Understanding the Effect of Listening to Music, Playing Music, and Singing on Brain Function: A Scoping Review of fNIRS Studies
Abstract
1. Introduction
1.1. Music Engagement Shapes Human Brain
1.2. Different Ways of Engagement and Divergent Impacts
1.3. The Current Study
2. Methods
2.1. Step 1: Research Question Definition
- (1)
- What are the effects of LSP on human brain function?
- (2)
- How do differences in musical features while LSP affect brain function?
- (3)
- What role do individual differences play in the effect of LSP on brain function?
- (4)
- What new insights has fNIRS brought to the study of the relationship between LSP and human brain function?
2.2. Step 2: Identification of Relevant Studies
2.3. Step 3: Study Selection
2.4. Step 4: Data Charting
2.5. Step 5: Data Collation, Summarization, and Reporting of Results
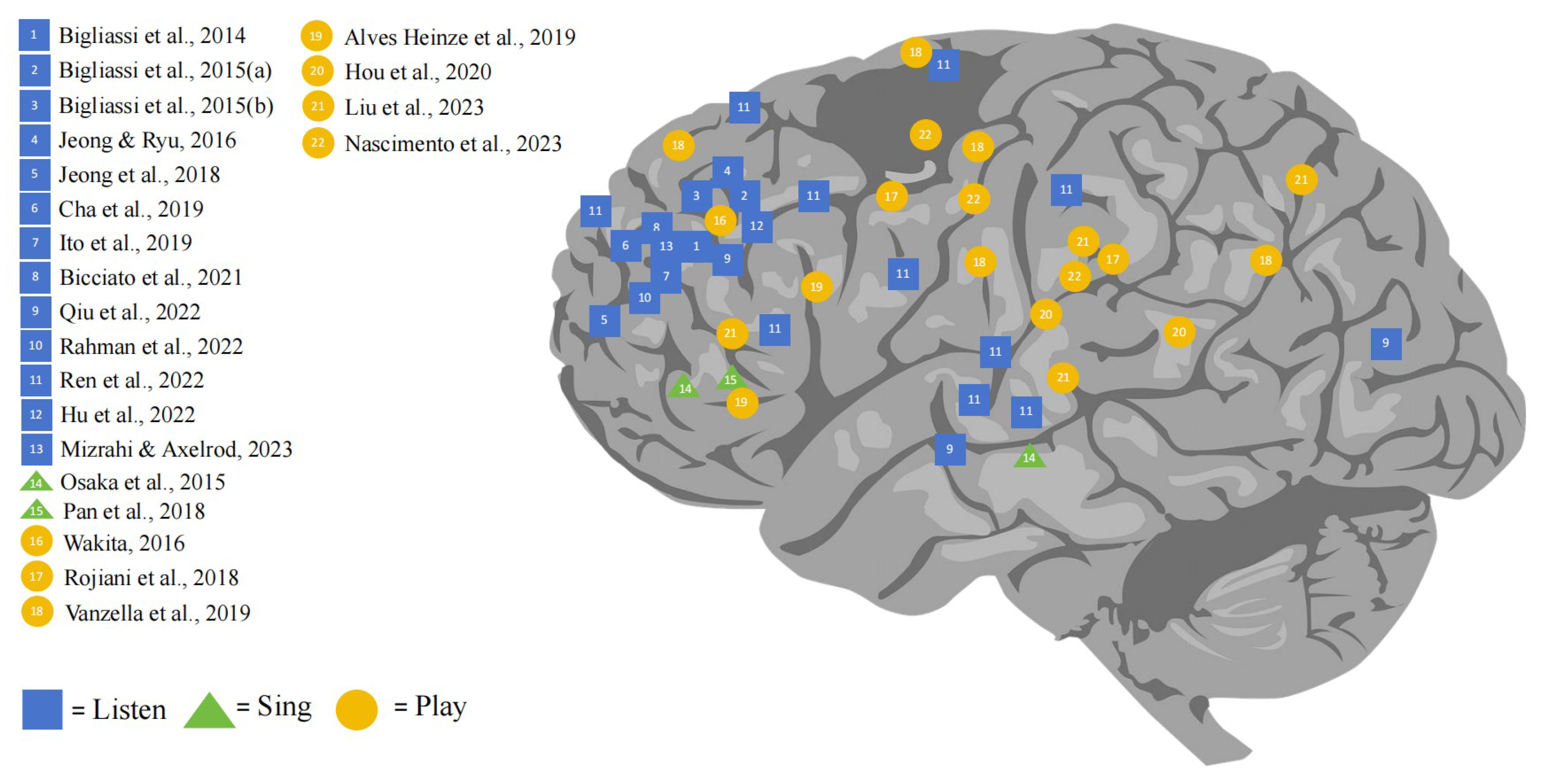
3. Results
3.1. The Effect of Listening to Music on Human Brain Function
3.1.1. The Effect of Musical Features on Brain Function
3.1.2. The Effect of Individual Differences on Brain Function
3.2. The Effect of Singing on Brain Function
3.3. The Effect of Playing Music on Brain Function
4. Discussion
4.1. The Responses of Passive and Active Music Experiences in Relevant Brain Areas
4.2. The Lateralized Brain in Music Perception and Processing
4.3. Individual Variations in Neural Responses to Music
4.4. Neural Synchronization in Musical Performance
5. Conclusions, Limitations, and Future Research
5.1. Conclusions
5.2. Limitations and Future Research
6. Implications for Future Studies
Author Contributions
Funding
Conflicts of Interest
References
- Crivelli, D.; Balconi, M. Near-Infrared Spectroscopy Applied to Complex Systems and Human Hyperscanning Networking. Appl. Sci. 2017, 7, 922. [Google Scholar] [CrossRef]
- Conard, N.J.; Malina, M.; Munzel, S.C. New flutes document the earliest musical tradition in southwestern Germany. Nature 2009, 460, 737–740. [Google Scholar] [CrossRef]
- Bottiroli, S.; Rosi, A.; Russo, R.; Vecchi, T.; Cavallini, E. The cognitive effects of listening to background music on older adults: Processing speed improves with upbeat music, while memory seems to benefit from both upbeat and downbeat music. Front. Aging Neurosci. 2014, 6, 284. [Google Scholar] [CrossRef] [PubMed]
- Atiwannapat, P.; Thaipisuttikul, P.; Poopityastaporn, P.; Katekaew, W. Active versus receptive group music therapy for major depressive disorder-A pilot study. Complement. Ther. Med. 2016, 26, 141–145. [Google Scholar] [CrossRef]
- Trimmer, C.; Tyo, R.; Pikard, J.; McKenna, C.; Naeem, F. Low-Intensity Cognitive Behavioural Therapy-Based Music Group (CBT-Music) for the Treatment of Symptoms of Anxiety and Depression: A Feasibility Study. Behav. Cogn. Psychother. 2018, 46, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Karmonik, C.; Brandt, A.; Elias, S.; Townsend, J.; Silverman, E.; Shi, Z.; Frazier, J.T. Similarity of individual functional brain connectivity patterns formed by music listening quantified with a data-driven approach. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Jäncke, L. Music drives brain plasticity. F1000 Biol. Rep. 2009, 1, 78. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.L.; Lerch, J.; Norton, A.; Forgeard, M.; Winner, E.; Evans, A.C.; Schlaug, G. Musical training shapes structural brain development. J. Neurosci. 2009, 29, 3019–3025. [Google Scholar] [CrossRef]
- Bangert, M.; Schlaug, G. Specialization of the specialized in features of external human brain morphology. Eur. J. Neurosci. 2006, 24, 1832–1834. [Google Scholar] [CrossRef]
- Koelsch, S.; Fritz, T.; Schulze, K.; Alsop, D.; Schlaug, G. Adults and children processing music: An fMRI study. NeuroImage 2005, 25, 1068–1076. [Google Scholar] [CrossRef]
- Whitehead, J.C.; Armony, J.L. Singing in the brain: Neural representation of music and voice as revealed by fMRI. Hum. Brain Mapp. 2018, 39, 4913–4924. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.M.Y.; Han, Y.M.Y. The functional brain networks activated by music listening: A neuroimaging meta-analysis and implications for treatment. Neuropsychology 2022, 36, 4–22. [Google Scholar] [CrossRef]
- Herholz, S.C.; Zatorre, R.J. Musical training as a framework for brain plasticity: Behavior, function, and structure. Neuron 2012, 76, 486–502. [Google Scholar] [CrossRef] [PubMed]
- Norman-Haignere, S.V.; Feather, J.; Boebinger, D.; Brunner, P.; Ritaccio, A.; McDermott, J.H.; Schalk, G.; Kanwisher, N. A neural population selective for song in human auditory cortex. Curr. Biol. 2022, 32, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Zarate, J.M.; Delhommeau, K.; Wood, S.; Zatorre, R.J. Vocal accuracy and neural plasticity following micromelody-discrimination training. PLoS ONE 2010, 5, e11181. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, A.M.; Gaca, M.; Herman, A.M.; Jednoróg, K.; Marchewka, A. How musical training shapes the adult brain: Predispositions and neuroplasticity. Front. Neurosci. 2021, 15, 630829. [Google Scholar] [CrossRef]
- Brattico, E.; Alluri, V.; Bogert, B.; Jacobsen, T.; Vartiainen, N.; Nieminen, S.; Tervaniemi, M. A functional MRI of happy and sad emotions in music with and without lyrics. Front. Psychol. 2011, 2, 16. [Google Scholar] [CrossRef]
- Kornysheva, K.; von Cramon, D.Y.; Jacobsen, T.; Schubotz, R.I. Tuning-in to the beat: Aesthetic appreciation of musical rhythms correlates with a premotor activity boost. Hum. Brain Mapp. 2010, 31, 48–64. [Google Scholar] [CrossRef]
- Sachs, M.E.; Damasio, A.; Habibi, A. The pleasures of sad music: A systematic review. Front. Hum. Neurosci. 2015, 9, 404. [Google Scholar] [CrossRef]
- Freitas, C.; Manzato, E.; Burini, A.; Taylor, M.J.; Lerch, J.P.; Anagnostou, E. Neural correlates of familiarity in music listening: A systematic review and a neuroimaging meta-analysis. Front. Neurosci. 2018, 12, 686. [Google Scholar] [CrossRef]
- Koelsch, S. Brain correlates of music-evoked emotions. Nat. Rev. Neurosci. 2014, 15, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.F.; Wong, Z.Y.; Thayala, N.V. The effectiveness of music listening in reducing depressive symptoms in adults: A systematic review. Complement. Ther. Med. 2011, 19, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Stegemann, T. Music listening for children and adolescents in health care contexts: A systematic review. Arts Psychother. 2016, 51, 72–85. [Google Scholar] [CrossRef]
- Schellenberg, E.G. Cognitive performance after listening to music: A review of the Mozart effect. In Music, Health, and Wellbeing; MacDonald, R., Kreutz, G., Mitchell, L., Eds.; OUP Oxford: Oxford, UK, 2012; pp. 324–338. [Google Scholar]
- Finn, S.; Fancourt, D. The biological impact of listening to music in clinical and nonclinical settings: A systematic review. Prog. Brain Res. 2018, 237, 173–200. [Google Scholar] [CrossRef]
- Daykin, N.; Mansfield, L.; Meads, C.; Julier, G.; Tomlinson, A.; Payne, A.; Victor, C. What works for wellbeing? A systematic review of wellbeing outcomes for music and singing in adults. Perspect. Public Health 2018, 138, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Zarate, J.M. The neural control of singing. Front. Hum. Neurosci. 2013, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Bigliassi, M.; Barreto-Silva, V.; Kanthack, T.F.D.; Altimari, L.R. Music and cortical blood flow: A functional near-infrared spectroscopy (fNIRS) study. Psychol. Neurosci. 2014, 7, 545–550. [Google Scholar] [CrossRef]
- Santosa, H.; Hong, M.J.; Hong, K.S. Lateralization of music processing with noises in the auditory cortex: An fNIRS study. Front. Behav. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef]
- Bigliassi, M.; Barreto-Silva, V.; Altimari, L.R.; Vandoni, M.; Codrons, E.; Buzzachera, C.F. How motivational and calm music may affect the prefrontal cortex area and emotional responses: A functional near-infrared spectroscopy (fNIRS) study. Percept. Mot. Skills 2015, 120, 202–218. [Google Scholar] [CrossRef]
- Bigliassi, M.; León-Domínguez, U.; Altimari, L.R. How does the prefrontal cortex “listen” to classical and techno music? A functional near-infrared spectroscopy (fNIRS) study. Psychol. Neurosci. 2015, 8, 246. [Google Scholar] [CrossRef]
- Jeong, E.; Ryu, H. Melodic contour identification reflects the cognitive threshold of aging. Front. Aging Neurosci. 2016, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Wakita, M. Interaction between perceived action and music sequences in the left prefrontal area. Front. Hum. Neurosci. 2016, 10, 656. [Google Scholar] [CrossRef] [PubMed]
- Jeong, E.; Ryu, H.; Jo, G.; Kim, J. Cognitive load changes during music listening and its implication in earcon design in public environments: An fNIRS study. Int. J. Environ. Res. Public Health 2018, 15, 2075. [Google Scholar] [CrossRef] [PubMed]
- Cha, K.C.; Suh, M.; Kwon, G.; Yang, S.; Lee, E.J. Young consumers’ brain responses to pop music on YouTube. Asia Pac. J. Mark. Logist. 2020, 32, 1132–1148. [Google Scholar] [CrossRef]
- Ito, S.I.; Ito, M.; Fukumi, M. Individual Differences in Brain Activities When Human Wishes to Listen to Music Continuously Using Near-Infrared Spectroscopy. Int. J. Adv. Sci. Technol. 2020, 29, 807–813. [Google Scholar]
- Bicciato, G.; Keller, E.; Wolf, M.; Brandi, G.; Schulthess, S.; Friedl, S.G.; Narula, G. Increase in low-frequency oscillations in fNIRS as cerebral response to auditory stimulation with familiar music. Brain Sci. 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Zhong, Y.; Xie, Q.; He, Z.; Wang, X.; Chen, Y.; Pan, J. Multi-modal integration of EEG-fNIRS for characterization of brain activity evoked by preferred music. Front. Neurorobot. 2022, 16, 823435. [Google Scholar] [CrossRef] [PubMed]
- Rahman, J.S.; Caldwell, S.; Jones, R.; Gedeon, T. Brain Melody Interaction: Understanding Effects of Music on Cerebral Hemodynamic Responses. Multimodal Technol. Interact. 2022, 6, 35. [Google Scholar] [CrossRef]
- Ren, H.; Jiang, X.; Meng, L.; Lu, C.; Wang, L.; Dai, C.; Chen, W. fNIRS-Based Dynamic Functional Connectivity Reveals the Innate Musical Sensing Brain Networks in Preterm Infants. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 1806–1816. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, M.; Liu, Y.; Wang, Z.; Cheng, X.; Pan, Y.; Hu, Y. Musical Meter Induces Interbrain Synchronization during Interpersonal Coordination. eNeuro 2022, 9, ENEURO.0504-21.2022. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, T.; Axelrod, V. Naturalistic auditory stimuli with fNIRS prefrontal cortex imaging: A potential paradigm for disorder of consciousness diagnostics (a study with healthy participants). Neuropsychologia 2023, 187, 108604. [Google Scholar] [CrossRef] [PubMed]
- Osaka, N.; Minamoto, T.; Yaoi, K.; Azuma, M.; Shimada, Y.M.; Osaka, M. How two brains make one synchronized mind in the inferior frontal cortex: fNIRS-based hyperscanning during cooperative singing. Front. Psychol. 2015, 6, 1811. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Novembre, G.; Song, B.; Li, X.; Hu, Y. Interpersonal synchronization of inferior frontal cortices tracks social interactive learning of a song. Neuroimage 2018, 183, 280–290. [Google Scholar] [CrossRef]
- Rojiani, R.; Zhang, X.; Noah, A.; Hirsch, J. Communication of emotion via drumming: Dual-brain imaging with functional near-infrared spectroscopy. Soc. Cogn. Affect. Neurosci. 2018, 13, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Vanzella, P.; Balardin, J.B.; Furucho, R.A.; Zimeo Morais, G.A.; Braun Janzen, T.; Sammler, D.; Sato, J.R. fNIRS responses in professional violinists while playing duets: Evidence for distinct leader and follower roles at the brain level. Front. Psychol. 2019, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Alves Heinze, R.; Vanzella, P.; Zimeo Morais, G.A.; Sato, J.R. Hand motor learning in a musical context and prefrontal cortex hemodynamic response: A functional near-infrared spectroscopy (fNIRS) study. Cogn. Process. 2019, 20, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Song, B.; Hu, Y.; Pan, Y.; Hu, Y. The averaged inter-brain coherence between the audience and a violinist predicts the popularity of violin performance. Neuroimage 2020, 211, 116655. [Google Scholar] [CrossRef]
- Liu, T.; Duan, L.; Dai, R.; Pelowski, M.; Zhu, C. Team-work, team-brain: Exploring synchrony and team interdependence in a nine-person drumming task via multiparticipant hyperscanning and inter-brain network topology with fNIRS. Neuroimage 2021, 237, 118147. [Google Scholar] [CrossRef]
- León Carrión, J.; Martín-Rodríguez, J.F.; Damas-López, J.; Pourrezai, K.; Izzetoglu, K.; Barroso y Martin, J.M.; Domínguez-Morales, M.R. A lasting poststimulus activation on dorsolateral prefrontal cortex is produced when processing valence and arousal in visual affective stimuli. Neurosci. Lett. 2007, 422, 147–152. [Google Scholar] [CrossRef]
- Hubbard, T.L. Auditory imagery: Empirical findings. Psychol. Bull. 2010, 136, 302. [Google Scholar] [CrossRef] [PubMed]
- Anwander, A.; Tittgemeyer, M.; von Cramon, D.Y.; Friederici, A.D.; Knösche, T.R. Connectivity-based parcellation of Broca’s area. Cereb. Cortex 2007, 17, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Avenanti, A.; Urgesi, C. Understanding ‘what’ others do: Mirror mechanisms play a crucial role in action perception. Soc. Cogn. Affect. Neurosci. 2011, 6, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Nomoto, Y.; Tanaka, S.; Shimada, S.; Tachibana, A.; Bronner, S.; Noah, J.A. Frontotemporal oxyhemoglobin dynamics predict performance accuracy of dance simulation gameplay: Temporal characteristics of top-down and bottom-up cortical activities. Neuroimage 2014, 85, 461–470. [Google Scholar] [CrossRef]
- Donaldson, P.H.; Rinehart, N.J.; Enticott, P.G. Noninvasive stimulation of the temporoparietal junction: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 547–572. [Google Scholar] [CrossRef] [PubMed]
- Shtyrov, Y.; Kujala, T.; Ahveninen, J.; Tervaniemi, M.; Alku, P.; Ilmoniemi, R.J.; Näätänen, R. Background acoustic noise and the hemispheric lateralization of speech processing in the human brain: Magnetic mismatch negativity study. Neurosci. Lett. 1998, 251, 141–144. [Google Scholar] [CrossRef]
- Zatorre, R.J.; Belin, P.; Penhune, V.B. Structure and function of auditory cortex: Music and speech. Trends Cogn. Sci. 2002, 6, 37–46. [Google Scholar] [CrossRef]
- Satoh, M.; Takeda, K.; Nagata, K.; Shimosegawa, E.; Kuzuhara, S. Positron-emission tomography of brain regions activated by recognition of familiar music. Am. J. Neuroradiol. 2006, 27, 1101–1106. [Google Scholar]
- Schellenberg, E.G. Music lessons enhance IQ. Psychol. Sci. 2004, 15, 511–514. [Google Scholar] [CrossRef]
- Gur, R.C.; Gunning-Dixon, F.; Bilker, W.B.; Gur, R.E. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb. Cortex 2002, 12, 998–1003. [Google Scholar] [CrossRef]

| Criterion | Inclusion | Exclusion |
|---|---|---|
| Scope of research | Empirical studies | Non-empirical studies (reviews, theoretical studies, or editorials) |
| Secondary data analysis | ||
| Type of documents | Peer-reviewed scholarly journal articles | Non-peer-reviewed scholarly journal articles |
| Language | English | Other languages than English |
| Participant | Healthy population | Unhealthy population |
| Method | fNIRS | Techniques other than fNIRS |
| Multimodal technologies including fNIRS |
| Contact Method | Author (year)/Country | Participants | Methods | Results | ||
|---|---|---|---|---|---|---|
| Sample Size and Age | Inclusion Criteria | Measurement of Brain Areas | Music Experiment Materials | |||
| Listen | Bigliassi et al. (2014)/America [29] | N = 18 (male = 10) Mage = 22.25 ± 2.34 yr. | Right-handed non-musicians | PFC | Rock: Iron Man Folk: Mr. Tambourine Man Trance: Acperience Classical: Air on G String Soft Rock: Tears in Heaven Subjects choose: 1 preferred song and 1 motivational song | PR, MO, and FO showed gender differences on PFC. |
| Santosa et al. (2014)/Korea [30] | N = 14 (male = 7) Mage = 28 ± 5 yr. | 12 right-handed; 2 left-handed | Auditory cortex | Für Elise (by Ludwig van Beethoven) | 1. Music processing: 75% R-HL; Noise processing: 65% R-HL. 2. Mixing noise in music: increased R-HL. 3. Noise level slightly lower than that of music: completely R-HL. | |
| Bigliassi et al. (2015a)/America [31] | N = 30 (male = 15) Male: Mage = 24.8 ± 2.4 yr. Female: Mage = 25.2 ± 3.1 yr. | Right-handed non-musicians | PFC | Two different songs in a random order: 1. A motivational song which they self-selected. 2. A calm song (Enya, May It Be; New Age style, 110 bpm). | Men exhibit stronger dlPFC activity while listening to music and residual sounds; Women have a higher level of excitement when listening to motivational music. | |
| Bigliassi et al. (2015b)/America [32] | N = 30 (male = 15) Male: Mage = 24.8 ± 2.4 yr. Female: Mage = 25.2 ± 3.1 yr. | Right-handed non-musicians | PFC | Classical music: L. Van Beethoven, Symphony no. 6 (Pastorale), Opera 68, 1806–1807 Techno Magnetiko | The activity of PFC is significantly influenced by exposure time, music type, and hemisphere. Classical music concerts generate more dlPFC activities (male>female). | |
| Jeong and Ryu (2016)/Korea [33] | N = 27 (college students = 13, male = 10, Mage = 23.54 yr.; olders = 14, male = 7, Mage = 56.07 yr.) | Right-handed non-musicians | PFC | Six contour stimuli: combination of three contours (rising, falling, and remaining unchanged), played by one of the three synthetic instruments (piano, flute, or strings). | 1. The ACC of the elderly group in CIT2 was significantly lower than that under environmental noise conditions. 2. The activity of RdlPFC in young group was significantly higher than that in elderly group in CIT2. | |
| Wakita (2016)/Japan [34] | With extensive training: N = 9 (male = 0), Mage = 29.2 yr. With little training: N = 9 (male = 2), Mage = 28.6 yr. | Right-handed; no visual or auditory disability | Left PFC | Mary Had a Little Lamb London Bridge Is Falling Down | Under uncoordinated conditions, participants with extensive training showed significantly higher activation in the left prefrontal region. | |
| Jeong et al. (2018)/Korea [35] | N = 16 (male = 10) Mage = 23.5 ± 1.7 yr. | Right-handed non-musicians | PFC | Six types of contour stimuli are paired with a combination of three contours (rising, falling, and remaining unchanged) and combined into 5 cognitive tasks (CITs). | The activity of BA10 increases with the difficulty of the task; The cognitive load suddenly increases between CIT4 and CIT5. | |
| Cha et al. (2019)/Korea [36] | N = 56 (male = 33) Mage = 24 yr. | N/A | mPFC | 5 popular songs: 2 songs higher than OSL (optimal sensory load), 2 songs lower than OSL, and 1 song at OSL. | Songs familiar to participants and songs higher than OSL can trigger more significant right side mPFC activity | |
| Ito et al. (2019)/Japan [37] | N = 6 Mage = 22.5 yr. | N/A | PFC | N/A | There are three different types of PFC activities that represent them wanting to listen to music. | |
| Bicciato et al. (2021)/Switzerland [38] | N = 6 (male = 4) Mage = 41.2 ± 12.6 yr. | Right-handed | PFC | Choose the music style that the subjects like | When listening to music for the first time, the LFO increases, which is positively correlated with PFC activation, especially in the left hemisphere. This performance disappeared the second time they listened to music. | |
| Qiu et al. (2022)/China [39] | N = 9 (male = 5) Mage = 31.25 yr. | Right-handed non-musicians | EEG: Occipital lobe fNIRS: frontal cortex temporal cortex parietal cortex | Neutral music, peferred music | Listening to their favorite and neutral music mainly activated their PFC (especially the right frontal lobe) and occipital lobe; In the same brain region, low-frequency bands (such as δ and θ) had more activation than high-frequency bands (such as β and γ). | |
| Rahman et al. (2022)/Australia [40] | N = 27 (male = 10) Mage = 19.4 ±1.5 yr. | N/A | PFC | Three types of music with 4 pieces each. | Classical music and instrumental music cause significant changes in the right and middle PFC; Sad music triggers stronger PFC activity. | |
| Ren et al. (2022)/China [41] | N = 10 (male = 8) Gestation age = 34.5 ± 1.0 weeks | Premature Apgar Score ≥ 7 Automated AABR | Frontal cortex temporal cortex parietal cortex | Mozart K.448 | Premature infants’ perception of music involves a wide range of functional connections between the two hemispheres at the brain network level: the functional performance of the frontal and temporal cortical regions shows right-sided hemisphere deviation, as well as synchronization between the bilateral temporal cortical regions. | |
| Hu et al. (2022)/China [42] | Expt. 1: N = 40 (female) Mage = 21.82 yr. Expt. 2: N = 32 (female) Mage = 20.64 yr. | Right-handed non-musicians who have studied fewer than 3 years | Frontal cortex | Expt. 1: a metered tone sequence and a non-metered tone sequence. Expt. 2: Four kinds of meters generated by manipulating the accent and the frequency of occurrence. | The interpersonal coordination effect is better with intervals and strong tone conditions. Among them, MFC-IBS is negatively correlated with the average interpersonal delay with intervals. | |
| Mizrahi and Axelrod (2023)/Israel [43] | N = 24 (male = 12) Mage = 22.2 ± 2.3 yr. | 2 left-handed | PFC | Concerto No. 1 by Tchaikovsky, Rhapsody on a Theme of Paganini by Rachmaninov, and Concerto No. 2 by Rachmaninov | There was no significant difference in the activity level of PFC when listening to two types of music. | |
| Sing | Osaka et al. (2015)/Japan [44] | Singing: N = 30 (15 pairs, M-M = 8) Mage = 22 yr. Humming: N = 28 (14 pairs, M-M = 9) Mage = 21 yr. | Not familiar with each other | IFC | Three popular Japanese nursery rhymes: Under the Spreading Chestnut Tree School of Killifish Sunset with the Evening Glow | Singing: The increased coherence between the left IFC and the right temporal cortex during collaboration is greater than that of a single individual. Humming: The increase in coherence during the collaboration process is greater than that of a single layer, including the left parietal cortex, bilateral IFC, right frontal cortex, and right temporal cortex, with FtF>FtW. |
| Pan et al. (2018)/China [45] | Student: N = 24 (male = 0) Mage = 20.58 ± 2.15 yr. Teacher: N = 1 (male = 0) Age = 24 yr. | Right-handed | IFC | The Moon Reflection (Lyrics: B. Peng, Music: Z. Liu and S. Yan) A Tune of Homesickness (Lyrics: C. Qu, Music: Q. Zheng). | In interactive learning tasks, IFC and IBS activity between learners and teachers is demonstrated, and this specific enhancement can predict learners’ behavioral performance. | |
| Play | Rojiani et al. (2018)/America [46] | N = 36 (male = 17) M-M = 5 pairs; M-Fe = 8 pairs; F-F = 5pairs Mage = 23.8 ± 3.2 yr. | 86% right-handed participants that can play drums | Frontal cortex temporal cortex parietal cortex | Drumming | The increase in drum frequency is related to the activation of TPJ in the listener’s nerves. Listening > Drumming: Right SMG, STG, BA39, BA22, and BA21. Drumming > Listening: Right BA6 and BA1,2,3; Left BA6. Drumming > Talking: Right BA40, BA22, and BA2; Left BA2 and BA40. |
| Vanzella et al. (2019)/Brazil [47] | N = 10 (duets = 5, male = 5) Mage = 32.8 ± 8 yr. | Right-handed professional musicians | Regions of the motor and sensorimotor corticesTPJ | No. 37 (Prelude and Canon) | The temporal parietal and sensorimotor cortices of second violinist are significantly more activated than those of first violinist. | |
| Alves Heinze et al. (2019)/Switzerland [48] | N = 15 (male = 10) Mage = 21.5 ± 2.4 tr. | Right-handedparticipants without experience in keyboard or musical instruments | PFC | Three arpeggiated chords (C major, A minor and F major) Playing the piano with the left hand | The activation level of PFC in the subjects significantly increased from the second block to the third block, reached its peak in the seventh block, and then stabilized and decreased. | |
| Hou et al. (2020) [49] | Audience: N = 16 (male = 0) Mage = 20.313 ± 1.922 yr. Violinist: N = 1 (male) Age = 21 yr. | Right-handed non-musician | Frontal cortex temporal cortex | Performance videos of 12 works recorded by violinists | 1. Violinist’s audience shows IBC in the left temporal cortex, right IFC, and central posterior cortex. IBC can show or predict popularity. 2. The correlation of the left temporal cortex is stronger when observed later (>50 s) than when observed earlier (0–50 s). | |
| Liu et al. (2021)/China [50] | N = 180 (male = 105) Mage = 23.14 ± 1.93 yr. | Right-handednon-musicians who werenot familiar with each other | mPFC TPJ | Played drums under 3 conditions: (1) Random drumming; (2) Team-centered conditions; (3) Focusing on metronome together | Under team-centered conditions, higher IBS was also observed in the left TPJ and mPFC; There is a positive correlation between target drum synchronization and TPJ. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, K.; Li, J.; Li, X.; Li, H. Understanding the Effect of Listening to Music, Playing Music, and Singing on Brain Function: A Scoping Review of fNIRS Studies. Brain Sci. 2024, 14, 751. https://doi.org/10.3390/brainsci14080751
Ding K, Li J, Li X, Li H. Understanding the Effect of Listening to Music, Playing Music, and Singing on Brain Function: A Scoping Review of fNIRS Studies. Brain Sciences. 2024; 14(8):751. https://doi.org/10.3390/brainsci14080751
Chicago/Turabian StyleDing, Keya, Jingwen Li, Xuemei Li, and Hui Li. 2024. "Understanding the Effect of Listening to Music, Playing Music, and Singing on Brain Function: A Scoping Review of fNIRS Studies" Brain Sciences 14, no. 8: 751. https://doi.org/10.3390/brainsci14080751
APA StyleDing, K., Li, J., Li, X., & Li, H. (2024). Understanding the Effect of Listening to Music, Playing Music, and Singing on Brain Function: A Scoping Review of fNIRS Studies. Brain Sciences, 14(8), 751. https://doi.org/10.3390/brainsci14080751







