Hyperacusis in Tinnitus Individuals Is Associated with Smaller Gray Matter Volumes in the Supplementary Motor Area Regardless of Hearing Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Data Acquisition
2.3. MRI Data Preprocessing
2.4. Volume-of-Interest Selection for the Supplementary Motor Area
2.5. Gray Matter Extraction from Volume-of-Interest Areas
2.6. Statistical Analyses
2.6.1. Demographic, Audiometric, and Questionnaire Data
2.6.2. Gray Matter Volume of the Supplementary Motor Area in Association with Hyperacusis
Effects of Hyperacusis and Hearing Loss on SMA Gray Matter Volume
Differentiating the Effects of the Attentional and Social Subscales of the Hyperacusis Questionnaire on SMA Gray Matter Volumes
Effects of Anxiety, Depression, or Tinnitus Burden
3. Results
3.1. Demographic, Audiometric, and Questionnaire Data
3.2. Association between Hyperacusis and SMA Gray Matter Volumes
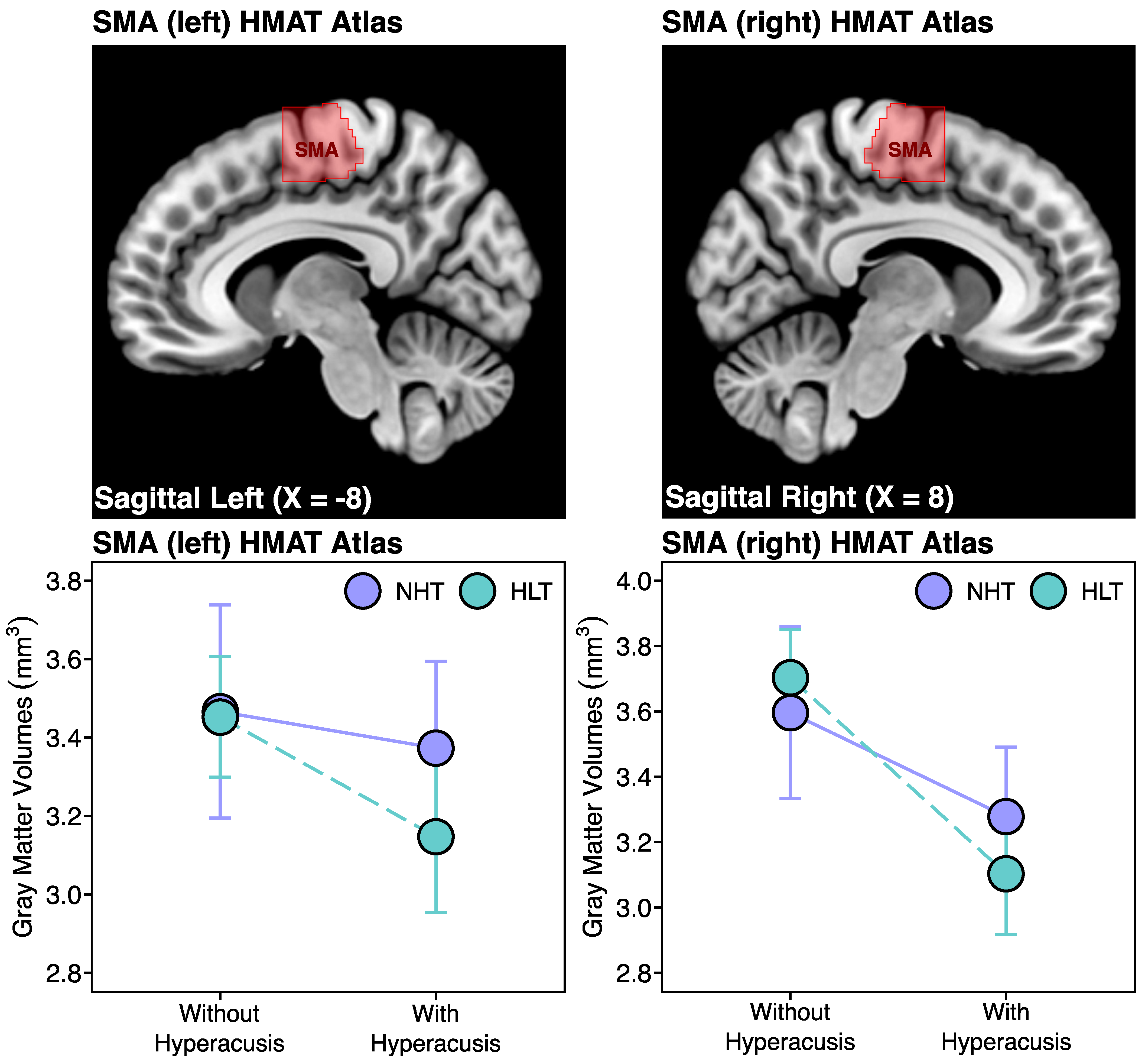
3.2.1. Human Motor Area Template (HMAT) Atlas Analysis
3.2.2. Human Brainnetome (HBN) Atlas Analysis
3.2.3. Smaller Gray Matter Volumes in Hyperacusis Are Associated with the Attentional Subscale of the Hyperacusis Questionnaire
3.2.4. Anxiety, Depression, or Tinnitus Burden Scores Do Not Drive Smaller SMA Gray Matter Volumes in Hyperacusis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anari, M.; Axelsson, A.; Eliasson, A.; Magnusson, L. Hypersensitivity to sound--questionnaire data, audiometry and classification. Scand. Audiol. 1999, 28, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Baguley, D.M. Hyperacusis. J. R. Soc. Med. 2003, 96, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Baguley, D.M.; Hoare, D.J. Hyperacusis: Major research questions. HNO 2018, 66, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xu, T.; Xiang, T.; Pu, J.; Liu, L.; Xiao, Y.; Lai, D. Prevalence of Hyperacusis in the General and Special Populations: A Scoping Review. Front. Neurol. 2021, 12, 706555. [Google Scholar] [CrossRef] [PubMed]
- Rosing, S.N.; Schmidt, J.H.; Wedderkopp, N.; Baguley, D.M. Prevalence of tinnitus and hyperacusis in children and adolescents: A systematic review. BMJ Open 2016, 6, e010596. [Google Scholar] [CrossRef]
- Paulin, J.; Andersson, L.; Nordin, S. Characteristics of hyperacusis in the general population. Noise Health 2016, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Borawska, B.; Bartnik, G.; Raj-Koziak, D.; Fabijańska, A.; Skarżyński, H.; Mrówka, M. Children with tinnitus and hyperacusis in the material of the tinnitus clinic, Warsaw. New Med. 2004, 3, 77–79. [Google Scholar]
- Aldè, M.; Di Berardino, F.; Ambrosetti, U.; Barozzi, S.; Piatti, G.; Zanetti, D.; Pignataro, L.; Cantarella, G. Audiological and vestibular symptoms following SARS-CoV-2 infection and COVID-19 vaccination in children aged 5–11 years. Am. J. Otolaryngol. 2023, 44, 103669. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, B.D.; Radziwon, K.; Salvi, R. Testing the Central Gain Model: Loudness Growth Correlates with Central Auditory Gain Enhancement in a Rodent Model of Hyperacusis. Neuroscience 2019, 407, 93–107. [Google Scholar] [CrossRef]
- Gu, J.W.; Halpin, C.F.; Nam, E.-C.; Levine, R.A.; Melcher, J.R. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J. Neurophysiol. 2010, 104, 3361–3370. [Google Scholar] [CrossRef]
- Koops, E.A.; van Dijk, P. Hyperacusis in tinnitus patients relates to enlarged subcortical and cortical responses to sound except at the tinnitus frequency. Hear. Res. 2021, 401, 108158. [Google Scholar] [CrossRef] [PubMed]
- Safazadeh, S.; Thioux, M.; Renken, R.J.; van Dijk, P. Sound-Evoked Neural Activity in Normal-Hearing Tinnitus: Effects of Frequency and Stimulated Ear Side. Brain Sci. 2024, 14, 544. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-J.; De Ridder, D.; Weisz, N.; Schlee, W.; Van de Heyning, P.; Vanneste, S. Hyperacusis-associated pathological resting-state brain oscillations in the tinnitus brain: A hyperresponsiveness network with paradoxically inactive auditory cortex. Brain Struct. Funct. 2014, 219, 1113–1128. [Google Scholar] [CrossRef]
- Makani, P.; Koops, E.A.; Pyott, S.J.; van Dijk, P.; Thioux, M. Hyperacusis is Associated with Smaller Gray Matter Volumes in the Supplementary Motor Area. SSRN Scholarly Paper. NeuroImage Clin. 2023, 38, 103425. [Google Scholar] [CrossRef]
- Zeng, F.-G. Tinnitus and hyperacusis: Central noise, gain and variance. Curr. Opin. Physiol. 2020, 18, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Makani, P.; Thioux, M.; Pyott, S.J.; van Dijk, P. A Combined Image- and Coordinate-Based Meta-Analysis of Whole-Brain Voxel-Based Morphometry Studies Investigating Subjective Tinnitus. Brain Sci. 2022, 12, 1192. [Google Scholar] [CrossRef] [PubMed]
- Langers, D.R.M.; de Kleine, E.; van Dijk, P. Tinnitus does not require macroscopic tonotopic map reorganization. Front. Syst. Neurosci. 2012, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Boyen, K.; Langers, D.R.M.; de Kleine, E.; van Dijk, P. Gray matter in the brain: Differences associated with tinnitus and hearing loss. Hear. Res. 2013, 295, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.A.; Langers, D.R.M. Tinnitus-related abnormalities in visual and salience networks during a one-back task with distractors. Hear. Res. 2015, 326, 15–29. [Google Scholar] [CrossRef]
- Koops, E.A.; de Kleine, E.; van Dijk, P. Gray matter declines with age and hearing loss, but is partially maintained in tinnitus. Sci. Rep. 2020, 10, 21801. [Google Scholar] [CrossRef]
- Khalfa, S.; Dubal, S.; Veuillet, E.; Perez-Diaz, F.; Jouvent, R.; Collet, L. Psychometric normalization of a hyperacusis questionnaire. ORL J. Otorhinolaryngol. Its Relat. Spec. 2002, 64, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Aazh, H.; Moore, B.C.J. Factors Associated with Depression in Patients with Tinnitus and Hyperacusis. Am. J. Audiol. 2017, 26, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Andersson, J.L.R.; Friston, K.J. Image registration using a symmetric prior—In three dimensions. Hum. Brain Mapp. 2000, 9, 212–225. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. NeuroImage 2007, 38, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Mayka, M.A.; Corcos, D.M.; Leurgans, S.E.; Vaillancourt, D.E. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta-analysis. NeuroImage 2006, 31, 1453–1474. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, H.; Zhuo, J.; Zhang, Y.; Wang, J.; Chen, L.; Yang, Z.; Chu, C.; Xie, S.; Laird, A.R.; et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 2016, 26, 3508–3526. [Google Scholar] [CrossRef] [PubMed]
- Fackrell, K.; Fearnley, C.; Hoare, D.J.; Sereda, M. Hyperacusis Questionnaire as a Tool for Measuring Hypersensitivity to Sound in a Tinnitus Research Population. BioMed Res. Int. 2015, 2015, 290425. [Google Scholar] [CrossRef]
- Nachev, P.; Kennard, C.; Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.F.; Krishnan, S.; Scott, S.K. Roles of supplementary motor areas in auditory processing and auditory imagery. Trends Neurosci. 2016, 39, 527–542. [Google Scholar] [CrossRef]
- Tanji, J.; Kurata, K. Contrasting neuronal activity in supplementary and precentral motor cortex of monkeys. I. Responses to instructions determining motor responses to forthcoming signals of different modalities. J. Neurophysiol. 1985, 53, 129–141. [Google Scholar] [CrossRef]
- Tanji, J.; Kurata, K. Comparison of movement-related activity in two cortical motor areas of primates. J. Neurophysiol. 1982, 48, 633–653. [Google Scholar] [CrossRef]
- Clayton, K.K.; Stecyk, K.S.; Guo, A.A.; Chambers, A.R.; Chen, K.; Hancock, K.E.; Polley, D.B. Sound elicits stereotyped facial movements that provide a sensitive index of hearing abilities in mice. Curr. Biol. CB 2024, 34, 1605–1620.e5. [Google Scholar] [CrossRef]
- Wong, E.; Radziwon, K.; Chen, G.D.; Liu, X.; Manno, F.A.; Manno, S.H.; Auerbach, B.; Wu, E.X.; Salvi, R.; Lau, C. Functional magnetic resonance imaging of enhanced central auditory gain and electrophysiological correlates in a behavioral model of hyperacusis. Hear. Res. 2020, 389, 107908. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.S.; Jahn, K.N.; Sugai, J.A.; Hancock, K.E.; Polley, D.B. The human pupil and face encode sound affect and provide objective signatures of tinnitus and auditory hypersensitivity disorders. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cerliani, L.; Rouw, R. Increased orbitofrontal connectivity in misophonia. bioRxiv 2020. [Google Scholar] [CrossRef]
- Hansen, H.A.; Stefancin, P.; Leber, A.B.; Saygin, Z.M. Neural evidence for non-orofacial triggers in mild misophonia. Front. Neurosci. 2022, 16, 880759. [Google Scholar] [CrossRef] [PubMed]
- Mudgal, V.; Mishra, S.; Mathur, R.; Dua, S. The Sound of Obsession: An Unusual Case report of Misophonia and OCD. Ann. Psychiatry Ment. Health 2023, 11, 1–3. [Google Scholar]
- Castro, A.A.; Lindberg, R.R.D.; Kim, G.; Brennan, C.; Jain, N.; Khan, R.A.; Husain, F.; Berenbaum, H. Obsessive-Compulsive Symptoms and Sound Sensitivities: Comparing Misophonia and Hyperacusis. bioRxiv 2023. [Google Scholar] [CrossRef]
- Hawken, E.R.; Dilkov, D.; Kaludiev, E.; Simek, S.; Zhang, F.; Milev, R. Transcranial Magnetic Stimulation of the Supplementary Motor Area in the Treatment of Obsessive-Compulsive Disorder: A Multi-Site Study. Int. J. Mol. Sci. 2016, 17, 420. [Google Scholar] [CrossRef]
- Yücel, M.; Harrison, B.J.; Wood, S.J.; Fornito, A.; Wellard, R.M.; Pujol, J.; Clarke, K.; Phillips, M.L.; Kyrios, M.; Velakoulis, D.; et al. Functional and Biochemical Alterations of the Medial Frontal Cortex in Obsessive-Compulsive Disorder. Arch. Gen. Psychiatry 2007, 64, 946–955. [Google Scholar] [CrossRef]
- Enzler, F.; Fournier, P.; Noreña, A.J. A psychoacoustic test for diagnosing hyperacusis based on ratings of natural sounds. Hear. Res. 2021, 400, 108124. [Google Scholar] [CrossRef]
- Vanneste, S.; Heyning, P.V.D.; Ridder, D.D. Tinnitus: A Large VBM-EEG Correlational Study. PLoS ONE 2015, 10, e0115122. [Google Scholar] [CrossRef]
- Schecklmann, M.; Lehner, A.; Poeppl, T.B.; Kreuzer, P.M.; Rupprecht, R.; Rackl, J.; Burger, J.; Frank, E.; Hajak, G.; Langguth, B.; et al. Auditory cortex is implicated in tinnitus distress: A voxel-based morphometry study. Brain Struct. Funct. 2013, 218, 1061–1070. [Google Scholar] [CrossRef]
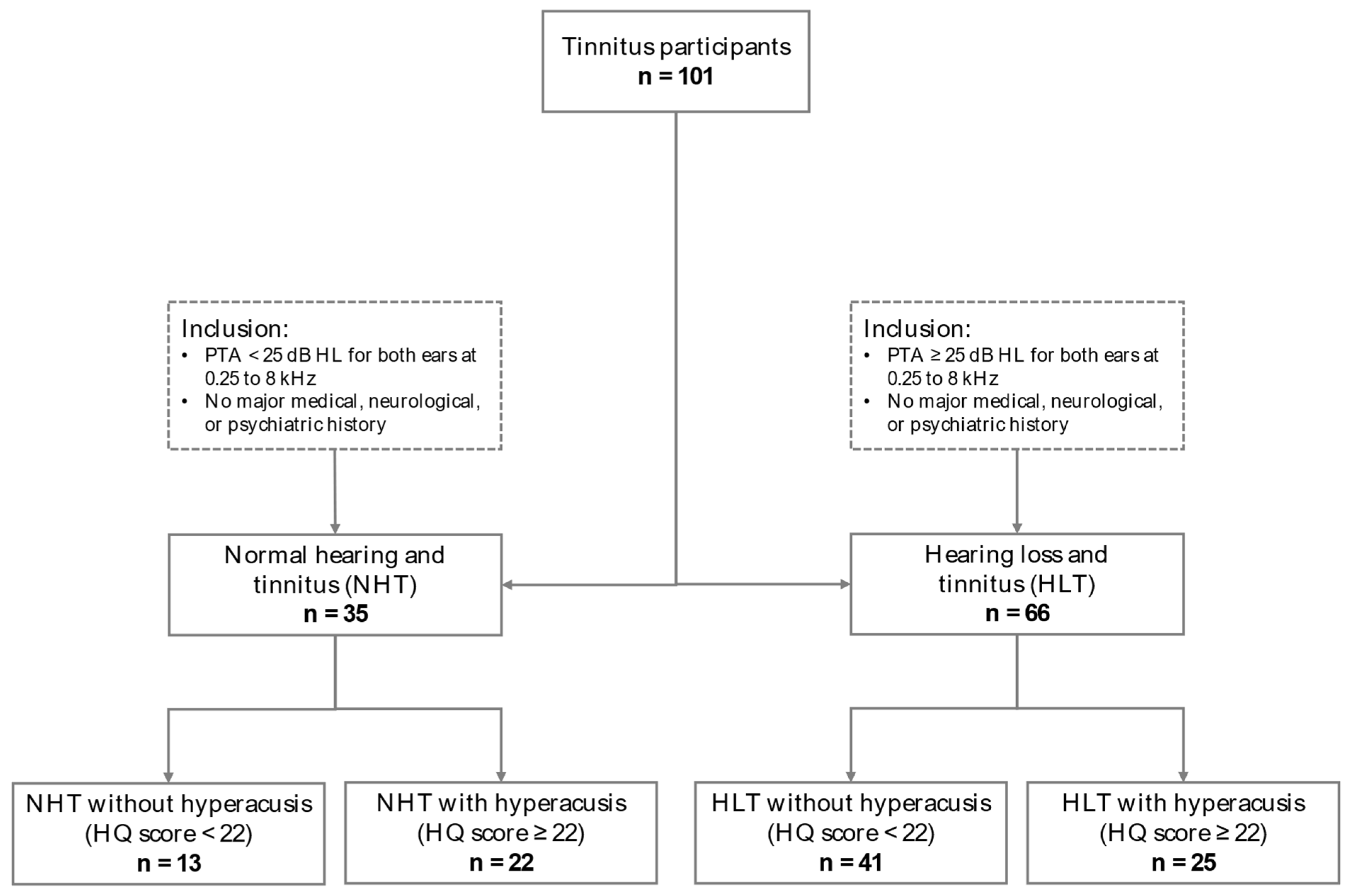


| Group | Normal Hearing and Tinnitus (NHT) | Hearing Loss and Tinnitus (HLT) | Statistic | ||
|---|---|---|---|---|---|
| Without Hyperacusis | With Hyperacusis | Without Hyperacusis | With Hyperacusis | ||
| Demographic | |||||
| n | 13 | 22 | 41 | 25 | - |
| Mean Age (years) | 45.5 ± 11.2 (29–62) c,d | 44.1 ± 12.2 (19–59) c,d | 58.3 ± 10.5 (27–76) a,b | 59.5 ± 7.9 (41–73) a,b | X2(3) = 30.0, p < 0.001 |
| Sex (male|female) | 8|5 | 10|12 | 32|9 | 15|10 | X2(3) = 7.0, p = 0.071 |
| Audiometric (for both ears 0.25 to 8 kHz) | |||||
| Mean PTA (dB HL) | 7.3 ± 5.4 c,d | 9.4 ± 7.3 c,d | 35.0 ± 8.6 a,b | 38.2 ± 12.3 a,b | X2(3) = 65.8, p < 0.001 |
| Questionnaires | |||||
| HQ | 16.5 ± 3.6 (10–21) b,d | 27.1 ± 4.1 (22–35) a,c | 13.2 ± 5.4 (0–21) b,d | 26.2 ± 3.7 (22–33) a,c | X2(3) = 76.0, p < 0.001 |
| HQ-Attentional ‡ | 9.2 ± 2.3 b,d | 13.5 ± 2.6 a,c | 6.5 ± 2.7 b,d | 13.5 ± 2.6 a,c | X2(3) = 57.5, p < 0.001 |
| HQ-Social ‡ | 1.8 ± 1.0 b,d | 5.9 ± 2.7 a,c | 2.6 ± 2.4 b,d | 5.2 ± 2.0 a,c | X2(3) = 31.3, p < 0.001 |
| HADS-Anxiety | 6.8 ± 4.2 (0–16) | 6.5 ± 3.4 (2–15) c | 3.7 ± 3.0 (0–11) b | 5.9 ± 4.6 (0–16) | X2(3) = 11.0, p = 0.011 |
| HADS-Depression | 5.0 ± 3.8 (0–14) | 4.9 ± 3.7 (0–15) | 3.2 ± 3.1 (0–10) d | 6.2 ± 4.7 (0–16) c | X2(3) = 8.4, p = 0.038 |
| THI | 33.7 ± 20.4 (4–66) | 44.4 ± 20.5 (10–88) c | 27.4 ± 19.3 (4–80) b,d | 41.8 ± 20.8 (6–82) c | X2(3) = 13.0, p = 0.005 |
| HQ-Attentional Score | HQ-Social Score | |
|---|---|---|
| HMAT VOIs | ||
| SMA left | rs(101) = −0.26, p = 0.010 | rs(101) = 0.10, p = 0.327 |
| SMA right | rs(101) = −0.33, p < 0.001 | rs(101) = −0.11, p = 0.273 |
| HBN VOIs | ||
| SMA_A6m left | rs(101) = −0.17, p = 0.089 | rs(101) = 0.13, p = 0.210 |
| SMA_A6m right | rs(101) = −0.30, p = 0.001 | rs(101) = −0.06, p = 0.569 |
| SMA_A4ll left | rs(101) = −0.32, p = 0.001 | rs(101) = −0.11, p = 0.259 |
| SMA_A4ll right | rs(101) = −0.35, p < 0.001 | rs(101) = −0.14, p = 0.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makani, P.; Thioux, M.; Koops, E.A.; Pyott, S.J.; van Dijk, P. Hyperacusis in Tinnitus Individuals Is Associated with Smaller Gray Matter Volumes in the Supplementary Motor Area Regardless of Hearing Levels. Brain Sci. 2024, 14, 726. https://doi.org/10.3390/brainsci14070726
Makani P, Thioux M, Koops EA, Pyott SJ, van Dijk P. Hyperacusis in Tinnitus Individuals Is Associated with Smaller Gray Matter Volumes in the Supplementary Motor Area Regardless of Hearing Levels. Brain Sciences. 2024; 14(7):726. https://doi.org/10.3390/brainsci14070726
Chicago/Turabian StyleMakani, Punitkumar, Marc Thioux, Elouise A. Koops, Sonja J. Pyott, and Pim van Dijk. 2024. "Hyperacusis in Tinnitus Individuals Is Associated with Smaller Gray Matter Volumes in the Supplementary Motor Area Regardless of Hearing Levels" Brain Sciences 14, no. 7: 726. https://doi.org/10.3390/brainsci14070726
APA StyleMakani, P., Thioux, M., Koops, E. A., Pyott, S. J., & van Dijk, P. (2024). Hyperacusis in Tinnitus Individuals Is Associated with Smaller Gray Matter Volumes in the Supplementary Motor Area Regardless of Hearing Levels. Brain Sciences, 14(7), 726. https://doi.org/10.3390/brainsci14070726









