Dorsal Anterior Cingulate Cortex Coordinates Contextual Mental Imagery for Single-Beat Manipulation during Rhythmic Sensorimotor Synchronization
Abstract
1. Introduction
2. Materials and Methods
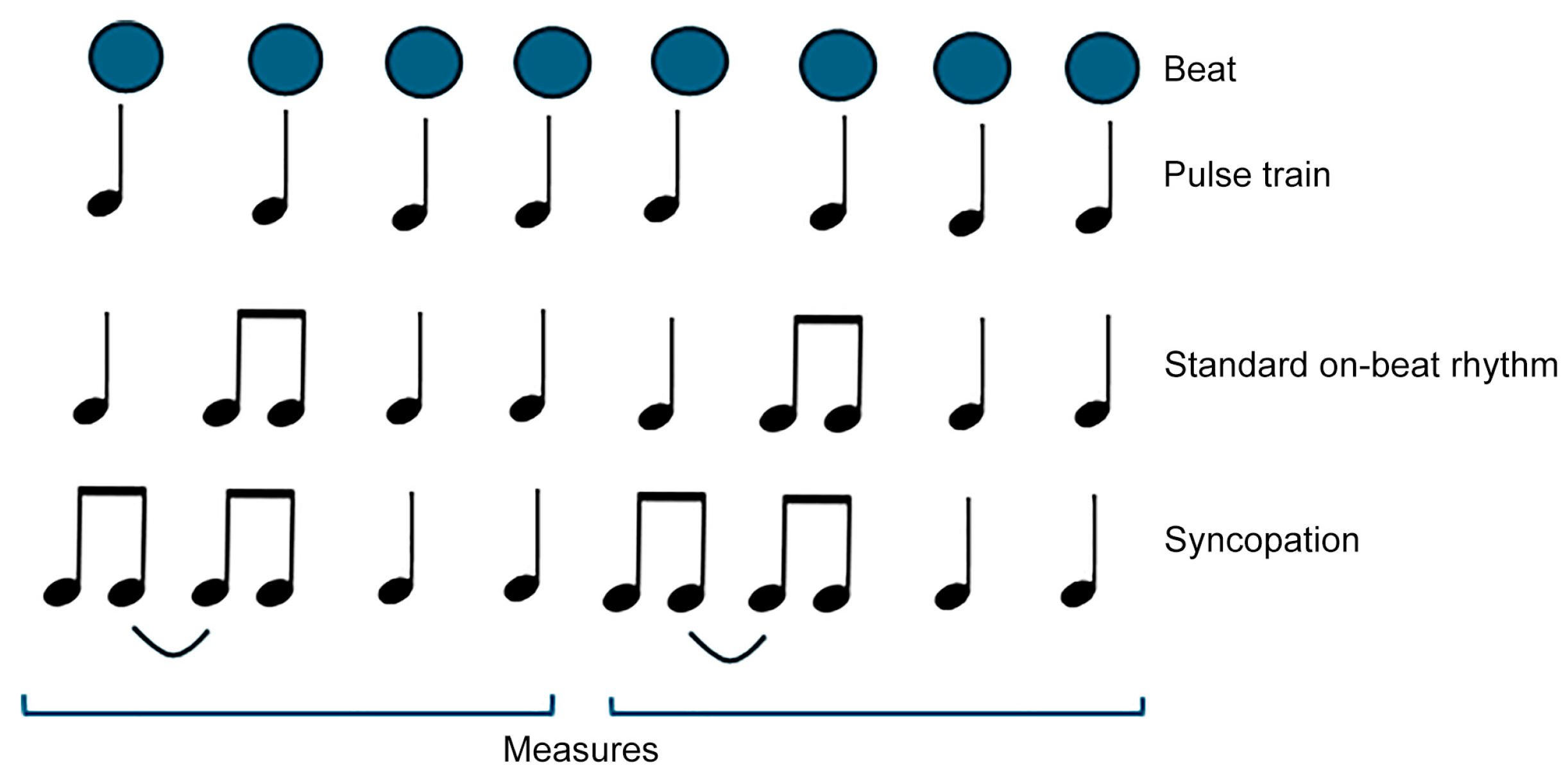
2.1. Stimuli and Experimental Procedure
2.2. Participants
2.3. Data Analysis
2.3.1. Behavioral Performance
2.3.2. Event-Related Deep-Brain Activity Method
2.3.3. ERP Trace Analysis
2.3.4. Statistics
3. Results
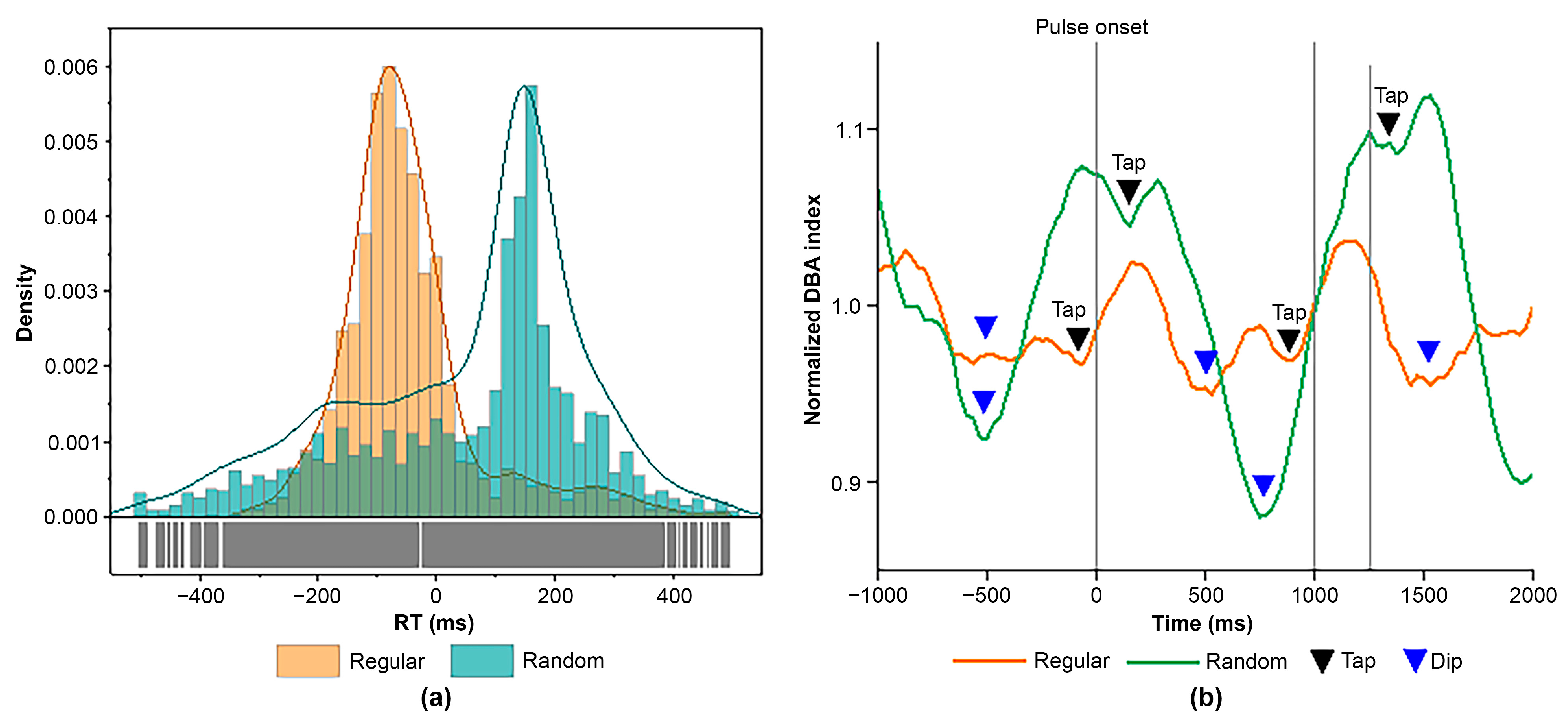
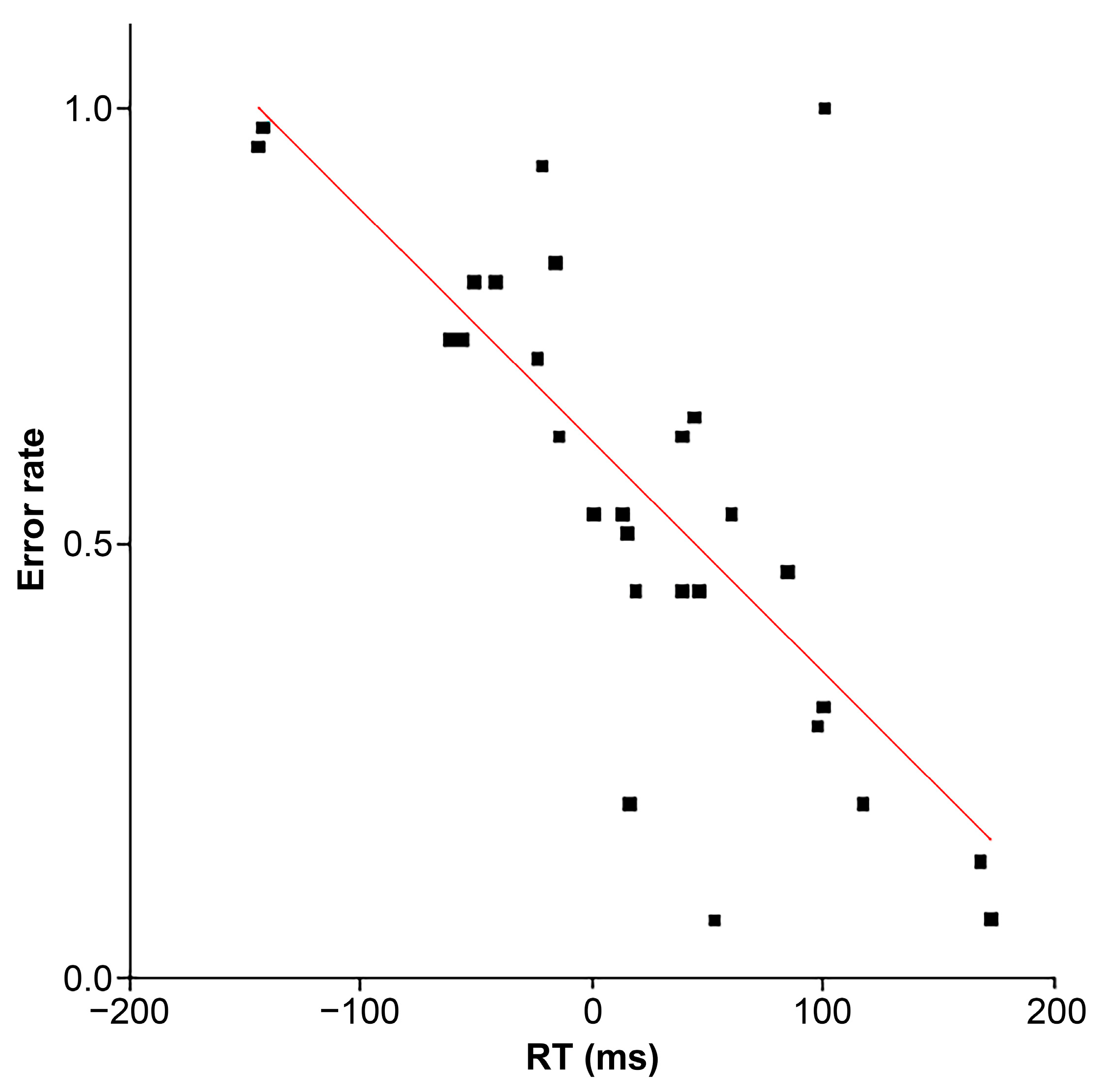
3.1. Behavioral Performance
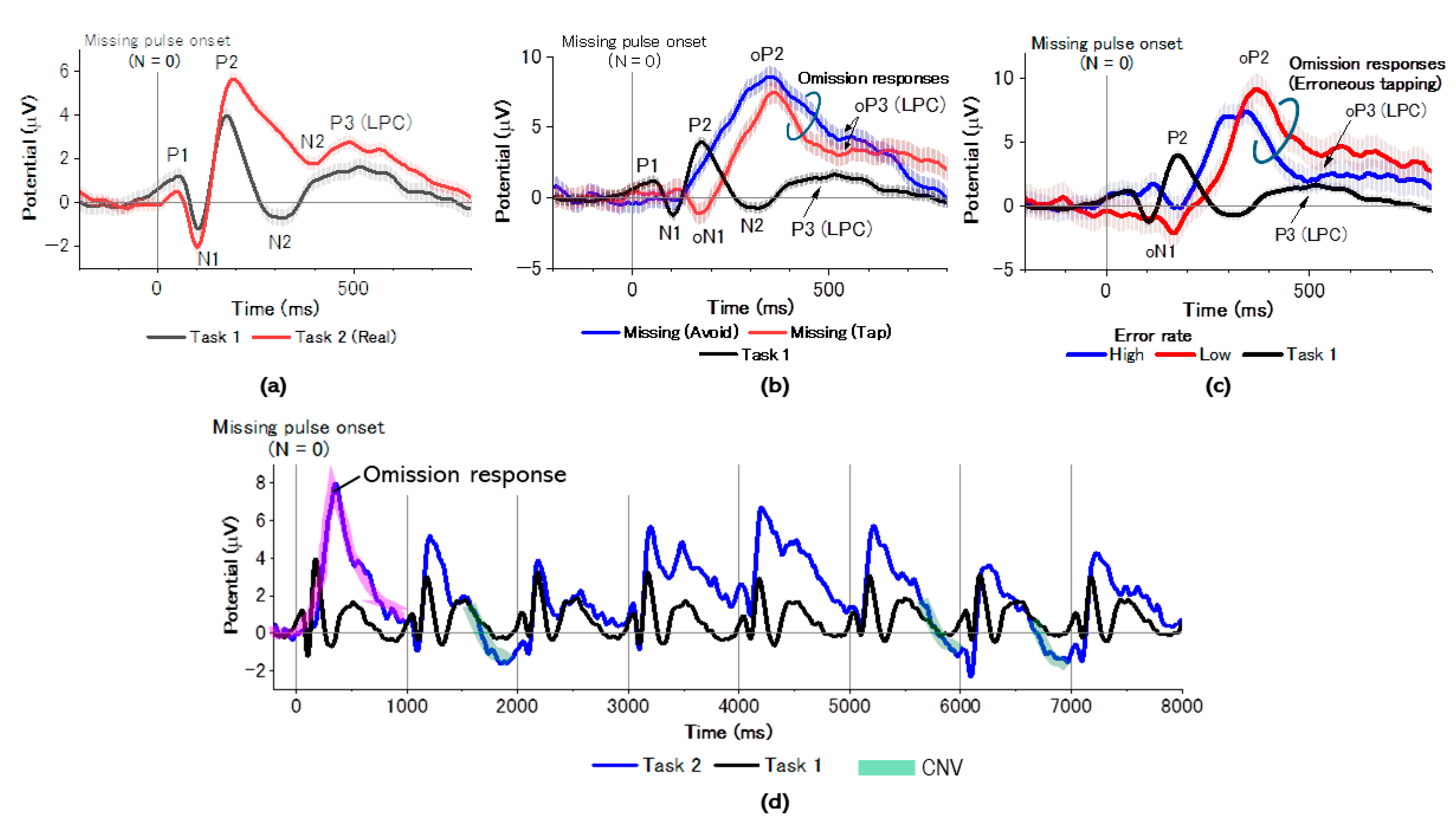
3.2. ER-DBA Trace Analysis
3.3. ERP Trace Analysis
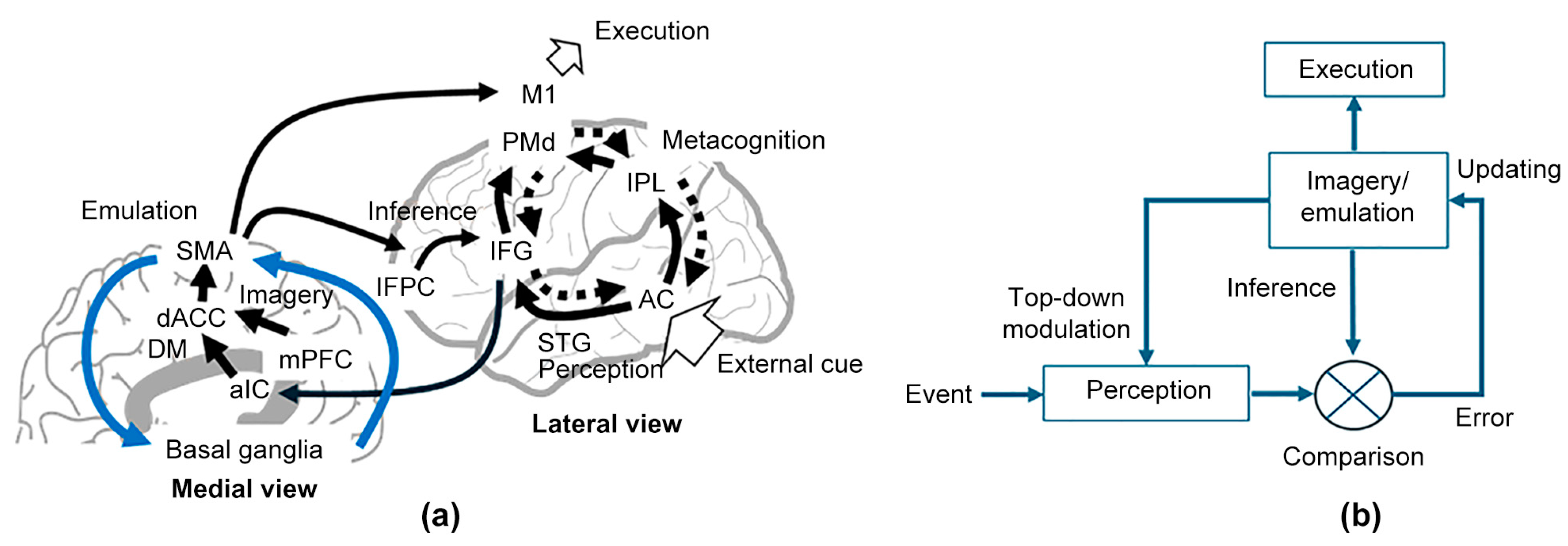
4. Discussion
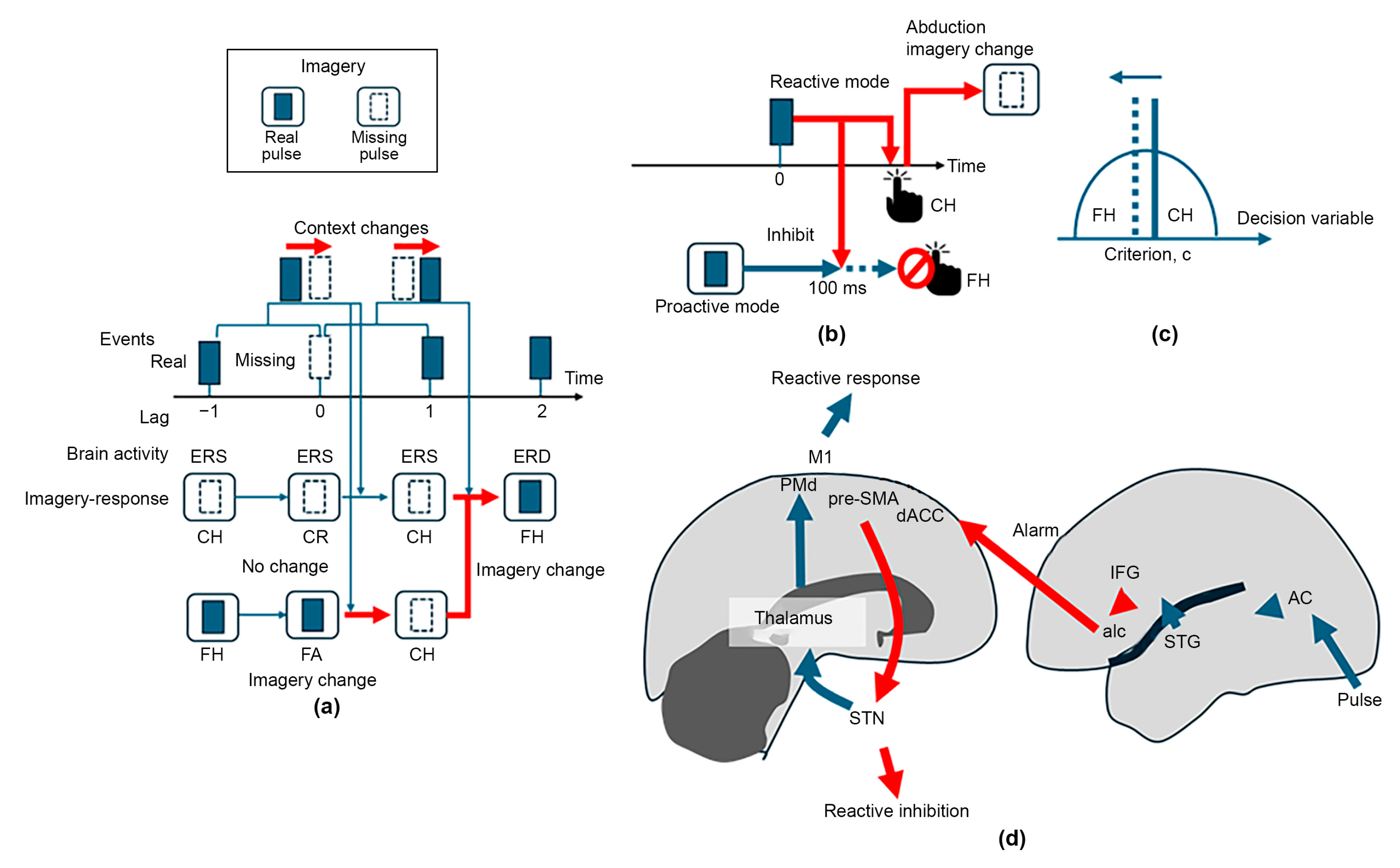
4.1. The DACC Utilizes Contextual Imagery for Dynamic Regulation of Cued-Tapping Mode
4.2. Maintenance of Mental Imagery
4.3. Is Modal Completion Caused by Fusion of Imagery and Perception?
4.4. Limitation and Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goebl, W.; Palmer, C. Synchronization of timing and motion among performing musicians. Music Percept. 2009, 26, 427–438. [Google Scholar] [CrossRef]
- Basso, J.C.; Satyal, M.K.; Rugh, R. Dance on the brain: Enhancing intra- and inter-brain synchrony. Front. Hum. Neurosci. 2020, 14, 584312. [Google Scholar] [CrossRef]
- Sioros, G.; Miron, M.; Davies, M.; Gouyon, F.; Madison, G. Syncopation creates the sensation of groove in synthesized music examples. Front. Psychol. 2014, 5, 1036. [Google Scholar] [CrossRef] [PubMed]
- Fiorin, G.; Delfitto, D. Syncopation as structure bootstrapping: The role of asymmetry in rhythm and language. Front. Psychol. 2024, 15, 1304485. [Google Scholar] [CrossRef] [PubMed]
- Bouwer, F.L.; Burgoyne, J.A.; Odijk, D.; Honing, H.; Grahn, J.A. What makes a rhythm complex? The influence of musical training and accent type on beat perception. PLoS ONE 2018, 13, e0190322. [Google Scholar] [CrossRef]
- Chen, J.L.; Penhune, V.B.; Zatorre, R.J. Listening to musical rhythms recruits motor regions of the brain. Cereb. Cortex 2008, 18, 2844–2854. [Google Scholar] [CrossRef]
- Large, E.W.; Herrera, J.A.; Velasco, M.J. Neural networks for beat perception in musical rhythm. Front. Syst. Neurosci. 2015, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Bouwer, F.L.; Van Zuijen, T.L.; Honing, H. Beat processing is pre-attentive for metrically simple rhythms with clear accents: An ERP study. PLoS ONE 2014, 9, e97467. [Google Scholar] [CrossRef] [PubMed]
- Large, E.W.; Roman, I.; Kim, J.C.; Cannon, J.; Pazdera, J.K.; Trainor, L.J.; Rinzel, J.; Bose, A. Dynamic models for musical rhythm perception and coordination. Front. Comput. Neurosci. 2023, 17, 1151895. [Google Scholar] [CrossRef]
- Large, E.W.; Snyder, J.S. Pulse and meter as neural resonance. Ann. N. Y. Acad. Sci. 2009, 1169, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Tal, I.; Large, E.W.; Rabinovitch, E.; Wei, Y.; Schroeder, C.E.; Poeppel, D.; Zion Golumbic, E. Neural entrainment to the beat: The “missing-pulse” phenomenon. J. Neurosci. 2017, 37, 6331–6341. [Google Scholar] [CrossRef] [PubMed]
- Ragazzoni, A.; Di Russo, F.; Fabbri, S.; Pesaresi, I.; Di Rollo, A.; Perri, R.L.; Barloscio, D.; Bocci, T.; Cosottini, M.; Sartucci, F. “Hit the missing stimulus”. A simultaneous EEG-fMRI study to localize the generators of endogenous ERPs in an omitted target paradigm. Sci. Rep. 2019, 9, 3684. [Google Scholar] [CrossRef] [PubMed]
- Nozaradan, S. Exploring how musical rhythm entrains brain activity with electroencephalogram frequency-tagging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130393. [Google Scholar] [CrossRef] [PubMed]
- Proksch, S.; Comstock, D.C.; Médé, B.; Pabst, A.; Balasubramaniam, R. Motor and predictive processes in auditory beat and rhythm perception. Front. Hum. Neurosci. 2020, 14, 578546. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.D.; Iversen, J.R. The evolutionary neuroscience of musical beat perception: The Action Simulation for Auditory Prediction (ASAP) hypothesis. Front. Syst. Neurosci. 2014, 8, 57. [Google Scholar] [CrossRef]
- Merchant, H.; Honing, H. Are non-human primates capable of rhythmic entrainment? Evidence for the gradual audiomotor evolution hypothesis. Front. Neurosci. 2013, 7, 274. [Google Scholar] [CrossRef]
- van der Steen, M.C.; Jacoby, N.; Fairhurst, M.T.; Keller, P.E. Sensorimotor synchronization with tempo-changing auditory sequences: Modeling temporal adaptation and anticipation. Brain Res. 2015, 1626, 66–87. [Google Scholar] [CrossRef] [PubMed]
- van der Steen, M.C.; Schwartze, M.; Kotz, S.A.; Keller, P.E. Modeling effects of cerebellar and basal ganglia lesions on adaptation and anticipation during sensorimotor synchronization. Ann. N. Y. Acad. Sci. 2015, 1337, 101–110. [Google Scholar] [CrossRef]
- van der Steen, M.C.; Keller, P.E. The ADaptation and Anticipation Model (ADAM) of sensorimotor synchronization. Front. Hum. Neurosci. 2013, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Harry, B.; Keller, P.E. Tutorial and simulations with ADAM: An adaptation and anticipation model of sensorimotor synchronization. Biol. Cybern. 2019, 113, 397–421. [Google Scholar] [CrossRef]
- Cannon, J. Expectancy-based rhythmic entrainment as continuous Bayesian inference. PLoS Comput. Biol. 2021, 17, e1009025. [Google Scholar] [CrossRef]
- Kaplan, T.; Cannon, J.; Jamone, L.; Pearce, M. Modeling enculturated bias in entrainment to rhythmic patterns. PLoS Comput. Biol. 2022, 18, e1010579. [Google Scholar] [CrossRef] [PubMed]
- Rohe, T.; Ehlis, A.C.; Noppeney, U. The neural dynamics of hierarchical Bayesian causal inference in multisensory perception. Nat. Commun. 2019, 10, 1907. [Google Scholar] [CrossRef] [PubMed]
- Rohe, T.; Noppeney, U. Cortical hierarchies perform Bayesian causal inference in multisensory perception. PLoS Biol. 2015, 13, e1002073. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.; Gross, J.; Thut, G. A new unifying account of the roles of neuronal entrainment. Curr. Biol. 2019, 29, R890–R905. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, N.; Kok, P.; Fleming, S.M. Imagery adds stimulus-specific sensory evidence to perceptual detection. J. Vis. 2022, 22, 11. [Google Scholar] [CrossRef]
- Morillon, B.; Schroeder, C.E.; Wyart, V.; Arnal, L.H. Temporal prediction in lieu of periodic stimulation. J. Neurosci. 2016, 36, 2342–2347. [Google Scholar] [CrossRef]
- Rimmele, J.M.; Morillon, B.; Poeppel, D.; Arnal, L.H. Proactive sensing of periodic and aperiodic auditory patterns. Trends Cogn. Sci. 2018, 22, 870–882. [Google Scholar] [CrossRef]
- Barry, D.N.; Barnes, G.R.; Clark, I.A.; Maguire, E.A. The neural dynamics of novel scene imagery. J. Neurosci. 2019, 39, 4375–4386. [Google Scholar] [CrossRef]
- Monk, A.M.; Dalton, M.A.; Barnes, G.R.; Maguire, E.A. The role of hippocampal-ventromedial prefrontal cortex neural dynamics in building mental representations. J. Cogn. Neurosci. 2021, 33, 89–103. [Google Scholar] [CrossRef]
- Na, C.H.; Jütten, K.; Forster, S.D.; Clusmann, H.; Mainz, V. Self-referential processing and resting-state functional MRI connectivity of cortical midline structures in glioma patients. Brain Sci. 2022, 12, 1463. [Google Scholar] [CrossRef] [PubMed]
- Madore, K.P.; Thakral, P.P.; Beaty, R.E.; Addis, D.R.; Schacter, D.L. Neural mechanisms of episodic retrieval support divergent creative thinking. Cereb. Cortex 2019, 29, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Beaty, R.E.; Benedek, M.; Kaufman, S.B.; Silvia, P.J. Default and executive network coupling supports creative idea production. Sci. Rep. 2015, 5, 10964. [Google Scholar] [CrossRef] [PubMed]
- Moulton, S.T.; Kosslyn, S.M. Imagining predictions: Mental imagery as mental emulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, X.J. Inhibitory control in the cortico-basal ganglia-thalamocortical loop: Complex regulation and interplay with memory and decision processes. Neuron 2016, 92, 1093–1105. [Google Scholar] [CrossRef]
- Aoki, S.; Smith, J.B.; Li, H.; Yan, X.; Igarashi, M.; Coulon, P.; Wickens, J.R.; Ruigrok, T.J.; Jin, X. An open cortico-basal ganglia loop allows limbic control over motor output via the nigrothalamic pathway. eLife 2019, 8, e49995. [Google Scholar] [CrossRef]
- Bizley, J.K.; Cohen, Y.E. The what, where and how of auditory-object perception. Nat. Rev. Neurosci. 2013, 14, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Su, J.; Ni, Y.; Bai, Y.; Zhang, X.; Li, X.; Wan, X. The neural system of metacognition accompanying decision-making in the prefrontal cortex. PLOS Biol. 2018, 16, e2004037. [Google Scholar] [CrossRef]
- Cichy, R.M.; Heinzle, J.; Haynes, J.D. Imagery and perception share cortical representations of content and location. Cereb. Cortex 2012, 22, 372–380. [Google Scholar] [CrossRef]
- Dijkstra, N.; Bosch, S.E.; van Gerven, M.A.J. Shared neural mechanisms of visual perception and imagery. Trends Cogn. Sci. 2019, 23, 423–434. [Google Scholar] [CrossRef]
- Colder, B. The basal ganglia select the expected sensory input used for predictive coding. Front. Comput. Neurosci. 2015, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Kral, A.; Yusuf, P.A.; Land, R. Higher–order auditory areas in congenital deafness: Top-down interactions and corticocortical decoupling. Hear. Res. 2017, 343, 50–63. [Google Scholar] [CrossRef]
- Bush, G.; Vogt, B.A.; Holmes, J.; Dale, A.M.; Greve, D.; Jenike, M.A.; Rosen, B.R. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc. Natl. Acad. Sci. USA 2002, 99, 523–528. [Google Scholar] [CrossRef]
- Marsh, A.A.; Blair, K.S.; Vythilingam, M.; Busis, S.; Blair, R.J. Response options and expectations of reward in decision-making: The differential roles of dorsal and rostral anterior cingulate cortex. Neuroimage 2007, 35, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Williams, Z.M.; Bush, G.; Rauch, S.L.; Cosgrove, G.R.; Eskandar, E.N. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat. Neurosci. 2004, 7, 1370–1375. [Google Scholar] [CrossRef]
- Walton, M.E.; Croxson, P.L.; Behrens, T.E.; Kennerley, S.W.; Rushworth, M.F. Adaptive decision making and value in the anterior cingulate cortex. Neuroimage 2007, 36, T142–T154. [Google Scholar] [CrossRef] [PubMed]
- Bryden, D.W.; Brockett, A.T.; Blume, E.; Heatley, K.; Zhao, A.; Roesch, M.R. Single neurons in anterior cingulate cortex signal the need to change action during performance of a stop-change task that induces response competition. Cereb. Cortex 2019, 29, 1020–1031. [Google Scholar] [CrossRef]
- Brockett, A.T.; Tennyson, S.S.; deBettencourt, C.A.; Gaye, F.; Roesch, M.R. Anterior cingulate cortex is necessary for adaptation of action plans. Proc. Natl. Acad. Sci. USA 2020, 117, 6196–6204. [Google Scholar] [CrossRef]
- Asemi, A.; Ramaseshan, K.; Burgess, A.; Diwadkar, V.A.; Bressler, S.L. Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Front. Hum. Neurosci. 2015, 9, 309. [Google Scholar] [CrossRef]
- Diwadkar, V.A.; Asemi, A.; Burgess, A.; Chowdury, A.; Bressler, S.L. Potentiation of motor sub-networks for motor control but not working memory: Interaction of dACC and SMA revealed by resting-state directed functional connectivity. PLoS ONE 2017, 12, e0172531. [Google Scholar] [CrossRef]
- Bush, K.A.; James, G.A.; Privratsky, A.A.; Fialkowski, K.P.; Kilts, C.D. Action-value processing underlies the role of the dorsal anterior cingulate cortex in performance monitoring during self-regulation of affect. PLoS ONE 2022, 17, e0273376. [Google Scholar] [CrossRef] [PubMed]
- van Noordt, S.J.; Segalowitz, S.J. Performance monitoring and the medial prefrontal cortex: A review of individual differences and context effects as a window on self-regulation. Front. Hum. Neurosci. 2012, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Segalowitz, S.J.; Santesso, D.L.; Murphy, T.I.; Homan, D.; Chantziantoniou, D.K.; Khan, S. Retest reliability of medial frontal negativities during performance monitoring. Psychophysiology 2010, 47, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; van Veen, V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cogn. Affect. Behav. Neurosci. 2007, 7, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.J.; Deng, X.P.; Zhao, N.; Jin, J.; Yue, J.; Hu, Y.S.; Jing, Y.; Wang, H.X.; Knösche, T.R.; Zang, Y.F.; et al. Resting-state fMRI functional connectivity strength predicts local activity change in the dorsal cingulate cortex: A multi-target focused rTMS study. Cereb. Cortex 2022, 32, 2773–2784. [Google Scholar] [CrossRef]
- Herremans, S.C.; De Raedt, R.; Van Schuerbeek, P.; Marinazzo, D.; Matthys, F.; De Mey, J.; Baeken, C. Accelerated HF-rTMS protocol has a rate-dependent effect on dacc activation in alcohol-dependent patients: An open-label feasibility study. Alcohol. Clin. Exp. Res. 2016, 40, 196–205. [Google Scholar] [CrossRef]
- Thanakamchokchai, J.; Tretriluxana, J.; Pakaprot, N.; Pisarnpong, A.; Fisher, B.E. Effects of high-frequency repetitive transcranial magnetic stimulation on reach-to-grasp performance in individuals with Parkinson’s disease: A preliminary study. Exp. Brain Res. 2020, 238, 1827–1837. [Google Scholar] [CrossRef]
- Moscatelli, F.; Toto, G.A.; Valenzano, A.; Cibelli, G.; Monda, V.; Limone, P.; Mancini, N.; Messina, A.; Marsala, G.; Messina, G.; et al. High frequencies (HF) repetitive transcranial magnetic stimulation (rTMS) increase motor coordination performances in volleyball players. BMC Neurosci. 2023, 24, 30. [Google Scholar] [CrossRef]
- Miyake, Y.; Onishi, Y.; Pöppel, E. Two types of anticipation in synchronization tapping. Acta Neurobiol. Exp. (Wars) 2004, 64, 415–426. [Google Scholar] [CrossRef]
- Yoles-Frenkel, M.; Avron, M.; Prut, Y. Impact of auditory context on executed motor actions. Front. Integr. Neurosci. 2016, 10, 1. [Google Scholar] [CrossRef]
- Ellena, G.; Contò, F.; Tosi, M.; Battelli, L. The effect of visual statistical learning on proactive motor control is modulated by transcranial random noise stimulation over frontoparietal cortex. J. Vis. 2023, 23, 5484. [Google Scholar] [CrossRef]
- Angelini, M.; Calbi, M.; Ferrari, A.; Sbriscia-Fioretti, B.; Franca, M.; Gallese, V.; Umiltà, M.A. Proactive control strategies for overt and covert go/NoGo tasks: An electrical neuroimaging study. PLoS ONE 2016, 11, e0152188. [Google Scholar] [CrossRef]
- Liebrand, M.; Kristek, J.; Tzvi, E.; Krämer, U.M. Ready for change: Oscillatory mechanisms of proactive motor control. PLoS ONE 2018, 13, e0196855. [Google Scholar] [CrossRef] [PubMed]
- Braver, T.S. The variable nature of cognitive control: A dual mechanisms framework. Trends Cogn. Sci. 2012, 16, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Schwarzkopp, T.; Mayr, U.; Jost, K. Early selection versus late correction: Age-related differences in controlling working memory contents. Psychol. Aging 2016, 31, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.C.; Shallice, T.; Dolan, R.J. The functional roles of prefrontal cortex in episodic memory. I. Encoding. Brain 1998, 121 Pt 7, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Scangos, K.W.; Stuphorn, V. Supplementary motor area exerts proactive and reactive control of arm movements. J. Neurosci. 2010, 30, 14657–14675. [Google Scholar] [CrossRef]
- Toma, K.; Ozawa, M.; Matsuo, K.; Nakai, T.; Fukuyama, H.; Sato, S. The role of the human supplementary motor area in reactive motor operation. Neurosci. Lett. 2003, 344, 177–180. [Google Scholar] [CrossRef]
- Irlbacher, K.; Kraft, A.; Kehrer, S.; Brandt, S.A. Mechanisms and neuronal networks involved in reactive and proactive cognitive control of interference in working memory. Neurosci. Biobehav. Rev. 2014, 46 Pt 1, 58–70. [Google Scholar] [CrossRef]
- Aben, B.; Buc Calderon, C.; Van den Bussche, E.; Verguts, T. Cognitive Effort Modulates Connectivity between Dorsal Anterior Cingulate Cortex and Task-Relevant Cortical Areas. J. Neurosci. 2020, 40, 3838–3848. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; de Hemptinne, C.; Miller, A.M.; Leibbrand, M.; Little, S.J.; Lim, D.A.; Larson, P.S.; Starr, P.A. Prefrontal-Subthalamic Hyperdirect Pathway Modulates Movement Inhibition in Humans. Neuron 2020, 106, 579–588.e3. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Meffre, J.; Duclos, Y.; Breysse, E.; Pelloux, Y. First evidence of a hyperdirect prefrontal pathway in the primate: Precise organization for new insights on subthalamic nucleus functions. Front. Comput. Neurosci. 2013, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Lieder, F.; Iwama, G. Toward a formal theory of proactivity. Cogn. Affect. Behav. Neurosci. 2021, 21, 490–508. [Google Scholar] [CrossRef] [PubMed]
- Mäki-Marttunen, V.; Hagen, T.; Espeseth, T. Proactive and reactive modes of cognitive control can operate independently and simultaneously. Acta Psychol. 2019, 199, 102891. [Google Scholar] [CrossRef]
- Marklund, P.; Persson, J. Context-dependent switching between proactive and reactive working memory control mechanisms in the right inferior frontal gyrus. Neuroimage 2012, 63, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Grisetto, F.; Delevoye-Turrell, Y.N.; Roger, C. Slower adaptation of control strategies in individuals with high impulsive tendencies. Sci. Rep. 2021, 11, 20368. [Google Scholar] [CrossRef]
- Wilson, E.C.; Reed, C.M.; Braida, L.D. Integration of auditory and vibrotactile stimuli: Effects of phase and stimulus-onset asynchrony. J. Acoust. Soc. Am. 2009, 126, 1960–1974. [Google Scholar] [CrossRef]
- Wilson, E.C.; Reed, C.M.; Braida, L.D. Integration of auditory and vibrotactile stimuli: Effects of frequency. J. Acoust. Soc. Am. 2010, 127, 3044–3059. [Google Scholar] [CrossRef]
- Di Luca, M.; Machulla, T.K.; Ernst, M.O. Recalibration of multisensory simultaneity: Cross-modal transfer coincides with a change in perceptual latency. J. Vis. 2009, 9, 7. [Google Scholar] [CrossRef]
- Cunillera, T.; Fuentemilla, L.; Brignani, D.; Cucurell, D.; Miniussi, C. A simultaneous modulation of reactive and proactive inhibition processes by anodal tDCS on the right inferior frontal cortex. PLoS ONE 2014, 9, e113537. [Google Scholar] [CrossRef] [PubMed]
- Grisetto, F.; Le Denmat, P.; Delevoye-Turrell, Y.N.; Vantrepotte, Q.; Davin, T.; Dinca, A.; Ghoulti, I.D.; Roger, C. Imbalanced weighting of proactive and reactive control as a marker of risk-taking propensity. PLoS ONE 2023, 18, e0277246. [Google Scholar] [CrossRef]
- Angelini, M.; Calbi, M.; Ferrari, A.; Sbriscia-Fioretti, B.; Franca, M.; Gallese, V.; Umiltà, M.A. Motor inhibition during overt and covert actions: An electrical neuroimaging study. PLoS ONE 2015, 10, e0126800. [Google Scholar] [CrossRef]
- Liu, C.C.; Watanabe, T. Accounting for speed-accuracy tradeoff in perceptual learning. Vision Res. 2012, 61, 107–114. [Google Scholar] [CrossRef]
- Vandierendonck, A. On the utility of integrated speed-accuracy measures when speed-accuracy trade-off is present. J. Cogn. 2021, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Standage, D.; Blohm, G.; Dorris, M.C. On the neural implementation of the speed-accuracy trade-off. Front. Neurosci. 2014, 8, 236. [Google Scholar] [CrossRef]
- Otani, Y.; Katagiri, Y.; Imai, E.; Kowa, H. Action-rule-based cognitive control enables efficient execution of stimulus-response conflict tasks: A model validation of Simon task performance. Front. Hum. Neurosci. 2023, 17, 1239207. [Google Scholar] [CrossRef]
- Di Liberto, G.M.; Marion, G.; Shamma, S.A. The music of silence: Part II: Music listening induces imagery responses. J. Neurosci. 2021, 41, 7449–7460. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Z. Curve fitting of the corporate recovery rates: The comparison of beta distribution estimation and kernel density estimation. PLoS ONE 2013, 8, e68238. [Google Scholar] [CrossRef]
- Jayakumar, V.; Simpson, T.L. Detectability and bias indices of pneumatic corneal stimuli using signal detection theory. Transl. Vis. Sci. Technol. 2020, 9, 17. [Google Scholar] [CrossRef]
- Locke, S.M.; Robinson, O.J. Affective bias through the lens of signal detection theory. Comput. Psychiatr. 2021, 5, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Omata, K.; Hanakawa, T.; Morimoto, M.; Honda, M. Spontaneous slow fluctuation of EEG alpha rhythm reflects activity in deep-brain structures: A simultaneous EEG-fMRI study. PLoS ONE 2013, 8, e66869. [Google Scholar] [CrossRef] [PubMed]
- Wianda, E.; Ross, B. The roles of alpha oscillation in working memory retention. Brain Behav. 2019, 9, e01263. [Google Scholar] [CrossRef] [PubMed]
- Zhozhikashvili, N.; Zakharov, I.; Ismatullina, V.; Feklicheva, I.; Malykh, S.; Arsalidou, M. Parietal Alpha Oscillations: Cognitive Loadalpha oscillations: Cognitive load and Mental Toughness.mental toughness. Brain Sci. 2022, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- Rektor, I.; Sochůrková, D.; Bocková, M. Intracerebral ERD/ERS in voluntary movement and in cognitive visuomotor task. Prog. Brain Res. 2006, 159, 311–330. [Google Scholar] [CrossRef]
- Hatsopoulos, N.G.; Xu, Q.; Amit, Y. Encoding of movement fragments in the motor cortex. J. Neurosci. 2007, 27, 5105–5114. [Google Scholar] [CrossRef] [PubMed]
- Pelaquim, A.; Sanfins, M.D.; Fornazieri, M.A. Standardization of latency and amplitude values of short, middle and long latency auditory evoked potentials in adults. Int. Arch. Otorhinolaryngol. 2023, 27, e278–e285. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.S.; Didoné, D.D.; Durante, A.S. Automated cortical auditory evoked potentials threshold estimation in neonates. Braz. J. Otorhinolaryngol. 2019, 85, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Toffolo, K.K.; Freedman, E.G.; Foxe, J.J. Evoking the N400 event-related potential (ERP) component using a publicly available novel set of sentences with semantically incongruent or congruent eggplants (endings). Neuroscience 2022, 501, 143–158. [Google Scholar] [CrossRef]
- Hagoort, P. Interplay between syntax and semantics during sentence comprehension: ERP effects of combining syntactic and semantic violations. J. Cogn. Neurosci. 2003, 15, 883–899. [Google Scholar] [CrossRef]
- Palolahti, M.; Leino, S.; Jokela, M.; Kopra, K.; Paavilainen, P. Event-related potentials suggest early interaction between syntax and semantics during on-line sentence comprehension. Neurosci. Lett. 2005, 384, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Wacongne, C.; Labyt, E.; van Wassenhove, V.; Bekinschtein, T.; Naccache, L.; Dehaene, S. Evidence for a hierarchy of predictions and prediction errors in human cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 20754–20759. [Google Scholar] [CrossRef] [PubMed]
- SanMiguel, I.; Widmann, A.; Bendixen, A.; Trujillo-Barreto, N.; Schröger, E. Hearing silences: Human auditory processing relies on preactivation of sound-specific brain activity patterns. J. Neurosci. 2013, 33, 8633–8639. [Google Scholar] [CrossRef]
- Braga, A.; Schönwiesner, M. Neural substrates and models of omission responses and predictive processes. Front. Neural Circuits 2022, 16, 799581. [Google Scholar] [CrossRef] [PubMed]
- Korka, B.; Widmann, A.; Waszak, F.; Darriba, Á.; Schröger, E. The auditory brain in action: Intention determines predictive processing in the auditory system-A review of current paradigms and findings. Psychon. Bull. Rev. 2022, 29, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Chennu, S.; Noreika, V.; Gueorguiev, D.; Shtyrov, Y.; Bekinschtein, T.A.; Henson, R. Silent expectations: Dynamic causal modeling of cortical prediction and attention to sounds that weren’t. J. Neurosci. 2016, 36, 8305–8316. [Google Scholar] [CrossRef]
- Begg, I.; Clark, J.M. Contextual imagery in meaning and memory. Mem. Cogn. 1975, 3, 117–122. [Google Scholar] [CrossRef]
- Schwanenflugel, P.J.; Akin, C.; Luh, W.M. Context availability and the recall of abstract and concrete words. Mem. Cogn. 1992, 20, 96–104. [Google Scholar] [CrossRef]
- Takano, K.; Miyake, Y. Two types of phase correction mechanism involved in synchronized tapping. Neurosci. Lett. 2007, 417, 196–200. [Google Scholar] [CrossRef]
- Yang, J.; Ouyang, F.; Holm, L.; Huang, Y.; Gan, L.; Zhou, L.; Chao, H.; Wang, M.; He, M.; Zhang, S.; et al. A mechanism of timing variability underlying the association between the mean and SD of asynchrony. Hum. Mov. Sci. 2019, 67, 102500. [Google Scholar] [CrossRef]
- Pollok, B.; Müller, K.; Aschersleben, G.; Schnitzler, A.; Prinz, W. The role of the primary somatosensory cortex in an auditorily paced finger tapping task. Exp. Brain Res. 2004, 156, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Repp, B.H. Sensorimotor synchronization: A review of the tapping literature. Psychon. Bull. Rev. 2005, 12, 969–992. [Google Scholar] [CrossRef]
- Jensen, O.; Mazaheri, A. Shaping functional architecture by oscillatory alpha activity: Gating by inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Berchicci, M.; Lucci, G.; Spinelli, D.; Di Russo, F. Stimulus onset predictability modulates proactive action control in a Go/No-go task. Front. Behav. Neurosci. 2015, 9, 101. [Google Scholar] [CrossRef] [PubMed]
- Bozhilova, N.; Kuntsi, J.; Rubia, K.; Asherson, P.; Michelini, G. Event-related brain dynamics during mind wandering in attention-deficit/hyperactivity disorder: An experience-sampling approach. NeuroImage Clin. 2022, 35, 103068. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, Y.; Ye, C.; Yang, J.; He, W.; Alpha, E.E. Alpha ERS-ERD pattern during divergent and convergent thinking depends on individual differences on metacontrol. J. Intell. 2023, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Acerbi, L.; Dokka, K.; Angelaki, D.E.; Ma, W.J. Bayesian comparison of explicit and implicit causal inference strategies in multisensory heading perception. PLOS Comput. Biol. 2018, 14, e1006110. [Google Scholar] [CrossRef]
- Gijsen, S.; Grundei, M.; Lange, R.T.; Ostwald, D.; Blankenburg, F. Neural surprise in somatosensory Bayesian learning. PLOS Comput. Biol. 2021, 17, e1008068. [Google Scholar] [CrossRef]
- Ostwald, D.; Spitzer, B.; Guggenmos, M.; Schmidt, T.T.; Kiebel, S.J.; Blankenburg, F. Evidence for neural encoding of Bayesian surprise in human somatosensation. Neuroimage 2012, 62, 177–188. [Google Scholar] [CrossRef]
- English, G.; Ghasemi Nejad, N.; Sommerfelt, M.; Yanik, M.F.; von der Behrens, W. Bayesian surprise shapes neural responses in somatosensory cortical circuits. Cell Rep. 2023, 42, 112009. [Google Scholar] [CrossRef] [PubMed]
- Klichowicz, A.; Lippoldt, D.E.; Rosner, A.; Krems, J.F. Information stored in memory affects abductive reasoning. Psychol. Res. 2021, 85, 3119–3133. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S. Modelplasticity and abductive decision making. Decis. Econ. Financ. 2023, 46, 255–276. [Google Scholar] [CrossRef]
- Hommel, B.; Müsseler, J.; Aschersleben, G.; Prinz, W. The Theory of Event Coding (TEC): A framework for perception and action planning. Behav. Brain Sci. 2001, 24, 849–878. [Google Scholar] [CrossRef] [PubMed]
- Arnal, L.H.; Kleinschmidt, A.; Spinelli, L.; Giraud, A.L.; Mégevand, P. The rough sound of salience enhances aversion through neural synchronisation. Nat. Commun. 2019, 10, 3671. [Google Scholar] [CrossRef]
- Trambaiolli, L.R.; Peng, X.; Lehman, J.F.; Linn, G.; Russ, B.E.; Schroeder, C.E.; Liu, H.; Haber, S.N. Anatomical and functional connectivity support the existence of a salience network node within the caudal ventrolateral prefrontal cortex. eLife 2022, 11, e76334. [Google Scholar] [CrossRef]
- van Wouwe, N.C.; Pallavaram, S.; Phibbs, F.T.; Martinez-Ramirez, D.; Neimat, J.S.; Dawant, B.M.; D’Haese, P.F.; Kanoff, K.E.; van den Wildenberg, W.P.M.; Okun, M.S.; et al. Focused stimulation of dorsal subthalamic nucleus improves reactive inhibitory control of action impulses. Neuropsychologia 2017, 99, 37–47. [Google Scholar] [CrossRef]
- Zhang, F.; Iwaki, S. Common neural network for different functions: An investigation of proactive and reactive inhibition. Front. Behav. Neurosci. 2019, 13, 124. [Google Scholar] [CrossRef]
- Rae, C.L.; Hughes, L.E.; Anderson, M.C.; Rowe, J.B. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J. Neurosci. 2015, 35, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Cervantes Constantino, F.; Simon, J.Z. Dynamic cortical representations of perceptual filling-in for missing acoustic rhythm. Sci. Rep. 2017, 7, 17536. [Google Scholar] [CrossRef]
- Horr, N.K.; Di Luca, M. Filling the blanks in temporal intervals: The type of filling influences perceived duration and discrimination performance. Front. Psychol. 2015, 6, 114. [Google Scholar] [CrossRef]
- Boltz, M. Perceiving the end: Effects of tonal relationships on melodic completion. J. Exp. Psychol. Hum. Percept. Perform. 1989, 15, 749–761. [Google Scholar] [CrossRef]
- Gerbino, W. Amodal completion revisited. Iperception 2020, 11, 2041669520937323. [Google Scholar] [CrossRef]
- Riecke, L.; Van Orstal, A.J.; Formisano, E. The auditory continuity illusion: A parametric investigation and filter model. Percept. Psychophys. 2008, 70, 1–12. [Google Scholar] [CrossRef]
- Riecke, L.; Micheyl, C.; Oxenham, A.J. Illusory auditory continuity despite neural evidence to the contrary. Adv. Exp. Med. Biol. 2013, 787, 483–489. [Google Scholar] [CrossRef]
- Petkov, C.I.; O’Connor, K.N.; Sutter, M.L. Encoding of illusory continuity in primary auditory cortex. Neuron 2007, 54, 153–165. [Google Scholar] [CrossRef]
- Berger, C.C.; Ehrsson, H.H. The fusion of mental imagery and sensation in the temporal association cortex. J. Neurosci. 2014, 34, 13684–13692. [Google Scholar] [CrossRef]
- Fram, N.R.; Berger, J. Syncopation as probabilistic expectation: Conceptual, computational, and experimental evidence. Cogn. Sci. 2023, 47, e13390. [Google Scholar] [CrossRef]
- Madison, G.; Sioros, G. What musicians do to induce the sensation of groove in simple and complex melodies, and how listeners perceive it. Front. Psychol. 2014, 5, 894. [Google Scholar] [CrossRef]
- Witek, M.A.G.; Popescu, T.; Clarke, E.F.; Hansen, M.; Konvalinka, I.; Kringelbach, M.L.; Vuust, P. Syncopation affects free body-movement in musical groove. Exp. Brain Res. 2017, 235, 995–1005. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, Q. Spatiotemporal dynamics of self-generated imagery reveal a reverse cortical hierarchy from cue-induced imagery. Cell Rep. 2023, 42, 113242. [Google Scholar] [CrossRef]
- Li, S.; Zeng, X.; Shao, Z.; Yu, Q. Neural Representations in Visual and Parietal Cortex Differentiate between Imagined, Perceived, and Illusory Experiences. J. Neurosci. 2023, 43, 6508–6524. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, N.; Ambrogioni, L.; Vidaurre, D.; van Gerven, M. Neural dynamics of perceptual inference and its reversal during imagery. eLife 2020, 9, e53588. [Google Scholar] [CrossRef] [PubMed]
- Ahissar, M.; Hochstein, S. The reverse hierarchy theory of visual perceptual learning. Trends Cogn. Sci. 2004, 8, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, H.; Hallett, M. What is the Bereitschaftspotential? Clin. Neurophysiol. 2006, 117, 2341–2356. [Google Scholar] [CrossRef] [PubMed]
- Azizian, A.; Freitas, A.L.; Parvaz, M.A.; Squires, N.K. Beware misleading cues: Perceptual similarity modulates the N2/P3 complex. Psychophysiology 2006, 43, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Dimoska, A.; Johnstone, S.J.; Barry, R.J. The auditory-evoked N2 and P3 components in the stop-signal task: Indices of inhibition, response-conflict or error-detection? Brain Cogn. 2006, 62, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhang, J.; Liu, J.; Chen, C.; Gao, X.; Chen, L.; Zhou, Z.; Zhou, H. Two-Hour Nicotine Withdrawal Improves Inhibitory Control Dysfunction in Male Smokers: Evidence from a Smoking-Cued Go/No-Go Task ERP Study. Neuropsychiatr. Dis. Treat 2024, 20, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Dercksen, T.T.; Widmann, A.; Noesselt, T.; Wetzel, N. Somatosensory omissions reveal action-related predictive processing. Hum. Brain Mapp. 2024, 45, e26550. [Google Scholar] [CrossRef]
- Kaas, A.; Goebel, R.; Valente, G.; Sorger, B. Topographic somatosensory imagery for real-time fMRI brain-computer interfacing. Front. Hum. Neurosci. 2019, 13, 427. [Google Scholar] [CrossRef]
- Panachakel, J.T.; Ramakrishnan, A.G. Decoding covert speech from EEG-A comprehensive review. Front. Neurosci. 2021, 15, 642251. [Google Scholar] [CrossRef]
- Alcaro, A.; Carta, S. The “instinct” of imagination. A neuro-ethological approach to the evolution of the reflective mind and its application to psychotherapy. Front. Hum. Neurosci. 2018, 12, 522. [Google Scholar] [CrossRef]
- Skottnik, L.; Linden, D.E.J. Mental imagery and brain regulation-new links between psychotherapy and neuroscience. Front. Psychiatry 2019, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Cordes, J.S.; Mathiak, K.A.; Dyck, M.; Alawi, E.M.; Gaber, T.J.; Zepf, F.D.; Klasen, M.; Zvyagintsev, M.; Gur, R.C.; Mathiak, K. Cognitive and neural strategies during control of the anterior cingulate cortex by fMRI neurofeedback in patients with schizophrenia. Front. Behav. Neurosci. 2015, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Liang, X.; Xue, C.; Qi, W.; Chen, S.; Song, Y.; Wu, H.; Zhang, X.; Xiao, C.; Chen, J. Altered anterior cingulate cortex subregional connectivity associated with cognitions for distinguishing the spectrum of pre-clinical Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1035746. [Google Scholar] [CrossRef]
- Dyck, M.S.; Mathiak, K.A.; Bergert, S.; Sarkheil, P.; Koush, Y.; Alawi, E.M.; Zvyagintsev, M.; Gaebler, A.J.; Shergill, S.S.; Mathiak, K. Targeting Treatment-Resistant Auditory Verbal Hallucinationstreatment-resistant auditory verbal hallucinations in Schizophreniaschizophrenia with fMRI-Based Neurofeedback—Exploring Different Casesbased neurofeedback—Exploring different cases of Schizophrenia.schizophrenia. Front. Psychiatry 2016, 7, 37. [Google Scholar] [CrossRef]
- Wincza, R.; Hartley, C.; Readman, M.; Linkenauger, S.; Crawford, T. Susceptibility to geometrical visual illusions in Parkinson’s disorder. Front. Psychol. 2023, 14, 1289160. [Google Scholar] [CrossRef]
- Jucevičiūtė, N.; Balnytė, R.; Laucius, O. Exploring the Spectrumspectrum of Visual Illusionsvisual illusions and Other Minor Hallucinationsother minor hallucinations in Patientspatients with Parkinson’s Diseasedisease in Lithuania. Medicina 2024, 60, 606. [Google Scholar] [CrossRef]







| Lag1 | RT (ms) | Lag2 | RT (ms) | Δ RT (ms) | p Value (Significant Level) |
| 0 | 20.3 | −2 | 86.0 | −65.7 | 0.009 NS |
| −1 | 103.7 | −83.4 | 0.001 *** | ||
| 1 | 159.8 | −139.5 | <0.0001 *** | ||
| 2 | 65.8 | −45.5 | 0.07 NS | ||
| 3 | 44.5 | −24.3 | 0.33 NS | ||
| 4 | 55.3 | −35.0 | 0.16 NS | ||
| 5 | 78.4 | −58.1 | 0.02 NS | ||
| 6 | 99.7 | −79.4 | 0.001 *** | ||
| 7 | 108.8 | −88.5 | 4.97761 × 10−4 *** | ||
| 8 | 129.3 | −109.0 | <0.0001 *** | ||
| 1 | 159.8 | −2 | 20.3 | 139.5 | <0.0001 *** |
| −1 | 103.7 | 56.1 | 0.001 NS | ||
| 0 | 20.3 | 139.5 | <0.0001 *** | ||
| 2 | 65.8 | 94.0 | 0.0001 *** | ||
| 3 | 44.5 | 115.3 | <0.0001 *** | ||
| 4 | 55.3 | 104.5 | <0.0001 *** | ||
| 5 | 78.4 | 81.4 | 0.001 NS | ||
| 6 | 99.7 | 60.1 | 0.001 NS | ||
| 7 | 108.8 | 51.0 | 0.001 *** | ||
| 8 | 129.3 | 30.5 | 0.002 NS | ||
| Lag1 | CH Ratio | Lag2 | CH Ratio | Δ CH Ratio | p Value (Significant Level) |
| 1 | 0.9 | −2 | 0.69898 | 0.2 | 0.00244 NS |
| −1 | 0.73711 | 0.2 | 0.00285 NS | ||
| 2 | 0.60 | 0.3 | 0.000242313 *** | ||
| 3 | 0.54 | 0.3 | <0.0001 *** | ||
| 4 | 0.51 | 0.4 | <0.0001 *** | ||
| 5 | 0.61 | 0.3 | 0.000339921 *** | ||
| 6 | 0.65 | 0.2 | 0.00199 ** | ||
| Lag1 | CH RT | Lag2 | CH RT | Δ CH RT | p Value (Significant Level) |
| 1 | 159.4 | −2 | 121.2 | 38.2 | 0.00205 NS |
| −1 | 132.0 | 27.4 | 0.00301 NS | ||
| 2 | 95.52 | 63.9 | 0.000677132 *** | ||
| 3 | 94.30 | 65.1 | 0.000479505 *** | ||
| 4 | 114.2 | 45.2 | 0.00465 NS | ||
| 5 | 126.4 | 33.1 | 0.0024 NS | ||
| 6 | 94.30 | 65.1 | 0.000479505 *** | ||
| Lag1 | FH RT | Lag2 | FH RT | Δ FH RT | p Value (Significant Level) |
| 1 | −9.8 | −2 | −11.50 | 1.7 | 0.88 NS |
| −1 | −23.52 | 13.7 | 0.23 NS | ||
| 2 | −25.30 | 15.5 | 0.18 NS | ||
| 3 | −27.10 | 17.2 | 0.11 NS | ||
| 4 | −42.51 | 32.7 | 0.06 NS | ||
| 5 | −29.70 | 19.8 | 0.08 NS | ||
| 6 | −25.57 | 15.7 | 0.84 NS |
| Lag1 | d-Prime1 | Lag2 | d-Prime2 | Δ d-Prime | p Value (Significant Level) |
| −2 | 0.67 | 1 | 1.32 | −0.65 | 0.00048 *** |
| 4 | 0.09 | 0.59 | 0.001 *** | ||
| −1 | 0.81 | 3 | 0.17 | 0.64 | 0.0006 *** |
| 4 | 0.09 | 0.73 | 0.0001 *** | ||
| 1 | 1.32 | 2 | 0.95 | 0.37 | <0.0001 *** |
| 3 | 1.05 | −1.05 | <0.0001 *** | ||
| 4 | 0.51 | −0.51 | <0.0001 *** | ||
| 5 | 0.56 | −0.56 | <0.0001 *** | ||
| 6 | 0.48 | −0.48 | <0.0001 *** | ||
| 2 | 0.95 | 7 | 0.53 | 0.42 | <0.0001 *** |
| 3 | 0.37 | 7 | 0.53 | −0.16 | <0.0001 *** |
| 4 | 0.51 | 7 | 0.53 | −0.02 | <0.0001 *** |
| Lag1 | Criterion1 | Lag2 | Criterion2 | Δ Criterion | p Value (Significant Level) |
| 1 | −0.63 | −2 | −0.31 | −0.33 | 0.15 NS |
| −1 | −0.37 | 0.37 | 0.25 NS | ||
| 2 | −0.16 | 0.16 | 0.03 NS | ||
| 3 | −0.05 | 0.05 | 0.01 NS | ||
| 4 | −0.01 | 0.01 | 0.006 NS | ||
| 5 | −0.17 | 0.17 | 0.04 NS | ||
| 6 | −0.23 | 0.23 | 0.08 NS | ||
| 7 | −0.45 | 0.45 | 0.41 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uemura, M.; Katagiri, Y.; Imai, E.; Kawahara, Y.; Otani, Y.; Ichinose, T.; Kondo, K.; Kowa, H. Dorsal Anterior Cingulate Cortex Coordinates Contextual Mental Imagery for Single-Beat Manipulation during Rhythmic Sensorimotor Synchronization. Brain Sci. 2024, 14, 757. https://doi.org/10.3390/brainsci14080757
Uemura M, Katagiri Y, Imai E, Kawahara Y, Otani Y, Ichinose T, Kondo K, Kowa H. Dorsal Anterior Cingulate Cortex Coordinates Contextual Mental Imagery for Single-Beat Manipulation during Rhythmic Sensorimotor Synchronization. Brain Sciences. 2024; 14(8):757. https://doi.org/10.3390/brainsci14080757
Chicago/Turabian StyleUemura, Maho, Yoshitada Katagiri, Emiko Imai, Yasuhiro Kawahara, Yoshitaka Otani, Tomoko Ichinose, Katsuhiko Kondo, and Hisatomo Kowa. 2024. "Dorsal Anterior Cingulate Cortex Coordinates Contextual Mental Imagery for Single-Beat Manipulation during Rhythmic Sensorimotor Synchronization" Brain Sciences 14, no. 8: 757. https://doi.org/10.3390/brainsci14080757
APA StyleUemura, M., Katagiri, Y., Imai, E., Kawahara, Y., Otani, Y., Ichinose, T., Kondo, K., & Kowa, H. (2024). Dorsal Anterior Cingulate Cortex Coordinates Contextual Mental Imagery for Single-Beat Manipulation during Rhythmic Sensorimotor Synchronization. Brain Sciences, 14(8), 757. https://doi.org/10.3390/brainsci14080757






