Identifying the Shared and Dissociable Neural Bases between Self-Worth and Moral Ambivalence
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. SAM Scale
2.3. RS-fMRI Data Acquisition
2.4. Data Preprocessing
2.4.1. fALFF–Behavior Correlation Analyses
2.4.2. RSFC–Behavior Correlation Analyses
3. Results
3.1. Behavior Data
3.2. Brain Regions and Functional Connectivity Related to Self-Worth Ambivalence
3.3. Brain Regions and Functional Connectivity Related to Moral Ambivalence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhar, S.S.; Kyrios, M. An investigation of self-ambivalence in obsessive-compulsive disorder. Behav. Res. Ther. 2007, 45, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Tisher, R.; Allen, J.S.; Crouch, W. The self-ambivalence measure: A psychometric investigation. Aust. J. Psychol. 2014, 66, 197–206. [Google Scholar] [CrossRef]
- Damiani, S.; Fusar-Poli, L.; Brondino, N.; Provenzani, U.; Baldwin, H.; Fusar-Poli, P.; Politi, P. World/self ambivalence: A shared mechanism in different subsets of psychotic experiences? Linking symptoms with resting-state fMRI. Psychiatry Res. Neuroimaging 2020, 299, 111068. [Google Scholar] [CrossRef] [PubMed]
- Bhar, S.S.; Kyrios, M.; Hordern, C. Self-Ambivalence in the Cognitive-Behavioural Treatment of Obsessive-Compulsive Disorder. Psychopathology 2015, 48, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Krause, R.J.; Rucker, D.D. Can Bad Be Good? The Attraction of a Darker Self. Psychol. Sci. 2020, 31, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, J.; Zeng, M.; Yang, J. Positive-Negative Asymmetry in Self-Related Processing. J. Individ. Differ. 2022, 43, 180–187. [Google Scholar] [CrossRef]
- Takano, K.; Iijima, Y.; Sakamoto, S.; Raes, F.; Tanno, Y. Is self-positive information more appealing than money? Individual differences in positivity bias according to depressive symptoms. Cogn. Emot. 2016, 30, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Bhar, S.S. Self-Ambivalence in Obsessive–Compulsive Disorder. Ph.D. Thesis, The University of Melbourne, Melbourne, Australia, 2004. [Google Scholar]
- Li, J.; Wang, S.; Zhao, X.; Yang, Y.; Cui, X.; Yang, J. A double-edged sword: Sub-dimensions of self-ambivalence play distinct roles in predicting mental health distinct roles in predicting mental health. 2024; submiss. [Google Scholar]
- Godwin, T.L.; Godwin, H.J.; Simonds, L.M. What is the relationship between fear of self, self-ambivalence, and obsessive–compulsive symptomatology? A systematic literature review. Clin. Psychol. Psychother. 2020, 27, 887–901. [Google Scholar] [CrossRef] [PubMed]
- Alicke, M.D.; Sedikides, C. Self-enhancement and self-protection: What they are and what they do. Eur. Rev. Soc. Psychol. 2009, 20, 1–48. [Google Scholar] [CrossRef]
- Seah, R.; Fassnacht, D.; Kyrios, M. Attachment anxiety and self-ambivalence as vulnerabilities toward Obsessive Compulsive Disorder. J. Obsessive. Compuls. Relat. Disord. 2018, 18, 40–46. [Google Scholar] [CrossRef]
- Ahern, C.; Kyrios, M.; Moulding, R. Self-Based Concepts and Obsessive-Compulsive Phenomena. Psychopathology 2015, 48, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Guidano, V. Complexity of the Self; Guilford Press: New York, NY, USA, 1987. [Google Scholar]
- Nohlen, H.U.; van Harreveld, F.; Rotteveel, M.; Lelieveld, G.J.; Crone, E.A. Evaluating ambivalence: Social-cognitive and affective brain regions associated with ambivalent decision-making. Soc. Cogn. Affect. Neurosci. 2014, 9, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Nohlen, H.U.; Van Harreveld, F.; Cunningham, W.A. Social evaluations under conflict: Negative judgments of conflicting information are easier than positive judgments. Soc. Cogn. Affect. Neurosci. 2019, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Park, H.J.; Jung, Y.C.; Chun, J.W.; Kim, H.S.; Seok, J.H.; Kim, N.W.; Park, I.H.; Oh, M.G.; Lee, J.D. Evaluative processing of ambivalent stimuli in patients with schizophrenia and depression: A [15O] H2O PET study. J. Int. Neuropsychol. Soc. 2009, 15, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Park, H.J.; Kim, J.J.; Chun, J.W.; Kim, H.S.; Kim, N.W.; Son, S.J.; Oh, M.G.; Lee, J.D. Reciprocal activation of the orbitofrontal cortex and the ventrolateral prefrontal cortex in processing ambivalent stimuli. Brain Res. 2008, 1246, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G.; Heinzel, A.; de Greck, M.; Bermpohl, F.; Dobrowolny, H.; Panksepp, J. Self-referential processing in our brain-A meta-analysis of imaging studies on the self. Neuroimage 2006, 31, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Liu, C.; Yang, Q.; Gu, Y.; Yin, S.; Chen, A. The neural basis of trait self-esteem revealed by the amplitude of low-frequency fluctuations and resting state functional connectivity. Soc. Cogn. Affect. Neurosci. 2016, 11, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, M.; Liu, M.; Zhao, X.; Hu, W.; Wang, C.; Deng, C.; Li, R.; Chen, H.; Yang, J. Multivariable pattern classification differentiates relational self-esteem from personal self-esteem. Soc. Cogn. Affect. Neurosci. 2021, 16, 726–735. [Google Scholar] [CrossRef]
- Jiang, K.; Wu, S.; Shi, Z.; Liu, M.; Peng, M.; Shen, Y.; Yang, J. Activations of the dorsolateral prefrontal cortex and thalamus during agentic self-evaluation are negatively associated with trait self-esteem. Brain Res. 2018, 1692, 134–141. [Google Scholar] [CrossRef]
- Aquino, K.; Americus, R. The self-importance of moral identity. J. Pers. Soc. Psychol. 2002, 83, 1423–1440. [Google Scholar] [CrossRef]
- Jennings, P.L.; Mitchell, M.S.; Hannah, S.T. The moral self: A review and integration of the literature. J. Organ. Behav. 2015, 60, 5–22. [Google Scholar] [CrossRef]
- Jordan, J.; Leliveld, M.C.; Tenbrunsel, A.E. The moral self-image scale: Measuring and understanding the malleability of the moral self. Front. Psychol. 2015, 6, 1878. [Google Scholar] [CrossRef] [PubMed]
- Frith, C.D.; Frith, U. The Neural Basis of Mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Fehlbaum, L.V.; Borbas, R.; Paul, K.; Eickhoff, S.B.; Raschle, N.M. Early and late neural correlates of mentalizing: ALE meta-analyses in adults, children and adolescents. Soc. Cogn. Affect. Neurosci. 2022, 17, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, J.; Zhao, Z.; Min, B.; Lu, J.; Li, K.; He, Y.; Jia, J. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: A resting-state fMRI study. Neuroimage 2011, 55, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Mennes, M.; Kelly, C.; Zuo, X.; Di Martino, A.; Biswal, B.; Castellanos, X.; Milham, M.P. Inter-individual differences in resting state functional connectivity. Neuroimage 2010, 50, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, S.; Dunn, J.; Li, H.; Qin, W.; Zhu, M.; Rao, L.L.; Song, M.; Yu, C.; Jiang, T. The neural correlates of risk propensity in males and females using resting-state fMRI. Front. Behav. Neurosci. 2014, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Hu, S.; Wang, X.; Song, Y.; Liu, J. Neural correlates of the happy life: The amplitude of spontaneous low frequency fluctuations predicts subjective well-being. Neuroimage 2015, 107, 136–145. [Google Scholar] [CrossRef]
- Cho, E. Making Reliability Reliable: A Systematic Approach to Reliability Coefficients. Organ. Res. Methods 2016, 19, 651–682. [Google Scholar] [CrossRef]
- Yan, C.G.; Wang, X.D.; Zuo, X.N.; Zang, Y.F. DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. Neuroinformatics 2016, 14, 339–351. [Google Scholar] [PubMed]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Yantis, S. A domain-independent source of cognitive control for task sets: Shifting spatial attention and switching categorization rules. J. Neurosci. 2009, 29, 3930–3938. [Google Scholar] [CrossRef]
- Yin, S.; Deák, G.; Chen, A. Coactivation of cognitive control networks during task switching. Neuropsychology 2018, 32, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chen, L.; Zhou, K. The role of the left posterior parietal lobule in top-down modulation on space-based attention: A transcranial magnetic stimulation study. Hum. Brain Mapp. 2012, 33, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Hohnsbehn, J.M.; Urschler, D.F.; Schneider, I.K. Torn but balanced: Trait ambivalence is negatively related to confirmation. Pers. Individ. Dif. 2022, 196, 111736. [Google Scholar] [CrossRef]
- Schneider, I.K.; Novin, S.; van Harreveld, F.; Genschow, O. Benefits of being ambivalent: The relationship between trait ambivalence and attribution biases. Br. J. Soc. Psychol. 2021, 60, 570–586. [Google Scholar] [CrossRef]
- Guarana, C.L.; Hernandez, M. Identified ambivalence: When cognitive conflicts can help individuals overcome cognitive traps. J. Appl. Psychol. 2016, 101, 1013–1029. [Google Scholar] [CrossRef]
- Rees, L.; Rothman, N.B.; Lehavy, R.; Sanchez-Burks, J. The ambivalent mind can be a wise mind: Emotional ambivalence increases judgment accuracy. J. Exp. Soc. Psychol. 2013, 49, 360–367. [Google Scholar] [CrossRef]
- Fong, C.T. The effects of emotional ambivalence on creativity. Acad. Manag. J. 2006, 49, 1016–1030. [Google Scholar] [CrossRef]
- Rothman, N.B.; Pratt, M.G.; Rees, L.; Vogus, T.J. Understanding the dual nature of ambivalence: Why and when ambivalence leads to good and bad outcomes. Acad. Manag. Ann. 2017, 11, 33–72. [Google Scholar] [CrossRef]
- Howard, J.D.; Kahnt, T. Identity-specific reward representations in orbitofrontal cortex are modulated by selective devaluation. J. Neurosci. 2017, 37, 2627–2638. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Cheng, W.; Feng, J. The orbitofrontal cortex: Reward, emotion and depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef] [PubMed]
- O’Doherty, J.; Critchley, H.; Deichmann, R.; Dolan, R.J. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J. Neurosci. 2003, 23, 7931–7939. [Google Scholar] [CrossRef]
- van Schie, C.C.; De Chiu, C.; Rombouts, S.A.R.B.; Heiser, W.J.; Elzinga, B.M. When compliments do not hit but critiques do: An fMRI study into self-esteem and self-knowledge in processing social feedback. Soc. Cogn. Affect. Neurosci. 2018, 13, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Will, G.J.; Rutledge, R.B.; Moutoussis, M.; Dolan, R.J. Neural and computational processes underlying dynamic changes in self-esteem. eLife 2017, 6, e28098. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Northoff, G. How is our self related to midline regions and the default-mode network? Neuroimage 2011, 57, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Sajonz, B.; Kahnt, T.; Margulies, D.S.; Park, S.Q.; Wittmann, A.; Stoy, M.; Ströhle, A.; Heinz, A.; Northoff, G.; Bermpohl, F. Delineating self-referential processing from episodic memory retrieval: Common and dissociable networks. Neuroimage 2010, 50, 1606–1617. [Google Scholar] [CrossRef]
- Fei, W.; Dian, C.; Jie, S. Trait dialectical thinking is associated with the strength of functional coupling between the dACC and the default mode network. Cogn. Affect. Behav. Neurosci. 2022, 22, 1021–1029. [Google Scholar] [CrossRef]
- Bzdok, D.; Schilbach, L.; Vogeley, K.; Schneider, K.; Laird, A.R.; Langner, R.; Eickhoff, S.B. Parsing the neural correlates of moral cognition: ALE meta-analysis on morality, theory of mind, and empathy. Brain Struct. Funct. 2012, 217, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Arioli, M.; Cattaneo, Z.; Ricciardi, E.; Canessa, N. Overlapping and specific neural correlates for empathizing, affective mentalizing, and cognitive mentalizing: A coordinate-based meta-analytic study. Hum. Brain Mapp. 2021, 42, 4777–4804. [Google Scholar] [CrossRef] [PubMed]
- Ellemers, N.; van der Toorn, J.; Paunov, Y.; van Leeuwen, T. The Psychology of Morality: A Review and Analysis of Empirical Studies Published from 1940 through 2017. Personal. Soc. Psychol. Rev. 2019, 23, 332–366. [Google Scholar] [CrossRef] [PubMed]
- Spencer-Rodgers, J.; Peng, K.; Wang, L.; Hou, Y. Dialectical self-esteem and east-west differences in psychological well-being. Personal. Soc. Psychol. Bull. 2004, 30, 1416–1432. [Google Scholar] [CrossRef] [PubMed]
- Spencer-Rodgers, J.; Williams, M.J.; Peng, K. Cultural differences in expectations of change and tolerance for contradiction: A decade of empirical research. Personal. Soc. Psychol. Rev. 2010, 14, 296–312. [Google Scholar] [CrossRef]
- Kitayama, S.; Yanagisawa, K.; Ito, A.; Ueda, R.; Uchida, Y.; Abe, N. Reduced orbitofrontal cortical volume is associated with interdependent self-construal. Proc. Natl. Acad. Sci. USA 2017, 114, 7969–7974. [Google Scholar] [CrossRef]
- Kim, B.H.; Shin, Y.B.; Kyeong, S.; Lee, S.K.; Kim, J.J. Neural basis of ambivalence towards ideal self-image in schizophrenia. Psychiatry Investig. 2020, 17, 452–459. [Google Scholar] [CrossRef]

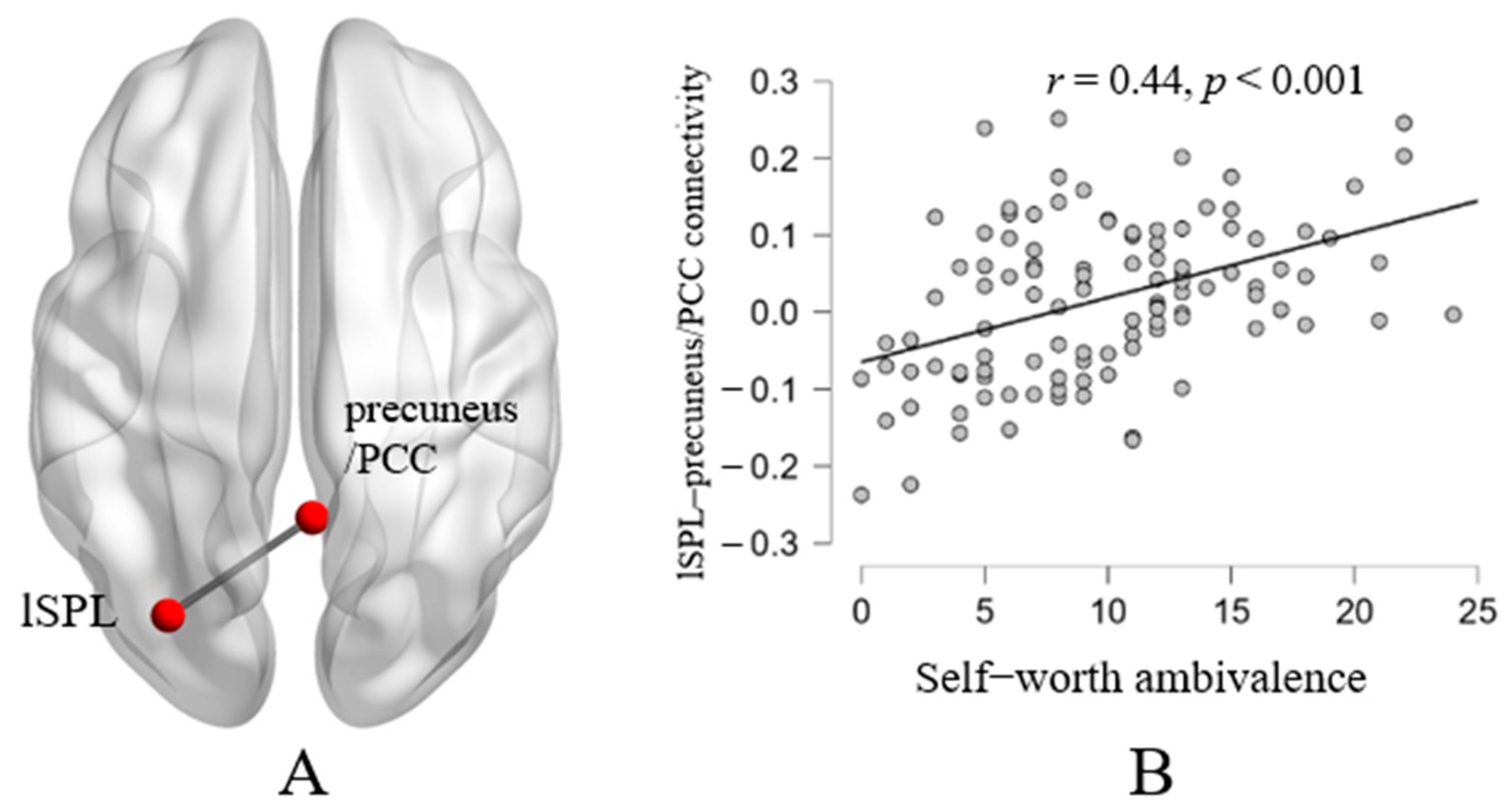

| Brain Regions | Peak | MNI | Coordinates | Peak r-Value | No. of Voxels |
|---|---|---|---|---|---|
| x | y | z | |||
| Left OFC | −12 | 69 | −3 | 0.37 | 56 |
| Left superior parietal lobule | −30 | −75 | 48 | 0.35 | 34 |
| Right cerebellum posterior lobe | 30 | −90 | −27 | −0.40 | 31 |
| Left occipital lobe | −3 | −93 | 27 | −0.38 | 32 |
| Right parietal lobe | 6 | −75 | 54 | −0.40 | 58 |
| Seed | Brain Regions | Peak | MNI | Coordinates | Cluster p FDR | Peak | No. of Voxels |
|---|---|---|---|---|---|---|---|
| x | y | z | p unc | ||||
| lSPL | |||||||
| Right precuneus/PCC | 4 | −52 | 36 | <.01 | <0.001 | 85 | |
| OFC | |||||||
| / |
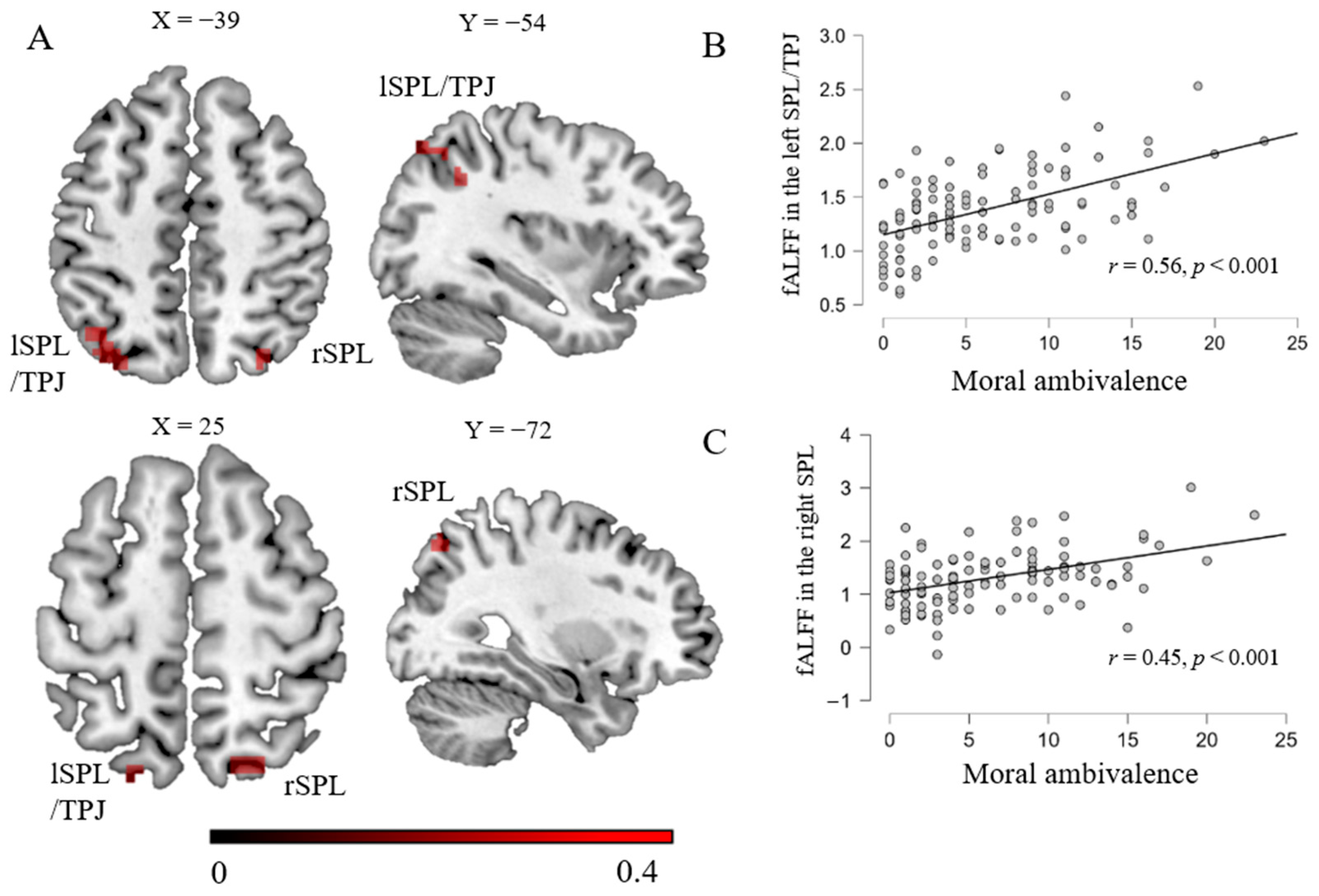
| Brain Regions | Peak | MNI | Coordinates | Peak r-Value | No. of Voxels |
|---|---|---|---|---|---|
| x | y | z | |||
| Left superior parietal lobule/TPJ | −39 | −54 | 42 | 0.42 | 49 |
| Right superior parietal lobule | 30 | −72 | 51 | 0.37 | 29 |
| Seed | Brain Regions | Peak | MNI | Coordinates | Cluster p FDR | Peak | No. of Voxels |
|---|---|---|---|---|---|---|---|
| x | y | z | p Unc | ||||
| lSPL/TPJ | |||||||
| Left OFC | −26 | 48 | −18 | <0.01 | <0.001 | 120 | |
| Left postcentral gyrus | −56 | −6 | 32 | <0.05 | <0.001 | 65 | |
| Left cerebellum | −32 | −52 | −58 | <0.05 | <0.001 | 66 | |
| rSPL | |||||||
| Right middle temporal gyrus | 70 | −28 | −10 | <0.001 | <0.001 | 162 | |
| Left inferior temporal gyrus | −56 | −60 | −28 | <0.05 | <0.001 | 74 | |
| Right inferior temporal gyrus | 60 | −48 | −16 | <0.05 | <0.001 | 73 | |
| Left middle temporal gyrus | −56 | −42 | −12 | <0.05 | <0.001 | 71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, S.; Du, T.; Tang, J.; Yang, J. Identifying the Shared and Dissociable Neural Bases between Self-Worth and Moral Ambivalence. Brain Sci. 2024, 14, 736. https://doi.org/10.3390/brainsci14070736
Li J, Wang S, Du T, Tang J, Yang J. Identifying the Shared and Dissociable Neural Bases between Self-Worth and Moral Ambivalence. Brain Sciences. 2024; 14(7):736. https://doi.org/10.3390/brainsci14070736
Chicago/Turabian StyleLi, Jiwen, Shuai Wang, Tengfei Du, Jianchao Tang, and Juan Yang. 2024. "Identifying the Shared and Dissociable Neural Bases between Self-Worth and Moral Ambivalence" Brain Sciences 14, no. 7: 736. https://doi.org/10.3390/brainsci14070736




