage Clin. 2014, 5, 93–99. [Google Scholar] [CrossRef]
- Tréhout, M.; Dollfus, S. Physical activity in patients with schizophrenia: From neurobiology to clinical benefits. Encephale 2018, 44, 538–547. [Google Scholar] [CrossRef]
- Sampaio-Baptista, C.; Johansen-Berg, H. White Matter Plasticity in the Adult Brain. Neuron 2017, 96, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Zemmoura, I.; Duffau, H. Functional Anatomy of the Inferior Longitudinal Fasciculus: From Historical Reports to Current Hypotheses. Front. Neuroanat. 2018, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-T.; Soya, H.; Yassa, M.A.; Li, R.-H.; Chu, C.-H.; Chen, A.-G.; Hung, C.-L.; Chang, Y.-K. Effects of Exercise Types on White Matter Microstructure in Late Midlife Adults: Preliminary Results from a Diffusion Tensor Imaging Study. Front. Aging Neurosci. 2022, 14, 943992. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Al-Sultan, F.; Jamea, A.A.; Almousa, A.; Alnafisah, M.; Alzahrani, M.; Abualait, T.; Yoo, W.-K. Physical Exercise Keeps the Brain Connected by Increasing White Matter Integrity in Healthy Controls. Medicine 2021, 100, e27015. [Google Scholar] [CrossRef] [PubMed]
- Frank, H.R.; Mulder, H.; Sriram, K.; Santanam, T.S.; Skinner, A.C.; Perrin, E.M.; Armstrong, S.C.; Peterson, E.D.; Pencina, M.; Wong, C.A. The Dose-Response Relationship between Physical Activity and Cardiometabolic Health in Young Adults. J. Adolesc. Health 2020, 67, 201–208. [Google Scholar] [CrossRef]
- Lee, I.M.; Skerrett, P.J. Physical Activity and All-Cause Mortality: What Is the Dose-Response Relation? Med. Sci. Sports Exerc. 2001, 33, S459–S471, discussion S493–S494. [Google Scholar] [CrossRef]

| Inclusion Criteria For all participants (SZPs and HCs): Age range: 18 to 60 years old Signed informed consent Ability to receive intervention: possession of a computer, internet access and a webcam Medical welfare affiliation For SZPs: Meeting DSM-IV-TR criteria for schizophrenia or schizoaffective disorders Stable psychotropic medication for at least 2 months prior to inclusion Guardian or trustee consent for protected majors |
| Exclusion criteria For all participants (SZPs and HCs): Under 18 or over 60 years old Participation in another biomedical research protocol during the present study Pregnancy MRI contraindications Progressive neurological diseases Physical restrictions on physical activity (moderate to severe heart failure, unstable coronary disease, severe valvular disease, recent pulmonary embolism or untreated deep venous thrombosis, uncontrolled hypertension, pulmonary arterial hypertension treated or not) Neuromuscular disorders, severe sensory and/or motor neuropathy Rheumatic and articular disorders; orthopedic/rheumatologic problems or bone lesions at risk of fracture that contraindicate physical activity History of stroke or myocardial infarction within 6 months prior to the selection visit For HCs: Lifetime diagnosis of schizophrenia or schizoaffective disorder according to the DSM-IV-TR criteria |
| Median (Quartile 1; Quartile 3) [Min–Max] | SZPs | HCs | p-Value |
|---|---|---|---|
| Number of participants, n | 15 | 8 | - |
| Gender (males, n (%)) | 11 (73.3) | 5 (62.5) | 0.59 |
| Handedness (right/left/both, n (%)) | 13/1/1 (86.7/6.7/6.7) | 6/1/1 (75/12.5/12.5) | 0.78 |
| Age (years) | 40.5 (32.8; 47.3) [25.8–55.1] | 39 (35.8; 46.6) [34.0–50.6] | 0.72 |
| Education level (years) | 12 (11; 16) [7–20] | 15 (12; 16.8) [11–20] | 0.16 |
| RG’s class (active/inactive, n (%)) | 9/6 (60/40) | 7/1 (87.5/12.5) | 0.17 |
| RG score | 20 (13; 25) [9–30] | 27.5 (22.5; 31.3) [13–33] | 0.045 |
| VO2max (mL/min/kg) | 22.5 (19.1; 30) [16.5–36.8] | 32.1 (24.7; 47.7) [16.4–49.7] | 0.076 |
| Age of disease onset (years) | 20 (19; 23) [17–30] | - | - |
| Illness duration (years) | 17 (11; 24) [4–35] | - | - |
| Chlorpromazine equivalent (mg/day) | 325 (140; 400) [50–800] | - | - |
| Total PANSS | 59 (48; 71) [44–96] | - | - |
| Positive PANSS | 13 (8; 15) [7–23] | - | - |
| Negative PANSS | 17 (12; 23) [10–32] | - | - |
| General PANSS | 31 (24; 35) [19–44] | - | - |
| SNS | 14 (10; 15) [3–23] | - | - |
| BNSS | 22 (10; 41) [5–53] | - | - |
| WM Fasciculi or Regions from JHU-ICBM Tracts and Labels Atlases Number of Voxels (>100) (Proportion in %) | FA—Baseline HCs > SZPs pFWE < 0.05, TFCE | FA-RV—Longitudinal SZPs > HCs p < 0.05, TFCE | SZPs Post-Intervention > Baseline p < 0.05, TFCE | |
|---|---|---|---|---|
| JHU-tracts | ||||
| L-anterior thalamic radiation | 353 (0.22) | 871 (0.89) | 140 (0.61) | |
| R-anterior thalamic radiation | 213 (0.12) | 1263 (1.83) | 254 (1.00) | |
| L-corticospinal tract | 102 (0.19) | 427 (0.59) | 168 (0.73) | |
| R-corticospinal tract | 411 (0.88) | 709 (0.68) | 462 (1.21) | |
| L-cingulum gyrus | 440 (0.36) | 286 (0.30) | - | |
| R-cingulum gyrus | 185 (0.19) | 174 (0.058) | - | |
| R-cingulum hippocampus | - | 339 (0.32) | 198 (1.29) | |
| Forceps major | 1091 (2.48) | 1286 (1.49) | - | |
| Forceps minor | 845 (4.06) | 525 (1.21) | - | |
| L-inferior fronto-occipital fasciculus | - | 794 (1.42) | - | |
| R-inferior fronto-occipital fasciculus | 575 (1.10) | 1837 (2.61) | 130 (0.82) | |
| L-inferior longitudinal fasciculus | 147 (0.17) | 763 (1.20) | 193 (1.06) | |
| R-inferior longitudinal fasciculus | 109 (0.52) | 1385 (1.20) | 322 (1.82) | |
| L-superior longitudinal fasciculus | 892 (2.25) | 1095 (0.90) | - | |
| R-superior longitudinal fasciculus | 1450 (3.32) | 2039 (1.94) | 241 (1.68) | |
| R-superior longitudinal fasciculus- part temporal | - | 110 (0.63) | 150 (0.83) | |
| L-uncinate fasciculus | - | 331 (0.54) | - | |
| R-uncinate fasciculus | - | 332 (0.45) | - | |
| JHU-labels | ||||
| Genu of the corpus callosum | 790 (8.00) | - | - | |
| Body of the corpus callosum | 1260 (12.78) | 132 (0.71) | - | |
| Splenium of the corpus callosum | 1125 (11.34) | 672 (3.65) | - | |
| L-corona radiata | 502 (5.05) | 250 (1.35) | - | |
| R-corona radiata | 143 (1.44) | 516 (2.79) | - | |
| L-sagittal stratum | - | 127 (0.69) | - | |
| R-sagittal stratum | - | 209 (1.13) | - | |
| L-external capsule | - | 276 (1.06) | - | |
| R-external capsule | - | 399 (1.01) | - | |
| R-anterior internal capsule | - | 231 (1.25) | - | |
| R-posterior internal capsule | - | 142 (0.77) | - | |
| L-retrolenticular part of internal capsule | - | 223 (1.21) | - | |
| Middle cerebellar peduncle | - | 174 (0.94) | 399 (10.07) | |
| L-cerebellar peduncle | - | 123 (0.66) | - | |
| R-cerebellar peduncle | - | 169 (0.92) | - | |
| WM Fasciculi or Regions from JHU-ICBM Tracts and Labels Atlases Number of Voxels (>100) (Proportion in %) | RD—Baseline HCs > SZPs pFWE < 0.05, TFCE | RD-RV—Longitudinal SZPs > HCs p < 0.05, TFCE | SZPs Post-Intervention > Baseline p < 0.05, TFCE | |
|---|---|---|---|---|
| JHU-tracts | ||||
| L-anterior thalamic radiation | 1096 (0.49) | 741 (1.26) | 629 (1.44) | |
| R-anterior thalamic radiation | 1555 (1.03) | 953 (1.38) | 554 (1.49) | |
| L-corticospinal tract | 334 (0.15) | 423 (0.47) | 855 (1.85) | |
| R-corticospinal tract | 1488 (0.86) | 662 (0.77) | 670 (1.30) | |
| L-cingulum gyrus | 1034 (0.40) | - | - | |
| R-cingulum gyrus | 559 (0.14) | - | - | |
| R-cingulum hippocampus | 137 (0.033) | 351 (0.50) | 231 (0.81) | |
| Forceps major | 2395 (1.38) | 1035 (1.92) | 221 (0.65) | |
| Forceps minor | 3009 (3.53) | - | - | |
| L-inferior fronto-occipital fasciculus | 993 (0.76) | 340 (0.91) | - | |
| R-inferior fronto-occipital fasciculus | 2875 (1.93) | 1050 (2.41) | 185 (0.81) | |
| L-inferior longitudinal fasciculus | 469 (0.31) | 418 (1.00) | 337 (1.50) | |
| R-inferior longitudinal fasciculus | 2043 (1.25) | 1071 (1.87) | 504 (1.55) | |
| L-superior longitudinal fasciculus | 3420 (1.82) | 310 (0.38) | 266 (0.56) | |
| R-superior longitudinal fasciculus | 4161 (2.10) | 1191 (1.36) | 196 (0.66) | |
| R-superior longitudinal fasciculus—part temporal | 122 (0.63) | 226 (0.62) | 120 (0.48) | |
| L-uncinate fasciculus | 276 (0.28) | - | - | |
| R-uncinate fasciculus | 531 (0.37) | 239 (0.48) | - | |
| JHU-labels | ||||
| Genu of the corpus callosum | 1176 (3.17) | - | - | |
| Body of the corpus callosum | 1899 (5.11) | - | - | |
| Splenium of the corpus callosum | 1491 (4.00) | 355 (3.04) | - | |
| L-corona radiata | 1504 (4.04) | - | - | |
| R-corona radiata | 1568 (4.21) | 237 (1.95) | - | |
| R-sagittal stratum | 184 (0.49) | 121 (0.99) | - | |
| L-external capsule | 300 (0.79) | - | - | |
| R-external capsule | 618 (1.17) | 438 (2.18) | - | |
| R-anterior internal capsule | 305 (0.80) | 116 (0.95) | - | |
| R-posterior internal capsule | 330 (0.88) | 117 (0.96) | - | |
| L-retrolenticular part of internal capsule | - | 115 (0.94) | - | |
| R-retrolenticular part of internal capsule | 199 (0.53) | - | - | |
| Middle cerebellar peduncle | - | 604 (4.95) | 1019 (15.68) | |
| R-cerebellar peduncle | 224 (0.59) | 155 (1.27) | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leroux, E.; Masson, L.; Tréhout, M.; Dollfus, S. Effects of Adapted Physical Activity on White Matter Integrity in Patients with Schizophrenia. Brain Sci. 2024, 14, 710. https://doi.org/10.3390/brainsci14070710
Leroux E, Masson L, Tréhout M, Dollfus S. Effects of Adapted Physical Activity on White Matter Integrity in Patients with Schizophrenia. Brain Sciences. 2024; 14(7):710. https://doi.org/10.3390/brainsci14070710
Chicago/Turabian StyleLeroux, Elise, Laura Masson, Maxime Tréhout, and Sonia Dollfus. 2024. "Effects of Adapted Physical Activity on White Matter Integrity in Patients with Schizophrenia" Brain Sciences 14, no. 7: 710. https://doi.org/10.3390/brainsci14070710
APA StyleLeroux, E., Masson, L., Tréhout, M., & Dollfus, S. (2024). Effects of Adapted Physical Activity on White Matter Integrity in Patients with Schizophrenia. Brain Sciences, 14(7), 710. https://doi.org/10.3390/brainsci14070710






