Dopamine Dysregulation in Reward and Autism Spectrum Disorder
Abstract
:1. Introduction
2. Alterations at the Genetic/Epigenetic Level Converging to ASD Pathways
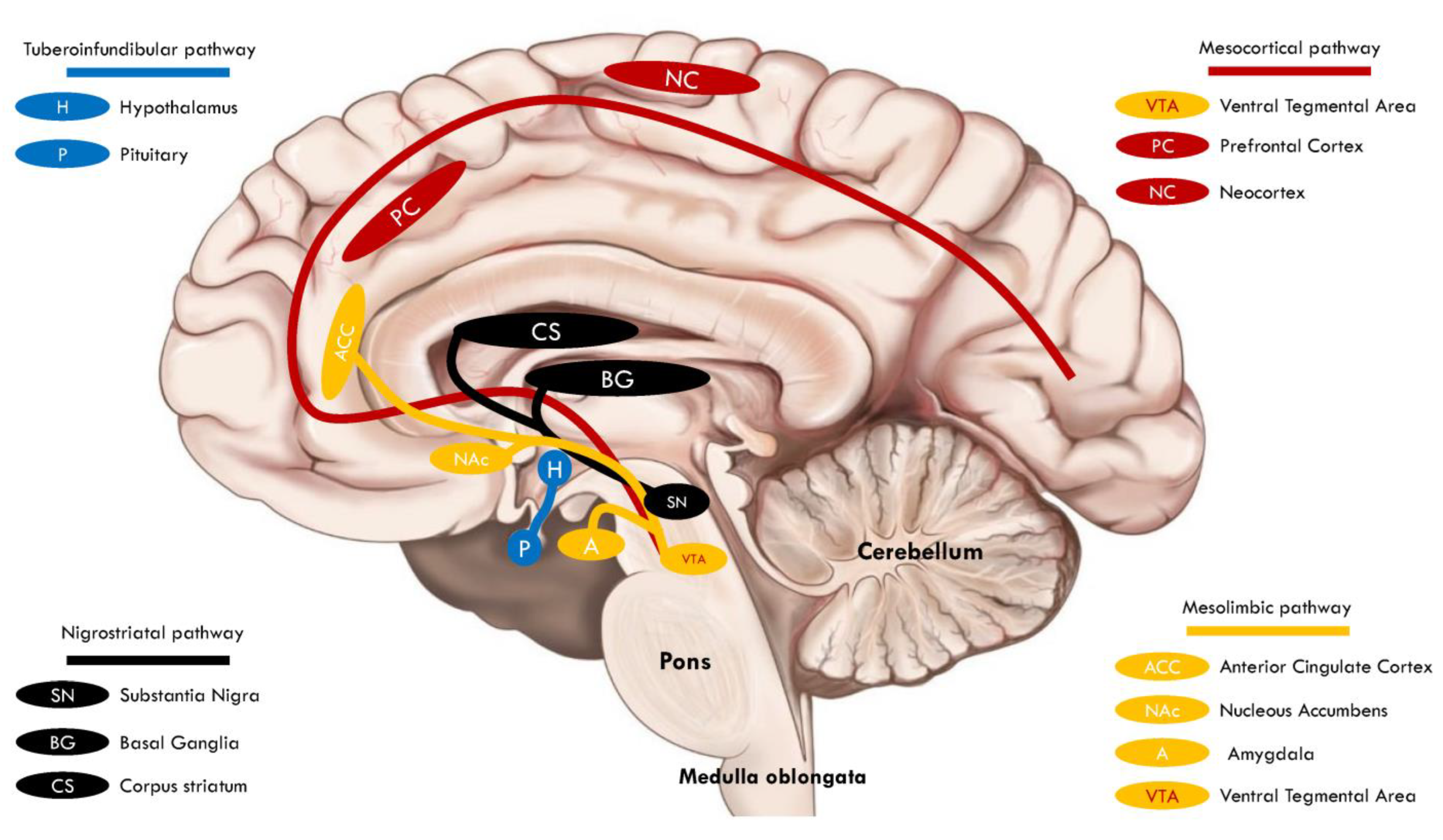
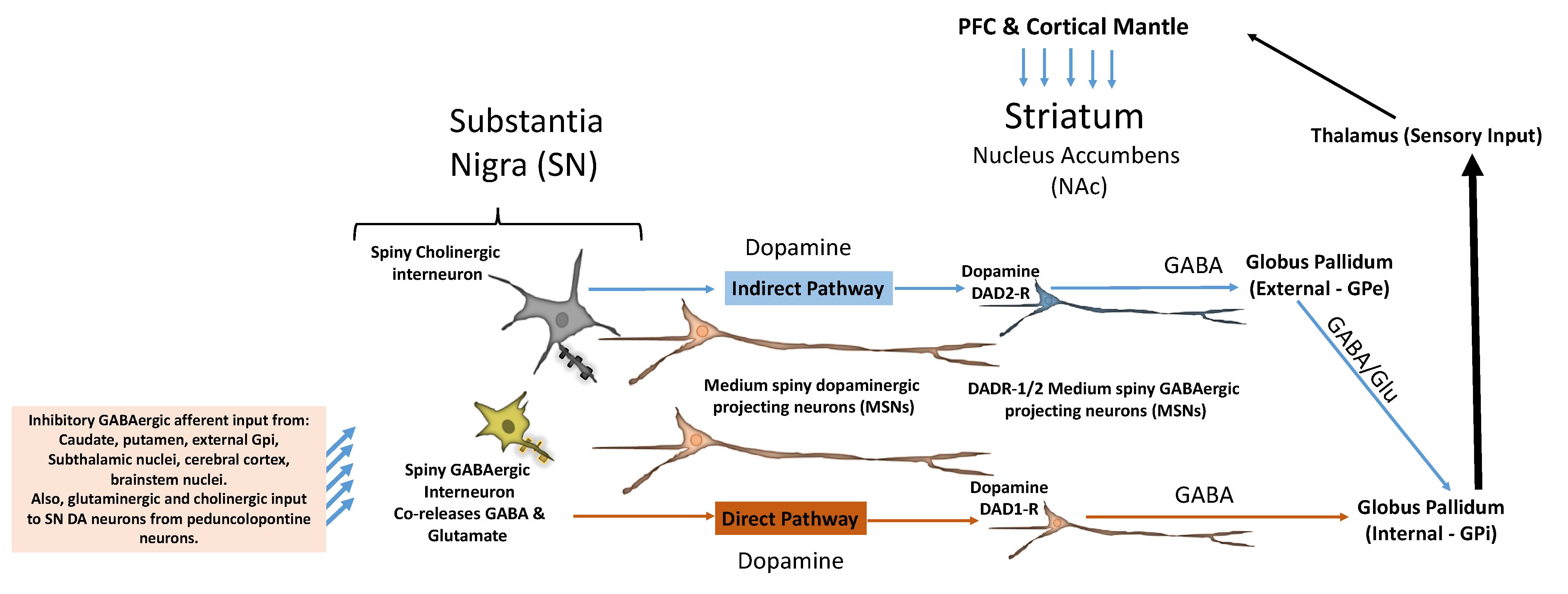
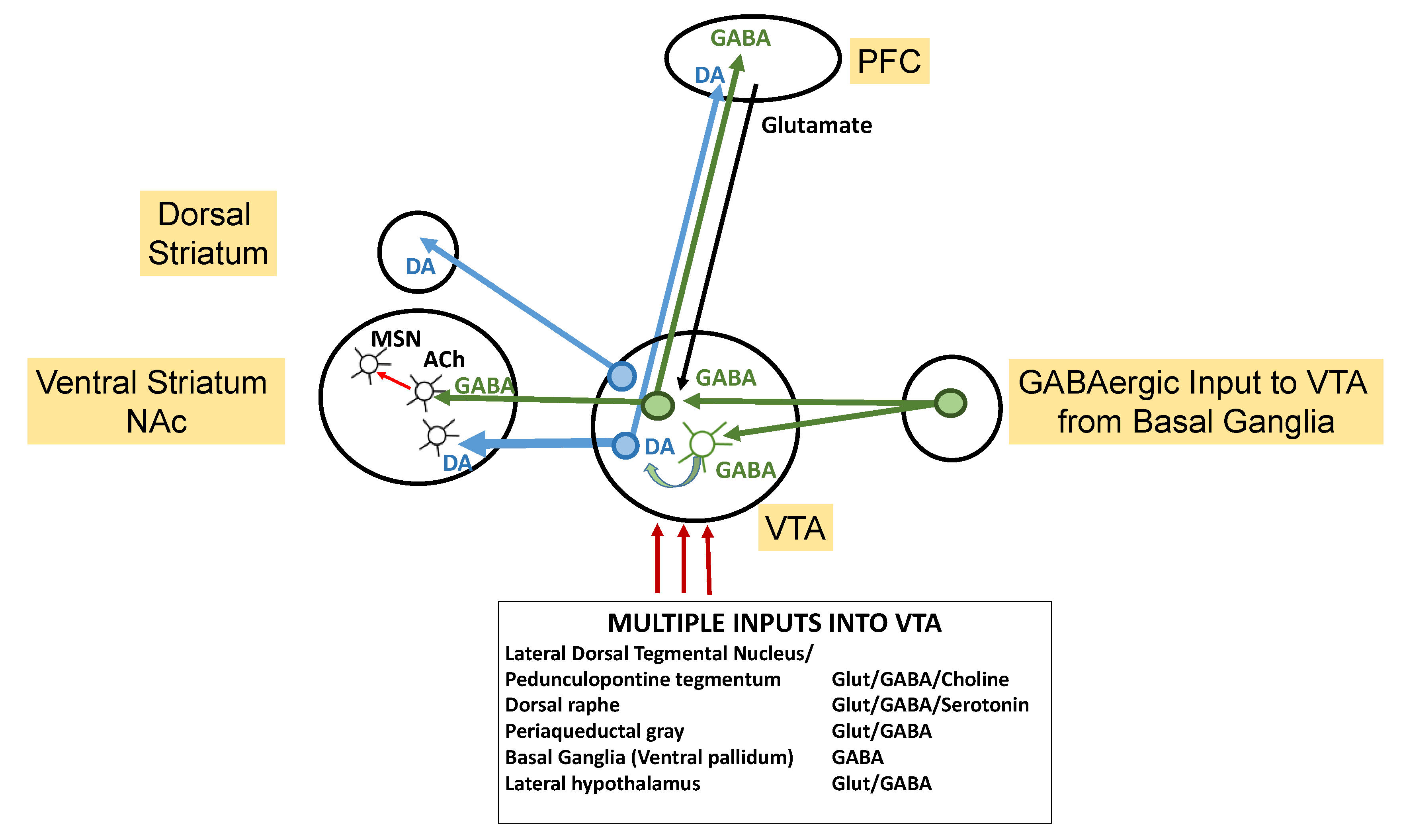
3. Mesolimbic DAergic Reward Pathway Disruption and ASD
4. Mesolimbic DAergic Social Reward, Brain Excitatory–Inhibitory Balance, and ASD
5. Dysregulated Neurotransmitter Signaling in ASD
6. DAergic Dysregulation and Neurocognitive Manifestations in ASD
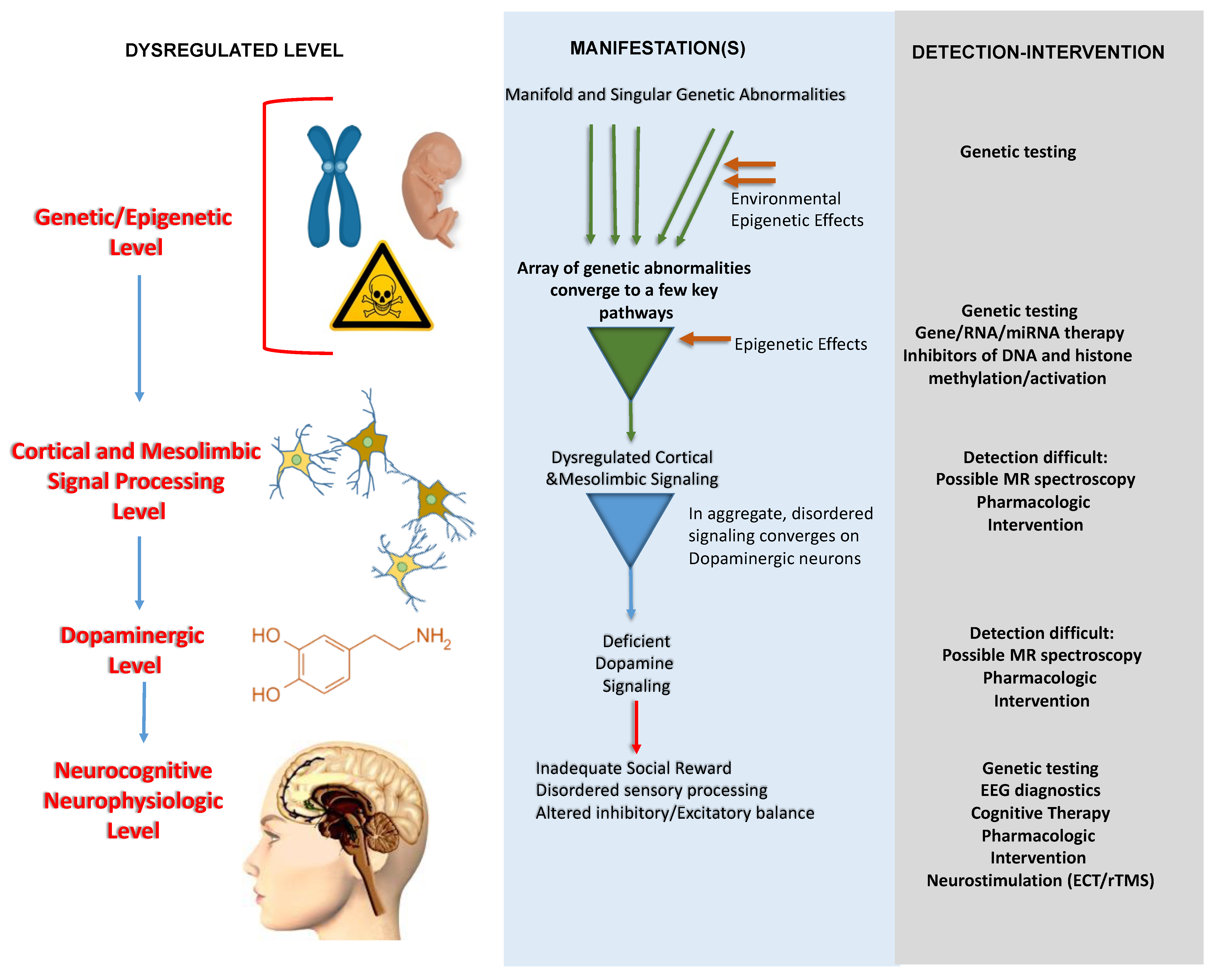
7. Conclusions and a Working Framework for ASD Pathogenesis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavăl, D. A Dopamine Hypothesis of Autism Spectrum Disorder. Dev. Neurosci. 2017, 39, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, J.; Fombonne, E.; Scorah, J.; Ibrahim, A.; Durkin, M.S.; Saxena, S.; Yusuf, A.; Shih, A.; Elsabbagh, M. Global prevalence of autism: A systematic review update. Autism Res. 2022, 15, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Dichter, G.S.; Felder, J.N.; Green, S.R.; Rittenberg, A.M.; Sasson, N.J.; Bodfish, J.W. Reward circuitry function in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 2012, 7, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.; Wiggins, L.; Christensen, D.L.; Maenner, M.J.; Daniels, J.; Warren, Z.; Kurzius-Spencer, M.; Zahorodny, W.; Robinson, C.R.; White, T.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. Surveill. Summ. 2018, 67, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Schalbroeck, R.; van Velden, F.H.P.; de Geus-Oei, L.-F.; Yaqub, M.; van Amelsvoort, T.; Booij, J.; Selten, J.-P. Striatal dopamine synthesis capacity in autism spectrum disorder and its relation with social defeat: An [18F]-FDOPA PET/CT study. Transl. Psychiatry 2021, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Arias-Carrión, O.; Caraza-Santiago, X.; Salgado-Licona, S.; Salama, M.; Machado, S.; Nardi, A.; Menéndez-González, M.; Murillo-Rodríguez, E. Orquestic regulation of neurotransmitters on reward-seeking behavior. Int. Arch. Med. 2014, 7, 29. [Google Scholar] [CrossRef]
- Baik, J.-H. Stress and the dopaminergic reward system. Exp. Mol. Med. 2020, 52, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Mineur, Y.S.; Picciotto, M.R. The role of acetylcholine in negative encoding bias: Too much of a good thing? Eur. J. Neurosci. 2019, 53, 114–125. [Google Scholar] [CrossRef]
- Coddington, L.T.; Lindo, S.E.; Dudman, J.T. Mesolimbic dopamine adapts the rate of learning from action. Nature 2023, 614, 294–302. [Google Scholar] [CrossRef]
- Dunigan, A.I.; Roseberry, A.G. Actions of feeding-related peptides on the mesolimbic dopamine system in regulation of natural and drug rewards. Addict. Neurosci. 2022, 2, 100011. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.Z.; Cheer, J.F.; Tonini, R. Modulating the Neuromodulators: Dopamine, Serotonin, and the Endocannabinoid System. Trends Neurosci. 2021, 44, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Paval, D. The dopamine hypothesis of autism spectrum disorder: A comprehensive analysis of the evidence. Int. Rev. Neurobiol. 2023, 173, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Kohls, G.; Peltzer, J.; Schulte-Rüther, M.; Kamp-Becker, I.; Remschmidt, H.; Herpertz-Dahlmann, B.; Konrad, K. Atypical Brain Responses to Reward Cues in Autism as Revealed by Event-Related Potentials: A Systematic Review and Meta-analysis. J. Autism Dev. Disord. 2011, 41, 1523–1533. [Google Scholar] [CrossRef] [PubMed]
- Matyjek, M.; Bayer, M.; Dziobek, I. Reward responsiveness in autism and autistic traits—Evidence from neuronal, autonomic, and behavioural levels. NeuroImage Clin. 2023, 38, 103442. [Google Scholar] [CrossRef] [PubMed]
- Clements, C.C.; Zoltowski, A.R.; Yankowitz, L.D.; Yerys, B.E.; Schultz, R.T.; Herrington, J.D. Evaluation of the Social Motivation Hypothesis of Autism.: A Systematic Review and Meta-analysis. JAMA Psychiatry 2018, 75, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Mandy, W.; Lai, M. Annual Research Review: The role of the environment in the developmental psychopathology of autism spectrum condition. J. Child Psychol. Psychiatry 2016, 57, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Mandic-Maravic, V.; Grujicic, R.; Milutinovic, L.; Munjiza-Jovanovic, A.; Pejovic-Milovancevic, M. Dopamine in Autism Spectrum Disorders—Focus on D2/D3 Partial Agonists and Their Possible Use in Treatment. Front. Psychiatry 2022, 12, 787097. [Google Scholar] [CrossRef] [PubMed]
- DiCarlo, G.E.; Wallace, M.T. Modeling dopamine dysfunction in autism spectrum disorder: From invertebrates to vertebrates. Neurosci. Biobehav. Rev. 2021, 133, 104494. [Google Scholar] [CrossRef]
- Christensen, J.; Grønborg, T.K.; Sørensen, M.J.; Schendel, D.; Parner, E.T.; Pedersen, L.H.; Vestergaard, M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA 2013, 309, 1696–1703. [Google Scholar] [CrossRef]
- Horder, J.; Petrinovic, M.M.; Mendez, M.A.; Bruns, A.; Takumi, T.; Spooren, W.; Barker, G.J.; Künnecke, B.; Murphy, D.G. Glutamate and GABA in autism spectrum disorder—A translational magnetic resonance spectroscopy study in man and rodent models. Transl. Psychiatry 2018, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, C.; Kohls, G.; Troiani, V.; Brodkin, E.S.; Schultz, R.T. The social motivation theory of autism. Trends Cogn. Sci. 2012, 16, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Whalley, K. Converging pathways. Nat. Rev. Neurosci. 2011, 12, 372. [Google Scholar] [CrossRef] [PubMed]
- Mahony, C.; O’Ryan, C. A molecular framework for autistic experiences: Mitochondrial allostatic load as a mediator between autism and psychopathology. Front. Psychiatry 2022, 13, 985713. [Google Scholar] [CrossRef]
- Jiang, C.C.; Lin, L.S.; Long, S.; Ke, X.Y.; Fukunaga, K.; Lu, Y.M.; Han, F. Signalling pathways in autism spectrum disorder: Mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 2022, 7, 229. [Google Scholar] [CrossRef]
- Port, R.G.; Gandal, M.J.; Roberts, T.P.L.; Siegel, S.J.; Carlson, G.C. Convergence of circuit dysfunction in ASD: A common bridge between diverse genetic and environmental risk factors and common clinical electrophysiology. Front. Cell. Neurosci. 2014, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Supekar, K.; Kochalka, J.; Schaer, M.; Wakeman, H.; Qin, S.; Padmanabhan, A.; Menon, V. Deficits in mesolimbic reward pathway underlie social interaction impairments in children with autism: A journal of neurology. Brain 2018, 141, 2795–2805. [Google Scholar] [CrossRef]
- Bailey, A.; Le Couteur, A.; Gottesman, I.; Bolton, P.; Simonoff, E.; Yuzda, E.; Rutter, M. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol. Med. 1995, 25, 63–77. [Google Scholar] [CrossRef]
- Constantino, J.N.; Zhang, Y.; Frazier, T.; Abbacchi, A.M.; Law, P. Sibling recurrence and the genetic epidemiology of autism. Am. J. Psychiatry 2010, 167, 1349–1356. [Google Scholar] [CrossRef]
- Fernandez, B.A.; Scherer, S.W. Syndromic autism spectrum disorders: Moving from a clinically defined to a molecularly defined approach. Dialog- Clin. Neurosci. 2017, 19, 353–371. [Google Scholar] [CrossRef]
- Geschwind, D.H. Autism: Many Genes, Common Pathways? Cell 2008, 135, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gomez, D.A.; Garcia-Guaqueta, D.P.; Charry-Sánchez, J.D.; Sarquis-Buitrago, E.; Blanco, M.; Velez-Van-Meerbeke, A.; Talero-Gutiérrez, C. A systematic review of common genetic variation and biological pathways in autism spectrum disorder. BMC Neurosci. 2021, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.M.; Ismail, S.; Zarouk, W.A.; Baky, O.A.; Sayed, A.A.; El-Hamid, S.A.; Salem, S. Genetic variants of neurotransmitter-related genes and miRNAs in Egyptian autistic patients. Sci. World J. 2013, 2013, 670621. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ching, C.R.K.; Lin, A.; Forsyth, J.K.; Kushan, L.; Vajdi, A.; Jalbrzikowski, M.; Hansen, L.; Villalon-Reina, J.E.; Qu, X.; et al. Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size. Mol. Psychiatry 2020, 25, 1822–1834. [Google Scholar] [CrossRef] [PubMed]
- Monks, S.; Niarchou, M.; Davies, A.R.; Walters, J.T.; Williams, N.; van den Owen, M.J.; Bree, M.B.v.D.; Murphy, K.C. Further evidence for high rates of schizophrenia in 22q11.2 deletion syndrome. Schizophr. Res. 2014, 153, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.S.; Wassman, E.R.; Baxter, A.L.; Hensel, C.H.; Martin, M.M.; Prasad, A.; Twede, H.; Vanzo, R.J.; Butler, M.G. Chromosomal Microarray Analysis of Consecutive Individuals with Autism Spectrum Disorders Using an Ultra-High Resolution Chromosomal Microarray Optimized for Neurodevelopmental Disorders. Int. J. Mol. Sci. 2016, 17, 2070. [Google Scholar] [CrossRef] [PubMed]
- Ousley, O.; Evans, A.N.; Fernandez-Carriba, S.; Smearman, E.L.; Rockers, K.; Morrier, M.J.; Evans, D.W.; Coleman, K.; Cubells, J. Examining the Overlap between Autism Spectrum Disorder and 22q11.2 Deletion Syndrome. Int. J. Mol. Sci. 2017, 18, 1071. [Google Scholar] [CrossRef] [PubMed]
- Michaelovsky, E.; Frisch, A.; Carmel, M.; Patya, M.; Zarchi, O.; Green, T.; Basel-Vanagaite, L.; Weizman, A.; Gothelf, D. Genotype-phenotype correlation in 22q11.2 deletion syndrome. BMC Med. Genet. 2012, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Grandy, D.K.; Marchionni, M.A.; Makam, H.; Stofko, R.E.; Alfano, M.; Frothingham, L.; Fischer, J.B.; Burke-Howie, K.J.; Bunzow, J.R.; Server, A.C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc. Natl. Acad. Sci. USA 1989, 86, 9762–9766. [Google Scholar] [CrossRef]
- Grandy, D.K.; Litt, M.; Allen, L.; Bunzow, J.R.; Marchionni, M.; Makam, H.; Reed, L.; Magenis, R.E.; Civelli, O. The human dopamine D2 receptor gene is located on chromosome 11 at q22-q23 and identifies a TaqI RFLP. Am. J. Hum. Genet. 1989, 45, 778–785. [Google Scholar]
- Ariza, M.; Garolera, M.; Jurado, M.A.; Garcia-Garcia, I.; Hernan, I.; Sánchez-Garre, C.; Vernet-Vernet, M.; Sender-Palacios, M.J.; Marques-Iturria, I.; Pueyo, R.; et al. Dopamine genes (DRD2/ANKK1-TaqA1 and DRD4-7R) and executive function: Their interaction with obesity. PLoS ONE 2012, 7, e41482. [Google Scholar] [CrossRef]
- Berlin, I.; de Brettes, B.; Aymard, G.; Diquet, B.; Arnulf, I.; Puech, A.J. Dopaminergic drug response and the genotype (Taq IA polymorphism) of the dopamine D2 receptor. Int. J. Neuropsychopharmacol. 2000, 3, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Thomas, N.; Singleton, A.; Piggot, M.; Lloyd, S.; Perry, E.K.; Morris, C.M.; Perry, R.H.; Ferrier, I.N.; A Court, J. D2 dopamine receptor gene (DRD2) Taql A polymorphism: Reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 1997, 7, 479–484. [Google Scholar] [CrossRef]
- Suzuki, G.; Harper, K.M.; Hiramoto, T.; Sawamura, T.; Lee, M.; Kang, G.; Tanigaki, K.; Buell, M.; Geyer, M.A.; Trimble, W.S.; et al. Sept5 deficiency exerts pleiotropic influence on affective behaviors and cognitive functions in mice. Hum. Mol. Genet. 2009, 18, 1652–1660. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, H.; Wang, P.; Cui, W.; Xu, K.; Chen, D.; Hu, M.; Li, Z.; Geng, X.; Wei, S. Oxytocin and serotonin in the modulation of neural function: Neurobiological underpinnings of autism-related behavior. Front. Neurosci. 2022, 16, 919890. [Google Scholar] [CrossRef] [PubMed]
- Kelemenova, S.; Schmidtova, E.; Ficek, A.; Celec, P.; Kubranska, A.; Ostatnikova, D. Polymorphisms of candidate genes in Slovak autistic patients. Psychiatr. Genet. 2010, 20, 137–139. [Google Scholar] [CrossRef]
- Lee, H.-J.; Macbeth, A.H.; Pagani, J.H.; Young, W.S., 3rd. Oxytocin: The great facilitator of life. Prog. Neurobiol. 2009, 88, 127–151. [Google Scholar] [CrossRef]
- Molosh, A.I.; Johnson, P.L.; Spence, J.P.; Arendt, D.; Federici, L.M.; Bernabe, C.; Janasik, S.P.; Segu, Z.M.; Khanna, R.; Goswami, C.; et al. Social learning and amygdala disruptions in Nf1 mice are rescued by blocking p21-activated kinase. Nat. Neurosci. 2014, 17, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.A.; LaSalle, J.M. Future Prospects for Epigenetics in Autism Spectrum Disorder. Mol. Diagn. Ther. 2022, 26, 569–579. [Google Scholar] [CrossRef]
- Eshraghi, A.A.; Liu, G.; Kay, S.-I.S.; Eshraghi, R.S.; Mittal, J.; Moshiree, B.; Mittal, R. Epigenetics and Autism Spectrum Disorder: Is There a Correlation? Front. Cell. Neurosci. 2018, 12, 78. [Google Scholar] [CrossRef]
- Breton, C.V.; Landon, R.; Kahn, L.G.; Enlow, M.B.; Peterson, A.K.; Bastain, T.; Braun, J.; Comstock, S.S.; Duarte, C.S.; Hipwell, A.; et al. Exploring the evidence for epigenetic regulation of environmental influences on child health across generations. Commun. Biol. 2021, 4, 769. [Google Scholar] [CrossRef]
- Golding, J.; Ellis, G.; Gregory, S.; Birmingham, K.; Iles-Caven, Y.; Rai, D.; Pembrey, M. Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Sci. Rep. 2017, 7, 46179. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, K.; Shiohama, T.; Takahashi, E. microRNA Biology on Brain Development and Neuroimaging Approach. Brain Sci. 2022, 12, 1366. [Google Scholar] [CrossRef] [PubMed]
- Vella, M.C.; Slack, F.J.C. elegans microRNAs. WormBook 2005. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.J.; Vissers, C.; Ming, G.L.; Song, H. Epigenetics and epitranscriptomics in temporal patterning of cortical neural progenitor competence. J. Cell Biol. 2018, 217, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Ma, K.; Wang, Z.J.; Hu, Z.; Matas, E.; Wei, J.; Yan, Z. Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition. Nat. Neurosci. 2018, 21, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Lintas, C. Linking genetics to epigenetics: The role of folate and folate-related pathways in neurodevelopmental disorders. Clin. Genet. 2019, 95, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Mao, X.; Zhu, C.; Zou, X.; Peng, F.; Yang, W.; Li, B.; Li, G.; Ge, T.; Cui, R. GABAergic System Dysfunction in Autism Spectrum Disorders. Front. Cell Dev. Biol. 2022, 9, 781327. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.J.; Hannan, A.J.; Craig, J.M. The Role of Epigenetic Change in Autism Spectrum Disorders. Front. Neurol. 2015, 6, 107. [Google Scholar] [CrossRef]
- Yip, J.; Soghomonian, J.J.; Blatt, G.J. Decreased GAD65 mRNA levels in select subpopulations of neurons in the cerebellar dentate nuclei in autism: An in situ hybridization study. Autism Res. 2009, 2, 50–59. [Google Scholar] [CrossRef]
- Yip, J.; Soghomonian, J.J.; Blatt, G.J. IncreasedGAD67 mRNA expression in cerebellar interneurons in autism: Implications for Purkinje cell dysfunction. J. Neurosci. Res. 2008, 86, 525–530. [Google Scholar] [CrossRef]
- Ginsberg, M.R.; Rubin, R.A.; Falcone, T.; Ting, A.H.; Natowicz, M.R. Brain Transcriptional and Epigenetic Associations with Autism. PLoS ONE 2012, 7, e44736. [Google Scholar] [CrossRef]
- Wang, W.; Kwon, E.J.; Tsai, L.-H. MicroRNAs in learning, memory, and neurological diseases. Learn. Mem. 2012, 19, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Havranek, T.; Bacova, Z.; Bakos, J. Oxytocin, GABA, and dopamine interplay in autism. Endocr. Regul. 2024, 58, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bukatova, S.; Renczes, E.; Reichova, A.; Filo, J.; Sadlonova, A.; Mravec, B.; Ostatnikova, D.; Bakos, J.; Bacova, Z. Shank3 Deficiency is Associated With Altered Profile of Neurotransmission Markers in Pups and Adult Mice. Neurochem. Res. 2021, 46, 3342–3355. [Google Scholar] [CrossRef]
- Yoo, T.; Cho, H.; Lee, J.; Park, H.; Yoo, Y.-E.; Yang, E.; Kim, J.Y.; Kim, H.; Kim, E. GABA Neuronal Deletion of Shank3 Exons 14–16 in Mice Suppresses Striatal Excitatory Synaptic Input and Induces Social and Locomotor Abnormalities. Front. Cell. Neurosci. 2018, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; De Tullio, A.; Iovino, M.; Disoteo, O.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. Dopamine in the Regulation of Glucose Homeostasis, Pathogenesis of Type 2 Diabetes, and Chronic Conditions of Impaired Dopamine Activity/Metabolism: Implication for Pathophysiological and Therapeutic Purposes. Biomedicines 2023, 11, 2993. [Google Scholar] [CrossRef] [PubMed]
- Poppi, L.A.; Ho-Nguyen, K.T.; Shi, A.; Daut, C.T.; Tischfield, M.A. Recurrent Implication of Striatal Cholinergic Interneurons in a Range of Neurodevelopmental, Neurodegenerative, and Neuropsychiatric Disorders. Cells 2021, 10, 907. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.C.; Shin, J.H.; Alvarez, V.A. Striatal Cholinergic Interneurons Are a Novel Target of Corticotropin Releasing Factor. J. Neurosci. 2019, 39, 5647–5661. [Google Scholar] [CrossRef]
- Le Merrer, J.; Detraux, B.; Gandía, J.; De Groote, A.; Fonteneau, M.; D’exaerde, A.d.K.; Becker, J.A. Balance Between Projecting Neuronal Populations of the Nucleus Accumbens Controls Social Behavior in Mice. Biol. Psychiatry 2024, 95, 123–135. [Google Scholar] [CrossRef]
- Schmidt, S.N.L.; Fenske, S.C.; Kirsch, P.; Mier, D. Nucleus accumbens activation is linked to salience in social decision making. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Francis, T.C.; Chandra, R.; Friend, D.M.; Finkel, E.; Dayrit, G.; Miranda, J.; Brooks, J.M.; Iñiguez, S.D.; O’donnell, P.; Kravitz, A.; et al. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol. Psychiatry 2015, 77, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Walle, R.; Petitbon, A.; Fois, G.R.; Varin, C.; Montalban, E.; Hardt, L.; Contini, A.; Angelo, M.F.; Potier, M.; Ortole, R.; et al. Nucleus accumbens D1- and D2-expressing neurons control the balance between feeding and activity-mediated energy expenditure. Nat. Commun. 2024, 15, 2543. [Google Scholar] [CrossRef] [PubMed]
- Bouarab, C.; Thompson, B.; Polter, A.M. VTA GABA Neurons at the Interface of Stress and Reward. Front. Neural Circuits 2019, 13, 78. [Google Scholar] [CrossRef]
- Sonne, J.; Reddy, V.; Beato, M.R. Neuroanatomy, Substantia Nigra. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Li, C.; Liu, S.; Lu, X.; Tao, F. Role of Descending Dopaminergic Pathways in Pain Modulation. Curr. Neuropharmacol. 2019, 17, 1176–1182. [Google Scholar] [CrossRef]
- Deperrois, N.; Moiseeva, V.; Gutkin, B. Minimal Circuit Model of Reward Prediction Error Computations and Effects of Nicotinic Modulations. Front. Neural Circuits 2019, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Loonen, A.J.M.; Ivanova, S.A. New insights into the mechanism of drug-induced dyskinesia. CNS Spectrums 2013, 18, 15–20. [Google Scholar] [CrossRef]
- Zinger, A.; Barcia, C.; Herrero, M.T.; Guillemin, G.J. The Involvement of Neuroinflammation and Kynurenine Pathway in Parkinson′ s Disease. Park. Dis. 2011, 2011, 716859. [Google Scholar]
- Grillner, S.; Robertson, B. The Basal Ganglia Over 500 Million Years. Curr. Biol. 2016, 26, R1088–R1100. [Google Scholar] [CrossRef]
- Pretegiani, E.; Optican, L.M. Eye Movements in Parkinson’s Disease and Inherited Parkinsonian Syndromes. Front. Neurol. 2017, 8, 592. [Google Scholar] [CrossRef]
- Partanen, J.; Achim, K. Neurons gating behavior—Developmental, molecular and functional features of neurons in the Substantia Nigra pars reticulata. Front. Neurosci. 2022, 16, 976209. [Google Scholar] [CrossRef]
- Walter, B.L.; Shaikh, A.G. Encyclopedia of the Neurological Sciences, 2nd ed.; Academic Press: Waltham, MA, USA, 2014; pp. 28–33. [Google Scholar]
- Galtieri, D.J.; Estep, C.M.; Wokosin, D.L.; Traynelis, S.; Surmeier, D.J. Pedunculopontine glutamatergic neurons control spike patterning in substantia nigra dopaminergic neurons. eLife 2017, 6, e30352. [Google Scholar] [CrossRef] [PubMed]
- Futami, T.; Takakusaki, K.; Kitai, S. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci. Res. 1995, 21, 331–342. [Google Scholar] [CrossRef]
- Collins, A.L.; Saunders, B.T. Heterogeneity in striatal dopamine circuits: Form and function in dynamic reward seeking. J. Neurosci. Res. 2020, 98, 1046–1069. [Google Scholar] [CrossRef] [PubMed]
- Skirzewski, M.; Princz-Lebel, O.; German-Castelan, L.; Crooks, A.M.; Kim, G.K.; Tarnow, S.H.; Reichelt, A.; Memar, S.; Palmer, D.; Li, Y.; et al. Continuous cholinergic-dopaminergic updating in the nucleus accumbens underlies approaches to reward-predicting cues. Nat. Commun. 2022, 13, 7924. [Google Scholar] [CrossRef]
- Suzuki, E.; Momiyama, T. M1 muscarinic acetylcholine receptor-mediated inhibition of GABA release from striatal medium spiny neurons onto cholinergic interneurons. Eur. J. Neurosci. 2021, 53, 796–813. [Google Scholar] [CrossRef]
- Myslivecek, J. Two Players in the Field: Hierarchical Model of Interaction between the Dopamine and Acetylcholine Signaling Systems in the Striatum. Biomedicines 2021, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Soares-Cunha, C.; Coimbra, B.; David-Pereira, A.; Borges, S.; Pinto, L.; Costa, P.; Sousa, N.; Rodrigues, A.J. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun. 2016, 7, 11829. [Google Scholar] [CrossRef]
- Gittis, A.H.; Kreitzer, A.C. Striatal microcircuitry and movement disorders. Trends Neurosci. 2012, 35, 557–564. [Google Scholar] [CrossRef]
- Gagnon, D.; Petryszyn, S.; Sanchez, M.G.; Bories, C.; Beaulieu, J.M.; De Koninck, Y.; Parent, A.; Parent, M. Striatal Neurons Expressing D1 and D2 Receptors are Morphologically Distinct and Differently Affected by Dopamine Denervation in Mice. Sci. Rep. 2017, 7, 41432. [Google Scholar] [CrossRef]
- Haber, S.N. The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 2014, 282, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef]
- Burton, A.C.; Nakamura, K.; Roesch, M.R. From ventral-medial to dorsal-lateral striatum: Neural correlates of reward-guided decision-making. Neurobiol. Learn. Mem. 2015, 117, 51–59. [Google Scholar] [CrossRef]
- Lipton, D.M.; Gonzales, B.J.; Citri, A. Dorsal Striatal Circuits for Habits, Compulsions and Addictions. Front. Syst. Neurosci. 2019, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.G.; Jang, S.H. Differences in neural connectivity between the substantia nigra and ventral tegmental area in the human brain. Front. Hum. Neurosci. 2014, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Martig, A.K.; Mizumori, S.J.Y. Ventral tegmental area and substantia nigra neural correlates of spatial learning. Learn. Mem. 2011, 18, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; McKenna, J.T. Turning a Negative into a Positive: Ascending GABAergic Control of Cortical Activation and Arousal. Front. Neurol. 2015, 6, 135. [Google Scholar] [CrossRef]
- Vadakkan, K.I. Framework for internal sensation of pleasure using constraints from disparate findings in nucleus accumbens. World J. Psychiatry 2021, 11, 681–695. [Google Scholar] [CrossRef]
- Li, A.; Zalesky, A.; Yue, W.; Howes, O.; Yan, H.; Liu, Y.; Fan, L.; Whitaker, K.J.; Xu, K.; Rao, G.; et al. A neuroimaging biomarker for striatal dysfunction in schizophrenia. Nat. Med. 2020, 26, 558–565. [Google Scholar] [CrossRef]
- Kosillo, P.; Bateup, H.S. Dopaminergic Dysregulation in Syndromic Autism Spectrum Disorders: Insights From Genetic Mouse Models. Front. Neural Circuits 2021, 15, 700968. [Google Scholar] [CrossRef]
- Howard, C.D.; Li, H.; Geddes, C.E.; Jin, X. Dynamic Nigrostriatal Dopamine Biases Action Selection. Neuron 2017, 93, 1436–1450.e8. [Google Scholar] [CrossRef] [PubMed]
- Watabe-Uchida, M.; Uchida, N. Multiple Dopamine Systems: Weal and Woe of Dopamine. Cold Spring Harb. Symp. Quant. Biol. 2018, 83, 83–95. [Google Scholar] [CrossRef] [PubMed]
- de Jong, J.W.; Afjei, S.A.; Dorocic, I.P.; Peck, J.R.; Liu, C.; Kim, C.K.; Tian, L.; Deisseroth, K.; Lammel, S. A Neural Circuit Mechanism for Encoding Aversive Stimuli in the Mesolimbic Dopamine System. Neuron 2019, 101, 133–151.e7. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.A.; Pettibone, J.R.; Mabrouk, O.S.; Hetrick, V.L.; Schmidt, R.; Weele, C.M.V.; Kennedy, R.T.; Aragona, B.J.; Berke, J.D. Mesolimbic dopamine signals the value of work. Nat. Neurosci. 2016, 19, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Mohebi, A.; Pettibone, J.R.; Hamid, A.A.; Wong, J.M.T.; Vinson, L.T.; Patriarchi, T.; Tian, L.; Kennedy, R.T.; Berke, J.D. Dissociable dopamine dynamics for learning and motivation. Nature 2019, 570, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.R.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Penzes, P. Common Mechanisms of Excitatory and Inhibitory Imbalance in Schizophrenia and Autism Spectrum Disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef] [PubMed]
- Bruining, H.; Hardstone, R.; Juarez-Martinez, E.L.; Sprengers, J.; Avramiea, A.-E.; Simpraga, S.; Houtman, S.J.; Poil, S.-S.; Dallares, E.; Palva, S.; et al. Measurement of excitation-inhibition ratio in autism spectrum disorder using critical brain dynamics. Sci. Rep. 2020, 10, 9195. [Google Scholar] [CrossRef] [PubMed]
- Stavropoulos, K.K.-M.; Carver, L.J. Oscillatory rhythm of reward: Anticipation and processing of rewards in children with and without autism. Mol. Autism 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Siegel-Ramsay, J.E.; Romaniuk, L.; Whalley, H.C.; Roberts, N.; Branigan, H.; Stanfield, A.C.; Lawrie, S.M.; Dauvermann, M.R. Glutamate and functional connectivity—support for the excitatory-inhibitory imbalance hypothesis in autism spectrum disorders. Psychiatry Res. Neuroimaging 2021, 313, 111302. [Google Scholar] [CrossRef]
- Smolders, I.; Bogaert, L.; Ebinger, G.; Michotte, Y. Muscarinic modulation of striatal dopamine, glutamate, and GABA release, as measured with in vivo microdialysis. J. Neurochem. 1997, 68, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Antal, M.; Beneduce, B.M.; Regehr, W.G. The substantia nigra conveys target-dependent excitatory and inhibitory outputs from the basal ganglia to the thalamus. J. Neurosci. 2014, 34, 8032–8042. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Roles for nigrostriatal—Not just mesocorticolimbic—Dopamine in reward and addiction. Trends Neurosci. 2009, 32, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Antoine, M.W.; Langberg, T.; Schnepel, P.; Feldman, D.E. Increased Excitation-Inhibition Ratio Stabilizes Synapse and Circuit Excitability in Four Autism Mouse Models. Neuron 2019, 101, 648–661.e4. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.X.; Huang, E.J. Dopaminergic Neurons and Brain Reward Pathways. Am. J. Pathol. 2016, 186, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.; Nonomura, S.; Kato, S.; Yoshida, J.; Matsushita, N.; Nambu, A.; Takada, M.; Hira, R.; Kobayashi, K.; Sakai, Y.; et al. Reward expectation enhances action-related activity of nigral dopaminergic and two striatal output pathways. Commun. Biol. 2023, 6, 914. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.P.F.; Malgady, J.M.; Chen, L.; Shi, K.W.; Cheng, E.; Plotkin, J.L.; Ge, S.; Xiong, Q. Nigrostriatal dopamine pathway regulates auditory discrimination behavior. Nat. Commun. 2022, 13, 5942. [Google Scholar] [CrossRef] [PubMed]
- Hollon, N.G.; Williams, E.W.; Howard, C.D.; Li, H.; Traut, T.I.; Jin, X. Nigrostriatal dopamine signals sequence-specific action-outcome prediction errors. Curr. Biol. 2021, 31, 5350–5363.e5. [Google Scholar] [CrossRef] [PubMed]
- Maestro, S.; Muratori, F.; Cavallaro, M.C.; Pei, F.; Stern, D.; Golse, B.; Palacio-Espasa, F. Attentional skills during the first 6 months of age in autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 1239–1245. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M. The Mysterious Motivational Functions of Mesolimbic Dopamine. Neuron 2012, 76, 470–485. [Google Scholar] [CrossRef]
- Mönnich, D.; Humphrys, L.J.; Höring, C.; Hoare, B.L.; Forster, L.; Pockes, S. Activation of Multiple G Protein Pathways to Characterize the Five Dopamine Receptor Subtypes Using Bioluminescence Technology. ACS Pharmacol. Transl. Sci. 2024, 7, 834–854. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Singh, S.; Shukla, S. Physiological and Functional Basis of Dopamine Receptors and Their Role in Neurogenesis: Possible Implication for Parkinson’s disease. J. Exp. Neurosci. 2018, 12, 1179069518779829. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, E.V.; Gainetdinov, R.R.; Gurevich, V.V. G protein-coupled receptor kinases as regulators of dopamine receptor functions. Pharmacol. Res. 2016, 111, 1–16. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, I.; Suárez-Pereira, I.; Santiago, M.; Pérez-Villegas, E.M.; Bravo, L.; López-Martín, C.; Roca-Ceballos, M.A.; García-Revilla, J.; Espinosa-Oliva, A.M.; Rodríguez-Gómez, J.A.; et al. Selective deletion of Caspase-3 gene in the dopaminergic system exhibits autistic-like behaviour. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2021, 104, 110030. [Google Scholar] [CrossRef]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [PubMed]
- South, M.; Rodgers, J.; Van Hecke, A. Anxiety and ASD: Current Progress and Ongoing Challenges. J. Autism Dev. Disord. 2017, 47, 3679–3681. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.L.; Liu, T.T.; Vollbrecht, M.S.; Mansour, M.H.; Nikodijevic, I.; Jadav, N.; Patibanda, N.; Dang, J.; Shekaran, G.; Reisler, R.C.; et al. Engrailed 2 deficiency and chronic stress alter avoidance and motivation behaviors. Behav. Brain Res. 2021, 413, 113466. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.M.; Palmiter, R.D. Reward without Dopamine. J. Neurosci. 2003, 23, 10827–10831. [Google Scholar] [CrossRef]
- Robertson, C.E.; Ratai, E.-M.; Kanwisher, N. Reduced GABAergic Action in the Autistic Brain. Curr. Biol. 2016, 26, 80–85. [Google Scholar] [CrossRef]
- Chao, H.-T.; Chen, H.; Samaco, R.C.; Xue, M.; Chahrour, M.; Yoo, J.; Gong, S.; Lu, H.-C.; Heintz, N.; Ekker, M.; et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 2010, 468, 263–269. [Google Scholar] [CrossRef]
- Ford, T.C.; Nibbs, R.; Crewther, D.P. Glutamate/GABA+ ratio is associated with the psychosocial domain of autistic and schizotypal traits. PLoS ONE 2017, 12, e0181961. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D.; Rustan, O.G.; Rooney, R.J.; Thuras, P.D. Downregulation of GABAA receptor protein subunits α6, β2, δ, ε, γ2, θ, and ρ2 in superior frontal cortex of subjects with autism. J. Autism Dev. Disord. 2014, 44, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Shinohe, A.; Hashimoto, K.; Nakamura, K.; Tsujii, M.; Iwata, Y.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Suzuki, K.; Sugihara, G.-I.; et al. Increased serum levels of glutamate in adult patients with autism. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2006, 30, 1472–1477. [Google Scholar] [CrossRef]
- Said, C.P.; Egan, R.D.; Minshew, N.J.; Behrmann, M.; Heeger, D.J. Normal binocular rivalry in autism: Implications for the excitation/inhibition imbalance hypothesis. Vis. Res. 2013, 77, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Chen, Q.; van der Goes, M.-S.; Hawrot, J.; Yao, A.Y.; Gao, X.; Lu, C.; Zang, Y.; Zhang, Q.; et al. Striatopallidal dysfunction underlies repetitive behavior in Shank3-deficient model of autism. J. Clin. Investig. 2017, 127, 1978–1990. [Google Scholar] [CrossRef] [PubMed]
- Sharp, B.M. Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl. Psychiatry 2017, 7, e1194. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.G.; Schumann, C.M.; Nordahl, C.W. Neuroanatomy of autism. Trends Neurosci. 2008, 31, 137–145. [Google Scholar] [CrossRef]
- Andersson, M.; Tangen, Ä.; Farde, L.; Bölte, S.; Halldin, C.; Borg, J.; Lundberg, J. Serotonin transporter availability in adults with autism—A positron emission tomography study. Mol. Psychiatry 2021, 26, 1647–1658. [Google Scholar] [CrossRef]
- Schain, R.J.; Freedman, D.X. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J. Pediatr. 1961, 58, 315–320. [Google Scholar] [CrossRef]
- Dölen, G.; Darvishzadeh, A.; Huang, K.W.; Malenka, R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013, 501, 179–184. [Google Scholar] [CrossRef]
- Cavalcante, L.E.; Zinn, C.G.; Schmidt, S.D.; Saenger, B.F.; Ferreira, F.F.; Furini, C.R.; Myskiw, J.C.; Izquierdo, I. Modulation of the storage of social recognition memory by neurotransmitter systems in the insular cortex. Behav. Brain Res. 2017, 334, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Barkus, E.; Badcock, J.C. A Transdiagnostic Perspective on Social Anhedonia. Front. Psychiatry 2019, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Dackis, C.A.; Gold, M.S. New concepts in cocaine addiction: The dopamine depletion hypothesis. Neurosci. Biobehav. Rev. 1985, 9, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Gold, M.S.; Febo, M.; Baron, D.; Modestino, E.J.; Elman, I.; Badgaiyan, R.D. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome RDS and anti- reward systems. Front. Biosci. 2018, 10, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.R.; Groome, A.; Taniguchi, L.; Eshel, N.; Bentzley, B.S. The role of dopamine in reward-related behavior: Shining new light on an old debate. J. Neurophysiol. 2020, 124, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Gardner, E.; Oscar-Berman, M.; Gold, M. “Liking” and “Wanting” Linked to Reward Deficiency Syndrome (RDS): Hypothesizing Differential Responsivity in Brain Reward Circuitry. Curr. Pharm. Des. 2012, 18, 113–118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bachevalier, J.; Loveland, K.A. The orbitofrontal–amygdala circuit and self-regulation of social–emotional behavior in autism. Neurosci. Biobehav. Rev. 2006, 30, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Zeeland, A.A.S.; Dapretto, M.; Ghahremani, D.G.; Poldrack, R.A.; Bookheimer, S.Y. Reward processing in autism. Autism Res. 2010, 3, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Masten, C.L.; Colich, N.L.; Rudie, J.D.; Bookheimer, S.Y.; Eisenberger, N.I.; Dapretto, M. An fMRI investigation of responses to peer rejection in adolescents with autism spectrum disorders. Dev. Cogn. Neurosci. 2011, 1, 260–270. [Google Scholar] [CrossRef]
- Brandenburg, C.; Soghomonian, J.-J.; Zhang, K.; Sulkaj, I.; Randolph, B.; Kachadoorian, M.; Blatt, G.J. Increased Dopamine Type 2 Gene Expression in the Dorsal Striatum in Individuals With Autism Spectrum Disorder Suggests Alterations in Indirect Pathway Signaling and Circuitry. Front. Cell. Neurosci. 2020, 14, 577858. [Google Scholar] [CrossRef]
- Gerfen, C.R. Segregation of D1 and D2 dopamine receptors in the striatal direct and indirect pathways: An historical perspective. Front. Synaptic Neurosci. 2023, 14, 1002960. [Google Scholar] [CrossRef] [PubMed]
- Soghomonian, J.J. The cortico-striatal circuitry in autism-spectrum disorders: A balancing act. Front. Cell. Neurosci. 2024, 17, 1329095. [Google Scholar] [CrossRef] [PubMed]
- Girgis, R.R.; Slifstein, M.; D’souza, D.; Lee, Y.; Periclou, A.; Ghahramani, P.; Laszlovszky, I.; Durgam, S.; Adham, N.; Nabulsi, N.; et al. Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [11C]-(+)-PHNO. Psychopharmacology 2016, 233, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Kohls, G.; Chevallier, C.; Troiani, V.; Schultz, R.T. Social ‘wanting’ dysfunction in autism: Neurobiological underpinnings and treatment implications. J. Neurodev. Disord. 2012, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Mano-Sousa, B.J.; Pedrosa, A.M.; Alves, B.C.; Galduróz, J.C.F.; Belo, V.S.; Chaves, V.E.; Duarte-Almeida, J.M. Effects of Risperidone in Autistic Children and Young Adults: A Systematic Review and Meta-Analysis. Curr. Neuropharmacol. 2021, 19, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Appiah-Kubi, P.; Olotu, F.A.; Soliman, M.E.S. Exploring the structural basis and atomistic binding mechanistic of the selective antagonist blockade at D3 dopamine receptor over D2 dopamine receptor. J. Mol. Recognit. 2021, 34, e2885. [Google Scholar] [CrossRef] [PubMed]
- Fung, L.K.; Mahajan, R.; Nozzolillo, A.; Bernal, P.; Krasner, A.; Jo, B.; Coury, D.; Whitaker, A.; Veenstra-Vanderweele, J.; Hardan, A.Y. Pharmacologic Treatment of Severe Irritability and Problem Behaviors in Autism: A Systematic Review and Meta-analysis. Pediatrics 2016, 137 (Suppl. S2), S124–S135. [Google Scholar] [CrossRef] [PubMed]
- Hermans, R.A.; Sassen, S.D.T.; Kloosterboer, S.M.; Reichart, C.G.; Kouijzer, M.E.J.; de Kroon, M.M.J.; Bastiaansen, D.; van Altena, D.; van Schaik, R.H.N.; Nasserinejad, K.; et al. Towards precision dosing of aripiprazole in children and adolescents with autism spectrum disorder: Linking blood levels to weight gain and effectiveness. Br. J. Clin. Pharmacol. 2023, 89, 3026–3036. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Bonnot, O.; Bodeau, N.; Consoli, A.; Laurent, C. Adverse effects of second-generation antipsychotics in children and adolescents: A Bayesian meta-analysis. J. Clin. Psychopharmacol. 2012, 32, 309–316. [Google Scholar] [CrossRef]
- Lee, A.; Choo, H.; Jeon, B. Serotonin Receptors as Therapeutic Targets for Autism Spectrum Disorder Treatment. Int. J. Mol. Sci. 2022, 23, 6515. [Google Scholar] [CrossRef]
- Eissa, N.; Venkatachalam, K.; Jayaprakash, P.; Yuvaraju, P.; Falkenstein, M.; Stark, H.; Sadek, B. Experimental Studies Indicate That ST-2223, the Antagonist of Histamine H3 and Dopamine D2/D3 Receptors, Restores Social Deficits and Neurotransmission Dysregulation in Mouse Model of Autism. Pharmaceuticals 2022, 15, 929. [Google Scholar] [CrossRef] [PubMed]
- Cosi, C.; Martel, J.-C.; Auclair, A.L.; Collo, G.; Cavalleri, L.; Heusler, P.; Leriche, L.; Gaudoux, F.; Sokoloff, P.; Moser, P.C.; et al. Pharmacology profile of F17464, a dopamine D3 receptor preferential antagonist. Eur. J. Pharmacol. 2021, 890, 173635. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Eissa, N.; Al Awad, M.; Jayaprakash, P.; Zhong, S.; Stölting, F.; Stark, H.; Sadek, B. The histamine H3R and dopamine D2R/D3R antagonist ST-713 ameliorates autism-like behavioral features in BTBR T+tf/J mice by multiple actions. Biomed. Pharmacother. 2021, 138, 111517. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.M.; Hill-Yardin, E.L.; Crack, P.J. The influence of neuroinflammation in Autism Spectrum Disorder. Brain Behav. Immun. 2019, 79, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Tamouza, R.; Volt, F.; Richard, J.-R.; Wu, C.-L.; Bouassida, J.; Boukouaci, W.; Lansiaux, P.; Cappelli, B.; Scigliuolo, G.M.; Rafii, H.; et al. Possible Effect of the use of Mesenchymal Stromal Cells in the Treatment of Autism Spectrum Disorders: A Review. Front. Cell Dev. Biol. 2022, 10, 809686. [Google Scholar] [CrossRef] [PubMed]
- Jembrek, M.; Vlainic, J. GABA Receptors: Pharmacological Potential and Pitfalls. Curr. Pharm. Des. 2015, 21, 4943–4959. [Google Scholar] [CrossRef] [PubMed]
- Silverman, J.L.; Pride, M.C.; Hayes, J.E.; Puhger, K.R.; Butler-Struben, H.M.; Baker, S.; Crawley, J.N. GABAB Receptor Agonist R-Baclofen Reverses Social Deficits and Reduces Repetitive Behavior in Two Mouse Models of Autism. Neuropsychopharmacology 2015, 40, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Cogram, P.; Deacon, R.M.J.; Warner-Schmidt, J.L.; von Schimmelmann, M.J.; Abrahams, B.S.; During, M.J. Gaboxadol Normalizes Behavioral Abnormalities in a Mouse Model of Fragile X Syndrome. Front. Behav. Neurosci. 2019, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.M.; Stein-O’brien, G.L.; Patino, A.V.; Nardou, R.; Grossman, C.D.; Brown, M.; Bangamwabo, B.; Ndiaye, N.; Giovinazzo, D.; Dardani, I.; et al. Parallel Social Information Processing Circuits Are Differentially Impacted in Autism. Neuron 2020, 108, 659–675.e6. [Google Scholar] [CrossRef]
- Pavăl, D.; Micluția, I.V. The The dopamine hypothesis of autism spectrum disorder revisited: Current status and future prospects. Dev. Neurosci. 2021, 43, 73–83. [Google Scholar] [CrossRef]
- Poulin, J.-F.; Gaertner, Z.; Moreno-Ramos, O.A.; Awatramani, R. Classification of Midbrain Dopamine Neurons Using Single-Cell Gene Expression Profiling Approaches. Trends Neurosci. 2020, 43, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Poulin, J.-F.; Zou, J.; Drouin-Ouellet, J.; Kim, K.-Y.A.; Cicchetti, F.; Awatramani, R.B. Defining midbrain dopaminergic neuron diversity by single-cell gene expression profiling. Cell Rep. 2014, 9, 930–943. [Google Scholar] [CrossRef] [PubMed]
- La Manno, G.; Gyllborg, D.; Codeluppi, S.; Nishimura, K.; Salto, C.; Zeisel, A.; Borm, L.E.; Stott, S.R.; Toledo, E.M.; Villaescusa, J.C.; et al. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell 2016, 167, 566–580.e19. [Google Scholar] [CrossRef] [PubMed]
- Hook, P.W.; McClymont, S.A.; Cannon, G.H.; Law, W.D.; Morton, A.J.; Goff, L.A.; McCallion, A.S. Single-Cell RNA-Seq of Mouse Dopaminergic Neurons Informs Candidate Gene Selection for Sporadic Parkinson Disease. Am. J. Hum. Genet. 2018, 102, 427–446. [Google Scholar] [CrossRef] [PubMed]
- Kramer, D.J.; Risso, D.; Kosillo, P.; Ngai, J.; Bateup, H.S. Combinatorial Expression of Grp and Neurod6 Defines Dopamine Neuron Populations with Distinct Projection Patterns and Disease Vulnerability. eneuro 2018, 5, ENEURO.0152-18.2018. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.; Macosko, E.Z.; Wysoker, A.; Goldman, M.; Krienen, F.M.; de Rivera, H.; Bien, E.; Baum, M.; Bortolin, L.; Wang, S.; et al. Molecular Diversity and Specializations among the Cells of the Adult Mouse Brain. Cell 2018, 174, 1015–1030.e16. [Google Scholar] [CrossRef] [PubMed]
- Tiklová, K.; Björklund, Å.K.; Lahti, L.; Fiorenzano, A.; Nolbrant, S.; Gillberg, L.; Volakakis, N.; Yokota, C.; Hilscher, M.M.; Hauling, T.; et al. Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat. Commun. 2019, 10, 581. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Modestino, E.J.; Gondre-Lewis, M.; Chapman, E.J.; Neary, J.; Siwicki, D.; Baron, D.; Hauser, M.; Smith, D.E.; Roy, A.K.; et al. The Benefits of Genetic Addiction Risk Score (GARS™) Testing in Substance Use Disorder (SUD). Int. J. Genom. Data Min. 2018, 2018, 115. [Google Scholar] [CrossRef] [PubMed]
- Blum, K.; Bowirrat, A.; Elman, I.; Baron, D.; Thanos, P.K.; Gold, M.S.; Hanna, C.; Makale, M.T.; Sunder, K.; Jafari, N.; et al. Evidence for the DRD2 Gene as a Determinant of Reward Deficiency Syndrome (RDS). Clin. Exp. Psychol. 2023, 9, 8–11. [Google Scholar]
- Dennen, C.A.; Blum, K.; Bowirrat, A.; Thanos, P.K.; Elman, I.; Ceccanti, M.; Badgaiyan, R.D.; McLaughlin, T.; Gupta, A.; Bajaj, A.; et al. Genetic Addiction Risk Severity Assessment Identifies Polymorphic Reward Genes as Antecedents to Reward Deficiency Syndrome (RDS) Hypodopaminergia’s Effect on Addictive and Non-Addictive Behaviors in a Nuclear Family. J. Pers. Med. 2022, 12, 1864. [Google Scholar] [CrossRef]
- Jensen, A.R.; Lane, A.L.; Werner, B.A.; McLees, S.E.; Fletcher, T.S.; Frye, R.E. Modern Biomarkers for Autism Spectrum Disorder: Future Directions. Mol. Diagn. Ther. 2022, 26, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Santarone, M.E.; Zambrano, S.; Zanotta, N.; Mani, E.; Minghetti, S.; Pozzi, M.; Villa, L.; Molteni, M.; Zucca, C. EEG Features in Autism Spectrum Disorder: A Retrospective Analysis in a Cohort of Preschool Children. Brain Sci. 2023, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.J.; Keegan, L.; Tager-Flusberg, H.; Gill, S.V. Neuroimaging Techniques as Descriptive and Diagnostic Tools for Infants at Risk for Autism Spectrum Disorder: A Systematic Review. Brain Sci. 2022, 12, 602. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blum, K.; Bowirrat, A.; Sunder, K.; Thanos, P.K.; Hanna, C.; Gold, M.S.; Dennen, C.A.; Elman, I.; Murphy, K.T.; Makale, M.T. Dopamine Dysregulation in Reward and Autism Spectrum Disorder. Brain Sci. 2024, 14, 733. https://doi.org/10.3390/brainsci14070733
Blum K, Bowirrat A, Sunder K, Thanos PK, Hanna C, Gold MS, Dennen CA, Elman I, Murphy KT, Makale MT. Dopamine Dysregulation in Reward and Autism Spectrum Disorder. Brain Sciences. 2024; 14(7):733. https://doi.org/10.3390/brainsci14070733
Chicago/Turabian StyleBlum, Kenneth, Abdalla Bowirrat, Keerthy Sunder, Panayotis K. Thanos, Colin Hanna, Mark S. Gold, Catherine A. Dennen, Igor Elman, Kevin T. Murphy, and Milan T. Makale. 2024. "Dopamine Dysregulation in Reward and Autism Spectrum Disorder" Brain Sciences 14, no. 7: 733. https://doi.org/10.3390/brainsci14070733





