Effect of Spatial and Temporal Prediction on Tactile Sensitivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Apparatus
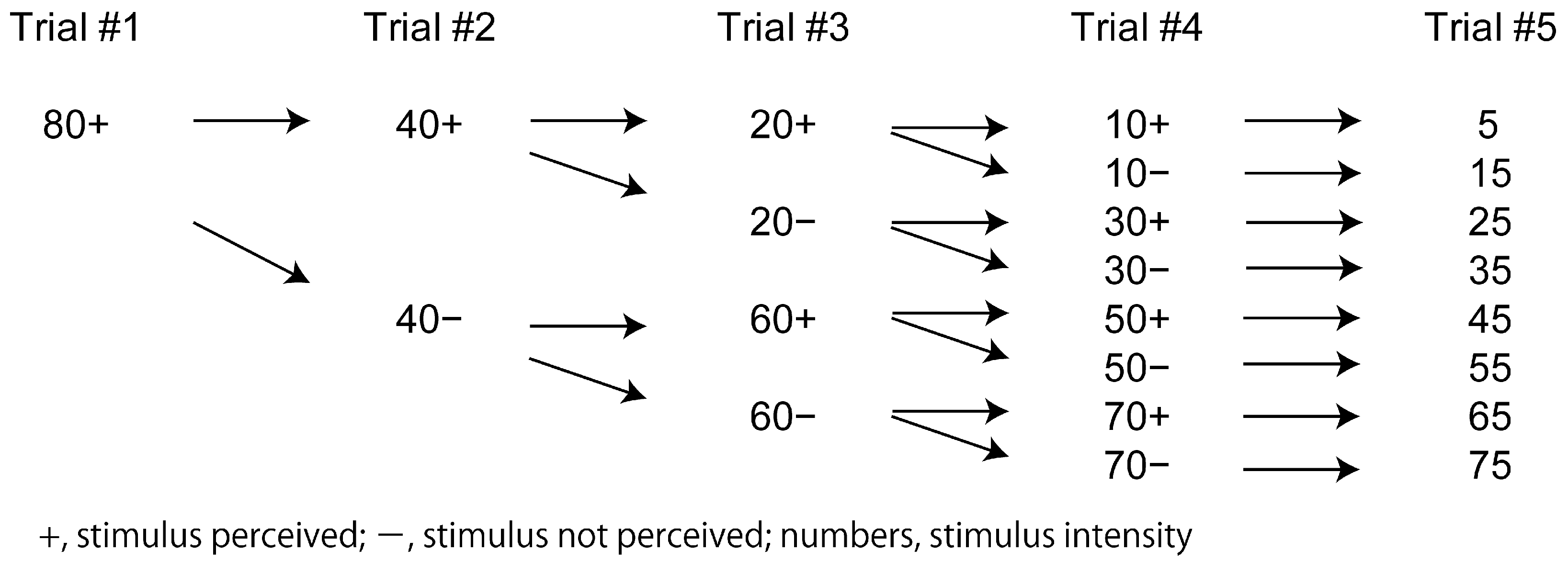
2.3. Testing Perceptual Threshold
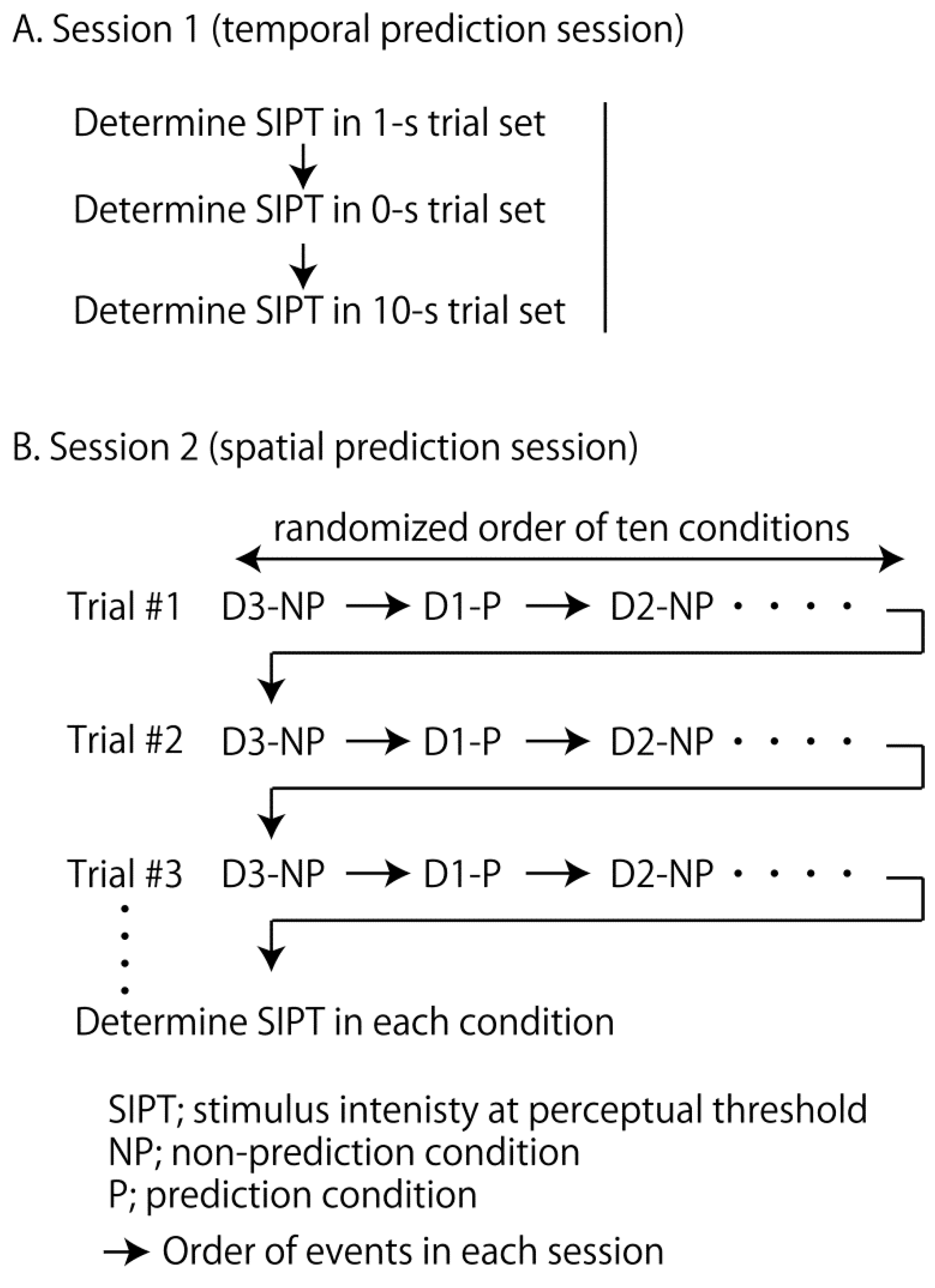
2.4. Temporal Prediction Session
2.5. Spatial Prediction Session
2.6. Statistical Analysis
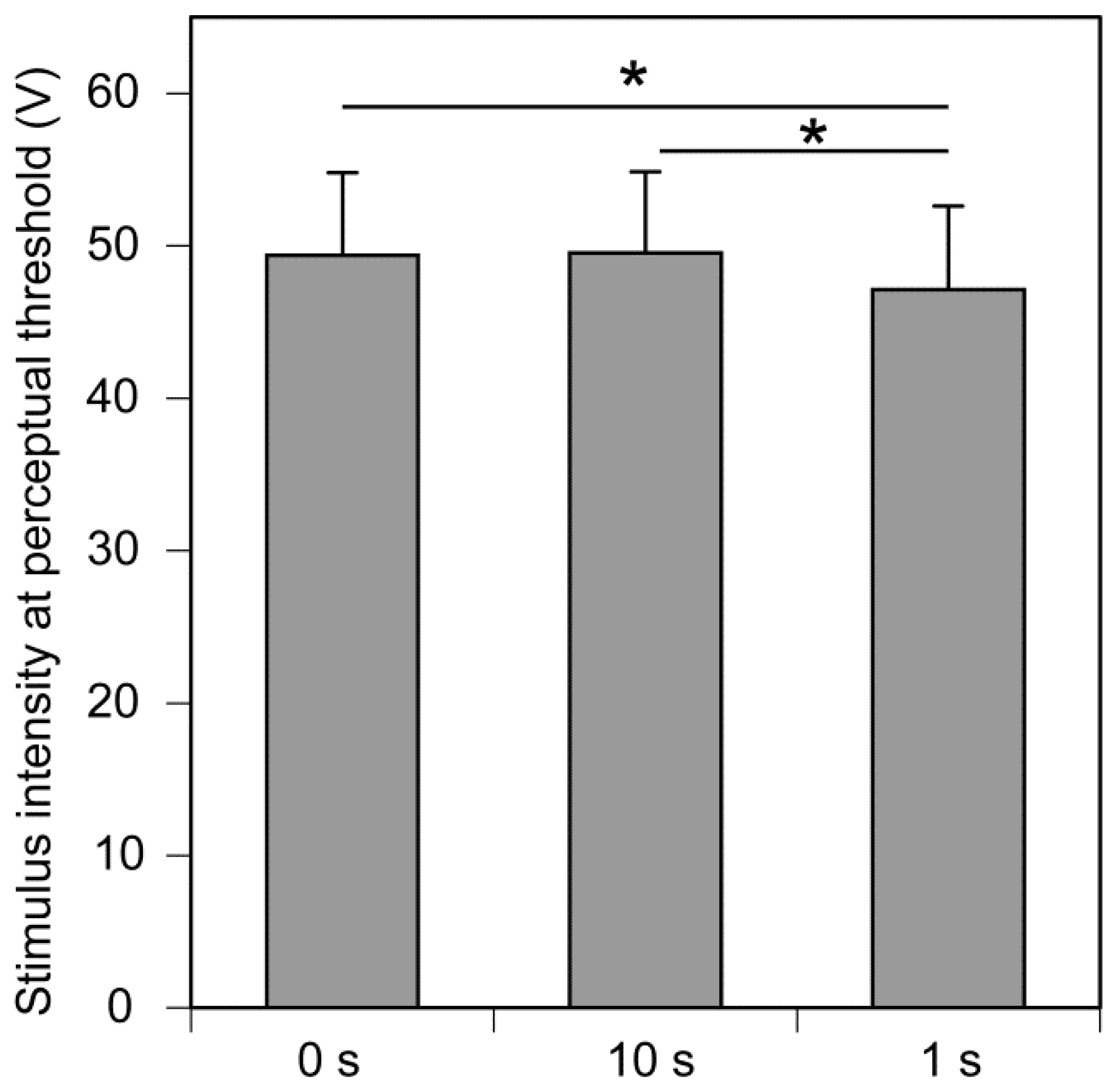
3. Results
4. Discussion
4.1. Temporal Prediction
4.2. Spatial Prediction
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hawkings, J.; Blakeslee, S. On Intelligence: How a New Understanding of the Brain Will Lead to the Creation of Truly Intelligent Machines; An Owl Book; Henry Holt and Company: New York, NY, USA, 2004. [Google Scholar]
- Zhou, A.B.; Sang, H.B.; Wang, A.J.; Zhang, M. Visual aperiodic temporal prediction increases perceptual sensitivity and reduces response latencies. Acta Psychol. 2020, 209, 103129. [Google Scholar] [CrossRef] [PubMed]
- Claxton, G. Why can’t we tickle ourselves? Percept. Mot. Ski. 1975, 41, 335–338. [Google Scholar] [CrossRef]
- Weiskrantz, L.; Elliott, J.; Darlington, C. Preliminary observations on tickling oneself. Nature 1971, 230, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Bays, P.M.; Flanagan, J.R.; Wolpert, D.M. Attenuation of self-generated tactile sensations is predictive, not postdictive. PLoS Biol. 2006, 4, e28. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.J.; Wolpert, D.; Frith, C. Why can’t you tickle yourself? Neuroreport 2000, 11, R11–R16. [Google Scholar] [CrossRef] [PubMed]
- Kilteni, K.; Engeler, P.; Ehrsson, H.H. Efference Copy Is Necessary for the Attenuation of Self-Generated Touch. iScience 2020, 23, 100843. [Google Scholar] [CrossRef] [PubMed]
- Van der Biest, L.; Legrain, V.; Paepe, A.D.; Crombez, G. Watching what’s coming near increases tactile sensitivity: An experimental investigation. Behav. Brain Res. 2016, 297, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Fuehrer, E.; Voudouris, D.; Lezkan, A.; Drewing, K.; Fiehler, K. Tactile suppression stems from specific sensorimotor predictions. Proc. Natl. Acad. Sci. USA 2022, 119, e2118445119. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kunimura, H.; Hiraoka, K. Effect of Time and Direction Preparation on Ankle Muscle Response During Backward Translation of a Support Surface in Stance. Mot. Control 2020, 24, 253–273. [Google Scholar] [CrossRef]
- Thomaschke, R.; Dreisbach, G. Temporal predictability facilitates action, not perception. Psychol. Sci. 2013, 24, 1335–1340. [Google Scholar] [CrossRef]
- Morillon, B.; Schroeder, C.E.; Wyart, V.; Arnal, L.H. Temporal Prediction in lieu of Periodic Stimulation. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Drevets, W.C.; Burton, H.; Videen, T.O.; Snyder, A.Z.; Simpson, J.R., Jr.; Raichle, M.E. Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature 1995, 373, 249–252. [Google Scholar] [CrossRef]
- Sathian, K.; Burton, H. The role of spatially selective attention in the tactile perception of texture. Percept. Psychophys. 1991, 50, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kennett, S.; Eimer, M.; Spence, C.; Driver, J. Tactile-visual links in exogenous spatial attention under different postures: Convergent evidence from psychophysics and ERPs. J. Cogn. Neurosci. 2001, 13, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Kennett, S.; Spence, C.; Driver, J. Visuo-tactile links in covert exogenous spatial attention remap across changes in unseen hand posture. Percept. Psychophys. 2002, 64, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Spence, C.; Nicholls, M.E.; Gillespie, N.; Driver, J. Cross-modal links in exogenous covert spatial orienting between touch, audition, and vision. Percept. Psychophys. 1998, 60, 544–557. [Google Scholar] [CrossRef]
- Spence, C.; Gallace, A. Recent developments in the study of tactile attention. Can. J. Exp. Psychol. = Rev. Can. De Psychol. Exp. 2007, 61, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, W.H.; Ehrenstein, A. Psychophysical Methods. In Modern Techniques in Neuroscience Research; Springer: Berlin/Heidelberg, Germany, 1999; pp. 1211–1241. [Google Scholar]
- Blankenburg, F.; Taskin, B.; Ruben, J.; Moosmann, M.; Ritter, P.; Curio, G.; Villringer, A. Imperceptible stimuli and sensory processing impediment. Science 2003, 299, 1864. [Google Scholar] [CrossRef]
- Taskin, B.; Holtze, S.; Krause, T.; Villringer, A. Inhibitory impact of subliminal electrical finger stimulation on SI representation and perceptual sensitivity of an adjacent finger. NeuroImage 2008, 39, 1307–1313. [Google Scholar] [CrossRef]
- Diederich, A. Intersensory facilitation of reaction time: Evaluation of counter and diffusion coactivation models. J. Math. Psychol. 1995, 39, 197–215. [Google Scholar] [CrossRef]
- Jacobs, J.V.; Horak, F.B. Cortical control of postural responses. J. Neural Transm. 2007, 114, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.V.; Fujiwara, K.; Tomita, H.; Furune, N.; Kunita, K.; Horak, F.B. Changes in the activity of the cerebral cortex relate to postural response modification when warned of a perturbation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2008, 119, 1431–1442. [Google Scholar] [CrossRef]
- Hamada, N.; Kunimura, H.; Matsuoka, M.; Oda, H.; Hiraoka, K. Advanced cueing of auditory stimulus to the head induces body sway in the direction opposite to the stimulus site during quiet stance in male participants. Front. Hum. Neurosci. 2022, 16, 1028700. [Google Scholar] [CrossRef]
- Graziano, M.S.; Cooke, D.F. Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia 2006, 44, 845–859. [Google Scholar] [CrossRef]
- Sherrington, C.S. Flexion-reflex of the limb, crossed extension-reflex, and reflex stepping and standing. J. Physiol. 1910, 40, 28–121. [Google Scholar] [CrossRef]
- Landis, C.; Hunt, W.A. The Startle Pattern; Farrar and Rinehart: New York, NY, USA, 1939. [Google Scholar]
- Schouenborg, J.; Weng, H.R.; Kalliomäki, J.; Holmberg, H. A survey of spinal dorsal horn neurones encoding the spatial organization of withdrawal reflexes in the rat. Exp. Brain Res. 1995, 106, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.W.; Harris, J. The organization of motor responses to noxious stimuli. Brain Research. Brain Res. Rev. 2004, 46, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Cooke, D.F.; Taylor, C.S.; Moore, T.; Graziano, M.S. Complex movements evoked by microstimulation of the ventral intraparietal area. Proc. Natl. Acad. Sci. USA 2003, 100, 6163–6168. [Google Scholar] [CrossRef]
- Brown, J. Some tests of the decay theory of immediate memory. Q. J. Exp. Psychol. 1958, 10, 12–21. [Google Scholar] [CrossRef]
- Fitts, P.M.; Posner, M.I. Human Performance; Brooks/Cole: Pacific Grove, CA, USA, 1967. [Google Scholar]
- Doeland, H.J.; Nauta, J.J.; van Zandbergen, J.B.; van der Eerden, H.A.; van Diemen, N.G.; Bertelsmann, F.W.; Heimans, J.J. The relationship of cold and warmth cutaneous sensation to age and gender. Muscle Nerve 1989, 12, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Hilz, M.J.; Axelrod, F.B.; Hermann, K.; Haertl, U.; Duetsch, M.; Neundörfer, B. Normative values of vibratory perception in 530 children, juveniles and adults aged 3–79 years. J. Neurol. Sci. 1998, 159, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Seah, S.A.; Griffin, M.J. Normal values for thermotactile and vibrotactile thresholds in males and females. Int. Arch. Occup. Environ. Health 2008, 81, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Wild, P.; Massin, N.; Lasfargues, G.; Baudin, V.; Unlu, D.; Donati, P. Vibrotactile perception thresholds in four non-exposed populations of working age. Ergonomics 2001, 44, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Gueorguiev, D.; Vezzoli, E.; Mouraux, A.; Lemaire-Semail, B.; Thonnard, J.L. The tactile perception of transient changes in friction. J. R. Soc. Interface 2017, 14, 20170641. [Google Scholar] [CrossRef]
- Yeon, J.; Kim, J.; Ryu, J.; Park, J.Y.; Chung, S.C.; Kim, S.P. Human Brain Activity Related to the Tactile Perception of Stickiness. Front. Hum. Neurosci. 2017, 11, 8. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunimura, H.; Oda, H.; Kawasaki, T.; Gao, H.; Fukuda, S.; Hiraoka, K. Effect of Spatial and Temporal Prediction on Tactile Sensitivity. Brain Sci. 2024, 14, 749. https://doi.org/10.3390/brainsci14080749
Kunimura H, Oda H, Kawasaki T, Gao H, Fukuda S, Hiraoka K. Effect of Spatial and Temporal Prediction on Tactile Sensitivity. Brain Sciences. 2024; 14(8):749. https://doi.org/10.3390/brainsci14080749
Chicago/Turabian StyleKunimura, Hiroshi, Hitoshi Oda, Taku Kawasaki, Han Gao, Shiho Fukuda, and Koichi Hiraoka. 2024. "Effect of Spatial and Temporal Prediction on Tactile Sensitivity" Brain Sciences 14, no. 8: 749. https://doi.org/10.3390/brainsci14080749
APA StyleKunimura, H., Oda, H., Kawasaki, T., Gao, H., Fukuda, S., & Hiraoka, K. (2024). Effect of Spatial and Temporal Prediction on Tactile Sensitivity. Brain Sciences, 14(8), 749. https://doi.org/10.3390/brainsci14080749






