Maximizing Participation in Olfactory Training in a Sample with Post-COVID-19 Olfactory Loss
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures and Procedures
2.3. Experiment Design
2.3.1. Experiment 1: Olfactory Training
2.3.2. Experiment 2: Comparative Analysis
2.4. Study Design
2.5. Statistical Analysis
3. Results
3.1. Experiment 1: Olfactory Training
3.2. Experiment 2: Comparative Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 2 August 2023).
- Ahmed, A.K.; Sayad, R.; Mahmoud, I.A.; El-Monem, A.M.A.; Badry, S.H.; Ibrahim, I.H.; Hafez, M.H.; El-Mokhtar, M.A.; Sayed, I.M. “Anosmia” the mysterious collateral damage of COVID-19. J. NeuroVirol. 2022, 28, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Cherry, G.; Rocke, J.; Chu, M.; Liu, J.; Lechner, M.; Lund, V.J.; Kumar, B.N. Loss of Smell and Taste: A New Marker of COVID-19? Tracking Reduced Sense of Smell during the Coronavirus Pandemic Using Search Trends. Expert Rev. Anti-Infect. Ther. 2020, 18, 1165–1170. [Google Scholar] [CrossRef]
- Lescure, F.-X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.-H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Hingrat, Q.L.; et al. Clinical and Virological Data of the First Cases of COVID-19 in Europe: A Case Series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and Gustatory Dysfunctions as a Clinical Presentation of Mild-to-Moderate Forms of the Coronavirus Disease (COVID-19): A Multicenter European Study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Osman, M.; Bouiller, K.; Tipirdamaz, C.; Gendrin, V.; Chirouze, C.; Lepiller, Q.; Bouvier, E.; Royer, P.; Pierron, A.; et al. Olfactory dysfunction in COVID-19, new insights from a cohort of 353 patients: The ANOSVID study. J. Med. Virol. 2022, 94, 4762–4775. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; Bilinska, K.; von Bartheld, C.S. Olfactory Dysfunction in COVID-19: New Insights into the Underlying Mechanisms. Trends Neurosci. 2023, 46, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.R.; Ralli, M.; Minni, A.; Greco, A.; De Vincentiis, M.; Allegra, E. Evaluation of olfactory dysfunction persistence after COVID-19: A prospective study. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1042–1048. [Google Scholar] [PubMed]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of Chemosensory Dysfunction and COVID-19 in Patients Presenting with Influenza-like Symptoms. Int. Forum Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Ferreli, F.; Gaino, F.; Russo, E.; Di Bari, M.; Rossi, V.; De Virgilio, A.; Di Stadio, A.; Spriano, G.; Mercante, G. Long-term olfactory dysfunction in COVID-19 patients: 18-month follow-up study. Int. Forum Allergy Rhinol. 2022, 12, 1078. [Google Scholar] [CrossRef]
- Alkholaiwi, F.M.; Altamimi, A.F.; Almalki, H.H.; Almughaiseeb, F.A.; Alsubaie, S.S.; Alsayahi, H.S.; Alhijli, F.W.; Alobaishi, R.S.; Agrawal, A.; Alqahtani, Z.A.; et al. Olfactory dysfunction among patients with COVID-19. Saudi Med. J. 2023, 44, 1085. [Google Scholar] [CrossRef]
- Wu, S.S.; Cabrera, C.I.; Quereshy, H.A.; Kocharyan, A.; D’Anza, B.; Otteson, T. Olfactory dysfunction incidence and resolution amongst 608 patients with COVID-19 infection. Am. J. Otolaryngol. 2023, 44, 103962. [Google Scholar] [CrossRef]
- Tan, B.K.J.; Han, R.; Zhao, J.J.; Tan, N.K.W.; Quah, E.S.H.; Tan, C.J.-W.; Chan, Y.H.; Teo, N.W.Y.; Charn, T.C.; See, A.; et al. Prognosis and persistence of smell and taste dysfunction in patients with COVID-19: Meta-analysis with parametric cure modelling of recovery curves. BMJ 2022, 378, e069503. [Google Scholar] [CrossRef]
- Fortunato, F.; Martinelli, D.; Iannelli, G.; Milazzo, M.; Farina, U.; Di Matteo, G.; De Nittis, R.; Ascatigno, L.; Cassano, M.; Lopalco, P.L.; et al. Self-Reported Olfactory and Gustatory Dysfunctions in COVID-19 Patients: A 1-Year Follow-up Study in Foggia District, Italy. BMC Infect. Dis. 2022, 22, 77. [Google Scholar] [CrossRef]
- Pendolino, A.L.; Tan, H.Q.M.; Choi, D.; Ottaviano, G.; Andrews, P.J. Long-Term Quality-of-Life Impairment in Patients with More than 1-Year COVID-19-Related Olfactory Dysfunction. Int. Forum Allergy Rhinol. 2023, 13, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Deng, Y.K.; Zeng, M.; Liu, Z. Long-term consequences of COVID-19: Chemosensory disorders. Curr. Allergy Asthma Rep. 2023, 23, 111–119. [Google Scholar] [CrossRef]
- Hu, B.; Gong, M.; Xiang, Y.; Qu, S.; Zhu, H.; Ye, D. Mechanism and treatment of olfactory dysfunction caused by coronavirus disease 2019. J. Transl. Med. 2023, 21, 829. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, S.; You, Y.; Tang, J.; Chen, C.; Wang, C.; Wen, W.; Wang, M.; Chen, J.; Luo, L.; et al. Olfactory dysfunction after COVID-19: Metanalysis reveals persistence in one-third of patients 6 months after initial infection. J. Infect. 2023, 86, 516. [Google Scholar] [CrossRef]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, K.W.; Bouysset, C.; Pirastu, N.; Dibattista, M.; et al. More Than Smell—COVID-19 Is Associated with Severe Impairment of Smell, Taste, and Chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto-Urata, M.; Urata, S.; Kagoya, R.; Imamura, F.; Nagayama, S.; Reyna, R.A.; Maruyama, J.; Yamasoba, T.; Kondo, K.; Hasegawa-Ishii, S.; et al. Prolonged and Extended Impacts of SARS-CoV-2 on the Olfactory Neurocircuit. Sci. Rep. 2022, 12, 5728. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Drozdzik, A.; Drozdzik, M. Oral Pathology in COVID-19 and SARS-CoV-2 Infection-Molecular Aspects. Int. J. Mol. Sci. 2022, 23, 1431. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Yang, L.; You, R. Physiological and Pathological Regulation of ACE2, the SARS-CoV-2 Receptor. Pharmacol. Res. 2020, 157, 104833. [Google Scholar] [CrossRef] [PubMed]
- Bilinska, K.; Jakubowska, P.; Von Bartheld, C.S.; Butowt, R. Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age. ACS Chem. Neurosci. 2020, 11, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, E.; El-Anwar, M.W.; Elghamry, R.M.; Abdallah, A.M.; Ibrahim, A.M. Anosmia in COVID-19 Patients: Can We Predict the Severity of Chest Manifestations? Int. Arch. Otorhinolaryngol. 2023, 27, e143–e151. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Brann, D.H.; Datta, S.R. Mechanisms of SARS-CoV-2-Associated Anosmia. Physiol. Rev. 2023, 103, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Zazhytska, M.; Kodra, A.; Hoagland, D.A.; Frere, J.; Fullard, J.F.; Shayya, H.; McArthur, N.G.; Moeller, R.; Uhl, S.; Omer, A.D.; et al. Non-Cell-Autonomous Disruption of Nuclear Architecture as a Potential Cause of COVID-19-Induced Anosmia. Cell 2022, 185, 1052–1064.e12. [Google Scholar] [CrossRef] [PubMed]
- Whitcroft, K.L.; Hummel, T. Clinical Diagnosis and Current Management Strategies for Olfactory Dysfunction: A Review. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.T.; Prem, B.; Besser, G.; Renner, B.; Mueller, C.A. Olfactory-Related Quality of Life Adjustments in Smell Loss during the Coronavirus-19 Pandemic. Am. J. Rhinol. Allergy 2022, 36, 253–260. [Google Scholar] [CrossRef]
- Auinger, A.B.; Besser, G.; Liu, D.T.; Renner, B.; Mueller, C.A. Long-Term Impact of Olfactory Dysfunction on Daily Life. Wien. Klin. Wochenschr. 2021, 133, 1004–1011. [Google Scholar] [CrossRef]
- Pekala, K.; Chandra, R.K.; Turner, J.H. Efficacy of Olfactory Training in Patients with Olfactory Loss: A Systematic Review and Meta-Analysis. Int. Forum Allergy Rhinol. 2016, 6, 299–307. [Google Scholar] [CrossRef]
- Bochicchio, V.; Mezzalira, S.; Maldonato, N.M.; Cantone, E.; Scandurra, C. Olfactory-related quality of life impacts psychological distress in people with COVID-19: The affective implications of olfactory dysfunctions. J. Affect. Disord. 2023, 323, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Abdelalim, A.A.; Mohamady, A.A.; Elsayed, R.A.; Elawady, M.A.; Ghallab, A.F. Corticosteroid Nasal Spray for Recovery of Smell Sensation in COVID-19 Patients: A Randomized Controlled Trial. Am. J. Otolaryngol. 2021, 42, 102884. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpoor, M.; Kabiri, M.; Rajati Haghi, M.; Ghadam Soltani, T.; Rezaei, A.; Faghfouri, A.; Poustchian Gholkhatmi, Z.; Bakhshaee, M. Intranasal Corticosteroid Treatment on Recovery of Long-Term Olfactory Dysfunction Due to COVID-19. Laryngoscope 2022, 132, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Altundag, A.; Yıldırım, D.; Tekcan Sanli, D.E.; Cayonu, M.; Kandemirli, S.G.; Sanli, A.N.; Arici Duz, O.; Saatci, O. Olfactory Cleft Measurements and COVID-19-Related Anosmia. Otolaryngol. Head Neck Surg. 2021, 164, 1337–1344. [Google Scholar] [CrossRef]
- Hwang, S.H.; Kim, S.W.; Basurrah, M.A.; Kim, D.H. The Efficacy of Olfactory Training as a Treatment for Olfactory Disorders Caused by Coronavirus Disease-2019: A Systematic Review and Meta-Analysis. Am. J. Rhinol. Allergy 2023, 37, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Tosch, C.; Wolz, M.; Klingelhoefer, L.; Fauser, M.; Storch, A.; Reichmann, H.; Hummel, T. Olfactory Training in Patients with Parkinson’s Disease. PLoS ONE 2013, 8, e61680. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Hanf, S.; Whitcroft, K.L.; Haehner, A.; Hummel, T. Examination of Olfactory Training Effectiveness in Relation to Its Complexity and the Cause of Olfactory Loss. Laryngoscope 2018, 128, 1518–1522. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Tsakiropoulou, E.; Constantinidis, J. Long Term Effects of Olfactory Training in Patients with Post-Infectious Olfactory Loss. Rhinology 2016, 54, 170–175. [Google Scholar] [CrossRef]
- Delgado-Lima, A.H.; Bouhaben, J.; Delgado-Losada, M.L. The efficacy of olfactory training in improving olfactory function: A meta-analysis. Eur. Arch. Oto-Rhino-Laryngol. 2024, 1–18. [Google Scholar] [CrossRef]
- Hummel, T.; Rissom, K.; Reden, J.; Hähner, A.; Weidenbecher, M.; Hüttenbrink, K.-B. Effects of Olfactory Training in Patients with Olfactory Loss. Laryngoscope 2009, 119, 496–499. [Google Scholar] [CrossRef]
- Saatci, O.; Altundag, A.; Duz, O.A.; Hummel, T. Olfactory training ball improves adherence and olfactory outcomes in post-infectious olfactory dysfunction. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2125–2132. [Google Scholar] [CrossRef]
- Lechien, J.R.; Vaira, L.A.; Saussez, S. Effectiveness of olfactory training in COVID-19 patients with olfactory dysfunction: A prospective study. Eur. Arch. Oto-Rhino-Laryngol. 2023, 280, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.L.; Henecke, S.; Thunell, E.; Swartz, M.; Martinsen, J.; Sahlstrand Johansson, P.; Lundstrom, J.N. Olfactory training using nasal inserts more effective due to increased adherence. medRxiv 2024. [Google Scholar] [CrossRef]
- Delgado-Losada, M.L.; Delgado-Lima, A.H.; Bouhaben, J. Spanish Validation for Olfactory Function Testing Using the Sniffin’ Sticks Olfactory Test: Threshold, Discrimination, and Identification. Brain Sci. 2020, 10, 943. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. “Sniffin” Sticks’: Olfactory Performance Assessed by the Combined Testing of Odor Identification, Odor Discrimination and Olfactory Threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Losada, M.L.; Bouhaben, J.; Delgado-Lima, A.H. Development of the spanish version of sniffin’s sticks olfactory identification test: Normative data and validity of parallel measures. Brain Sci. 2021, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Sorokowska, A.; Drechsler, E.; Karwowski, M.; Hummel, T. Effects of Olfactory Training: A Meta-Analysis. Rhinology 2017, 55, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Thomas, B.J.; Gilbert, D.D.; Tong, S.; Teynor, N.J.; Friedman, N.R.; Herrmann, B.W.; Gitomer, S.A. Olfactory Dysfunction and Training in Children with COVID-19 Infection: A Prospective Study Post-COVID Pediatric Olfactory Training. Int. J. Pediatr. Otorhinolaryngol. 2024, 176, 111799. [Google Scholar] [CrossRef] [PubMed]
- Alarfaj, A.A.; Aldrweesh, A.K.; Aldoughan, A.F.; Alarfaj, S.M.; Alabdulqader, F.K.; Alyahya, K.A. Olfactory Dysfunction Following COVID-19 and the Potential Benefits of Olfactory Training. J. Clin. Med. 2023, 12, 4761. [Google Scholar] [CrossRef] [PubMed]
- Kollndorfer, K.; Fischmeister, F.P.S.; Kowalczyk, K.; Hoche, E.; Mueller, C.A.; Trattnig, S.; Schöpf, V. Olfactory Training Induces Changes in Regional Functional Connectivity in Patients with Long-Term Smell Loss. NeuroImage Clin. 2015, 9, 401–410. [Google Scholar] [CrossRef]
- Hosseini, K.; Zare-Sadeghi, A.; Sadigh-Eteghad, S.; Mirsalehi, M.; Khezerloo, D. Effects of Olfactory Training on Resting-state Effective Connectivity in Patients with Posttraumatic Olfactory Dysfunction. Acta Neurobiol. Exp. 2020, 80, 381–388. [Google Scholar] [CrossRef]
- Vandersteen, C.; Payne, M.; Dumas, L.-É.; Cancian, É.; Plonka, A.; D’Andréa, G.; Chirio, D.; Demonchy, É.; Risso, K.; Askenazy-Gittard, F.; et al. Olfactory Training in Post-COVID-19 Persistent Olfactory Disorders: Value Normalization for Threshold but Not Identification. J. Clin. Med. 2022, 11, 3275. [Google Scholar] [CrossRef] [PubMed]
- Yaylacı, A.; Azak, E.; Önal, A.; Aktürk, D.R.; Karadenizli, A. Effects of Classical Olfactory Training in Patients with COVID-19-Related Persistent Loss of Smell. Eur. Arch. Otorhinolaryngol. 2023, 280, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Altundag, A.; Cayonu, M.; Kayabasoglu, G.; Salihoglu, M.; Tekeli, H.; Saglam, O.; Hummel, T. Modified Olfactory Training in Patients with Postinfectious Olfactory Loss. Laryngoscope 2015, 125, 1763–1766. [Google Scholar] [CrossRef] [PubMed]
- Gudziol, V.; Buschhüter, D.; Abolmaali, N.; Gerber, J.; Rombaux, P.; Hummel, T. Increasing olfactory bulb volume due to treatment of chronic rhinosinusitis—A longitudinal study. Brain 2009, 132, 3096–3101. [Google Scholar] [CrossRef] [PubMed]
- Damm, M.; Pikart, L.K.; Reimann, H.; Burkert, S.; Göktas, Ö.; Haxel, B.; Frey, S.; Charalampakis, I.; Beule, A.; Renner, B.; et al. Olfactory Training Is Helpful in Postinfectious Olfactory Loss: A Randomized, Controlled, Multicenter Study: Olfactory Training. Laryngoscope 2014, 124, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.L.; Schöpf, V. Olfactory Loss and Regain: Lessons for Neuroplasticity. Neuroscientist 2018, 24, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Oleszkiewicz, A.; Heyne, L.; Sienkiewicz-Oleszkiewicz, B.; Cuevas, M.; Haehner, A.; Hummel, T. Odours Count: Human Olfactory Ecology Appears to Be Helpful in the Improvement of the Sense of Smell. Sci. Rep. 2021, 11, 16888. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.-J.; Fletcher, R.B.; et al. Non-Neuronal Expression of SARS-CoV-2 Entry Genes in the Olfactory System Suggests Mechanisms Underlying COVID-19-Associated Anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory Transmucosal SARS-CoV-2 Invasion as a Port of Central Nervous System Entry in Individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Fleiner, F.; Lau, L.; Göktas, Ö. Active Olfactory Training for the Treatment of Smelling Disorders. Ear Nose Throat J. 2012, 91, 198–215. [Google Scholar] [CrossRef]
- Genetzaki, S.; Tsakiropoulou, E.; Nikolaidis, V.; Markou, K.; Konstantinidis, I. Postinfectious Olfactory Dysfunction: Oral Steroids and Olfactory Training versus Olfactory Training Alone: Is There Any Benefit from Steroids? ORL J. Otorhinolaryngol. Relat. Spec. 2021, 83, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Jiramongkolchai, P.; Jones, M.S.; Peterson, A.; Lee, J.J.; Liebendorfer, A.; Klatt-Cromwell, C.N.; Schneider, J.S.; Drescher, A.J.; Ogden, M.A.; Brunworth, J.D.; et al. Association of Olfactory Training with Neural Connectivity in Adults with Postviral Olfactory Dysfunction. JAMA Otolaryngol. Head Neck Surg. 2021, 147, 502. [Google Scholar] [CrossRef] [PubMed]
- Lamira, J.M.; Soler, Z.M.; Schlosser, R.J. A Pilot Study of Olfactory Training in Older Hyposmic Adults. Am. J. Rhinol. Allergy 2019, 33, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Mahmut, M.K.; Oelschlägel, A.; Haehner, A.; Hummel, T. The Impact of Olfactory Training Using a Nasal Clip and Extended Periods of Odor Exposure. J. Sens. Stud. 2022, 37, e12721. [Google Scholar] [CrossRef]
- Gürbüz, D.; Kesimli, M.C.; Bilgili, A.M.; Durmaz, H.Ö. Olfactory Rehabilitation and Olfactory Bulb Volume Changes in Patients after Total Laryngectomy: A Prospective Randomized Study. Braz. J. Otorhinolaryngol. 2022, 88, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kollndorfer, K.; Kowalczyk, K.; Hoche, E.; Mueller, C.A.; Pollak, M.; Trattnig, S.; Schöpf, V. Recovery of Olfactory Function Induces Neuroplasticity Effects in Patients with Smell Loss. Neural Plast. 2014, 2014, 140419. [Google Scholar] [CrossRef] [PubMed]
- Le Bon, S.-D.; Konopnicki, D.; Pisarski, N.; Prunier, L.; Lechien, J.R.; Horoi, M. Efficacy and Safety of Oral Corticosteroids and Olfactory Training in the Management of COVID-19-Related Loss of Smell. Eur. Arch. Otorhinolaryngol. 2021, 278, 3113–3117. [Google Scholar] [CrossRef]
- Morquecho-Campos, P.; Larsson, M.; Boesveldt, S.; Olofsson, J.K. Achieving Olfactory Expertise: Training for Transfer in Odor Identification. Chem. Senses 2019, 44, 197–203. [Google Scholar] [CrossRef]
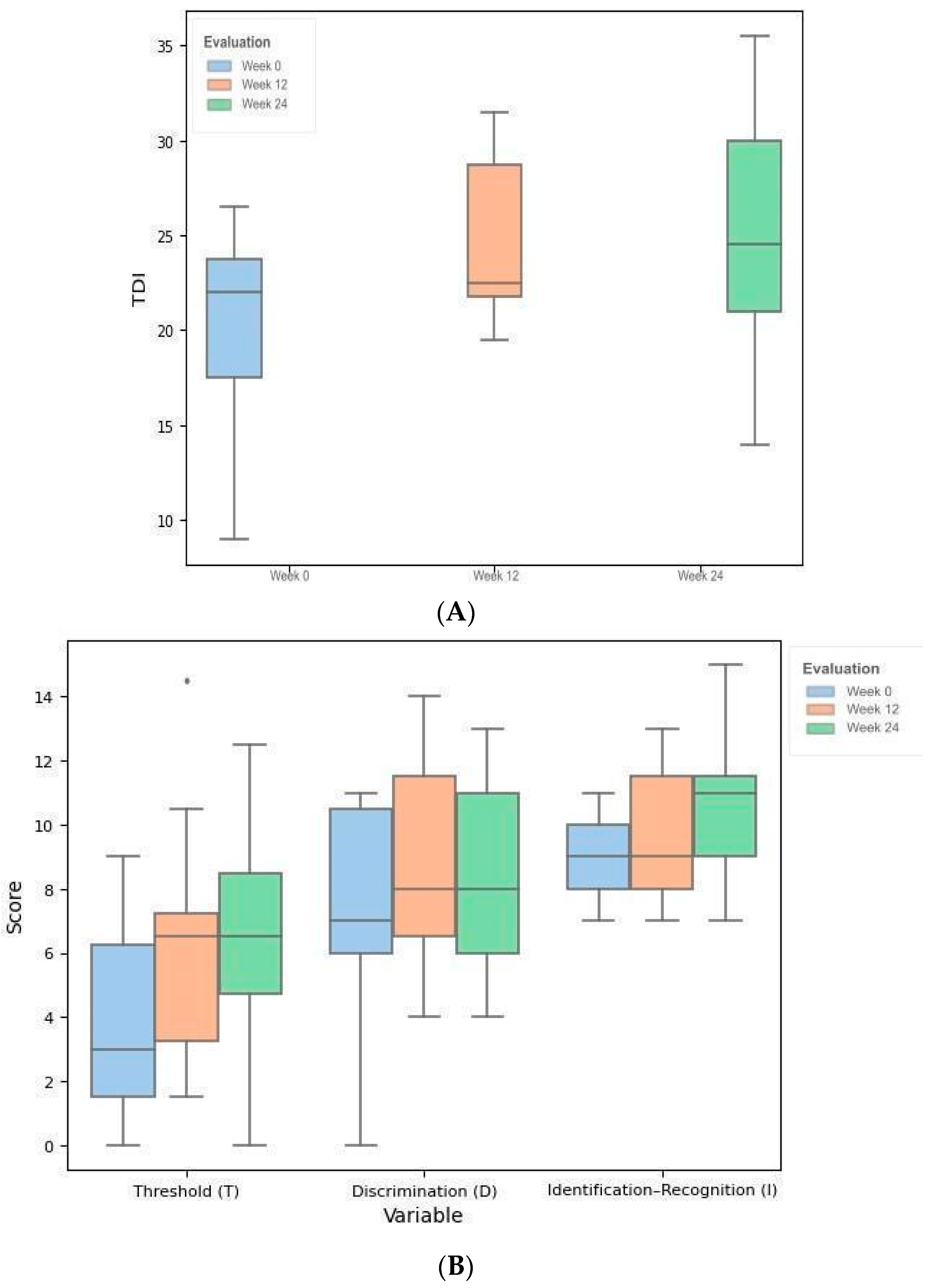

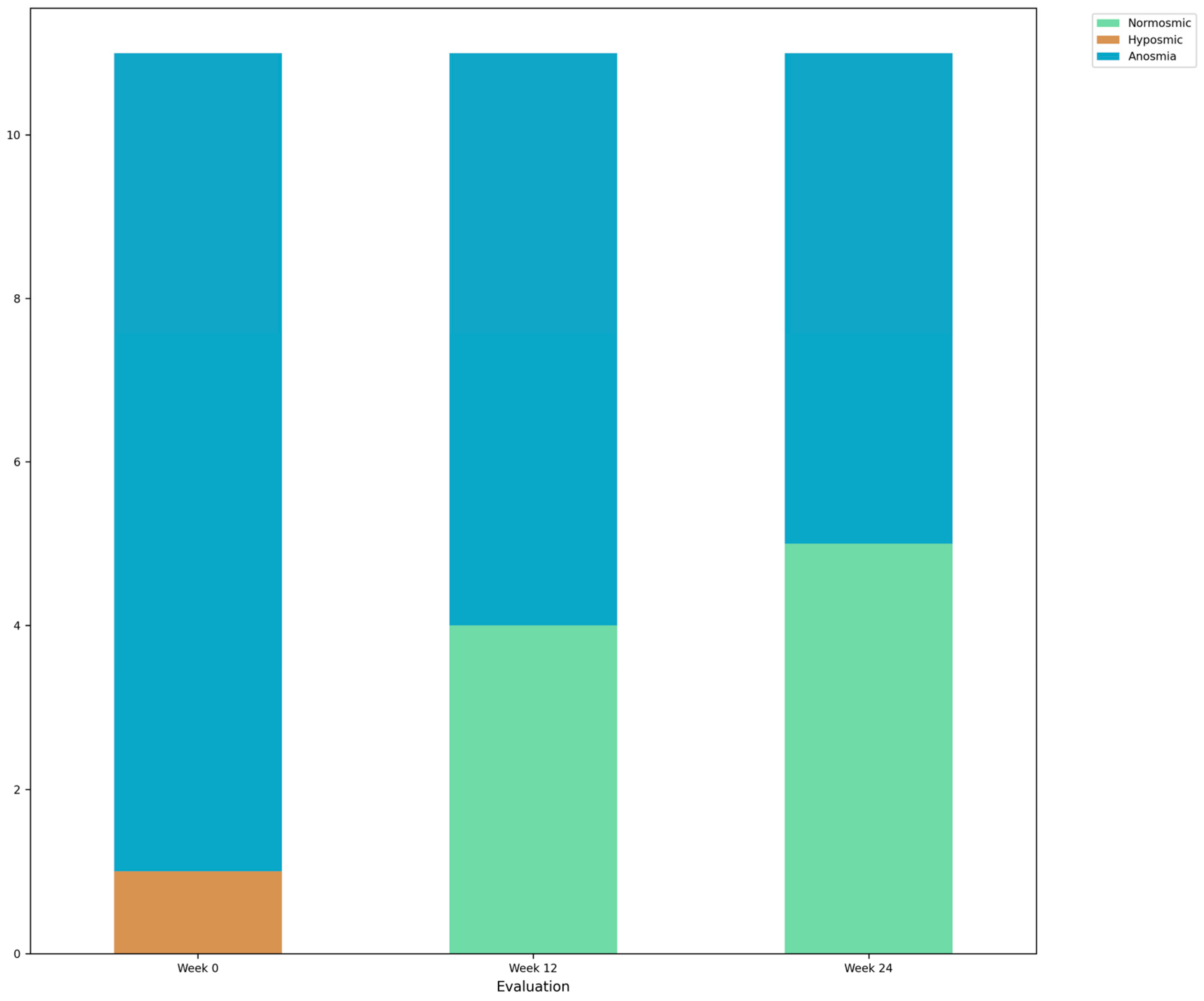
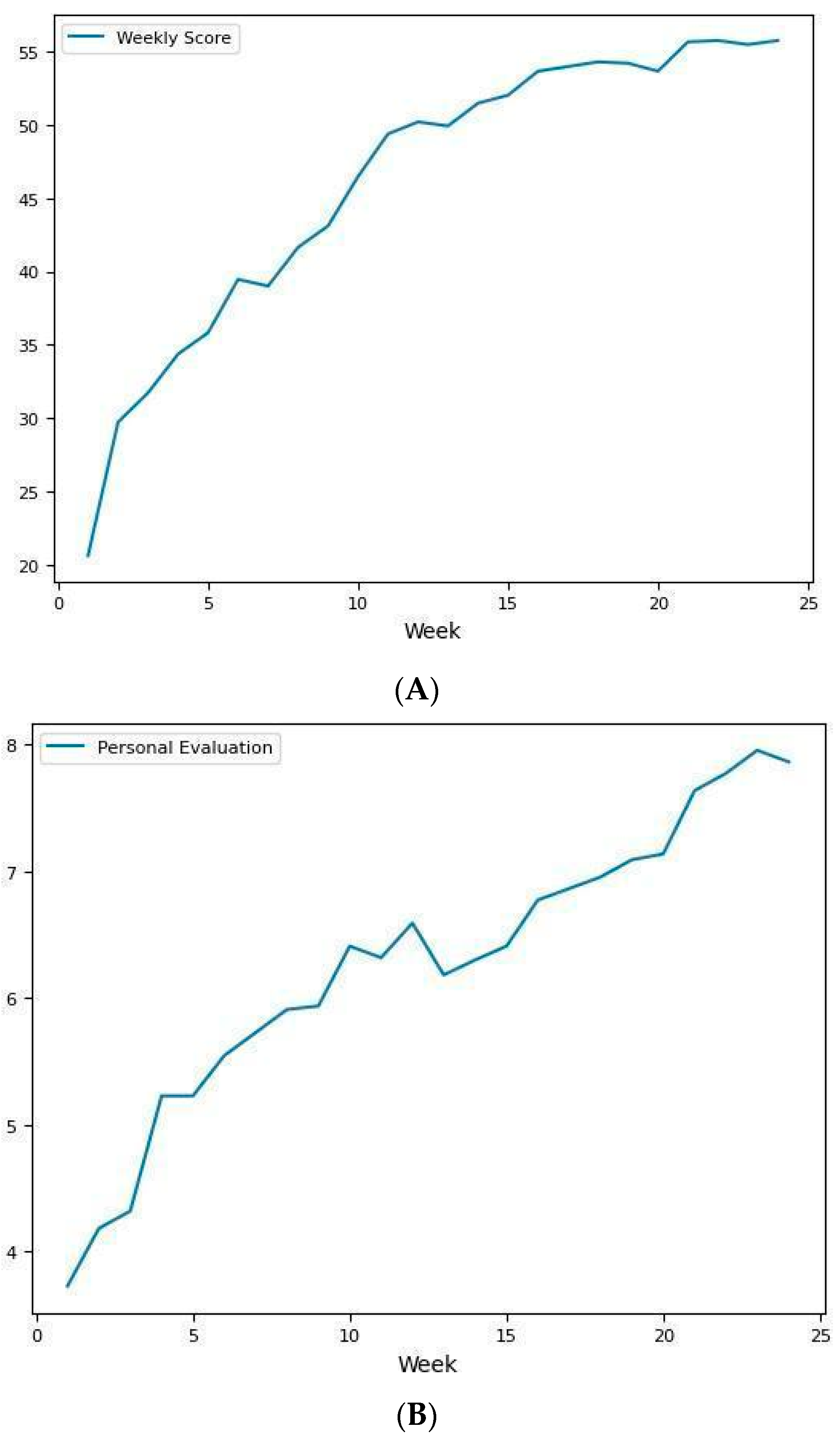
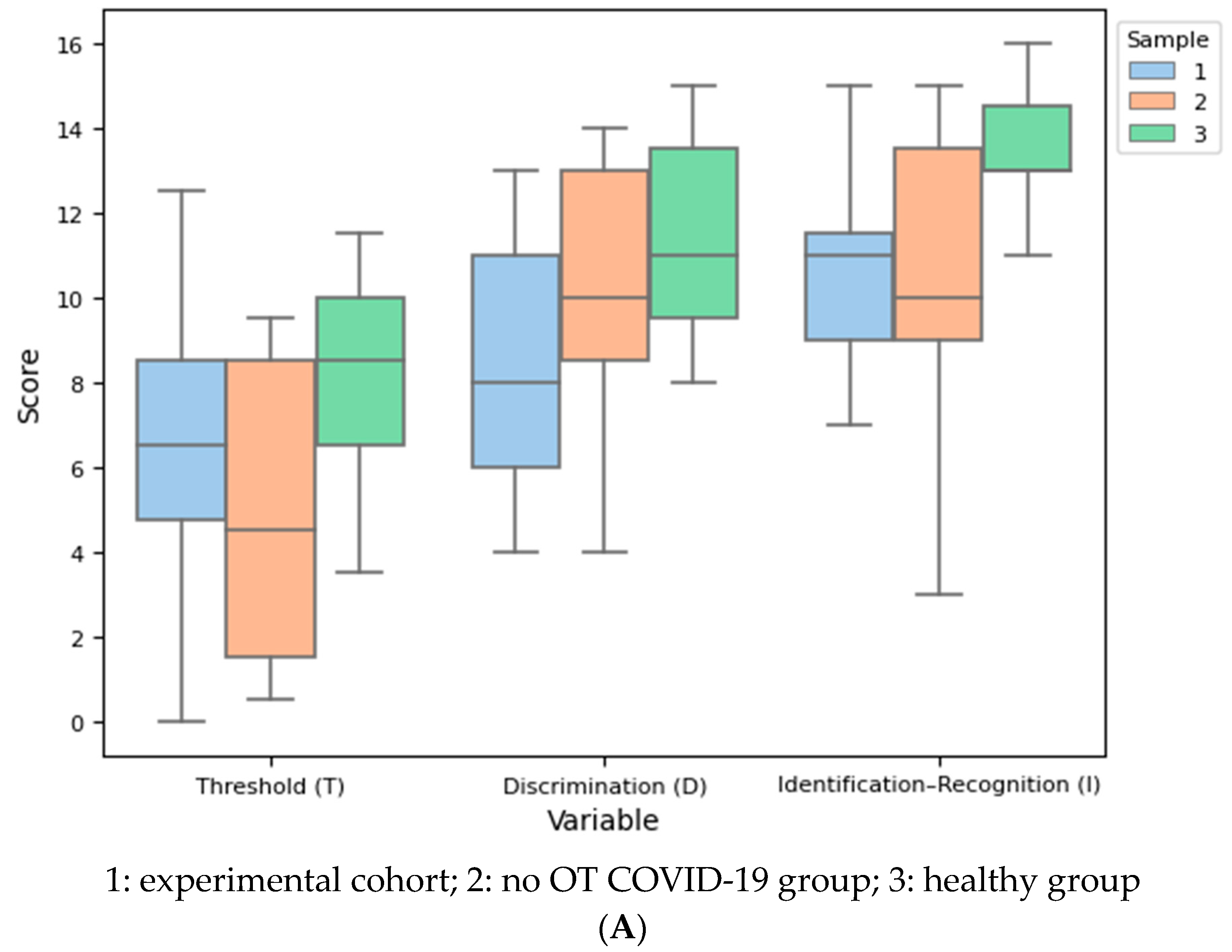

| Week 0 | Week 12 | Week 24 | df | F | Test | p | SMD (1, 2) | SMD (1, 3) | SMD (2, 3) | |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 11 | 11 | 11 | |||||||
| Threshold (T), mean (SD) | 3.8 (3.3) | 6.2 (3.8) | 6.5 (3.4) | [2, 20] | 3.368 | ANOVA RM | 0.055 | - | - | - |
| Discrimination (D), mean (SD) | 7.5 (3.4) | 8.9 (3.2) | 8.3 (3.1) | [2, 20] | 0.647 | ANOVA RM | 0.534 | - | - | - |
| Identification (I), mean (SD) | 8.9 (1.3) | 9.5 (2.1) | 10.5 (2.3) | [2, 20] | 2.190 | ANOVA RM | 0.138 | - | - | - |
| TDI, mean (SD) | 20.3 (5.6) | 24.6 (4.3) | 25.4 (6.2) | [2, 20] | 5.115 | ANOVA RM | 0.016 * | 0.869 (0.041 *) | 0.859 (0.041 *) | 0.136 (0.657) |
| EG | CG | HG | df | F | Test | p | SMD (EG, CG) | SMD (EG, HG) | SMD (CG, HG) | |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 11 | 11 | 11 | |||||||
| Age | 34.55 | 33.00 | 32.55 | |||||||
| Threshold (T), mean (SD) | 6.5 (3.4) | 4.9 (3.5) | 8.2 (2.5) | 8 | 2.928 | One-way ANOVA | 0.069 | −0.475 (0.457) | 0.549 (0.457) | 1.064 (0.055) |
| Discrimination (D), mean (SD) | 8.3 (3.1) | 10.5 (3.3) | 11.5 (2.3) | 8 | 3.391 | One-way ANOVA | 0.047 * | 0.685 (0.205) | 1.158 (0.042 *) | 0.352 (0.706) |
| Identification (I), mean (SD) | 10.5 (2.3) | 10.6 (3.5) | 13.5 (1.4) | 8 | 4.903 | One-way ANOVA | 0.014 * | 0.031 (0.996) | 1.545 (0.026 *) | 1.095 (0.032 *) |
| TDI, mean (SD) | 25.4 (6.2) | 26.0 (8.6) | 33.2 (4.0) | 8 | 4.819 | One-way ANOVA | 0.015 * | 0.085 (0.972) | 1.493 (0.024 *) | 1.069 (0.040 *) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Lima, A.H.; Bouhaben, J.; Delgado-Losada, M.L. Maximizing Participation in Olfactory Training in a Sample with Post-COVID-19 Olfactory Loss. Brain Sci. 2024, 14, 730. https://doi.org/10.3390/brainsci14070730
Delgado-Lima AH, Bouhaben J, Delgado-Losada ML. Maximizing Participation in Olfactory Training in a Sample with Post-COVID-19 Olfactory Loss. Brain Sciences. 2024; 14(7):730. https://doi.org/10.3390/brainsci14070730
Chicago/Turabian StyleDelgado-Lima, Alice Helena, Jaime Bouhaben, and María Luisa Delgado-Losada. 2024. "Maximizing Participation in Olfactory Training in a Sample with Post-COVID-19 Olfactory Loss" Brain Sciences 14, no. 7: 730. https://doi.org/10.3390/brainsci14070730





