Resting-State Functional Connectivity Profile of Insular Subregions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
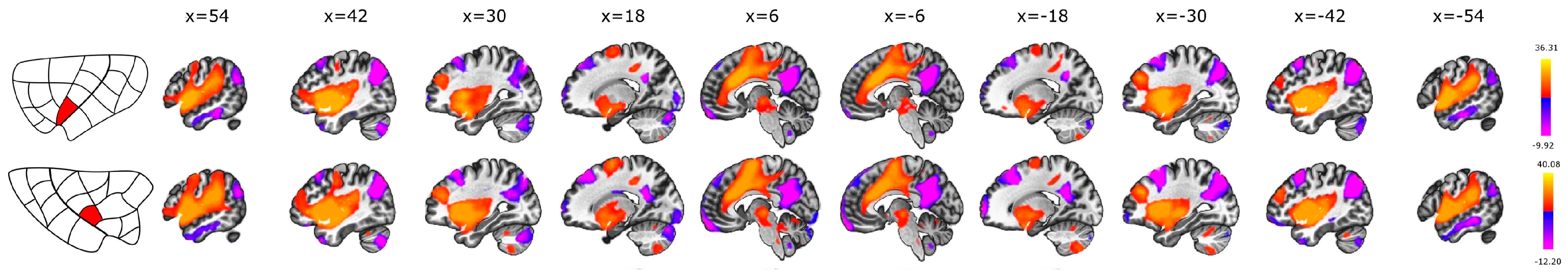
3.1. Seed-to-Voxel
3.2. Intrinsic Intra-Insular Connectivity
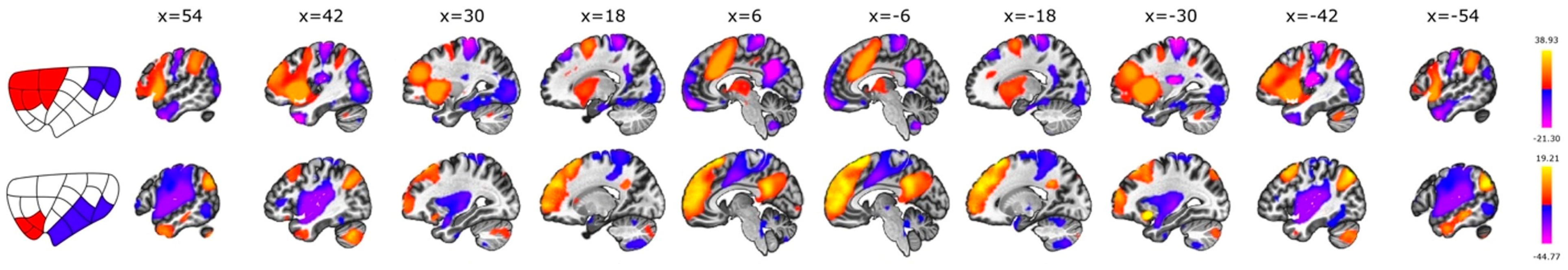
3.3. Anterior vs. Posterior
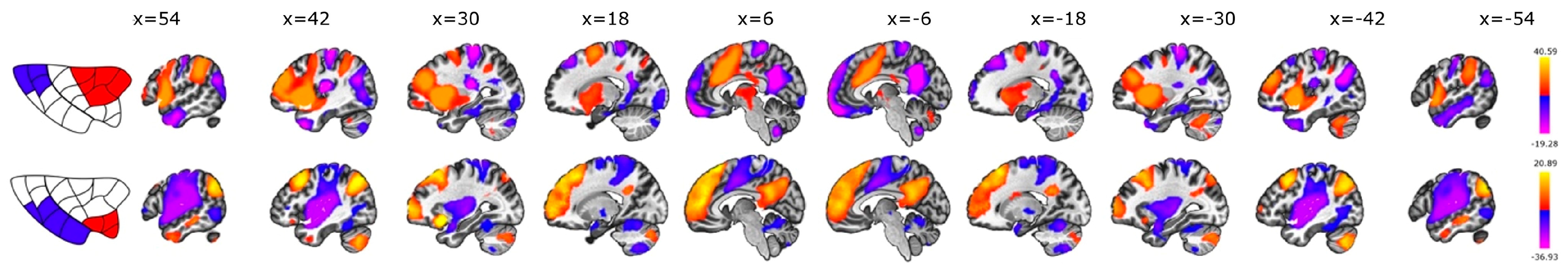
3.4. Dorsal vs. Ventral
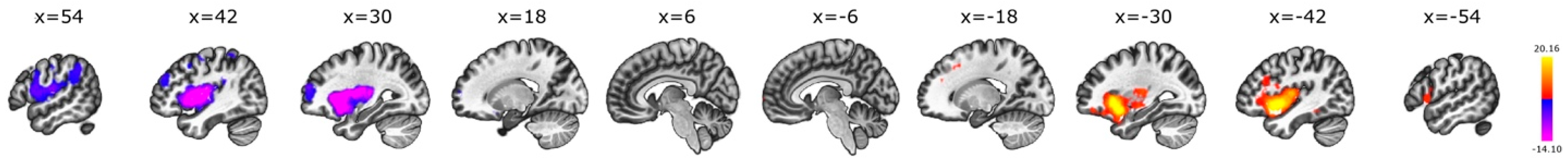
3.5. Interhemispheric
4. Discussion
4.1. Cortical Functional Connectivity
4.2. Limbic and Subcortical Connectivity
4.3. Comparison with Structural Connectivity
4.4. Subdivisions of the Insula
4.5. Functional Significance of Insular Subregions in Relation to Brain Function and Disease
4.6. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephani, C.; Fernandez-Baca Vaca, G.; Maciunas, R.; Koubeissi, M.; Luders, H.O.; Lüders, H.O. Functional Neuroanatomy of the Insular Lobe. Brain Struct. Funct. 2011, 216, 137–149. [Google Scholar] [CrossRef]
- Ture, U.; Yasargil, D.C.; Al-Mefty, O.; Yasargil, M.G.; Türe, U.; Yaşargil, D.C.; Al-Mefty, O.; Yaşargil, M.G. Topographic Anatomy of the Insular Region. J. Neurosurg. 1999, 90, 720–733. [Google Scholar] [CrossRef]
- Flynn, F.G. Anatomy of the Insula Functional and Clinical Correlates. Aphasiology 1999, 13, 55–78. [Google Scholar] [CrossRef]
- Augustine, J.R. The Insular Lobe in Primates Including Humans. Neurol. Res. 1985, 7, 2–10. [Google Scholar] [CrossRef]
- Cloutman, L.L.; Binney, R.J.; Drakesmith, M.; Parker, G.J.M.; Lambon Ralph, M.A. The Variation of Function across the Human Insula Mirrors Its Patterns of Structural Connectivity: Evidence from in Vivo Probabilistic Tractography. Neuroimage 2012, 59, 3514–3521. [Google Scholar] [CrossRef]
- Cerliani, L.; Thomas, R.M.; Jbabdi, S.; Siero, J.C.W.; Nanetti, L.; Crippa, A.; Gazzola, V.; D’Arceuil, H.; Keysers, C. Probabilistic Tractography Recovers a Rostrocaudal Trajectory of Connectivity Variability in the Human Insular Cortex. Hum. Brain Mapp. 2012, 33, 2005–2034. [Google Scholar] [CrossRef]
- Jakab, A.; Molnár, P.P.; Bogner, P.; Béres, M.; Berényi, E.L.; Molnar, P.P.; Bogner, P.; Beres, M.; Berenyi, E.L. Connectivity-Based Parcellation Reveals Interhemispheric Differences in the Insula. Brain Topogr. 2012, 25, 264–271. [Google Scholar] [CrossRef]
- Ghaziri, J.; Tucholka, A.; Girard, G.; Boucher, O.; Houde, J.C.; Descoteaux, M.; Obaid, S.; Gilbert, G.; Rouleau, I.; Nguyen, D.K. Subcortical Structural Connectivity of Insular Subregions. Sci. Rep. 2018, 8, 8596. [Google Scholar] [CrossRef]
- Ghaziri, J.; Tucholka, A.; Girard, G.; Houde, J.C.; Boucher, O.; Gilbert, G.; Descoteaux, M.; Lippé, S.; Rainville, P.; Nguyen, D.K. The Corticocortical Structural Connectivity of the Human Insula. Cereb. Cortex 2017, 27, 1216–1228. [Google Scholar] [CrossRef]
- Nomi, J.S.S.; Schettini, E.; Broce, I.; Dick, A.S.S.; Uddin, L.Q.Q. Structural Connections of Functionally Defined Human Insular Subdivisions. Cereb. Cortex 2017, 28, 3445–3456. [Google Scholar] [CrossRef]
- Deco, G.; Jirsa, V.K.; McIntosh, A.R. Emerging Concepts for the Dynamical Organization of Resting-State Activity in the Brain. Nat. Rev. Neurosci. 2011, 12, 43–56. [Google Scholar] [CrossRef]
- Honey, C.J.; Sporns, O.; Cammoun, L.; Gigandet, X.; Thiran, J.P.; Meuli, R.; Hagmann, P. Predicting Human Resting-State Functional Connectivity from Structural Connectivity. Proc. Natl. Acad. Sci. USA 2009, 106, 2035–2040. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Mandl, R.C.W.W.; Kahn, R.S.; Hulshoff Pol, H.E.; Mandl, C.W.; Kahn, S.; Heuvel, M.P.; van den Pol, H.E.H.; van den Heuvel, M.P.; Mandl, R.C.W.W.; et al. Functionally Linked Resting-State Networks Reflect the Underlying Structural Connectivity Architecture of the Human Brain. Hum. Brain Mapp. 2009, 30, 3127–3141. [Google Scholar] [CrossRef]
- Taylor, K.S.; Seminowicz, D.A.; Davis, K.D. Two Systems of Resting State Connectivity between the Insula and Cingulate Cortex. Hum. Brain Mapp. 2009, 30, 2731–2745. [Google Scholar] [CrossRef]
- Cauda, F.; Agata, F.D.; Sacco, K.; Duca, S.; Geminiani, G.; Vercelli, A.; D’Agata, F.; Sacco, K.; Duca, S.; Geminiani, G.; et al. Functional Connectivity of the Insula in the Resting Brain. Neuroimage 2011, 55, 8–23. [Google Scholar] [CrossRef]
- Deen, B.; Pitskel, N.B.; Pelphrey, K.A. Three Systems of Insular Functional Connectivity Identified with Cluster Analysis. Cereb. Cortex 2011, 21, 1498–1506. [Google Scholar] [CrossRef]
- Kelly, C.; Toro, R.; Di Martino, A.; Cox, C.L.; Bellec, P.; Castellanos, F.X.; Milham, M.P. A Convergent Functional Architecture of the Insula Emerges across Imaging Modalities. Neuroimage 2012, 61, 1129–1142. [Google Scholar] [CrossRef]
- Chang, L.J.; Yarkoni, T.; Khaw, M.W.; Sanfey, A.G. Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cereb. Cortex 2013, 23, 739–749. [Google Scholar] [CrossRef]
- Kurth, F.; Zilles, K.; Fox, P.T.; Laird, A.R.; Eickhoff, S.B. A Link between the Systems: Functional Differentiation and Integration within the Human Insula Revealed by Meta-Analysis. Brain Struct. Funct. 2010, 214, 519–534. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Kinnison, J.; Pessoa, L.; Anderson, M.L. Beyond the Tripartite Cognition–Emotion–Interoception Model of the Human Insular Cortex. J. Cogn. Neurosci. 2014, 26, 16–27. [Google Scholar] [CrossRef]
- Cauda, F.; Costa, T.; Torta, D.M.E.; Sacco, K.; D’Agata, F.; Duca, S.; Geminiani, G.; Fox, P.T.; Vercelli, A. Meta-Analytic Clustering of the Insular Cortex: Characterizing the Meta-Analytic Connectivity of the Insula When Involved in Active Tasks. Neuroimage 2012, 62, 343–355. [Google Scholar] [CrossRef]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A Multi-Modal Parcellation of Human Cerebral Cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef]
- Morel, A.; Gallay, M.N.; Baechler, A.; Wyss, M.; Gallay, D.S. The Human Insula: Architectonic Organization and Postmortem MRI Registration. Neuroscience 2013, 236, 117–135. [Google Scholar] [CrossRef]
- Vercelli, U.G.O.; Diano, M.; Costa, T.; Nani, A.; Duca, S.; Geminiani, G.; Vercelli, A.; Cauda, F. Node Detection Using High-Dimensional Fuzzy Parcellation Applied to the Insular Cortex. Neural Plast. 2015, 2016, 1938292. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Paulus, M.P.; Stein, M.B. An Insular View of Anxiety. Biol. Psychiatry 2006, 60, 383–387. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect 2012, 2, 125–141. [Google Scholar] [CrossRef]
- Calhoun, V.D.; Wager, T.D.; Krishnan, A.; Rosch, K.S.; Seymour, K.E.; Nebel, M.B.; Mostofsky, S.H.; Nyalakanai, P.; Kiehl, K. The Impact of T1 versus EPI Spatial Normalization Templates for FMRI Data Analyses. Hum. Brain Mapp. 2017, 38, 5331–5342. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Unified Segmentation. Neuroimage 2005, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J. A Fast Diffeomorphic Image Registration Algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Hutton, C.; Ashburner, J.; Turner, R.; Friston, K. Modeling Geometric Deformations in EPI Time Series. Neuroimage 2001, 13, 903–919. [Google Scholar] [CrossRef]
- Friston, K.J.; Ashburner, J.; Frith, C.D.; Poline, J.-B.; Heather, J.D.; Frackowiak, R.S.J. Spatial Registration and Normalization of Images. Hum. Brain Mapp. 1995, 3, 165–189. [Google Scholar] [CrossRef]
- Henson, R.; Buchel, C.; Josephs, O.; Friston, K. The Slice-Timing Problem in Event-Related FMRI. Neuroimage 1999, 9, 125. [Google Scholar]
- Sladky, R.; Friston, K.J.; Tröstl, J.; Cunnington, R.; Moser, E.; Windischberger, C. Slice-Timing Effects and Their Correction in Functional MRI. Neuroimage 2011, 58, 588–594. [Google Scholar] [CrossRef]
- Power, J.D.; Mitra, A.; Laumann, T.O.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Methods to Detect, Characterize, and Remove Motion Artifact in Resting State FMRI. Neuroimage 2014, 84, 320–341. [Google Scholar] [CrossRef]
- Demertzi, A.; Antonopoulos, G.; Heine, L.; Voss, H.U.; Crone, J.S.; de Los Angeles, C.; Bahri, M.A.; Di Perri, C.; Vanhaudenhuyse, A.; Charland-Verville, V.; et al. Intrinsic Functional Connectivity Differentiates Minimally Conscious from Unresponsive Patients. Brain 2015, 138, 2619–2631. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. FMRI Denoising Pipeline. In Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020; pp. 17–25. [Google Scholar]
- Behzadi, Y.; Restom, K.; Liau, J.; Liu, T.T. A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based FMRI. Neuroimage 2007, 37, 90–101. [Google Scholar] [CrossRef]
- Chai, X.J.; Castañón, A.N.; Öngür, D.; Whitfield-Gabrieli, S. Anticorrelations in Resting State Networks without Global Signal Regression. Neuroimage 2012, 59, 1420–1428. [Google Scholar] [CrossRef]
- Friston, K.J.; Williams, S.; Howard, R.; Frackowiak, R.S.J.; Turner, R. Movement-Related Effects in FMRI Time-Series. Magn. Reson. Med. 1996, 35, 346–355. [Google Scholar] [CrossRef]
- Hallquist, M.N.; Hwang, K.; Luna, B. The Nuisance of Nuisance Regression: Spectral Misspecification in a Common Approach to Resting-State FMRI Preprocessing Reintroduces Noise and Obscures Functional Connectivity. Neuroimage 2013, 82, 208–225. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional Connectivity in the Resting Brain: A Network Analysis of the Default Mode Hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The Human Brain Is Intrinsically Organized into Dynamic, Anticorrelated Functional Networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. Preparing FMRI Data for Statistical Analysis. arXiv 2022. [Google Scholar] [CrossRef]
- Biswal, B.B.; Mennes, M.; Zuo, X.-N.; Gohel, S.; Kelly, C.; Smith, S.M.; Beckmann, C.F.; Adelstein, J.S.; Buckner, R.L.; Colcombe, S.; et al. Toward Discovery Science of Human Brain Function. Proc. Natl. Acad. Sci. USA 2010, 107, 4734–4739. [Google Scholar] [CrossRef]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the Brain’s Functional Architecture during Activation and Rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. Functional Connectivity Measures. In Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020; pp. 26–62. ISBN 9780578644004. [Google Scholar]
- Worsley, K.J.; Marrett, S.; Neelin, P.; Vandal, A.C.; Friston, K.J.; Evans, A.C.; Brain, M. A Unified Statistical Approach for Determining Significant Signals in Images of Cerebral Activation. Hum. Brain Mapp. 1996, 4, 58–73. [Google Scholar] [CrossRef]
- Nieto-Castanon, A. Cluster-Level Inferences. In Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020; pp. 83–104. ISBN 9780578644004. [Google Scholar]
- Nieto-Castanon, A. General Linear Model. In Handbook of Functional Connectivity Magnetic Resonance Imaging Methods in CONN; Hilbert Press: Boston, MA, USA, 2020; pp. 63–82. ISBN 9780578644004. [Google Scholar]
- Chumbley, J.; Worsley, K.; Flandin, G.; Friston, K. Topological FDR for Neuroimaging. Neuroimage 2010, 49, 3057–3064. [Google Scholar] [CrossRef]
- Makris, N.; Goldstein, J.M.; Kennedy, D.; Hodge, S.M.; Caviness, V.S.; Faraone, S.V.; Tsuang, M.T.; Seidman, L.J. Decreased Volume of Left and Total Anterior Insular Lobule in Schizophrenia. Schizophr. Res. 2006, 83, 155–171. [Google Scholar] [CrossRef]
- Frazier, J.A.; Chiu, S.; Breeze, J.L.; Makris, N.; Lange, N.; Kennedy, D.N.; Herbert, M.R.; Bent, E.K.; Koneru, V.K.; Dieterich, M.E.; et al. Structural Brain Magnetic Resonance Imaging of Limbic and Thalamic Volumes in Pediatric Bipolar Disorder. Am. J. Psychiatry 2005, 162, 1256–1265. [Google Scholar] [CrossRef]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An Automated Labeling System for Subdividing the Human Cerebral Cortex on MRI Scans into Gyral Based Regions of Interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Seidman, L.J.; Makris, N.; Ahern, T.; O’Brien, L.M.; Caviness, V.S.; Kennedy, D.N.; Faraone, S.V.; Tsuang, M.T. Hypothalamic Abnormalities in Schizophrenia: Sex Effects and Genetic Vulnerability. Biol. Psychiatry 2007, 61, 935–945. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Miezin, F.M.; Cohen, A.L.; Wenger, K.K.; Dosenbach, R.A.T.; Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E.; et al. Distinct Brain Networks for Adaptive and Stable Task Control in Humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11073–11078. [Google Scholar] [CrossRef]
- Lamichhane, B.; Dhamala, M. The Salience Network and Its Functional Architecture in a Perceptual Decision: An Effective Connectivity Study. Brain Connect 2015, 5, 362–370. [Google Scholar] [CrossRef]
- Kelly, A.M.C.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Competition between Functional Brain Networks Mediates Behavioral Variability. Neuroimage 2008, 39, 527–537. [Google Scholar] [CrossRef]
- Ackermann, H.; Riecker, A. The Contribution(s) of the Insula to Speech Production: A Review of the Clinical and Functional Imaging Literature. Brain Struct. Funct. 2010, 214, 419–433. [Google Scholar] [CrossRef]
- Oh, A.; Duerden, E.G.; Pang, E.W. The Role of the Insula in Speech and Language Processing. Brain Lang. 2014, 135, 96–103. [Google Scholar] [CrossRef]
- Boucher, O.; Rouleau, I.; Escudier, F.; Malenfant, A.; Denault, C.; Charbonneau, S.; Finet, P.; Lassonde, M.; Lepore, F.; Bouthillier, A.; et al. Neuropsychological Performance before and after Partial or Complete Insulectomy in Patients with Epilepsy. Epilepsy Behav. 2015, 43, 53–60. [Google Scholar] [CrossRef]
- van den Heuvel, M.P.; Hulshoff Pol, H.E. Exploring the Brain Network: A Review on Resting-State FMRI Functional Connectivity. Eur. Neuropsychopharmacol. 2010, 20, 519–534. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Nomi, J.S.; Hébert-Seropian, B.; Ghaziri, J.; Boucher, O. Structure and Function of the Human Insula. J. Clin. Neurophysiol. 2017, 34, 300–306. [Google Scholar] [CrossRef]
- Tracey, I.; Mantyh, P.W. The Cerebral Signature for Pain Perception and Its Modulation. Neuron 2007, 55, 377–391. [Google Scholar] [CrossRef]
- Singer, T.; Seymour, B.; O’Doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for Pain Involves the Affective but Not Sensory Components of Pain. Science 2004, 303, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Lutz, A.; McFarlin, D.R.; Perlman, D.M.; Salomons, T.V.; Davidson, R.J. Altered Anterior Insula Activation during Anticipation and Experience of Painful Stimuli in Expert Meditators. Neuroimage 2013, 64, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Wiech, K.; Lin, C.S.; Brodersen, K.H.; Bingel, U.; Ploner, M.; Tracey, I. Anterior Insula Integrates Information about Salience into Perceptual Decisions about Pain. J. Neurosci. 2010, 30, 16324–16331. [Google Scholar] [CrossRef] [PubMed]
- Kucyi, A.; Davis, K.D. The Dynamic Pain Connectome. Trends Neurosci. 2015, 38, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L.; Mauguière, F.; Isnard, J. Functional Mapping of the Human Insula: Data from Electrical Stimulations. Rev. Neurol. 2019, 175, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, W.; Wang, S.; Zhou, Q.; Wang, H.; Zhang, B.; Huang, J.; Hong, B.; Wang, X. The Roles of Subdivisions of Human Insula in Emotion Perception and Auditory Processing. Cereb. Cortex 2019, 29, 517–528. [Google Scholar] [CrossRef]
- Bamiou, D.-E.E.; Musiek, F.E.; Luxon, L.M. The Insula (Island of Reil) and Its Role in Auditory Processing. Literature Review. Brain Res. Brain Res. Rev. 2003, 42, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.; Daquin, G.; Milandre, L.; Royere, M.L.; Rey, M.; Lanteri, A.; Salamon, G.; Khalil, R. Mutism and Auditory Agnosia Due to Bilateral Insular Damage--Role of the Insula in Human Communication. Neuropsychologia 1995, 33, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Golay, L.; Schnider, A.; Ptak, R. Cortical and Subcortical Anatomy of Chronic Spatial Neglect Following Vascular Damage. Behav. Brain Funct. 2008, 4, 43. [Google Scholar] [CrossRef]
- Singer, T.; Critchley, H.D.; Preuschoff, K. A Common Role of Insula in Feelings, Empathy and Uncertainty. Trends Cogn. Sci. 2009, 13, 334–340. [Google Scholar] [CrossRef]
- Cheng, X.; Zheng, L.; Li, L.; Zheng, Y.; Guo, X.; Yang, G. Anterior Insula Signals Inequalities in a Modified Ultimatum Game. Neuroscience 2017, 348, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.A.; Levin, I.P.; Shiv, B.; Bechara, A. The Effects of Insula Damage on Decision-Making for Risky Gains and Losses. Soc. Neurosci. 2009, 4, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Gasquoine, P.G. Contributions of the Insula to Cognition and Emotion. Neuropsychol. Rev. 2014, 24, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Cauda, F.; Geminiani, G.; D’Agata, F.; Sacco, K.; Duca, S.; Bagshaw, A.P.; Cavanna, A.E.; Cavanna, A.E. Functional Connectivity of the Posteromedial Cortex. PLoS ONE 2010, 5, e13107. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q. Salience Processing and Insular Cortical Function and Dysfunction. Nat. Rev. Neurosci. 2014, 16, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.C.W.; Zambreanu, L.; Godinez, A.; Craig, A.D.; Tracey, I. Somatotopic Organisation of the Human Insula to Painful Heat Studied with High Resolution Functional Imaging. Neuroimage 2005, 27, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J. The Anatomy of Language: A Review of 100 FMRI Studies Published in 2009. Ann. N. Y. Acad. Sci. 2009, 1191, 62–88. [Google Scholar] [CrossRef] [PubMed]
- Akkermans, S.E.A.; Luijten, M.; van Rooij, D.; Franken, I.H.A.; Buitelaar, J.K. Putamen Functional Connectivity during Inhibitory Control in Smokers and Non-Smokers. Addict. Biol. 2018, 23, 359–368. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Rudrauf, D.; Damasio, H.; Bechara, A. Damage to the Insula Disrupts Addiction to Cigarette Smoking. Science 2007, 315, 531–534. [Google Scholar] [CrossRef]
- Christopher, L.; Koshimori, Y.; Lang, A.E.; Criaud, M.; Strafella, A.P. Uncovering the Role of the Insula in Non-Motor Symptoms of Parkinson’s Disease. Brain 2014, 137, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Criaud, M.; Christopher, L.; Boulinguez, P.; Ballanger, B.; Lang, A.E.; Cho, S.S.; Houle, S.; Strafella, A.P. Contribution of Insula in Parkinson’s Disease: A Quantitative Meta-Analysis Study. Hum. Brain Mapp. 2016, 37, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- Isnard, J.; Guénot, M.; Ostrowsky, K.; Sindou, M.; Mauguière, F. The Role of the Insular Cortex in Temporal Lobe Epilepsy. Ann. Neurol. 2000, 48, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Isnard, J.; Guénot, M.; Sindou, M.; Mauguière, F. Clinical Manifestations of Insular Lobe Seizures: A Stereo-Electroencephalographic Study. Epilepsia 2004, 45, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Kunimatsu, A.; Yasaka, K.; Akai, H.; Kunimatsu, N.; Abe, O. MRI Findings in Posttraumatic Stress Disorder. J. Magn. Reson. Imaging 2020, 52, 380–396. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, J.M.; Rogers, B.P.; Blackford, J.U.; Heckers, S.; Woodward, N.D. Insula Functional Connectivity in Schizophrenia. Schizophr. Res. 2020, 220, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Testa, N.; Jordan, R.; Elyan, R.; Kanekar, S.; Wang, J.; Eslinger, P.; Yang, Q.X.; Zhang, B.; Karunanayaka, P.R. Functional Connectivity between the Resting-State Olfactory Network and the Hippocampus in Alzheimer’s Disease. Brain Sci. 2019, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Williams, D. Basal Ganglia Local Field Potential Activity: Character and Functional Significance in the Human. Clin. Neurophysiol. 2005, 116, 2510–2519. [Google Scholar] [CrossRef]
- Wise, R.J.; Greene, J.; Büchel, C.; Scott, S.K. Brain Regions Involved in Articulation. Lancet 1999, 353, 1057–1061. [Google Scholar] [CrossRef]
- Manes, J.L.; Parkinson, A.L.; Larson, C.R.; Greenlee, J.D.; Eickhoff, S.B.; Corcos, D.M.; Robin, D.A. Connectivity of the Subthalamic Nucleus and Globus Pallidus Pars Interna to Regions within the Speech Network: A Meta-Analytic Connectivity Study. Hum. Brain Mapp. 2014, 35, 3499–3516. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Laird, A.R.; Glahn, D.C.; Blangero, J.; Sanghera, M.K.; Pessoa, L.; Fox, P.M.; Uecker, A.; Friehs, G.; Young, K.A.; et al. The Functional Connectivity of the Human Caudate: An Application of Meta-Analytic Connectivity Modeling with Behavioral Filtering. Neuroimage 2012, 60, 117–129. [Google Scholar] [CrossRef] [PubMed]
- di Martino, A.; Scheres, A.; Margulies, D.S.; Kelly, A.M.C.; Uddin, L.Q.; Shehzad, Z.; Biswal, B.; Walters, J.R.; Castellanos, F.X.; Milham, M.P. Functional Connectivity of Human Striatum: A Resting State FMRI Study. Cereb. Cortex 2008, 18, 2735–2747. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Wang, D.; Pan, R.; Holt, D.J.; Liu, H.; RW, K. Abnormalities in Hemispheric Specialization of Caudate Nucleus Connectivity in Schizophrenia. JAMA Psychiatry 2015, 72, 552. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and Emotional Control of Pain and Its Disruption in Chronic Pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Borsook, D.; Upadhyay, J.; Chudler, E.H.; Becerra, L. A Key Role of the Basal Ganglia in Pain and Analgesia—Insights Gained through Human Functional Imaging. Mol. Pain 2010, 6, 1744-8069-6-27. [Google Scholar] [CrossRef]
- Emmert, K.; Breimhorst, M.; Bauermann, T.; Birklein, F.; van de Ville, D.; Haller, S. Comparison of Anterior Cingulate vs. Insular Cortex as Targets for Real-Time FMRI Regulation during Pain Stimulation. Front. Behav. Neurosci. 2014, 8, 350. [Google Scholar] [CrossRef]
- Phillips, R.G.; LeDoux, J.E. Differential Contribution of Amygdala and Hippocampus to Cued and Contextual Fear Conditioning. Behav. Neurosci. 1992, 106, 274–285. [Google Scholar] [CrossRef]
- Roy, A.K.; Shehzad, Z.; Margulies, D.S.; Kelly, A.M.C.; Uddin, L.Q.; Gotimer, K.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional Connectivity of the Human Amygdala Using Resting State FMRI. Neuroimage 2009, 45, 614–626. [Google Scholar] [CrossRef]
- Baur, V.; Hänggi, J.; Langer, N.; Jäncke, L. Resting-State Functional and Structural Connectivity within an Insula-Amygdala Route Specifically Index State and Trait Anxiety. Biol. Psychiatry 2013, 73, 85–92. [Google Scholar] [CrossRef]
- Viinikainen, M.; Jääskeläinen, I.P.; Alexandrov, Y.; Balk, M.H.; Autti, T.; Sams, M. Nonlinear Relationship between Emotional Valence and Brain Activity: Evidence of Separate Negative and Positive Valence Dimensions. Hum. Brain Mapp. 2009, 31, 1030–1040. [Google Scholar] [CrossRef]
- Smith, B.W.; Mitchell, D.G.V.; Hardin, M.G.; Jazbec, S.; Fridberg, D.; Blair, R.J.R.; Ernst, M. Neural Substrates of Reward Magnitude, Probability, and Risk during a Wheel of Fortune Decision-Making Task. Neuroimage 2009, 44, 600–609. [Google Scholar] [CrossRef]
- Adolfi, F.; Couto, B.; Richter, F.; Decety, J.; Lopez, J.; Sigman, M.; Manes, F.; Ibáñez, A. Convergence of Interoception, Emotion, and Social Cognition: A Twofold FMRI Meta-Analysis and Lesion Approach. Cortex 2017, 88, 124–142. [Google Scholar] [CrossRef]
- Salgado, S.; Kaplitt, M.G. The Nucleus Accumbens: A Comprehensive Review. Ster. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef]
- Dambacher, F.; Sack, A.T.; Lobbestael, J.; Arntz, A.; Brugman, S.; Schuhmann, T. Out of Control: Evidence for Anterior Insula Involvement in Motor Impulsivity and Reactive Aggression. Soc. Cogn. Affect Neurosci. 2013, 10, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Nachev, P.; Lopez-Sosa, F.; Gonzalez-Rosa, J.J.; Galarza, A.; Avecillas, J.; Pineda-Pardo, J.A.; Lopez-Ibor, J.J.; Reneses, B.; Barcia, J.A.; Strange, B. Dynamic Risk Control by Human Nucleus Accumbens. Brain 2015, 138, 3496–3502. [Google Scholar] [CrossRef]
- Liu, X.; Hairston, J.; Schrier, M.; Fan, J. Common and Distinct Networks Underlying Reward Valence and Processing Stages: A Meta-Analysis of Functional Neuroimaging Studies. Neurosci. Biobehav. Rev. 2011, 35, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.; Studer, B.; Bruss, J.; Tranel, D.; Bechara, A. Damage to Insula Abolishes Cognitive Distortions during Simulated Gambling. Proc. Natl. Acad. Sci. USA 2014, 111, 6098–6103. [Google Scholar] [CrossRef]
- Craig, A.D. Human Feelings: Why Are Some More Aware than Others? Trends Cogn. Sci. 2004, 8, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Interoception: The Sense of the Physiological Condition of the Body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Craig, A.D. How Do You Feel--Now? The Anterior Insula and Human Awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Tian, Y.; Zalesky, A. Characterizing the Functional Connectivity Diversity of the Insula Cortex: Subregions, Diversity Curves and Behavior. Neuroimage 2018, 183, 716–733. [Google Scholar] [CrossRef]
- Ryvlin, P.; Nguyen, D.K. Insular Seizures and Epilepsies: Ictal Semiology and Minimal Invasive Surgery. Curr. Opin. Neurol. 2021, 34, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Yen Tran, T.P.; Boucher, O.; Bouthillier, A.; Nguyen, D.K. Operculo-Insular Epilepsy: Scalp and Intracranial Electroencephalographic Findings. J. Clin. Neurophysiol. 2017, 34, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Hébert-Seropian, B.; Boucher, O.; Jutras-Aswad, D.; Nguyen, D.K. Uncommon Case of Complete Loss of Hunger Following an Isolated Left Insular Stroke. Neurocase 2021, 27, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Hébert-Seropian, B.; Boucher, O.; Citherlet, D.; Roy-Côté, F.; Gravel, V.; Obaid, S.; Bouthillier, A.; Nguyen, D.K. Decreased Self-Reported Appetite Following Insular Cortex Resection in Patients with Epilepsy. Appetite 2021, 166, 105479. [Google Scholar] [CrossRef] [PubMed]
- Roy-Côté, F.; Zahal, R.; Frasnelli, J.; Nguyen, D.K.; Boucher, O. Insula and Olfaction: A Literature Review and Case Report. Brain Sci. 2021, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Boucher, O.; Turgeon, C.; Champoux, S.; Menard, L.; Rouleau, I.; Lassonde, M.; Lepore, F.; Nguyen, D.K. Hyperacusis Following Unilateral Damage to the Insular Cortex: A Three-Case Report. Brain Res. 2015, 1606, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Denis, D.J.; Marouf, R.; Rainville, P.; Bouthillier, A.; Nguyen, D.K. Effects of Insular Stimulation on Thermal Nociception. Eur. J. Pain 2016, 20, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Von Siebenthal, Z.; Boucher, O.; Rouleau, I.; Lassonde, M.; Lepore, F.; Nguyen, D.K. Decision-Making Impairments Following Insular and Medial Temporal Lobe Resection for Drug-Resistant Epilepsy. Soc. Cogn. Affect. Neurosci. 2017, 12, 128–137. [Google Scholar] [CrossRef]
- Citherlet, D.; Boucher, O.; Gravel, V.; Roy-Côté, F.; Bouthillier, A.; Nguyen, D.K. The Effects of Insular and Mesiotemporal Lesions on Affective Information Processing: Preliminary Evidence from Patients with Epilepsy Surgery. Epilepsy Behav. 2020, 111, 107264. [Google Scholar] [CrossRef]
- Boucher, O.; Rouleau, I.; Lassonde, M.; Lepore, F.; Bouthillier, A.; Nguyen, D.K. Social Information Processing Following Resection of the Insular Cortex. Neuropsychologia 2015, 71, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, D.; Obaid, S.; Fournier-Gosselin, M.P.; Bouthillier, A.; Nguyen, D.K. Deep Brain Stimulation of the Posterior Insula in Chronic Pain: A Theoretical Framework. Brain Sci. 2021, 11, 639. [Google Scholar] [CrossRef]
- Zangen, A.; Moshe, H.; Martinez, D.; Barnea-Ygael, N.; Vapnik, T.; Bystritsky, A.; Duffy, W.; Toder, D.; Casuto, L.; Grosz, M.L.; et al. Repetitive Transcranial Magnetic Stimulation for Smoking Cessation: A Pivotal Multicenter Double-Blind Randomized Controlled Trial. World Psychiatry 2021, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Krienen, F.M.; Yeo, B.T.T. Opportunities and Limitations of Intrinsic Functional Connectivity MRI. Nat. Neurosci. 2013, 16, 832–837. [Google Scholar] [CrossRef] [PubMed]
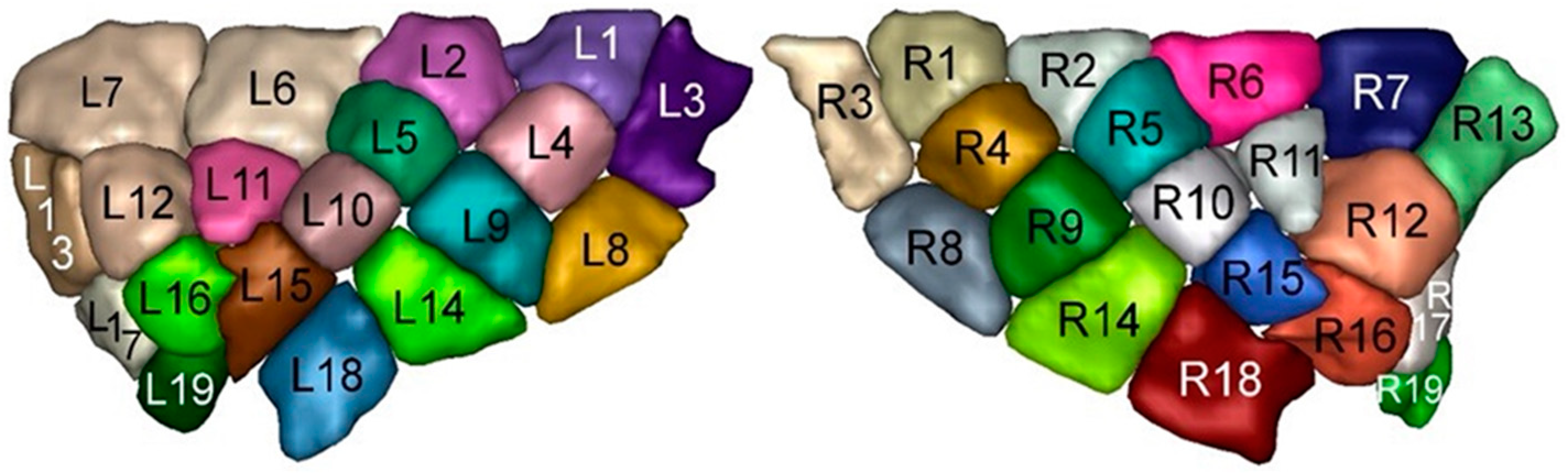
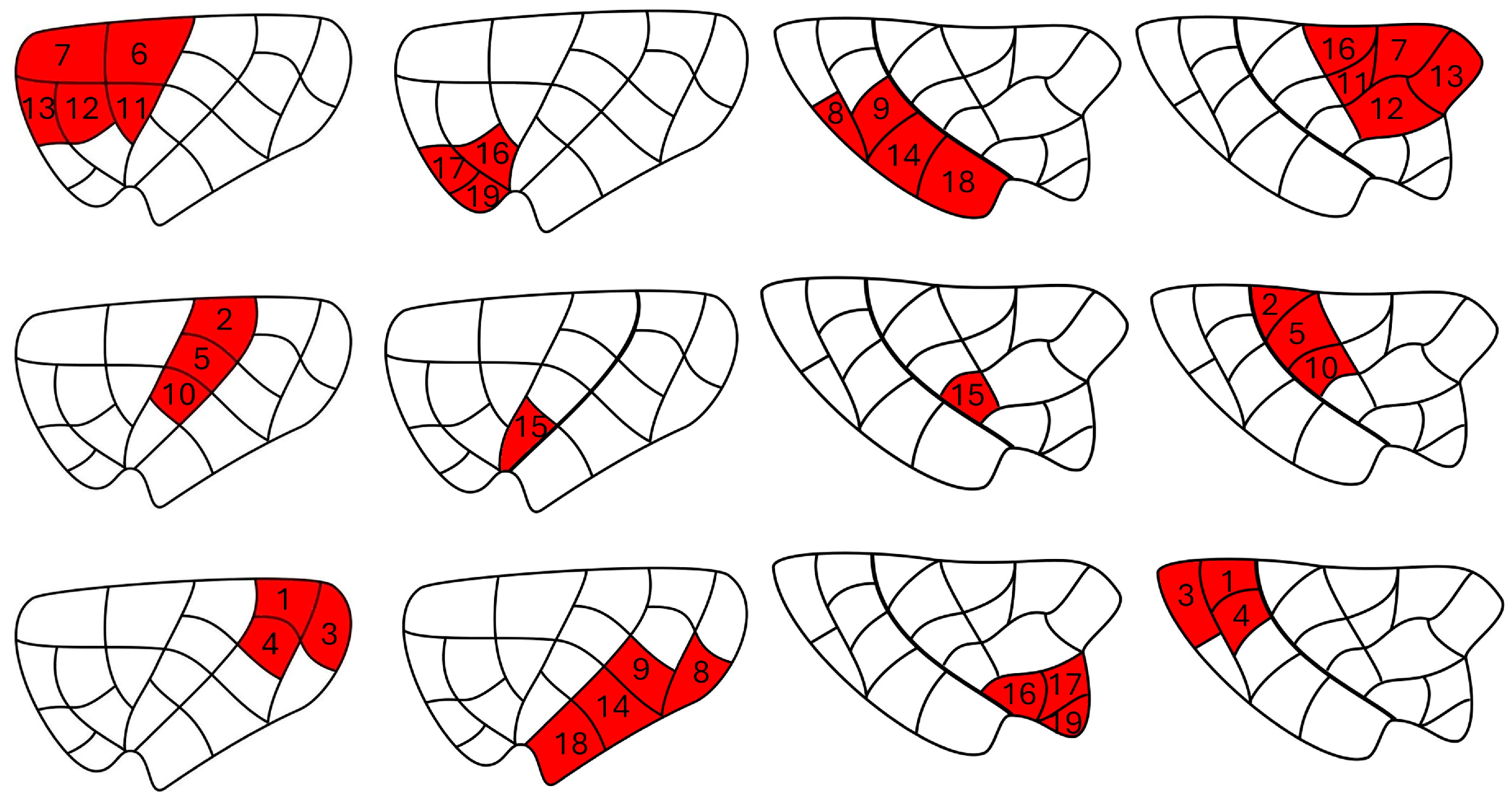

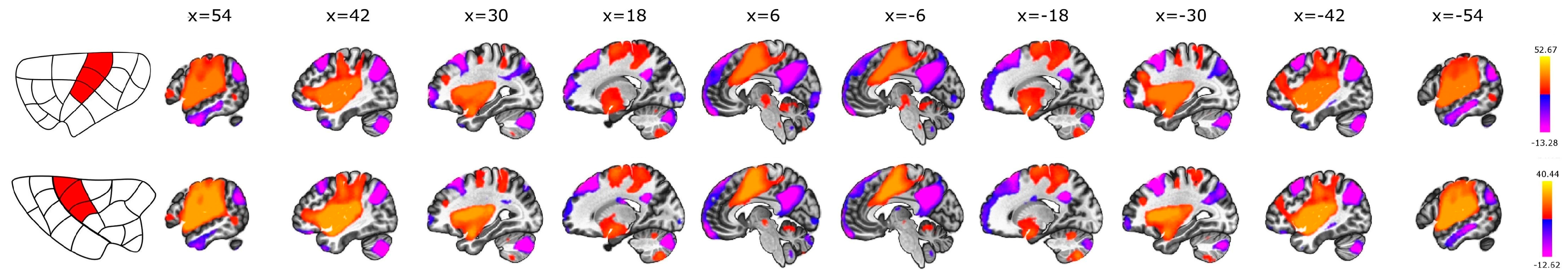

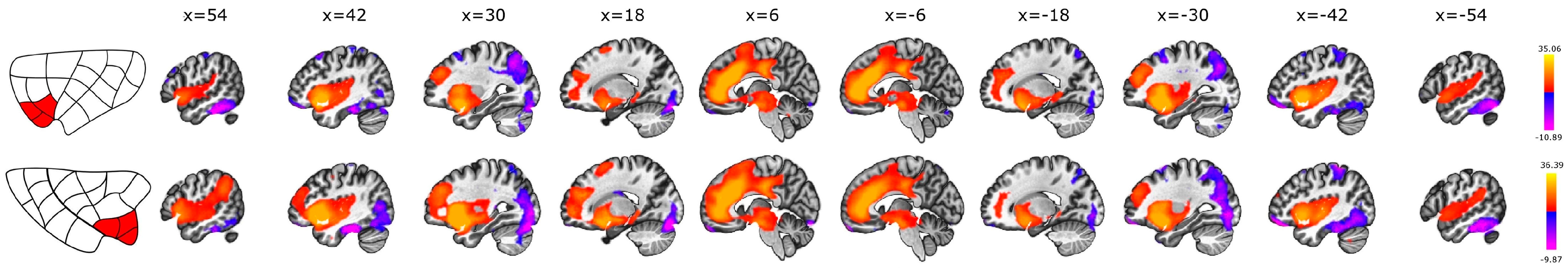


| Insular Subregion | ROI |
|---|---|
| Dorsal anterior | 6, 7, 11, 12, 13 |
| Ventral anterior | 16, 17, 19 |
| Dorsal middle | 2, 5, 10 |
| Ventral middle | 15 |
| Dorsal posterior | 1, 3, 4 |
| Ventral posterior | 8, 9, 14, 18 |
| Multimodal Insular Subdivision (Glasser et al. 2016 [22]) | Current Insular Subdivision |
|---|---|
| AVI (anterior ventral insular area) | dAI |
| AAIC (anterior agranular insular complex) | vAI |
| Middle insular area (MI) | dMI |
| Insula granular (Ig) | dPI |
| Posterior insular areas (Pol1 and Pol2) | Part of vMI and vPI |
| Parainsular cortex (PI) | vPI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaziri, J.; Fei, P.; Tucholka, A.; Obaid, S.; Boucher, O.; Rouleau, I.; Nguyen, D.K. Resting-State Functional Connectivity Profile of Insular Subregions. Brain Sci. 2024, 14, 742. https://doi.org/10.3390/brainsci14080742
Ghaziri J, Fei P, Tucholka A, Obaid S, Boucher O, Rouleau I, Nguyen DK. Resting-State Functional Connectivity Profile of Insular Subregions. Brain Sciences. 2024; 14(8):742. https://doi.org/10.3390/brainsci14080742
Chicago/Turabian StyleGhaziri, Jimmy, Phillip Fei, Alan Tucholka, Sami Obaid, Olivier Boucher, Isabelle Rouleau, and Dang K. Nguyen. 2024. "Resting-State Functional Connectivity Profile of Insular Subregions" Brain Sciences 14, no. 8: 742. https://doi.org/10.3390/brainsci14080742





