Individual Deviation-Based Functional Hypergraph for Identifying Subtypes of Autism Spectrum Disorder
Abstract
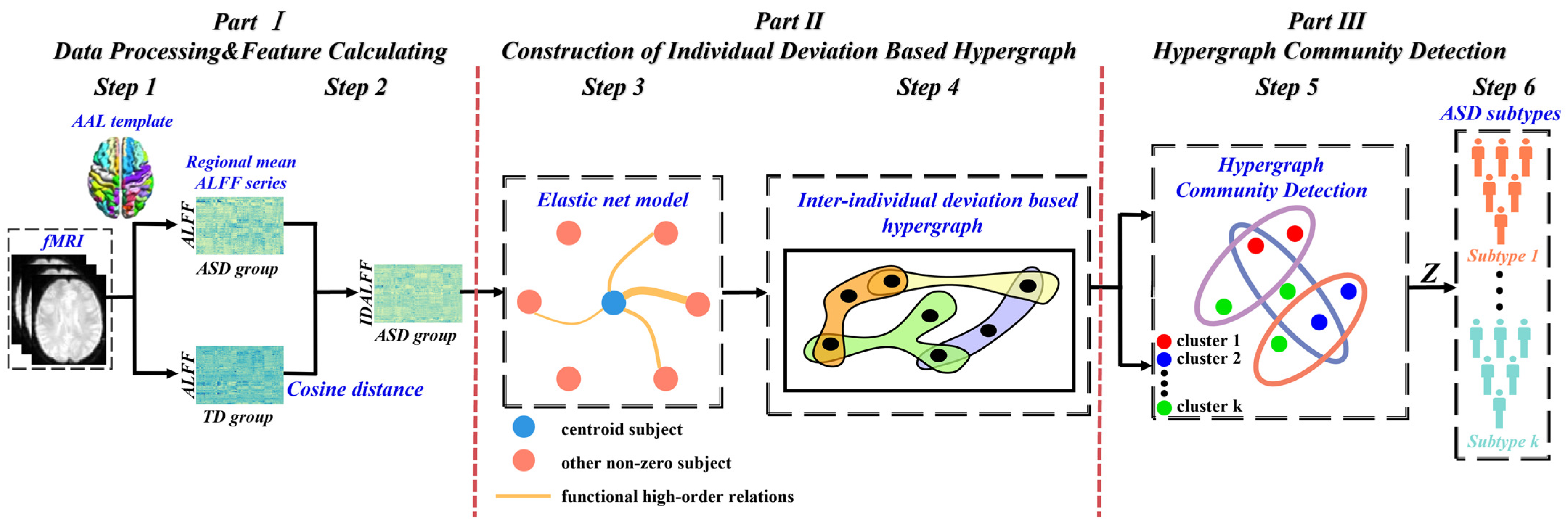
:1. Introduction
- We proposed an individual deviation-based hypergraph (ID-Hypergraph) model, which characterizes high-order relationships among individuals, to parse the neuroactivational heterogeneity of ASD;
- We identified four ASD subtypes with heterogeneous changes in both brain activity and behavior domains;
- The identified ASD subtypes were highly separable and were reproducible across different datasets.
2. Materials
2.1. Participants
2.2. Image Preprocessing
3. Methods
3.1. Preliminaries on Hypergraph
3.2. Construction of Inter-Individual Deviation-Based Hypergraph
3.2.1. Inter-Individual Deviation of ALFF (IDALFF)
3.2.2. Inter-Individual Deviation Based Hypergraph (ID-Hypergraph)
3.3. Hypergraph Community Detection
3.4. SVM Classifier
3.5. Statistical Analysis
3.6. Reproducibility Analysis
4. Results
4.1. Altered ALFF between ASD and TD Group
4.2. Subtyping ASD Based on ID-Hypergraph
4.3. Classification Between ASD Subtypes
4.4. Characterization of the ASD Subtypes
4.5. Clinical Symptoms of the ASD Subtypes
4.6. Reproducibility of the ASD Subtypes
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Zhang, F.; Jia, X.; Wang, X.; Zhang, H.; Ying, S.; Wang, Q.; Shi, J.; Shen, D. Multi-class ASD classification via label distribution learning with class-shared and class-specific decomposition. Med. Image Anal. 2022, 75, 102294. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Risi, S.; Lambrecht, L.; Cook, E.H.; Leventhal, B.L.; DiLavore, P.C.; Pickles, A.; Rutter, M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000, 30, 205–223. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; Text Revision; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643. [Google Scholar]
- Lombardo, M.V.; Lai, M.-C.; Baron-Cohen, S. Big data approaches to decomposing heterogeneity across the autism spectrum. Mol. Psychiatry 2019, 24, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Udhnani, M.; Lecavalier, L. Heterogeneity in autism spectrum disorder explained by social-communicative and restricted repetitive behavior balance subgroups. Res. Autism Spectr. Disord. 2024, 114, 102387. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Pramparo, T.; Gazestani, V.; Warrier, V.; Bethlehem, R.A.; Carter Barnes, C.; Lopez, L.; Lewis, N.E.; Eyler, L.; Pierce, K. Large-scale associations between the leukocyte transcriptome and BOLD responses to speech differ in autism early language outcome subtypes. Nat. Neurosci. 2018, 21, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Li, J.; Ju, Y.; Xiao, C.; Li, K.; Shi, B.; Zheng, W.; Zhang, Y. Altered Relationship between Functional Connectivity and Fiber-Bundle Structure in High-Functioning Male Adults with Autism Spectrum Disorder. Brain Sci. 2023, 13, 1098. [Google Scholar] [CrossRef]
- King, J.B.; Prigge, M.B.; King, C.K.; Morgan, J.; Weathersby, F.; Fox, J.C.; Dean, D.C.; Freeman, A.; Villaruz, J.A.M.; Kane, K.L.; et al. Generalizability and reproducibility of functional connectivity in autism. Mol. Autism 2019, 10, 1–23. [Google Scholar] [CrossRef]
- Yao, Z.; Hu, B.; Xie, Y.; Zheng, F.; Liu, G.; Chen, X.; Zheng, W. Resting-state time-varying analysis reveals aberrant variations of functional connectivity in autism. Front. Hum. Neurosci. 2016, 10, 463. [Google Scholar] [CrossRef]
- Zheng, W.; Eilam-Stock, T.; Wu, T.; Spagna, A.; Chen, C.; Hu, B.; Fan, J. Multi-Feature Based Network Revealing the Structural Abnormalities in Autism Spectrum Disorder. IEEE Trans. Affect. Comput. 2021, 12, 732–742. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Zhang, Z.; Liu, T.; Zhang, Y.; Fan, J.; Wu, D. Developmental pattern of the cortical topology in high-functioning individuals with autism spectrum disorder. Hum. Brain Mapp. 2021, 42, 660–675. [Google Scholar] [CrossRef] [PubMed]
- Tartarisco, G.; Cicceri, G.; Di Pietro, D.; Leonardi, E.; Aiello, S.; Marino, F.; Chiarotti, F.; Gagliano, A.; Arduino, G.M.; Apicella, F. Use of machine learning to investigate the quantitative checklist for autism in toddlers (Q-CHAT) towards early autism screening. Diagnostics 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Safdar, S.; Zafar, S.; Zafar, N.; Khan, N.F. Machine learning based decision support systems (DSS) for heart disease diagnosis: A review. Artif. Intell. Rev. 2018, 50, 597–623. [Google Scholar] [CrossRef]
- Haar, S.; Berman, S.; Behrmann, M.; Dinstein, I. Anatomical abnormalities in autism? Cereb. Cortex 2016, 26, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.E.; Ghassemi, M.M.; Nemati, S.; Niehaus, K.E.; Clifton, D.A.; Clifford, G.D. Machine learning and decision support in critical care. Proc. IEEE 2016, 104, 444–466. [Google Scholar] [CrossRef] [PubMed]
- Mottron, L.; Bzdok, D. Autism spectrum heterogeneity: Fact or artifact? Mol. Psychiatry 2020, 25, 3178–3185. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Weng, Z.; Zhang, Y.; Tao, Y.; Hu, B. Three-Stream Convolutional Neural Network for Depression Detection with Ocular Imaging. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 4921–4930. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-J.; Vogelstein, J.T.; Gozzi, A.; Bernhardt, B.C.; Yeo, B.T.; Milham, M.P.; Di Martino, A. Toward neurosubtypes in autism. Biol. Psychiatry 2020, 88, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhai, G.; Liu, J.; Cao, Y.; Zhang, X.; Cui, D.; Gao, L. Inter-individual heterogeneity of functional brain networks in children with autism spectrum disorder. Mol. Autism 2022, 13, 1–13. [Google Scholar] [CrossRef]
- Hong, S.-J.; Valk, S.L.; Di Martino, A.; Milham, M.P.; Bernhardt, B.C. Multidimensional neuroanatomical subtyping of autism spectrum disorder. Cereb. Cortex 2018, 28, 3578–3588. [Google Scholar] [CrossRef]
- Ren, P.; Bi, Q.; Pang, W.; Wang, M.; Zhou, Q.; Ye, X.; Li, L.; Xiao, L. Stratifying ASD and characterizing the functional connectivity of subtypes in resting-state fMRI. Behav. Brain Res. 2023, 449, 114458. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Uddin, L.Q.; Guo, X.; Wang, J.; Wang, R.; Wang, X.; Duan, X.; Chen, H. Parsing brain structural heterogeneity in males with autism spectrum disorder reveals distinct clinical subtypes. Hum. Brain Mapp. 2019, 40, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hoogman, M.; Roth Mota, N.; Buitelaar, J.K.; Vasquez, A.A.; Franke, B.; van Rooij, D.; ENIGMA-ASD Working Group. Dissecting the heterogeneous subcortical brain volume of autism spectrum disorder using community detection. Autism Res. 2022, 15, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Wang, Y.; Li, J.; Cai, H.; Zhang, X.; Yao, Z.; Yang, M.; Zheng, W. Decomposing Neuroanatomical Heterogeneity of Autism Spectrum Disorder Across Different Developmental Stages Using Morphological Multiplex Network Model. IEEE Trans. Comput. Soc. Syst. 2024. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Gao, X.; Jie, B.; Kim, M.; Yap, P.-T.; Wee, C.-Y.; Shen, D. Multimodal hyper-connectivity of functional networks using functionally-weighted LASSO for MCI classification. Med. Image Anal. 2019, 52, 80–96. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Feng, Y.; Ji, S.; Ji, R. HGNN+: General hypergraph neural networks. IEEE Trans. Pattern Anal. Mach. Intell. 2022, 45, 3181–3199. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Yao, Z.; Xie, Y.; Fan, J.; Hu, B. Identification of Alzheimer’s disease and mild cognitive impairment using networks constructed based on multiple morphological brain features. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Jie, B.; Wee, C.-Y.; Shen, D.; Zhang, D. Hyper-connectivity of functional networks for brain disease diagnosis. Med. Image Anal. 2016, 32, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, X.; Chen, H.; Zhai, G.; Cao, Y.; Zhang, T.; Gao, L. Exploring the heterogeneity of brain structure in autism spectrum disorder based on individual structural covariance network. Cereb. Cortex 2023, 33, 7311–7321. [Google Scholar] [CrossRef]
- Qi, S.; Morris, R.; Turner, J.A.; Fu, Z.; Jiang, R.; Deramus, T.P.; Zhi, D.; Calhoun, V.D.; Sui, J. Common and unique multimodal covarying patterns in autism spectrum disorder subtypes. Mol. Autism 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Choi, H.; Byeon, K.; Park, B.-y.; Lee, J.-e.; Valk, S.L.; Bernhardt, B.; Di Martino, A.; Milham, M.; Hong, S.-J.; Park, H. Diagnosis-informed connectivity subtyping discovers subgroups of autism with reproducible symptom profiles. NeuroImage 2022, 256, 119212. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ming, Y.; Zhao, W.; Feng, R.; Zhou, Y.; Wu, L.; Wang, J.; Xiao, J.; Li, L.; Shan, X. Developmental prediction modeling based on diffusion tensor imaging uncovering age-dependent heterogeneity in early childhood autistic brain. Mol. Autism 2023, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Kanai, R.; Rees, G. The structural basis of inter-individual differences in human behaviour and cognition. Nat. Rev. Neurosci. 2011, 12, 231–242. [Google Scholar] [CrossRef]
- Kernbach, J.M.; Satterthwaite, T.D.; Bassett, D.S.; Smallwood, J.; Margulies, D.; Krall, S.; Shaw, P.; Varoquaux, G.; Thirion, B.; Konrad, K. Shared endo-phenotypes of default mode dysfunction in attention deficit/hyperactivity disorder and autism spectrum disorder. Transl. Psychiatry 2018, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Huang, J.; Schölkopf, B. Learning with hypergraphs: Clustering, classification, and embedding. Adv. Neural Inf. Process. Syst. 2006, 19, 1601–1608. [Google Scholar]
- Ma, Y.; Ma, Y.; Jiang, X. Hypergraph clustering based on modularity feature projection for high-order relationship community detection of microorganisms. Methods 2022, 203, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Chodrow, P.S.; Veldt, N.; Benson, A.R. Generative hypergraph clustering: From blockmodels to modularity. Sci. Adv. 2021, 7, eabh1303. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Yan, C.-G.; Li, Q.; Denio, E.; Castellanos, F.X.; Alaerts, K.; Anderson, J.S.; Assaf, M.; Bookheimer, S.Y.; Dapretto, M. The autism brain imaging data exchange: Towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol. Psychiatry 2014, 19, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; O’connor, D.; Chen, B.; Alaerts, K.; Anderson, J.S.; Assaf, M.; Balsters, J.H.; Baxter, L.; Beggiato, A.; Bernaerts, S. Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Sci. Data 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Strehl, A.; Ghosh, J. Cluster ensembles—A knowledge reuse framework for combining multiple partitions. J. Mach. Learn. Res. 2002, 3, 583–617. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V. Scikit-learn: Machine Learn-ing in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yi, H.G.; Leonard, M.K.; Chang, E.F. The encoding of speech sounds in the superior temporal gyrus. Neuron 2019, 102, 1096–1110. [Google Scholar] [CrossRef] [PubMed]
- Olson, I.R.; Plotzker, A.; Ezzyat, Y. The enigmatic temporal pole: A review of findings on social and emotional processing. Brain 2007, 130, 1718–1731. [Google Scholar] [CrossRef] [PubMed]
- Insel, T.; Cuthbert, B.; Garvey, M.; Heinssen, R.; Pine, D.S.; Quinn, K.; Sanislow, C.; Wang, P. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am. Psychiatr. Assoc. 2010, 167, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010, 33, 1. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, J.; Kassani, P.H.; Zhang, Y.; Bai, Y.; Stephen, J.M.; Wilson, T.W.; Calhoun, V.D.; Wang, Y.-P. Multi-hypergraph learning-based brain functional connectivity analysis in fMRI data. IEEE Trans. Med. Imaging 2019, 39, 1746–1758. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Kong, W.; Liu, H.; Huang, B.; Wang, Y. High-order Brain Network Analysis of Depression Based on Dynamic Functional Connectivity. In Proceedings of the 2023 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Istanbul, Turkiye, 5–8 December 2023; IEEE: Piscataway, NJ, USA, 2023; pp. 2579–2586. [Google Scholar]
- Ogutu, J.O.; Schulz-Streeck, T.; Piepho, H.-P. Genomic selection using regularized linear regression models: Ridge regression, lasso, elastic net and their extensions. BMC Proc. 2012, 6, S10. [Google Scholar] [CrossRef]
- Sandin, S.; Yip, B.H.; Yin, W.; Weiss, L.A.; Dougherty, J.D.; Fass, S.; Constantino, J.N.; Hailin, Z.; Turner, T.N.; Marrus, N. Examining sex differences in autism heritability. JAMA Psychiatry 2024, 81, 673–680. [Google Scholar] [CrossRef]
- Warrier, V.; Greenberg, D.M.; Weir, E.; Buckingham, C.; Smith, P.; Lai, M.-C.; Allison, C.; Baron-Cohen, S. Elevated rates of autism, other neurodevelopmental and psychiatric diagnoses, and autistic traits in transgender and gender-diverse individuals. Nat. Commun. 2020, 11, 3959. [Google Scholar] [CrossRef] [PubMed]
- Bargiela, S.; Steward, R.; Mandy, W. The experiences of late-diagnosed women with autism spectrum conditions: An investigation of the female autism phenotype. J. Autism Dev. Disord. 2016, 46, 3281–3294. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wei, Y.; Feng, Y.; Cao, J.; Gao, Y. Dynamic Hypergraph Neural Networks. In Proceedings of the Twenty-Eighth International Joint Conference on Artificial Intelligence, IJCAI 2019, Macao, China, 10–16 August 2019; pp. 2635–2641. [Google Scholar]
- Liu, Y.; Luo, Q.; Xiao, M.; Yu, D.; Chen, H.; Cheng, X. Reordering and Compression for Hypergraph Processing. IEEE Trans. Comput. 2024, 73, 1486–1499. [Google Scholar] [CrossRef]
- Ebisch, S.J.; Gallese, V.; Willems, R.M.; Mantini, D.; Groen, W.B.; Romani, G.L.; Buitelaar, J.K.; Bekkering, H. Altered intrinsic functional connectivity of anterior and posterior insula regions in high-functioning participants with autism spectrum disorder. Hum. Brain Mapp. 2011, 32, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Banker, S.M.; Gu, X.; Schiller, D.; Foss-Feig, J.H. Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci. 2021, 44, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Duan, X.; Long, Z.; Chen, H.; Wang, Y.; Zheng, J.; Zhang, Y.; Li, R.; Chen, H. Decreased amygdala functional connectivity in adolescents with autism: A resting-state fMRI study. Psychiatry Res. Neuroimaging 2016, 257, 47–56. [Google Scholar] [CrossRef]
- Nair, A.; Treiber, J.M.; Shukla, D.K.; Shih, P.; Müller, R.-A. Impaired thalamocortical connectivity in autism spectrum disorder: A study of functional and anatomical connectivity. Brain 2013, 136, 1942–1955. [Google Scholar] [CrossRef]
- Spagna, A.; Dufford, A.J.; Wu, Q.; Wu, T.; Zheng, W.; Coons, E.E.; Hof, P.R.; Hu, B.; Wu, Y.; Fan, J. Gray matter volume of the anterior insular cortex and social networking. J. Comp. Neurol. 2018, 526, 1183–1194. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and function of the human insula. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 2017, 34, 300. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, L.; Zhao, Z.; Liu, T.; Hu, B.; Wu, D. Spatiotemporal Developmental Gradient of Thalamic Morphology, Microstructure, and Connectivity from the Third Trimester to Early Infancy. J. Neurosci. 2023, 43, 559–570. [Google Scholar] [CrossRef]
- Biesbroek, J.M.; Verhagen, M.G.; van der Stigchel, S.; Biessels, G.J. When the central integrator disintegrates: A review of the role of the thalamus in cognition and dementia. Alzheimers Dement. 2024, 20, 2209–2222. [Google Scholar] [CrossRef] [PubMed]




| Discovery (ABIDE-I) | Replication (ABIDE-II) | |||||
|---|---|---|---|---|---|---|
| Groups | ASD (n = 147) | TD (n = 125) | p Value | ASD (n = 134) | TD (n = 132) | p Value |
| Age | 16.43 ± 8.15 | 17.73 ± 6.41 | 0.900 a | 15.72 ± 7.79 | 17.93 ± 6.28 | 0.326 a |
| Gender | Male | Male | - | Male | Male | - |
| Handedness | Right | Right | - | Right | Right | - |
| IQ | 110.75 ± 14.47 | 115.67 ± 12.74 | 0.009 a | 110.54 ± 16.96 | 114.27 ± 11.95 | 0.040 a |
| Groups | ACC | SPE | SEN | AUC |
|---|---|---|---|---|
| Subtype1 vs. Subtype2 | 0.8643 | 0.8607 | 0.8333 | 0.8658 |
| Subtype1 vs. Subtype3 | 0.8500 | 0.9267 | 0.7967 | 0.8467 |
| Subtype1 vs. Subtype4 | 0.8125 | 0.7583 | 0.8233 | 0.8317 |
| Subtype2 vs. Subtype3 | 0.7429 | 0.7517 | 0.7767 | 0.7075 |
| Subtype2 vs. Subtype4 | 0.7428 | 0.7633 | 0.8017 | 0.7517 |
| Subtype3 vs. Subtype4 | 0.8321 | 0.8767 | 0.8600 | 0.8233 |
| Average result | 0.8074 | 0.8229 | 0.8153 | 0.8045 |
| Information | Subtype1 | Subtype2 | Subtype3 | Subtype4 |
|---|---|---|---|---|
| Age | 23.33 ± 11.16 | 14.86 ± 3.97 | 18.41 ± 6.23 | 16.50 ± 7.27 |
| IQ | 107.70 ± 18.79 | 112.99 ± 11.71 | 113.13 ± 16.06 | 108.82 ± 12.38 |
| ADOS | ||||
| Communication | 3.57 ± 1.43 | 3.00 ± 1.18 | 4.06 ± 1.61 | 3.73 ± 2.00 |
| Social | 7.52 ± 2.60 | 7.07 ± 2.62 | 8.25 ± 3.07 | 7.45 ± 2.98 |
| Behavior | 1.83 ± 0.79 | 2.36 ± 1.43 | 2.56 ± 1.67 | 2.00 ± 1.26 |
| Total | 10.83 ± 3.87 | 10.78 ± 3.60 | 12.13 ± 4.35 | 11.18 ± 4.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zheng, W.; Fu, X.; Zhang, Y.; Yang, S.; Wang, Y.; Zhang, Z.; Hu, B.; Xu, G. Individual Deviation-Based Functional Hypergraph for Identifying Subtypes of Autism Spectrum Disorder. Brain Sci. 2024, 14, 738. https://doi.org/10.3390/brainsci14080738
Li J, Zheng W, Fu X, Zhang Y, Yang S, Wang Y, Zhang Z, Hu B, Xu G. Individual Deviation-Based Functional Hypergraph for Identifying Subtypes of Autism Spectrum Disorder. Brain Sciences. 2024; 14(8):738. https://doi.org/10.3390/brainsci14080738
Chicago/Turabian StyleLi, Jialong, Weihao Zheng, Xiang Fu, Yu Zhang, Songyu Yang, Ying Wang, Zhe Zhang, Bin Hu, and Guojun Xu. 2024. "Individual Deviation-Based Functional Hypergraph for Identifying Subtypes of Autism Spectrum Disorder" Brain Sciences 14, no. 8: 738. https://doi.org/10.3390/brainsci14080738







