Neuromodulation Treatments Targeting Pathological Synchrony for Tinnitus in Adults: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion/Exclusion Criteria
2.2. Search Strategy
2.3. Selection of Studies
2.4. Data Extraction
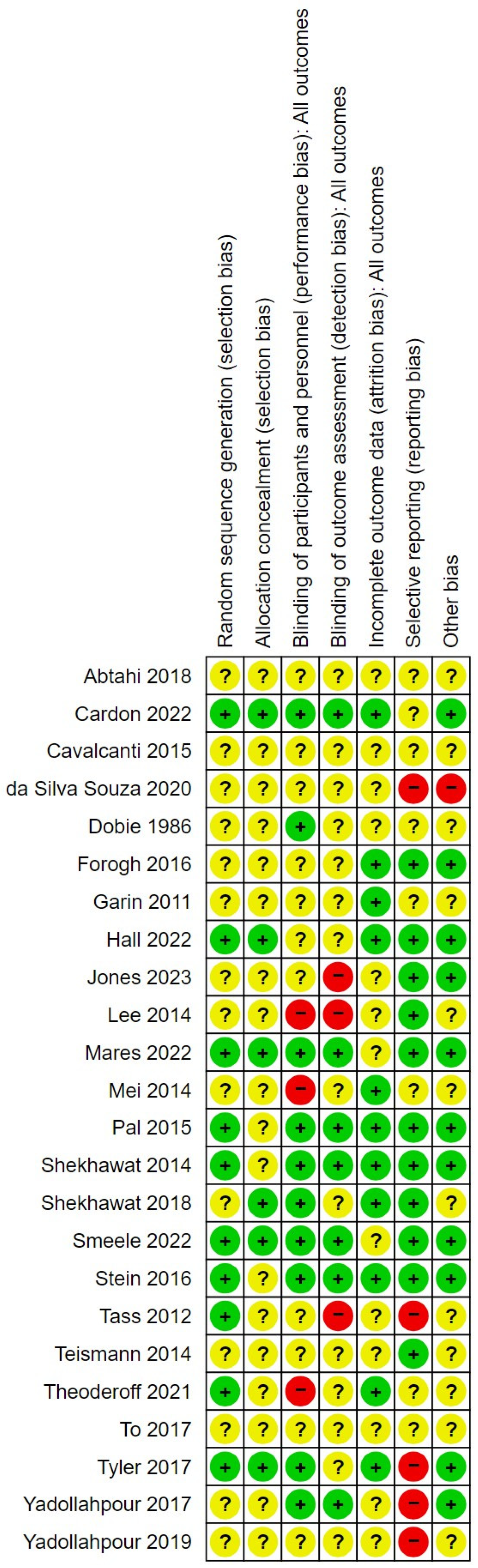
2.5. Risk of Bias
2.6. Analysis
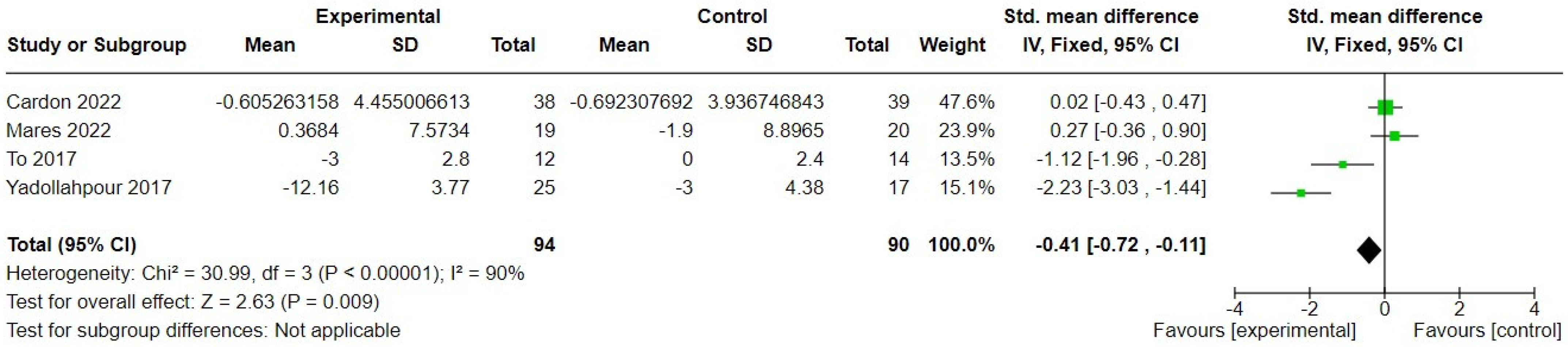
3. Results
3.1. Risk of Bias in the Included Studies
3.2. Synthesis of Findings
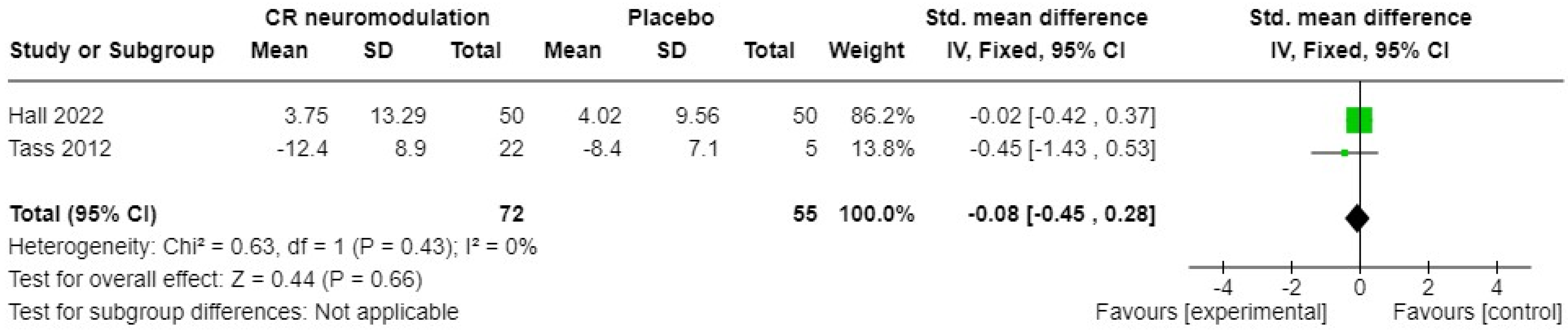
3.2.1. Acoustic Neuromodulation Interventions
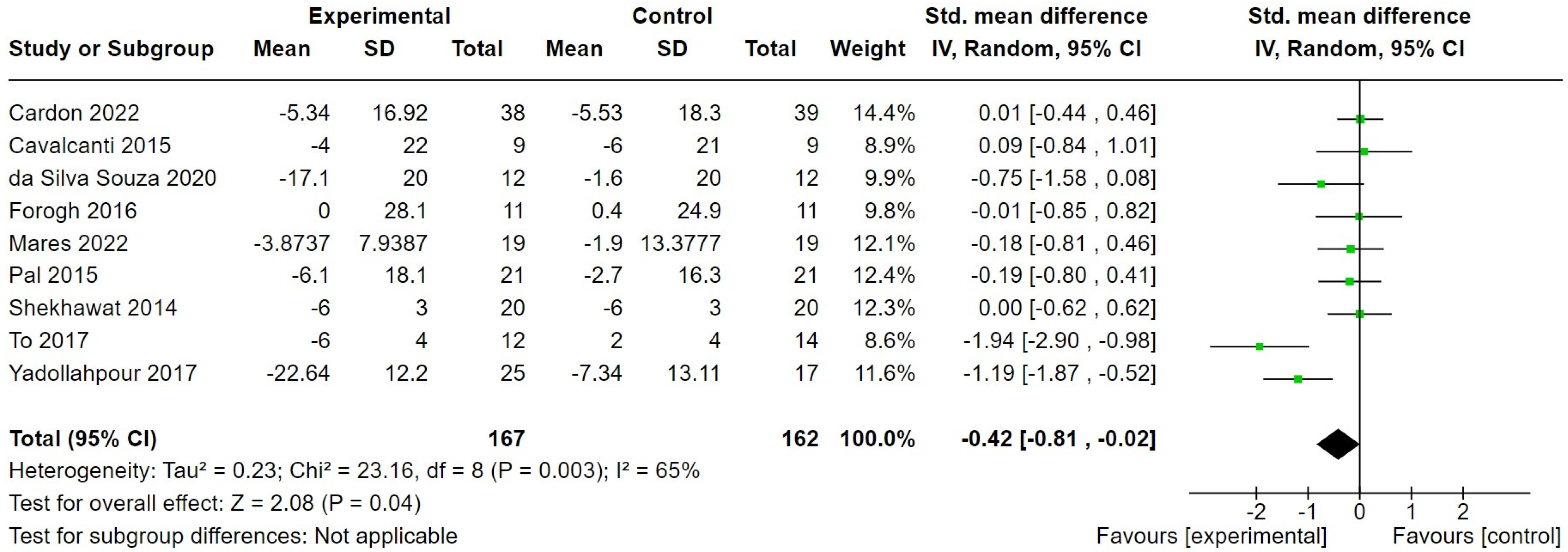
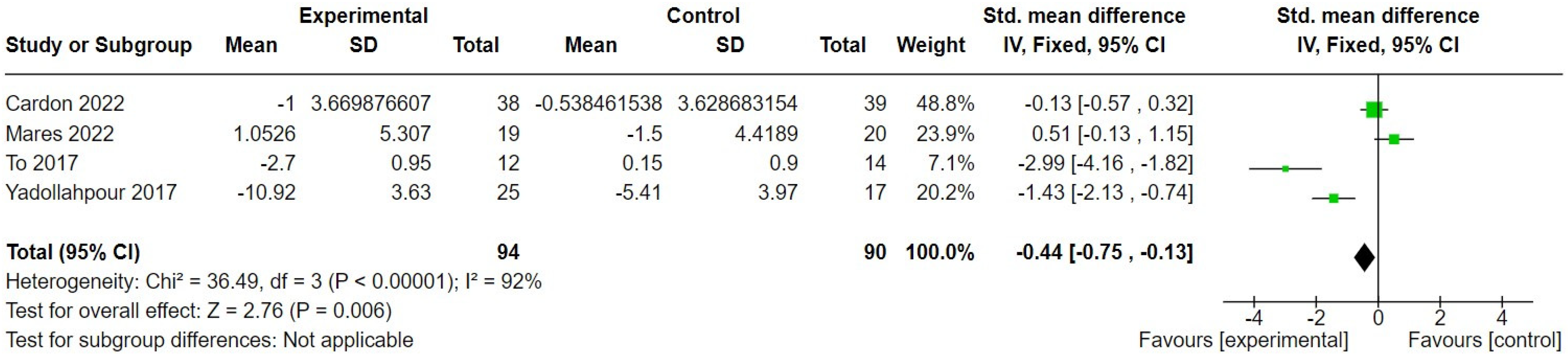
3.2.2. Transcranial Direct Current Stimulation
3.2.3. Vagus Nerve Stimulation
3.2.4. Alternating Current Stimulation
3.2.5. Combined Auditory and Somatosensory Stimulation
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- De Ridder, D.; Schlee, W.; Vanneste, S.; Londero, A.; Weisz, N.; Kleinjung, T.; Shekhawat, G.S.; Elgoyhen, A.B.; Song, J.J.; Andersson, G.; et al. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 2021, 260, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Jarach, C.M.; Lugo, A.; Scala, M.; van den Brandt, P.A.; Cederroth, C.R.; Odone, A.; Garavello, W.; Schlee, W.; Langguth, B.; Gallus, S. Global prevalence and incidence of tinnitus: A systematic review and meta-analysis. JAMA Neurol. 2022, 79, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, T.G.; Ferrari, G.M.S. The control of tinnitus through hearing aids: Suggestions for optimal use. Pró-Fono Rev. Atual. Cient. 2002, 14, 111–118. [Google Scholar]
- Fuller, T.; Cima, R.; Langguth, B.; Mazurek, B.; Vlaeyen, J.W.S.; Hoare, D.J. Cognitive behavioural therapy for tinnitus. Cochrane Database Syst. Rev. 2020, 1, CD012614. [Google Scholar] [CrossRef] [PubMed]
- Fuller, T.; Hoare, D.J. CBT for Tinnitus. In Textbook of Tinnitus, 2nd ed.; Schlee, W., Langguth, B., De Ridder, D., Vanneste, S., Kleinjung, T., Møller, A.R., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 545–561. [Google Scholar]
- Handscomb, L.; Shorter, G.W.; Hoare, D.J.; Hall, D.A. Evaluation of a cognitive behavioral model of tinnitus distress: A cross-sectional study using structural equation modeling. Ear Hear. 2020, 41, 1028–1039. [Google Scholar] [CrossRef]
- Weisz, N.; Moratti, S.; Meinzer, M.; Dohrmann, K.; Elbert, T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005, 2, e153. [Google Scholar] [CrossRef]
- Weisz, N.; Muller, S.; Schlee, W.; Dohrmann, K.; Hartmann, T.; Elbert, T. The neural code of auditory phantom perception. J. Neurosci. 2007, 27, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Kahlbrock, N.; Weisz, N. Transient reduction of tinnitus intensity is marked by concomitant reductions of delta band power. BMC Biol. 2008, 6, 4. [Google Scholar] [CrossRef]
- Adjamian, P.; Sereda, M.; Zobay, O.; Hall, D.A.; Palmer, A.R. Neuromagnetic indicators of tinnitus and tinnitus masking in patients with and without hearing loss. J. Assos. Res. Otolaryngol. 2012, 13, 715–731. [Google Scholar] [CrossRef]
- Schlee, W.; Schecklmann, M.; Lehner, A.; Kreuzer, P.M.; Vielsmeier, V.; Poeppl, T.B.; Langguth, B. Reduced variability of auditory alpha activity in chronic tinnitus. Neural Plast. 2014, 1, 436146. [Google Scholar] [CrossRef]
- Tass, P.A.; Popovych, O.V. Unlearning tinnitus-related cerebral synchrony with acoustic coordinated reset stimulation: Theoretical concept and modelling. Biol. Cybern. 2012, 106, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ashton, H.; Reid, K.; Marsh, R.; Johnson, I.; Alter, K.; Griffiths, T. High frequency localised “hot spots” in temporal lobes of patients with intractable tinnitus: A quantitative electroencephalographic (QEEG) study. Neurosci. Lett. 2007, 426, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Antal, A.; Varga, E.T.; Kincses, T.Z.; Nitsche, M.A.; Paulus, W. Oscillatory brain activity and transcranial direct current stimulation in humans. Neuroreport 2004, 15, 1307–1310. [Google Scholar] [CrossRef] [PubMed]
- Ironside, M.; Walsh, V. A double blind, placebo controlled exploration of the effect of repeated sessions of transcranial alternating current stimulation on tinnitus loudness and distress. Clin. Neurophysiol. 2013, 124, e184–e185. [Google Scholar] [CrossRef]
- Vanneste, S.; De Ridder, D. Noninvasive and invasive neuromodulation for the treatment of tinnitus: An overview. Neuromodulation 2012, 15, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Adamchic, I.; Hauptmann, C.; Barnikol, U.B.; Pawelczyk, N.; Popovych, O.; Barnikol, T.T.; Silchenko, A.; Volkmann, J.; Deuschl, G.; Meissner, W.G.; et al. Coordinated reset neuromodulation for Parkinson’s disease: Proof-of-concept study. Mov. Disord. 2014, 29, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Tass, P.A.; Hauptmann, C. Anti-kindling achieved by stimulation targeting slow synaptic dynamics. Restor. Neurol. Neurosci. 2009, 27, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Engineer, N.D.; Riley, J.R.; Seale, J.D.; Vrana, W.A.; Shetake, J.A.; Sudanagunta, S.P.; Borland, M.S.; Kilgard, M.P. Reversing pathological neural activity using targeted plasticity. Nature 2011, 470, 101–104. [Google Scholar] [CrossRef]
- Engineer, N.D.; Møller, A.R.; Kilgard, M.P. Directing neural plasticity to understand and treat tinnitus. Hear. Res. 2013, 295, 58–66. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Engineer, N.D.; Kilgard, M.P. Safety and efficacy of vagus nerve stimulation paired with tones for the treatment of tinnitus: A case series. Neuromodulation 2012, 17, 170–179. [Google Scholar] [CrossRef]
- Lehtimäki, J.; Hyvärinen, P.; Ylikoski, M.; Bergholm, M.; Mäkelä, J.P. Transcutaneous vagus nerve stimulation in tinnitus: A pilot study. Acta Otolaryngol. 2013, 133, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, P.M.; Landgrebe, M.; Resch, M.; Husser, O.; Schecklmann, M.; Geisreiter, F.; Poeppl, T.B.; Prasser, S.J.; Hajak, G.; Rupprecht, R.; et al. Feasibility, safety and efficacy of transcutaneous vagus nerve stimulation in chronic tinnitus: An open pilot study. Brain Stimul. 2014, 7, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Hoare, D.J.; Whitham, D.; Henry, J.A.; Shorter, G.W. Neuromodulation (desynchronisation) for tinnitus in adults. Cochrane Database Syst. Rev. 2015, 6, CD011760. [Google Scholar] [CrossRef]
- Sereda, M.; McFerran, D.; Axon, E.; Baguley, D.M.; Hall, D.A.; Potgieter, I.; Cima, R.; Cox, S.; Hoare, D.J. A process for prioritising systematic reviews in tinnitus. Int. J. Audiol. 2020, 59, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0.; The Cochrane Collaboration; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Huedo-Medina, T.B.; Sànchez-Meca, J.; Marín-Martinez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, H.; Okhovvat, A.; Heidari, S.; Gharagazarloo, A.; Mirdamadi, M.; Nilforoush, M.H.; Ghazavi, H. Effect of transcranial direct current stimulation on short-term and long-term treatment of chronic tinnitus. Am. J. Otolaryngol. 2018, 39, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Cardon, E.; Jacquemin, L.; Vermeersch, H.; Joossen, I.; Moyaert, J.; Mertens, G.; Vanderveken, O.M.; Lammers, M.J.; Van de Heyning, P.; Van Rompaey, V.; et al. Dual-site transcranial direct current stimulation to treat tinnitus: A randomized controlled trial. Brain 2022, 145, 4222–4231. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, K.; Brasil-Neto, J.P.; Allam, N.; Boechat-Barros, R. A double-blind, placebo-controlled study of the effects of daily tDCS sessions targeting the dorsolateral prefrontal cortex on tinnitus handicap inventory and visual analog scale scores. Brain Stimul. 2015, 8, 978–980. [Google Scholar] [CrossRef] [PubMed]
- da Silva Souza, D.; Almeidaa, A.A.; dos Santos Andrade, S.M.; da Silva Machado, D.G.; Leitão, M.; Sanchez, T.G.; da Rosa, M.R.D. Transcranial direct current stimulation improves tinnitus perception and modulates cortical electrical activity in patients with tinnitus: A randomized clinical trial. Clin. Neurophysiol. 2020, 50, 289–300. [Google Scholar] [CrossRef]
- Dobie, R.A.; Hoberg, K.E.; Rees, T.S. Electrical tinnitus suppression: A double-blind crossover study. Otolaryngol. Head Neck Surg. 1986, 95, 319–323. [Google Scholar] [CrossRef]
- Forogh, B.; Mirshaki, Z.; Raissi, G.R.; Shirazi, A.; Mansoori, K.; Ahadi, T. Repeated sessions of transcranial direct current stimulation for treatment of chronic subjective tinnitus: A pilot randomized controlled trial. Neurol. Sci. 2016, 37, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Garin, P.; Gilain, C.; Van Damme, J.P.; de Fays, K.; Jamart, J.; Ossemann, M.; Vandermeeren, Y. Short-and long-lasting tinnitus relief induced by transcranial direct current stimulation. J. Neurol. 2011, 258, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Pierzycki, R.H.; Thomas, H.; Greenberg, D.; Sereda, M.; Hoare, D.J. Systematic evaluation of the T30 neurostimulator treatment for tinnitus: A double-blind randomised placebo-controlled trial with open-label extension. Brain Sci. 2022, 12, 317. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.R.; Martel, D.T.; Riffle, T.L.; Errickson, J.; Souter, J.R.; Basura, G.J.; Stucken, E.; Schvartz-Leyzac, K.C.; Shore, S.E. Reversing synchronized brain circuits using targeted auditory-somatosensory stimulation to treat phantom percepts: A randomized clinical trial. JAMA Netw. Open 2023, 6, e2315914. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Chung, H.; Chung, J.H.; Yeo, S.G.; Park, M.S.; Byun, J.Y. Effectiveness of transcutaneous electrical stimulation for chronic tinnitus. Acta Otolaryngol. 2014, 134, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Mares, T.; Albrecht, J.; Buday, J.; Podgorna, G.; Le, T.H.; Magyarova, E.; Poshor, K.; Halik, J.; Buna, J.; Capek, V.; et al. Long-term effect of transcranial direct current stimulation in the treatment of chronic tinnitus: A randomized, placebo-controlled trial. Front. Psychiatry 2022, 13, 969800. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.G.; Yang, S.B.; Cai, S.J.; Lei, H.P.; Chuang, Z.H.; Guo, Y.H.; Zhang, D.Q. Treatment of tinnitus with electrical stimulation on acupoint in the distribution area of ear vagus nerve combining with sound masking: Randomized controlled trial. World J. Acupunct. Moxibustion 2014, 24, 30–35. [Google Scholar] [CrossRef]
- Pal, N.; Maire, R.; Stephan, M.A.; Herrmann, F.R.; Benninger, D.H. Transcranial direct current stimulation for the treatment of chronic tinnitus: A randomized controlled study. Brain Stimul. 2015, 8, 1101–1107. [Google Scholar] [CrossRef]
- Shekhawat, G.S.; Searchfield, G.D.; Stinear, C.M. Randomized trial of transcranial direct current stimulation and hearing aids for tinnitus management. Neurorehabilit. Neural Repair 2014, 28, 410–419. [Google Scholar] [CrossRef]
- Shekhawat, G.S.; Vanneste, S. High-definition transcranial direct current stimulation of the dorsolateral prefrontal cortex for tinnitus modulation: A preliminary trial. J. Neural Transm. 2018, 125, 163–171. [Google Scholar] [CrossRef]
- Smeele, S.J.; Adhia, D.B.; De Ridder, D. Feasibility and safety of high-definition infraslow pink noise stimulation for treating chronic tinnitus—A randomized placebo-controlled trial. Neuromodulation 2023, 26, 801–816. [Google Scholar] [CrossRef]
- Stein, A.; Wunderlich, R.; Lau, P.; Engell, A.; Wollbrink, A.; Shaykevich, A.; Kuhn, J.T.; Holling, H.; Rudack, C.; Pantev, C. Clinical trial on tonal tinnitus with tailor made notched music training. BMC Neurol. 2016, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Tass, P.A.; Adamchic, I.; Freunda, H.J.; von Stackelberg, T.; Hauptmann, C. Counteracting tinnitus by acoustic coordinated reset neuromodulation. Restor. Neurol. Neurosci. 2012, 30, 137–159. [Google Scholar] [CrossRef]
- Teismann, H.; Wollbrink, A.; Okamoto, H.; Schlaug, G.; Rudack, C.; Pantev, C. Combining transcranial direct current stimulation and tailor-made notched music training to decrease tinnitus-related distress–A pilot study. PLoS ONE 2014, 9, e89904. [Google Scholar] [CrossRef]
- Theodoroff, S.M.; McMillan, G.P.; Schmidt, C.J.; Dann, S.M.; Hauptmann, C.; Goodworth, M.C.; Leibowitz, R.Q.; Random, C.; Henry, J.A. Randomised controlled trial of interventions for bothersome tinnitus: DesyncraTM versus cognitive behavioural therapy. Int. J. Audiol. 2022, 61, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- To, W.T.; Ost, J.; Hart, J.; De Ridder, D.; Vanneste, S. The added value of auditory cortex transcranial random noise stimulation (tRNS) after bifrontal transcranial direct current stimulation (tDCS) for tinnitus. J. Neural Transm. 2017, 124, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Tyler, R.; Cacace, A.; Stocking, C.; Tarver, B.; Engineer, N.; Martin, J.; Deshpande, A.; Stecker, N.; Pereira, M.; Kilgard, M.; et al. Vagus nerve stimulation paired with tones for the treatment of tinnitus: A prospective randomized double-blind controlled pilot study in humans. Sci. Rep. 2017, 7, 11960. [Google Scholar] [CrossRef]
- Yadollahpour, A.; Bayat, A.; Rashidi, S.; Saki, N.; Karimi, M. Dataset of acute repeated sessions of bifrontal transcranial direct current stimulation for treatment of intractable tinnitus: A randomized controlled trial. Data Brief 2017, 15, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yadollahpour, A.; Rashidi, S.; Jaberzade, S. Single session anodal, cathodal and placebo bifrontal tDCS for treatment of intractable chronic tinnitus: A randomized controlled clinical trial. Brain Stimul. 2019, 12, 406. [Google Scholar] [CrossRef]
- Meikle, M.B.; Henry, J.A.; Griest, S.E.; Stewart, B.J.; Abrams, H.B.; McArdle, R.; Myers, P.J.; Newman, C.W.; Sandridge, S.; Turk, D.C.; et al. The tinnitus functional index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear. 2012, 33, 153–176. [Google Scholar] [CrossRef]
- Newman, C.; Jacobson, G.; Spitzer, B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Kuk, F.K.; Tyler, R.S.; Russell, D.; Jordan, H. The psychometric properties of a tinnitus handicap questionnaire. Ear Hear. 1990, 11, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Hiller, W.; Goebel, G. Factors influencing tinnitus loudness and annoyance. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Ward, C.; Mendelson, M.; Mock, J.; Erbaugh, J.J.A.G.P. Beck depression inventory (BDI). Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Whoqol Group. Development of the WHOQOL: Rationale and current status. Int. J. Ment. Health 1994, 23, 24–56. [Google Scholar] [CrossRef]
- Staehr, J.K. The use of well-being measures in primary health care-the DepCare project. In World Health Organization, Regional Office for Europe: Well-Being Measures in Primary Health Care-the DepCare Project; World Health Organization: Geneva, Switzerland, 1998. [Google Scholar]
- Herdman, M.; Gudex, C.; Lloyd, A.; Janssen, M.F.; Kind, P.; Parkin, D.; Bonsel, G.; Badia, X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual. Life Res. 2011, 20, 1727–1736. [Google Scholar] [CrossRef]
- Jacquemin, L.; Shekhawat, G.S.; Van de Heyning, P.; Mertens, G.; Fransen, E.; Van Rompaey, V.; Topsakal, V.; Moyaert, J.; Beyers, J.; Gilles, A. Effects of electrical stimulation in tinnitus patients: Conventional versus high-definition tDCS. Neurorehab. Neural Repair 2018, 32, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893. [Google Scholar] [CrossRef]
- Biggs, J.T.; Wylie, L.T.; Ziegler, V.E. Validity of the Zung self-rating depression scale. Br. J. Psychiatry 1978, 132, 381–385. [Google Scholar] [CrossRef]
- Whoqol Group. Development of the world health organization WHOQOL-BREF quality of life assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar] [CrossRef]
- Lewis, P.M.; Thomson, R.H.; Rosenfeld, J.V.; Fitzgerald, P.B. Brain neuromodulation techniques: A review. Neuroscientist 2016, 22, 406–421. [Google Scholar] [CrossRef]
- Cima, R.F.; Kikidis, D.; Mazurek, B.; Haider, H.; Cederroth, C.R.; Norena, A.; Lapira, A.; Bibas, A.; Hoare, D.J. Tinnitus healthcare: A survey revealing extensive variation in opinion and practices across Europe. BMJ Open 2020, 10, e029346. [Google Scholar] [CrossRef] [PubMed]
- Sereda, M.; Xia, J.; El Refaie, A.; Hall, D.A.; Hoare, D.J. Sound therapy (using amplification devices and/or sound generators) for tinnitus. Cochrane Database Syst. Rev. 2018, 12, CD013094. [Google Scholar] [CrossRef]
- Labree, B.; Hoare, D.J.; Gascoyne, L.E.; Scutt, P.; Del Giovane, C.; Sereda, M. Determining the effects of transcranial direct current stimulation on tinnitus, depression, and anxiety: A systematic review. Brain Sci. 2022, 12, 484. [Google Scholar] [CrossRef]
- Ciechanski, P.; Carlson, H.L.; Yu, S.S.; Kirton, A. Modeling transcranial direct-current stimulation-induced electric fields in children and adults. Front. Hum. Neurosci. 2018, 12, 268. [Google Scholar] [CrossRef]
- Lee, J.S.A.; Bestmann, S.; Evans, C. A Future of Current Flow Modelling for Transcranial Electrical Stimulation? Curr. Behav. Neurosci. Rep. 2021, 8, 150–159. [Google Scholar] [CrossRef]
- Evans, C.; Bachmann, C.; Lee, J.S.; Gregoriou, E.; Ward, N.; Bestmann, S. Dose-controlled tDCS reduces electric field intensity variability at a cortical target site. Brain Stim. 2020, 13, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, S.; Boutron, I.; Moher, D. CONSORT and Its Extensions for Reporting Clinical Trials. In Principles and Practice of Clinical Trials; Piantadosi, S., Meinert, C., Eds.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Puljak, L.; Ramic, I.; Naharro, C.A.; Brezova, J.; Lin, Y.C.; Surdila, A.A.; Tomajkova, E.; Farias Medeiros, I.; Nikolovska, M.; Poklepovic Pericic, T.; et al. Cochrane risk of bias tool was used inadequately in the majority of non-Cochrane systematic reviews. J. Clin. Epidemiol. 2020, 123, 114–119. [Google Scholar] [CrossRef]
- Labree, B.; Hoare, D.J.; Fackrell, K.; Hall, D.A.; Gascoyne, L.E.; Sereda, M. Establishing a Core Domain Set for early-phase clinical trials of electrical stimulation interventions for tinnitus in adults: Protocol for an online Delphi study. Trials 2022, 23, 1039. [Google Scholar] [CrossRef]
- Hall, D.A.; Smith, H.; Hibbert, A.; Colley, V.; Haider, H.F.; Horobin, A.; Londero, A.; Mazurek, B.; Thacker, B.; Fackrell, K.; et al. Core Outcome Measures in Tinnitus (COMiT) initiative. The COMiT’ID study: Developing core outcome domains sets for clinical trials of sound-, psychology-, and pharmacology-based interventions for chronic subjective tinnitus in adults. Trends Hear. 2018, 22, 2331216518814384. [Google Scholar] [CrossRef] [PubMed]


| Participants | Intervention | Comparison | Main Outcome | |
|---|---|---|---|---|
| Acoustic | ||||
| Tass 2012 [45] | 44 male, 19 female. Chronic (≥6 months) tonal tinnitus. Mean age 45.7 years (SD = 10.8) in intervention and 57.6 years (SD = 6.3) in control group. | Acoustic CR(r) neuromodulation (4 tones per sequence above and below dominant tinnitus pitch) for 4–6 h a day for 12 weeks. | Placebo sound stimulation using tones distant from the dominant tinnitus pitch. Maximum of 1 h per day, for 12 weeks. | Tinnitus symptom severity |
| Hall 2022 [35] | 46 male and 54 female. Chronic subjective tinnitus for >3 months. Mean age was 49.1 years (SD 11.3) in intervention group and 51.8 (SD 12.2) in control group. | Acoustic neuromodulation, 6 h per day for 12 weeks (blinded), then 4 h per day for 24 weeks (unblinded). | Placebo sound 6 h per day for 12 weeks (blinded), then 4 h per day for 24 weeks (unblinded). | Tinnitus symptom severity. |
| Theodoroff 2021 [47] | 38 male and 23 female. Tonal tinnitus for >6 months. Mean age 53.9 (SD = 12.6) in intervention group and 55.8 (SD = 10.4) in control group. | Desyncra™ (Bad Neuenahr, Germany) 4–6 h per day for ~24 weeks. | Cognitive behavioural therapy, 6 sessions × 60 min over 8 weeks. | Tinnitus symptom severity. |
| Stein 2016 [44] | 67 male and 33 female. Chronic (≥3 months), tonal tinnitus. Mean age 47.7 (SD 9.9) in the intervention and 47.1 (SD 11.7) in control group. | Tailor-made notched music (set according to tinnitus pitch). Participants listened to music 2 h per day for 12 weeks. | Placebo notched music (moving notch). Participants listened to music 2 h per day for 12 weeks. | Tinnitus symptom severity. |
| Smeele 2022 [43] | 17 male and 6 female. Chronic (≥3 months), tonal tinnitus. Mean age 57.4 (SD = 16.4) in the intervention and 59.9 (SD = 8.5) in control group. | High-definition infraslow pink noise stimulation, 12 × 30 min over 4 weeks. | Acti-sham, stimulation shunted to induce negligible electric field in target areas, 12 × 30 min over 4 weeks. | Feasibility, safety, resting-state EEG. |
| transcranial Direct Current Stimulation | ||||
| Abtahi 2018 [28] | 46 male, 23 female. Tinnitus > 1 year Mean age 47.4 years and 46.9 (SD = 14.6) in intervention groups and 45.4 (SD = 13.2) in control group. | tDCS (anodal or cathodal) one session of 2 mA stimulation for 20 min. | Sham stimulation. One session of 2 mA stimulation for 20 min. Dose not described. | Tinnitus intensity on a VAS. |
| Cardon 2022 [29] | 43 males, 34 females. Chronic (>6 months) subjective tinnitus. Mean age 53.47 (14.39) in intervention and 51.95 (13.87) in control group. | High-definition tDCS, 6 × 30 min sessions over 3 weeks, each at 2 mA for 30 min. | Sham stimulation, 6 × 30 min sessions over 3 weeks, but constant current was only applied for the first 20 s. | Tinnitus symptom severity. |
| Cavalcanti 2015 [30] | 9 males, 9 females with sensorineural hearing loss and chronic tinnitus. Mean age of all 54.72 years. | tDCS, 2 mA stimulation for 20 min daily for 5 days. | Sham stimulation, 20 min daily for 5 days but with only 10 s initial stimulation. | Tinnitus symptom severity. |
| da Silva Souza 2020 [31] | 8 males, 16 females. Chronic tinnitus ≥ 6 months. Mean age 44.58 years (SD = 16.20) in intervention and 50.50 (SD = 9.72) in control group. | tDCS, 2 mA current for 20 min daily for 5 days. | Sham stimulation, 20 min daily for 5 days but with only 30 s initial stimulation. | Tinnitus symptom severity. |
| Dobie 1986 [32] | 15 males, 5 females. Sensorineural hearing loss and tinnitus severe enough to justify participation. Mean age of all 50.25 years (SD = 12.26). | tDCS, 60 kHz carrier frequency modulated by a continuously swept 3 V signal across 5 kΩ load, ≤5 h per day for 7 days. | Sham stimulation. Device not connected to current. ≤5 h per day for 7 days. | Self-reported use and improvements (% scales). |
| Forogh 2016 [33] | 14 males, 8 females. Chronic tinnitus (≥6 months). Mean age 49.8 years (SD = 4.1) in intervention and 46.6 years (SD = 5.3) in control group. | tDCS, 2 mA stimulation for 20 min daily for 5 days. | Sham stimulation, 20 min daily for 5 days but with only 30 s initial stimulation. | Tinnitus symptom severity. |
| Garin 2018 [34] | 15 males, 5 females. Stable tinnitus for at least 2 months. Mean age of all was 50.9 years (SD = 12.9) | tDCS. Anodal or cathodal, 1 mA stimulation for 20 min. | Sham stimulation, 110 uA over 15 ms delivered every 550 ms over 20 min. | Self-report of improvement (single-item scales). |
| Mares 2022 [38] | 23 males, 16 females. Mean age 49 (SD = 16.73) in intervention and 46.15 years (SD = 18.5) in control group. | tDCS, 1.5 mA for 20 min, 6 times over 2 weeks. | Sham stimulation. Device-specific preprogrammed sham protocol. | Tinnitus symptom severity. |
| Pal 2015 [40] | 24 males, 18 females. Chronic (≥1) year non-pulsatile subjective tinnitus. Mean age 51.6 years (SD = 12.2) in intervention and 48.0 (SD = 9.9) in control group. | tDCS, 2 mA stimulation for 20 min daily for 5 days. | Sham stimulation, 20 min daily for 5 days but with only 90 s initial stimulation at 1 mA. | Tinnitus symptom severity. |
| Shekhawat 2014 [41] | 36 males, 4 females. Chronic tinnitus (>2 years). Mean age 59.9 years (SD = 9.6) in intervention and 58.5 (SD = 6.4) in control group. | tDCS, 2 mA current, 20 min per day over 5 days. | Sham stimulation, 20 min daily for 5 days but with only 30 s initial stimulation at 2 mA. | Tinnitus symptom severity. |
| Shekhawat 2018 [42] | 13 males. Chronic tinnitus (>2 years). Mean age of all 53.6 years (SD 11.7). | High-definition tDCS, 2 mA current for a single 20-min session. | Sham stimulation, 20-min session but with only 30 s initial stimulation at 2 mA. | Tinnitus symptom severity. |
| To 2017 [48] | 22 males, 18 females. Chronic tinnitus (>1 year). Mean age 49.17 years (SD = 13.32) and 47.29 years (SD = 9.15) in interventions groups and 48.64 years (SD = 10.49) in control group. | tDCS, 1.5 mA for 20 min per session, two sessions per week for 4 weeks, with or without the addition of transcranial random noise stimulation. | Waiting list. | Tinnitus symptom severity. |
| Yadollahpour 2017 [50] | 19 males, 23 females. Chronic tinnitus (≥6 months). Mean age 44.68 years (SD = 6.87) in the intervention and 47.53 years (SD = 7.56) in the control group. | tDCS, 4 mA current (maximum output) for 20 min per day over 5 days. | Sham stimulation, 20 min session per day for 5 days but with only 30 s initial stimulation at (max) 4 mA. | Tinnitus symptom severity. |
| Yadollahpour 2019 [51] | Sex not reported. Chronic intractable tinnitus. Mean age of all 46.5 years (SD = 1.4). | tDCS (single session) anodal or cathodal. 2 mA current for 20 min. | Sham stimulation. 20-min session but with only 30 s initial stimulation at 2 mA. | Tinnitus symptom severity. |
| Teismann 2014 [46] | Sex not reported. Chronic (≥3 months), tonal tinnitus. Mean age 42.9 years (SD = 6.87) and 44.45 years (SD = 13.29) in intervention groups and 44.91 years (SD = 9.92) in control group. | tDCS Anodal or cathodal, 2 mA for 30-min sessions over 5, paired with notched music which also was listed to for 2 h post tDCS. | Sham stimulation. 30-min session but with only 30 s initial stimulation at 2 mA. | Tinnitus symptom severity. |
| Vagus nerve | ||||
| Tyler 2017 [49] | 25 males, 5 females. Tinnitus for ≥1 year. Mean age 55.9 years (SD = 7.6) in intervention and 54.9 years (SD = 9.1) in control group. | Vagus nerve stimulation, 15 × 0.8 mA pulses every 30 s for 2.5 h paired with tones. Daily for 6 weeks. | Vagus nerve stimulation not paired with tones, 2.5 h daily for 6 weeks. | Tinnitus symptom severity. |
| Mei 2014 [39] | 29 males, 34 females. Tinnitus recurrent over 1 month or persistent for ≥5 days. Mean age of all 41.1 years (SD = 12.6). | Auricular acupuncture and sound masking, 1 mA current, pulse frequency of 20 Hz, pulse width of 1 ms; 20 min, twice per day for 8 weeks. | Flunarizine hydrochloride (5 mg orally before bed), and oryzanol 20 mg orally three times per day, for 8 weeks. | Tinnitus symptom severity. |
| transcranial Alternating Current Stimulation | ||||
| Lee 2014 [37] | 39 males, 26 females. Subjective, unilateral tinnitus > 6 months. Mean age 46.6 years (SD = 13.0) in intervention and 45.6 (SD = 11.0) in control group. | Alternating current stimulation, 15 mA stimulation for 30 s at 5 sites on external earl 8 sessions over 4 weeks. | Sham stimulation. Identical sessions but power supply switched off. | Tinnitus symptom severity. |
| Bimodal | ||||
| Jones 2023 [36] | 59 males, 49 females. Tinnitus > 6 months. Mean age 47.0 (SD = 1.8) in intervention and 47.1 (SD = 1.8) in control group. | Bisensory (auditory and somatosensory) stimulation, 3 biphasic squarewave pulses; 150 microseconds per phase paired with auditory stimulation, 30 min daily for 6 weeks | Auditory stimulation only, 30 min daily for 6 weeks. | Tinnitus symptom severity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoare, D.J.; Shorter, G.W.; Shekhawat, G.S.; El Refaie, A.; Labree, B.; Sereda, M. Neuromodulation Treatments Targeting Pathological Synchrony for Tinnitus in Adults: A Systematic Review. Brain Sci. 2024, 14, 748. https://doi.org/10.3390/brainsci14080748
Hoare DJ, Shorter GW, Shekhawat GS, El Refaie A, Labree B, Sereda M. Neuromodulation Treatments Targeting Pathological Synchrony for Tinnitus in Adults: A Systematic Review. Brain Sciences. 2024; 14(8):748. https://doi.org/10.3390/brainsci14080748
Chicago/Turabian StyleHoare, Derek J., Gillian W. Shorter, Giriraj S. Shekhawat, Amr El Refaie, Bas Labree, and Magdalena Sereda. 2024. "Neuromodulation Treatments Targeting Pathological Synchrony for Tinnitus in Adults: A Systematic Review" Brain Sciences 14, no. 8: 748. https://doi.org/10.3390/brainsci14080748
APA StyleHoare, D. J., Shorter, G. W., Shekhawat, G. S., El Refaie, A., Labree, B., & Sereda, M. (2024). Neuromodulation Treatments Targeting Pathological Synchrony for Tinnitus in Adults: A Systematic Review. Brain Sciences, 14(8), 748. https://doi.org/10.3390/brainsci14080748






