BOLD Cerebrovascular Reactivity and NOVA Quantitative MR Angiography in Adult Patients with Moyamoya Vasculopathy Undergoing Cerebral Bypass Surgery
Abstract
:1. Introduction
- (1)
- (2)
2. Methods
2.1. Patient Selection
2.2. Quantitative Advanced MRI Techniques
2.2.1. BOLD-CVR Measurement and Analysis
2.2.2. qMRA NOVA
2.3. Direct STA-MCA Bypass and Indirect Revascularization
2.4. Statistical Analysis
3. Results
3.1. Study Population Characteristics
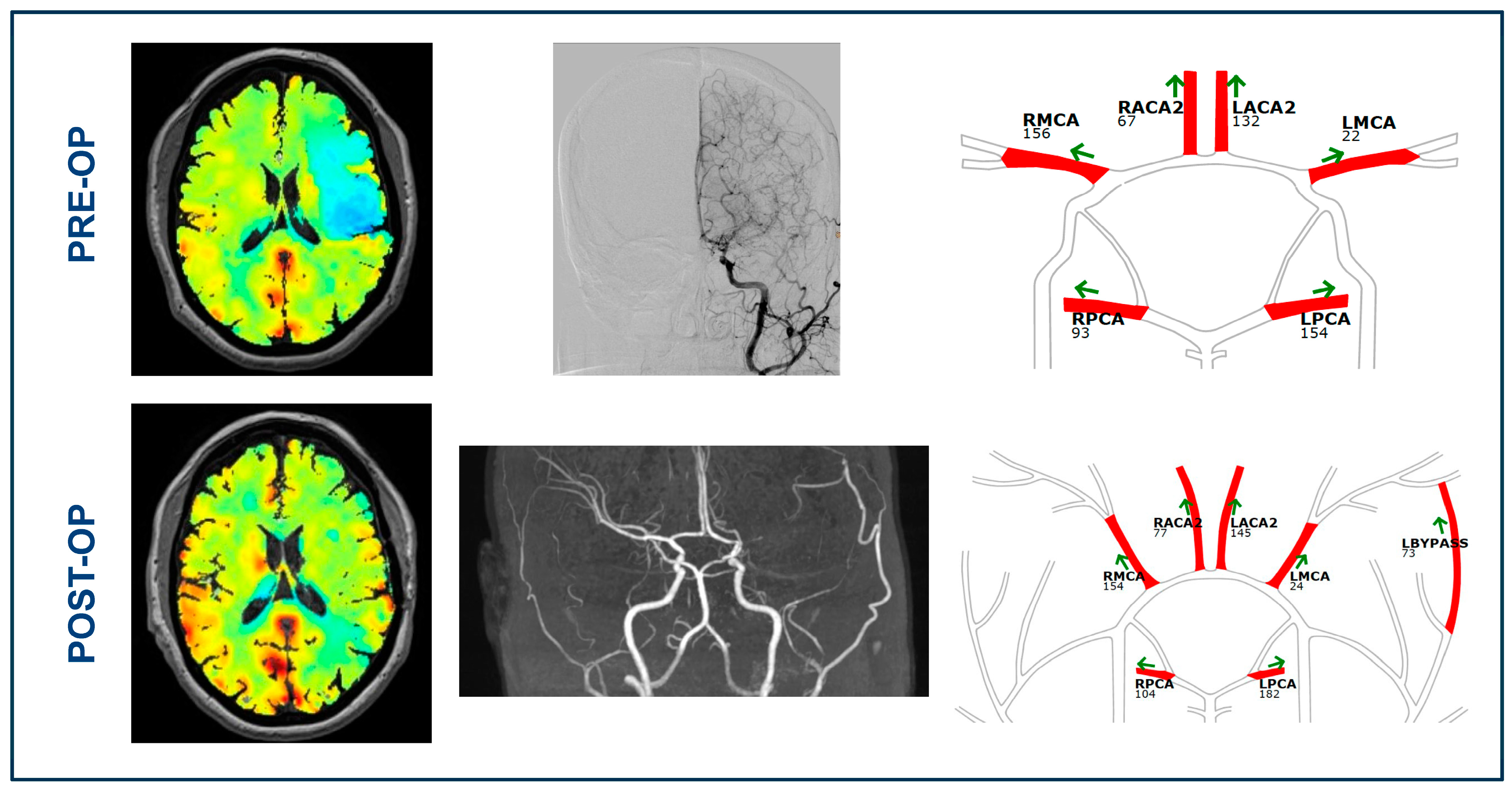
3.2. BOLD-CVR and qMRA-NOVA Imaging Data before Bypass Surgery
3.3. BOLD Cerebrovascular Reactivity after Bypass
3.4. qMRA-NOVA Values after Surgical Revascularization
4. Discussion
4.1. Cerebral Hemodynamics in Patients with Moyamoya Vasculopathy
4.2. Quantitative Flow Analysis in Patients with Moyamoya Vasculopathy
4.3. Uncovering the Link between Impaired Hemodynamics and Blood Flow in Moyamoya Vasculopathy
4.4. Limitations
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Scott, R.M.; Smith, E.R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 2009, 360, 1226–1237. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Zhou, D.; Ya, J.; Li, S.; Yang, Q.; Ding, Y.; Ji, X.; Meng, R. Progress in moyamoya disease. Neurosurg. Rev. 2020, 43, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.M.; Burke, A.M.; Sherma, A.K.; Hurley, M.C.; Batjer, H.H.; Bendok, B.R. Moyamoya disease: A summary. Neurosurg. Focus 2009, 26, E11. [Google Scholar] [CrossRef]
- Liu, J.J.; Steinberg, G.K. Direct Versus Indirect Bypass for Moyamoya Disease. Neurosurg. Clin. N. Am. 2017, 28, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Finkenstaedt, S.; Guida, L.; Regli, L.; Esposito, G. Surgical revascularization of frontal areas in pediatric Moyamoya vasculopathy: A systematic review. J. Neurosurg. Sci. 2021, 65, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Kronenburg, A.; Fierstra, J.; Braun, K.P.; Klijn, C.J.; van der Zwan, A.; Regli, L. “STA-MCA bypass with encephalo-duro-myo-synangiosis combined with bifrontal encephalo-duro-periosteal-synangiosis” as a one-staged revascularization strategy for pediatric moyamoya vasculopathy. Childs Nerv. Syst. 2015, 31, 765–772. [Google Scholar] [CrossRef]
- Esposito, G.; Amin-Hanjani, S.; Regli, L. Role of and Indications for Bypass Surgery After Carotid Occlusion Surgery Study (COSS)? Stroke 2016, 47, 282–290. [Google Scholar] [CrossRef]
- Esposito, G.; Sebök, M.; Amin-Hanjani, S.; Regli, L. Cerebral Bypass Surgery: Level of Evidence and Grade of Recommendation. Acta Neurochir. Suppl. 2018, 129, 73–77. [Google Scholar]
- Grüter, B.E.; Tosic, L.; Voglis, S.; Vasella, F.; Mutschler, V.; Bichsel, O.; Scherrer, N.; Regli, L.; Esposito, G. Trends in Literature on Cerebral Bypass Surgery: A Systematic Review. Cerebrovasc. Dis. 2022, 51, 102–113. [Google Scholar] [CrossRef]
- Bersano, A.; Khan, N.; Fuentes, B.; Acerbi, F.; Canavero, I.; Tournier-Lasserve, E.; Vajcoczy, P.; Zedde, M.L.; Hussain, S.; Lémeret, S.; et al. European Stroke Organisation (ESO) Guidelines on Moyamoya angiopathy Endorsed by Vascular European Reference Network (VASCERN). Eur. Stroke J. 2023, 8, 55–84. [Google Scholar] [CrossRef]
- Lehman, V.T.; Cogswell, P.M.; Rinaldo, L.; Brinjikji, W.; Huston, J.; Klaas, J.P.; Lanzino, G. Contemporary and emerging magnetic resonance imaging methods for evaluation of moyamoya disease. Neurosurg. Focus 2019, 47, E6. [Google Scholar] [CrossRef]
- Du, L.; Jiang, H.; Li, J.; Duan, T.; Zhou, C.; Yan, F. Imaging methods for surgical revascularization in patients with moyamoya disease: An updated review. Neurosurg. Rev. 2022, 45, 343–356. [Google Scholar] [CrossRef]
- Fierstra, J.; van Niftrik, C.; Warnock, G.; Wegener, S.; Piccirelli, M.; Pangalu, A.; Esposito, G.; Valavanis, A.; Buck, A.; Luft, A.; et al. Staging Hemodynamic Failure With Blood Oxygen-Level-Dependent Functional Magnetic Resonance Imaging Cerebrovascular Reactivity: A Comparison Versus Gold Standard (15O-)H2O-Positron Emission Tomography. Stroke 2018, 49, 621–629. [Google Scholar] [CrossRef]
- Yuxue, S.; Yan, W.; Bingqian, X.; Hao, L.; Chaoyue, L. Arterial spin labeling for moyamoya angiopathy: A preoperative and postoperative evaluation method. Transl. Neurosci. 2023, 14, 20220288. [Google Scholar] [CrossRef]
- Sebök, M.; Esposito, G.; Niftrik, C.; Fierstra, J.; Schubert, T.; Wegener, S.; Held, J.; Kulcsár, Z.; Luft, A.R.; Regli, L. Flow augmentation STA-MCA bypass evaluation for patients with acute stroke and unilateral large vessel occlusion: A proposal for an urgent bypass flowchart. J. Neurosurg. 2022, 137, 1047–1055. [Google Scholar] [CrossRef]
- Sebök, M.; van Niftrik, C.H.B.; Winklhofer, S.; Wegener, S.; Esposito, G.; Stippich, C.; Luft, A.; Regli, L.; Fierstra, J. Mapping Cerebrovascular Reactivity Impairment in Patients with Symptomatic Unilateral Carotid Artery Disease. J. Am. Heart Assoc. 2021, 10, e020792. [Google Scholar] [CrossRef]
- Sebök, M.; van der Wouden, F.; Mader, C.; Pangalu, A.; Treyer, V.; Fisher, J.A.; Mikulis, D.J.; Hüllner, M.; Regli, L.; Fierestra, J.; et al. Hemodynamic Failure Staging With Blood Oxygenation Level-Dependent Cerebrovascular Reactivity and Acetazolamide-Challenged (15O-)H2O-Positron Emission Tomography Across Individual Cerebrovascular Territories. J. Am. Heart Assoc. 2023, 12, e029491. [Google Scholar] [CrossRef]
- White, T.; Gandhi, S.; Langer, D.J.; Katz, J.M.; Dehdashti, A.R. Does Advanced Imaging Aid in the Preoperative Evaluation of Patients with Moyamoya Disease? Cureus 2022, 14, e29816. [Google Scholar] [CrossRef]
- Amin-Hanjani, S.; Singh, A.; Rifai, H.; Thulborn, K.R.; Alaraj, A.; Aletich, V.; Charbel, F.T. Combined direct and indirect bypass for moyamoya: Quantitative assessment of direct bypass flow over time. Neurosurgery 2013, 73, 962–967; discussion 967–968. [Google Scholar] [CrossRef]
- Sebök, M.; van Niftrik, C.H.B.; Piccirelli, M.; Bozinov, O.; Wegener, S.; Esposito, G.; Pangalu, A.; Valavanis, A.; Buck, A.; Luft, A.R.; et al. BOLD cerebrovascular reactivity as a novel marker for crossed cerebellar diaschisis. Neurology 2018, 91, e1328–e1337. [Google Scholar] [CrossRef]
- Sebök, M.; van Niftrik, C.H.B.; Piccirelli, M.; Muscas, G.; Pangalu, A.; Wegener, S.; Stippich, C.; Regli, L.; Fierstra, J. Crossed Cerebellar Diaschisis in Patients with Symptomatic Unilateral Anterior Circulation Stroke Is Associated with Hemodynamic Impairment in the Ipsilateral MCA Territory. J. Magn. Reson. Imaging 2021, 53, 1190–1197. [Google Scholar] [CrossRef]
- Sebök, M.; van Niftrik, C.H.B.; Wegener, S.; Luft, A.; Regli, L.; Fierstra, J. Agreement of novel hemodynamic imaging parameters for the acute and chronic stages of ischemic stroke: A matched-pair cohort study. Neurosurg. Focus 2021, 51, E12. [Google Scholar] [CrossRef] [PubMed]
- Slessarev, M.; Han, J.; Mardimae, A.; Prisman, E.; Preiss, D.; Volgyesi, G.; Ansel, C.; Duffin, J.; Fisher, J.A. Prospective targeting and control of end-tidal CO2 and O2 concentrations. J. Physiol. 2007, 581 Pt 3, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Sebök, M.; Höbner, L.M.; Fierstra, J.; Schubert, T.; Wegener, S.; Kulcsár, Z.; Luft, A.R.; Regli, L.; Esposito, G. Flow-augmentation STA-MCA bypass for acute and subacute ischemic stroke due to internal carotid artery occlusion and the role of advanced neuroimaging with hemodynamic and flow-measurement in the decision-making: Preliminary data. Quant. Imaging Med. Surg. 2024, 14, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Kronenburg, A.; Esposito, G.; Fierstra, J.; Braun, K.P.; Regli, L. Combined Bypass Technique for Contemporary Revascularization of Unilateral MCA and Bilateral Frontal Territories in Moyamoya Vasculopathy. Acta Neurochir. Suppl. 2014, 119, 65–70. [Google Scholar] [PubMed]
- Esposito, G.; Burkhardt, J.K.; Bozinov, O.; Regli, L. Indocyanine green videoangiography for the identification of superficial temporal artery branches in EC-IC bypass surgery. Acta Neurochir. 2016, 158, 565–570. [Google Scholar] [CrossRef] [PubMed]
- van Niftrik, C.H.B.; Sebök, M.; Nicholson, P.; Olijnyk, L.; Thurner, P.; Venkatraghavan, L.; Schaafsma, J.; Radovanovic, I.; Fisher, J.A.; Krings, T.; et al. A dual-center validation of the PIRAMD scoring system for assessing the severity of ischemic Moyamoya disease. Quant. Imaging Med. Surg. 2023, 13, 4618–4632. [Google Scholar] [CrossRef]
- Nemoto, E.M.; Yonas, H.; Kuwabara, H.; Pindzola, R.R.; Sashin, D.; Meltzer, C.C.; Price, J.C.; Chang, Y.; Johnson, D.W. Identification of hemodynamic compromise by cerebrovascular reserve and oxygen extraction fraction in occlusive vascular disease. J. Cereb. Blood Flow Metab. 2004, 24, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Derdeyn, C.P. Hemodynamics and oxygen extraction in chronic large artery steno-occlusive disease: Clinical applications for predicting stroke risk. J. Cereb. Blood Flow Metab. 2018, 38, 1584–1597. [Google Scholar] [CrossRef]
- Zhu, F.; Qian, Y.; Xu, B.; Gu, Y.; Karunanithi, K.; Zhu, W.; Chen, L.; Mao, Y.; Morgan, M.K. Quantitative assessment of changes in hemodynamics of the internal carotid artery after bypass surgery for moyamoya disease. J. Neurosurg. 2018, 129, 677–683. [Google Scholar] [CrossRef]
- Han, J.S.; Abou-Hamden, A.; Mandell, D.M.; Poublanc, J.; Crawley, A.P.; Fisher, J.A.; Mikulis, D.J.; Tymianski, M. Impact of extracranial-intracranial bypass on cerebrovascular reactivity and clinical outcome in patients with symptomatic moyamoya vasculopathy. Stroke 2011, 42, 3047–3054. [Google Scholar] [CrossRef] [PubMed]
- Conklin, J.; Fierstra, J.; Crawley, A.P.; Han, J.S.; Poublanc, J.; Mandell, D.M.; Silver, F.L.; Tymianski, M.; Fisher, J.A.; Mikulis, D.J. Impaired cerebrovascular reactivity with steal phenomenon is associated with increased diffusion in white matter of patients with Moyamoya disease. Stroke 2010, 41, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Mesiwala, A.H.; Sviri, G.; Fatemi, N.; Britz, G.W.; Newell, D.W. Long-term outcome of superficial temporal artery-middle cerebral artery bypass for patients with moyamoya disease in the US. Neurosurg. Focus 2008, 24, E15. [Google Scholar] [CrossRef] [PubMed]
- Sam, K.; Poublanc, J.; Sobczyk, O.; Han, J.S.; Battisti-Charbonney, A.; Mandell, D.M.; Tymianski, M.; Crawley, A.P.; A Fisher, J.; Mikulis, D.J. Assessing the effect of unilateral cerebral revascularisation on the vascular reactivity of the non-intervened hemisphere: A retrospective observational study. BMJ Open 2015, 5, e006014. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Lober, R.M.; Ostergren, L.; Petralia, J.; Bell-Stephens, T.; Navarro, R.; Feroze, A.; Steinberg, G.K. Measuring Cerebral Blood Flow in Moyamoya Angiopathy by Quantitative Magnetic Resonance Angiography Noninvasive Optimal Vessel Analysis. Neurosurgery 2017, 81, 921–927. [Google Scholar] [CrossRef]
- Kim, T.; Oh, C.W.; Bang, J.S.; Kim, J.E.; Cho, W.S. Moyamoya Disease: Treatment and Outcomes. J. Stroke 2016, 18, 21–30. [Google Scholar] [CrossRef]
- Sebök, M.; Niftrik, C.; Lohaus, N.; Esposito, G.; Amki, M.E.; Winklhofer, S.; Wegener, S.; Regli, L.; Fierstra, J. Leptomeningeal collateral activation indicates severely impaired cerebrovascular reserve capacity in patients with symptomatic unilateral carotid artery occlusion. J. Cereb. Blood Flow Metab. 2021, 41, 3039–3051. [Google Scholar] [CrossRef]
- Strother, M.K.; Anderson, M.D.; Singer, R.J.; Du, L.; Moore, R.D.; Shyr, Y.; Ladner, T.; Arteaga, D.; Day, M.; Clemmons, P.; et al. Cerebrovascular collaterals correlate with disease severity in adult North American patients with Moyamoya disease. Am. J. Neuroradiol. 2014, 35, 1318–1324. [Google Scholar] [CrossRef]
- Heyn, C.; Poublanc, J.; Crawley, A.; Mandell, D.; Han, J.S.; Tymianski, M.; Terbrugge, K.; Fisher, J.; Mikulis, D. Quantification of cerebrovascular reactivity by blood oxygen level-dependent MR imaging and correlation with conventional angiography in patients with Moyamoya disease. Am. J. Neuroradiol. 2010, 31, 862–867. [Google Scholar] [CrossRef]
- Hauser, T.K.; Seeger, A.; Bender, B.; Klose, U.; Thurow, J.; Ernemann, U.; Tatagiba, M.; Meyer, P.T.; Khan, N.; Roder, C. Hypercapnic BOLD MRI compared to H215O PET/CT for the hemodynamic evaluation of patients with Moyamoya Disease. Neuroimage Clin. 2019, 22, 101713. [Google Scholar] [CrossRef]
- Mikulis, D.J.; Krolczyk, G.; Desal, H.; Logan, W.; Deveber, G.; Dirks, P.; Tymianski, M.; Crawley, A.; Vesely, A.; Kassner, A.; et al. Preoperative and postoperative mapping of cerebrovascular reactivity in moyamoya disease by using blood oxygen level-dependent magnetic resonance imaging. J. Neurosurg. 2005, 103, 347–355. [Google Scholar] [CrossRef] [PubMed]


| Total Patient Cohort (n = 12) | |
|---|---|
| Age (mean ± SD) | 50.25 ± 13.34 |
| Gender: male, n (%) | 7 (58.3) |
| Smoking, n (%) | 5 (41.7) |
| Arterial hypertension, n (%) | 3 (25.0) |
| Dyslipidemia, n (%) | 2 (16.7) |
| Diabetes mellitus, n (%) | 4 (33.3) |
| mRS (median (IQR)) | |
| Before surgery | 1 (2) |
| After surgery | 0 (1) |
| NIHSS (median (IQR)) | |
| Before surgery | 0 (2) |
| After surgery | 0 (0) |
| BOLD-CVR (%BOLD/mmHgCO2) | Mean ± SD |
|---|---|
| CVR whole brain | 0.11 ± 0.07 |
| CVR symptomatic hemisphere | 0.09 ± 0.07 |
| CVR non-symptomatic hemisphere | 0.12 ± 0.08 |
| CVR symptomatic ACA territory | 0.08 ± 0.08 |
| CVR non-symptomatic ACA territory | 0.11 ± 0.08 |
| CVR symptomatic MCA territory | 0.03 ± 0.07 |
| CVR non-symptomatic MCA territory | 0.10 ± 0.11 |
| CVR symptomatic PCA territory | 0.23 ± 0.06 |
| CVR non-symptomatic PCA territory | 0.25 ± 0.06 |
| qMRA-NOVA (mL/min): | Mean ± SD |
|---|---|
| VFR symptomatic A2-ACA vessel | 96.69 ± 53.94 |
| VFR non-symptomatic A2-ACA vessel | 97.23 ± 70.46 |
| VFR symptomatic M1-MCA vessel | 16.15 ± 18.45 |
| VFR non-symptomatic M1-MCA vessel | 101.38 ± 86.97 |
| VFR symptomatic P2-PCA vessel | 164.69 ± 63.20 |
| VFR non-symptomatic P2-PCA vessel | 123.54 ± 61.59 |
| VFR symptomatic hemisphere | 282.77 ± 89.70 |
| VFR non-symptomatic hemisphere | 322.15 ± 109.05 |
| BOLD-CVR (%BOLD/mmHgCO2) | |||
|---|---|---|---|
| (Mean ± SD) | Pre-Bypass (n = 12) | Post-Bypass (n = 12) | p-Value |
| CVR whole brain | 0.11 ± 0.07 | 0.12 ± 0.04 | 0.52 |
| CVR symptomatic hemisphere | 0.09 ± 0.07 | 0.12 ± 0.03 | 0.31 |
| CVR non-symptomatic hemisphere | 0.12 ± 0.08 | 0.14 ± 0.06 | 0.71 |
| CVR ACA symptomatic | 0.08 ± 0.08 | 0.09 ± 0.05 | 0.62 |
| CVR ACA non-symptomatic | 0.11 ± 0.08 | 0.13 ± 0.06 | 0.46 |
| CVR MCA symptomatic | 0.03 ± 0.07 | 0.07 ± 0.06 | 0.09 |
| CVR MCA non-symptomatic | 0.10 ± 0.11 | 0.10 ± 0.09 | 0.86 |
| CVR PCA symptomatic | 0.23 ± 0.06 | 0.26 ± 0.07 | 0.29 |
| CVR PCA non-symptomatic | 0.25 ± 0.06 | 0.28 ± 0.10 | 0.37 |
| qMRA-NOVA (mL/min) | |||
|---|---|---|---|
| (Mean ± SD) | Pre-Bypass (n = 10) | Post-Bypass (n = 10) | p-Value |
| VFR A2 affected | 96.69 ± 53.94 | 117.10 ± 56.90 | 0.39 |
| VFR A2 unaffected | 97.23 ± 70.46 | 112.40 ± 60.58 | 0.59 |
| VFR M1 affected | 16.15 ± 18.45 | 11.00 ± 16.07 | 0.49 |
| VFR M1 unaffected | 101.38 ± 86.97 | 115.10 ± 111.99 | 0.74 |
| VFR P2 affected | 164.69 ± 63.20 | 175.40 ± 32.49 | 0.63 |
| VFR P2 unaffected | 123.54 ± 61.59 | 113.30 ± 32.69 | 0.64 |
| VFR affected hemisphere * | 282.77 ± 89.70 | 383.70 ± 40.37 | 0.003 |
| VFR unaffected hemisphere | 322.15 ± 109.05 | 349.00 ± 102.96 | 0.56 |
| VFR bypass | / | 86.70 ± 30.32 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbani Nerini, L.; Bellomo, J.; Höbner, L.M.; Stumpo, V.; Colombo, E.; van Niftrik, C.H.B.; Schubert, T.; Kulcsár, Z.; Wegener, S.; Luft, A.; et al. BOLD Cerebrovascular Reactivity and NOVA Quantitative MR Angiography in Adult Patients with Moyamoya Vasculopathy Undergoing Cerebral Bypass Surgery. Brain Sci. 2024, 14, 762. https://doi.org/10.3390/brainsci14080762
Garbani Nerini L, Bellomo J, Höbner LM, Stumpo V, Colombo E, van Niftrik CHB, Schubert T, Kulcsár Z, Wegener S, Luft A, et al. BOLD Cerebrovascular Reactivity and NOVA Quantitative MR Angiography in Adult Patients with Moyamoya Vasculopathy Undergoing Cerebral Bypass Surgery. Brain Sciences. 2024; 14(8):762. https://doi.org/10.3390/brainsci14080762
Chicago/Turabian StyleGarbani Nerini, Loris, Jacopo Bellomo, Lara Maria Höbner, Vittorio Stumpo, Elisa Colombo, Christiaan Hendrik Bas van Niftrik, Tilman Schubert, Zsolt Kulcsár, Susanne Wegener, Andreas Luft, and et al. 2024. "BOLD Cerebrovascular Reactivity and NOVA Quantitative MR Angiography in Adult Patients with Moyamoya Vasculopathy Undergoing Cerebral Bypass Surgery" Brain Sciences 14, no. 8: 762. https://doi.org/10.3390/brainsci14080762






