Abstract
Substance use disorders (SUDs) are complex biopsychosocial diseases that cause neurocognitive deficits and neurological impairments by altering the gene expression in reward-related brain areas. Repeated drug use gives rise to alterations in DNA methylation, histone modifications, and the expression of microRNAs in several brain areas that may be associated with the development of psychotic symptoms. The first section of this review discusses how substance use contributes to the development of psychotic symptoms via epigenetic alterations. Then, we present more evidence about the link between SUDs and brain epigenetic alterations. The next section presents associations between paternal and maternal exposure to substances and epigenetic alterations in the brains of offspring and the role of maternal diet in preventing substance-induced neurological impairments. Then, we introduce potential therapeutic agents/approaches such as methyl-rich diets to modify epigenetic alterations for alleviating psychotic symptoms or depression in SUDs. Next, we discuss how substance use–gut microbiome interactions contribute to the development of neurological impairments through epigenetic alterations and how gut microbiome-derived metabolites may become new therapeutics for normalizing epigenetic aberrations. Finally, we address possible challenges and future perspectives for alleviating psychotic symptoms and depression in patients with SUDs by modulating diets, the epigenome, and gut microbiome.
1. Introduction
Substance use disorder (SUD) is defined as a chronic state of the uncontrolled exploration and use of drugs that exert detrimental effects on the family, society, and professional aspects of a patient’s life. The high prevalence of psychotic symptoms such as hallucinations and delusions has been reported in patients with substance use [1]. For instance, cannabis and amphetamine users exhibit a higher prevalence of psychotic disorders and cognitive symptoms like schizophrenia [2,3,4]. Moreover, a greater frequency of both opioid and cocaine use has been reported in individuals with psychotic symptoms compared to individuals with nonpsychotic symptoms [5]. Similar to other neuropsychiatric diseases, the etiology of SUDs is complex and multifactorial, in which a variety of responsible genes interplay among each other and with the environment. This interplay alerts neuronal function and structure in different brain areas and gives rise to continuous changes at the cellular, molecular, and behavioral levels [6]. For example, it appears that amphetamine affects brain function via interplay with nerve terminals that utilize indoleamines, like serotonin and catecholamines, including norepinephrine and dopamine as multifunctional neurotransmitters [7]. The prevalence of psychotic symptoms in substance users may be due to the burst release of dopamine in the striatum and subsequently excessive secretion of glutamate into the brain cortex, which further leads to the injury of cortical interneurons and the disruption of thalamocortical signals [8]. It has been found that there is also a potent relationship between any type of SUD and the polygenic risk score for schizophrenia [9]. Genetic factors with a heritability of almost 50% (h2 = ~50%) are associated with SUDs and their adverse consequences. For example, alcohol-related tendencies can be affected by loci in alcohol-metabolizing genes (e.g., ADH1B and ALDH2), and nicotine-related tendencies can be influenced by loci within the CHRNA5–CHRNA3–CHRNB4 gene cluster [10]. More information about the genetics of SUDs is provided in a review written by Gelernter and Polimanti [11].
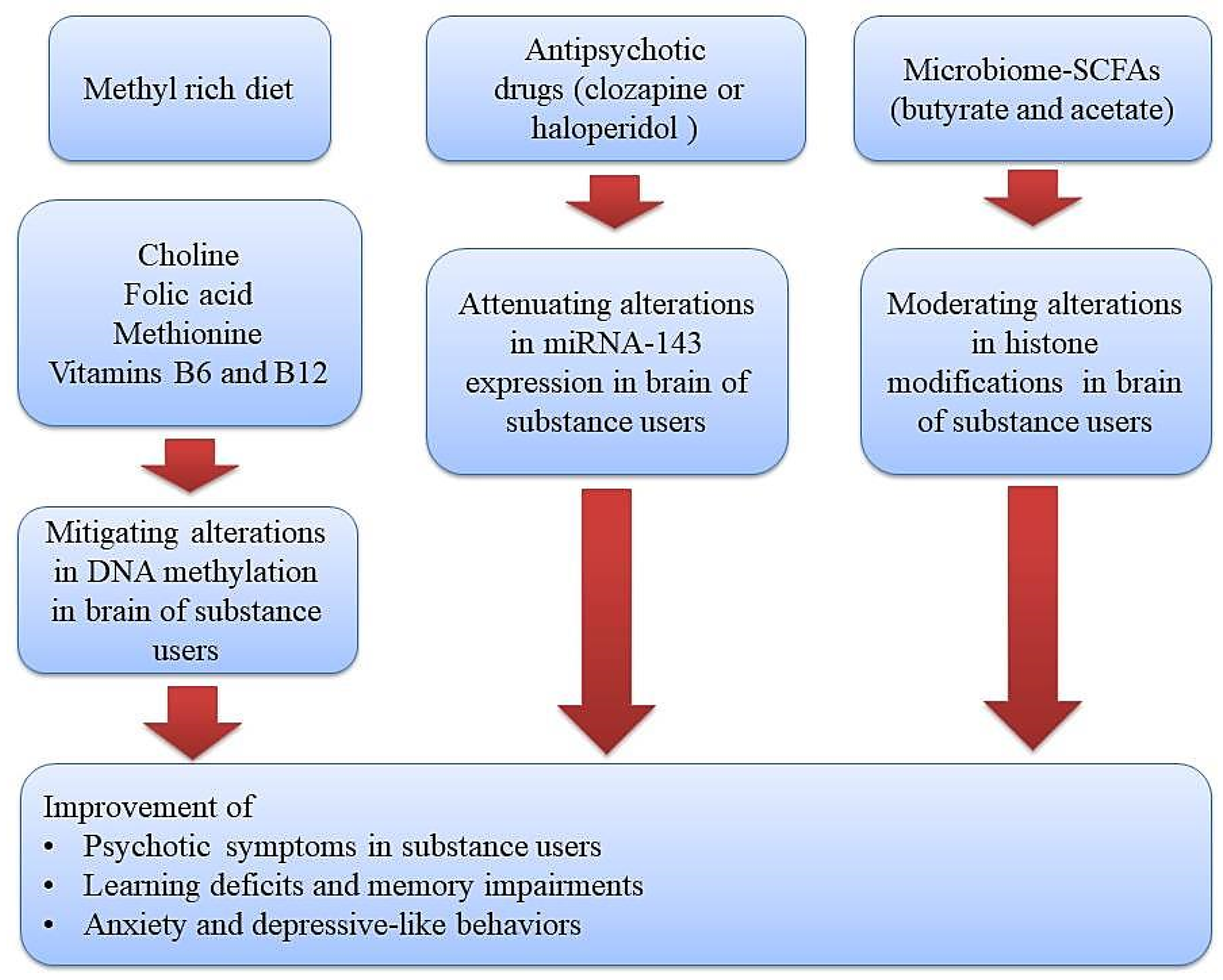
The tight relationship among genes and environmental factors in the development of psychotic symptoms in substance users can be mediated by epigenetic mechanisms as well [12]. During this process, transcription factors and some specific enzymatic protein complexes play a critical role in modulating gene expression and creating long-lasting alterations through modification of chromatin structure [13,14]. These chromatin-modifying mechanisms or other epigenetic alterations have the capacity to alter gene expression without changing DNA sequences. Moreover, drug use or the toxic effects of alcohol may create disturbances in the absorption of micronutrients (omega–3, choline, vitamins, and folic acid) and hence imbalances in the levels of methyl donors, which further give rise to the development of neuropsychiatric diseases via brain epigenetic changes, especially DNA methylation [15]. Therefore, diet modifications and using supplementations with adequate levels of methyl donors is a promising strategy in alleviating the development of psychotic symptoms and neurological impairments in substance users (Figure 1).
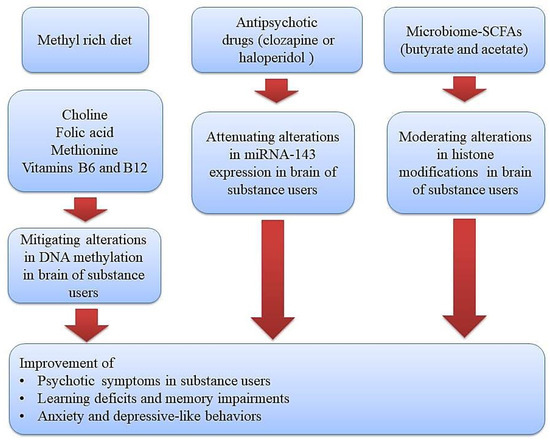
Figure 1.
Therapeutic approaches using diet or epigenetic drugs (methyl donor micronutrients, antipsychotic drugs, and gut microbiome-derived metabolites) for improving substance-induced neurological impairments via normalizing epigenetic aberrations. Methyl donor nutrients, such as methionine, choline, folate, and some B vitamins, participate in one-carbon metabolism and hence could serve as potential epigenetic diets to reduce substance-induced neurological impairments. Likewise, antipsychotic drugs and gut microbiome–derived metabolites like butyrate and acetate can target a gene for epigenetic regulation and thus could serve as potential epigenetic modifiers to improve psychotic symptoms, learning and memory impairments, and depressive-like behaviors in substance users.
This narrative review aims to elaborate links between substance use-induced neuropsychiatric impairments and epigenetic alterations in brain tissue and the role of diet modifications in alleviating such deficits via normalizing epigenetic aberrations. To this end, we briefly present associations between substance use and the development of psychotic symptoms and neuropsychiatric diseases via epigenetic aberrations. We will discuss studies that support the link between substance use and epigenetic alterations, including DNA methylation, histone modifications, and microRNAs (miRNAs), in particular, in the brain tissue. Note that in DNA methylation, a methyl group is added to a cytosine residue, or less frequently, to an adenine residue that is followed by guanine. This process, mediated by different enzymes, results in methylated cytosines acting as targets for DNA-binding proteins (e.g., MeCP2, MBD1, MBD3, and MBD4), which mediate chromatin condensation and gene silencing. Histone modifications are another type of epigenetic regulation, during which different amino acids of histone tail proteins can become acetylated or methylated (mediated by various enzymes). These modifications affect the positive electric charge of histone proteins and thus the intensity of their binding to DNA, which has a negative electric charge. Histone acetylation generally decreases chromatin condensation and stimulates gene expression, whereas histone methylation can either increase or decrease gene expression depending on the identity or location of the methylated amino acids of histone tail proteins. Additionally, in RNA interference, another type of epigenetic regulation, miRNAs—small non-coding RNAs approximately 20 bases in length—bind to their cognate RNAs and inhibit gene transcription or promote RNA degradation [16].
In this work, we will summarize those studies that show the impact of paternal and maternal exposure to substances on epigenetic alterations in the brains of offspring and the role of maternal diet on the prevention of substance induced neurological impairments notably psychosis, possibly via epigenetic mechanisms. In addition, we provide an overview on the use of different types of diet, especially the methyl-rich diet, for alleviating psychotic symptoms and depressive-like behaviors in patients with SUDs. The next step involves determining how substance use–gut microbiome interactions contribute to the development of psychotic symptoms and depressive-like behaviors through epigenetic alterations and how gut microbiome-derived metabolites help researchers in the design of new therapies based on normalizing epigenetic aberrations. The last section discusses potential challenges and presents future perspectives relevant to alleviating psychotic symptoms and depressive-like behaviors in patients with SUDs using diet modifications and modulation of the gut microbiome.
2. Association between Substance Use and the Development of Psychotic Symptoms and Depressive-Like Behaviors
There is increasing evidence that drug use is associated with the development of psychotic symptoms in various contexts encompassing substance withdrawal, acute or chronic intoxication, in the form of substance-induced psychosis, and delirium [17]. Substance-induced psychosis is described as a condition in which the onset of psychosis appears to be due to substance use, but it remains for days, weeks, or even months in the absence of substance use [18]. Long-term neuropsychiatric deficits induced by substance use are mainly attributed to the activation of different signaling pathways relevant to triggering and the progression of oxidative stress and inflammation [19,20]. For example, methamphetamine-induced psychosis is related to changes in the balance of the immune system, the activation of a variety of chemokines and cytokines (e.g., IL-1α, CCL11, and CCL27), elevated lipid peroxidation, and decreased antioxidant defenses [21,22]. Persistent psychotic symptoms can be induced by amphetamines, cannabis, and alcohol [23]. It is estimated that almost 40% of methamphetamine users suffer from psychotic symptoms like hallucinations and delusions in addition to violence, impulsivity, and cognitive disturbance [24,25].
Mechanistically, the emergence of psychotic symptoms in patients with SUDs could be linked to epigenetic alterations leading to gene expression dysregulation. For example, it was found that decreased DNA methylation at a particular dopamine receptor type 4 (DRD4) CpG2.3 unit was associated with paranoid symptoms in patients with methamphetamine use disorder, and higher methylation levels at the catechol-O-methyltransferase (COMT) CpG 51.52 unit was linked to reduced motor-impulsivity scores in the same set of patients [26]. In another study, Veerasakul et al. found a significant elevation in parvalbumin (PVALB) DNA methylation in methamphetamine-induced psychosis, indicating that methamphetamine dependence confers the GABAergic deficits by epigenetic changes [27]. Kalayasiri et al. reported a strong link between methamphetamine-induced paranoia and alterations in long interspersed element-1 methylation patterns, which modulate the immune and neuro-oxidative pathways [28]. In addition to DNA methylation and histone modifications, substance-induced psychosis is connected to alterations in miRNAs. In this line, an interesting study demonstrated that patients with methamphetamine-induced psychosis exhibited significant differences in the levels of miR-let-7d, miR-let-7e, miR-15b, and miR-181a compared to control subjects [29]. In a more recent study, Chen et al. reported that psychological comorbidities in substance users are linked to the dysregulation of some crucial exosomal miRNAs connected to changes in the levels of certain neurotransmitters, such as serotonin, in these patients [30]. In another study by the same group, they found a negative correlation between the expression levels of exosomal miR-92a-3p, miR-16-5p, miR-129-5p, and miR-363-3p and Hamilton Anxiety/Depression scores in methamphetamine-dependent patients as well as heroin-dependent patients.
In the following sections, we address more details pertaining to the link between substance use and substance-induced psychiatric diseases. Overall, current findings demonstrate that substance use is associated with the development of psychotic symptoms and depressive-like behaviors, and such malfunctions are mediated by epigenetic shifts causing gene expression dysregulation.
3. The Effects of Substance Use on Changing Brain Functions via Epigenetic Alterations
Epigenetic regulatory mechanisms such as DNA methylation, histone modifications, and miRNAs play powerful roles in adaptive alterations in neuroplasticity following prolonged drug use [31,32]. Previous studies have shown that neuronal functions relevant to learning, memory, and synaptic plasticity can be dynamically regulated by DNA methylation and histone modifications in individuals with SUDs [33,34]. For example, heroin-induced remodeling of the actin cytoskeleton via alterations in DNA methylation levels might participate in behavioral plasticity [35]. In this process, the proteasomal degradation of DNA methyltransferase DNMT3a by the E2 ubiquitin-conjugating enzyme contributes to the initiation of CaMKK1 gene transcription and the elevation of CaMKK1 protein expression via decreasing DNA methylation of its promoter region and thereby facilitates actin polymerization through the activation of the CaMKIα/βPIX/Rac1 pathway in the dorsal hippocampus [35]. Likewise, there is an interesting association between DNA hypermethylation of the dopamine transporter gene (DAT1) and dopamine release in individuals addicted to psychoactive substances [36]. Furthermore, reduced levels of brain-derived neurotrophic factor (BDNF) methylation in CpG 5–11 have been found in subjects with tobacco use and depression compared to those who did not consume tobacco with or without depression [37]. In addition to DNA methylation, histone modifications may play important roles in altering neuronal functions in subjects with SUDs [38]. For example, human primary astrocytes treated with opioid and psychostimulants exhibited elevated levels of global acetylation of H3 histone lysine residues, except for the acetylation of the 14th lysine residue [39]. It is hypothesized that illicit drugs like ∆9-tetrahydrocannabinol have the capacity for the activation of histone deacetylases, causing heterochromatic sequences of genes involved in cognitive functions, and higher risks of schizophrenia development and aggravation [40]. Similarly, the retrieval of heroin-related memories is associated with changes in histone acetylation during reconsolidation, and hence interventions to modulate histone acetylation can serve as valid approaches to cure SUD and hamper relapses [41]. Another study showed that adolescent-intermittent ethanol exposure diminishes the level of H3 acetylation in the hippocampus, reduces the expression of BDNF, and, subsequently, suppresses neurogenesis in this brain region [42]. Figure 2 illustrates how drug use and alcohol consumption increase the risk of psychosis, anxiety, depressive-like behaviors, and learning and memory deficits in users via epigenetic alterations.
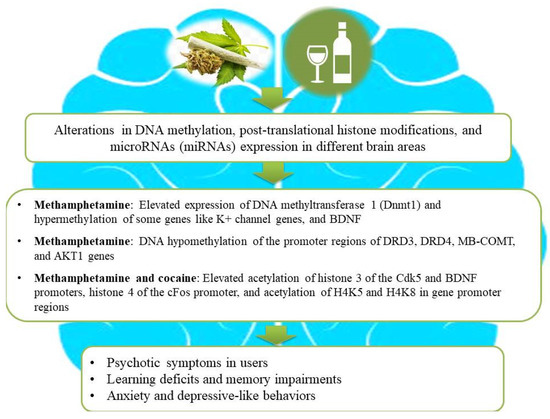
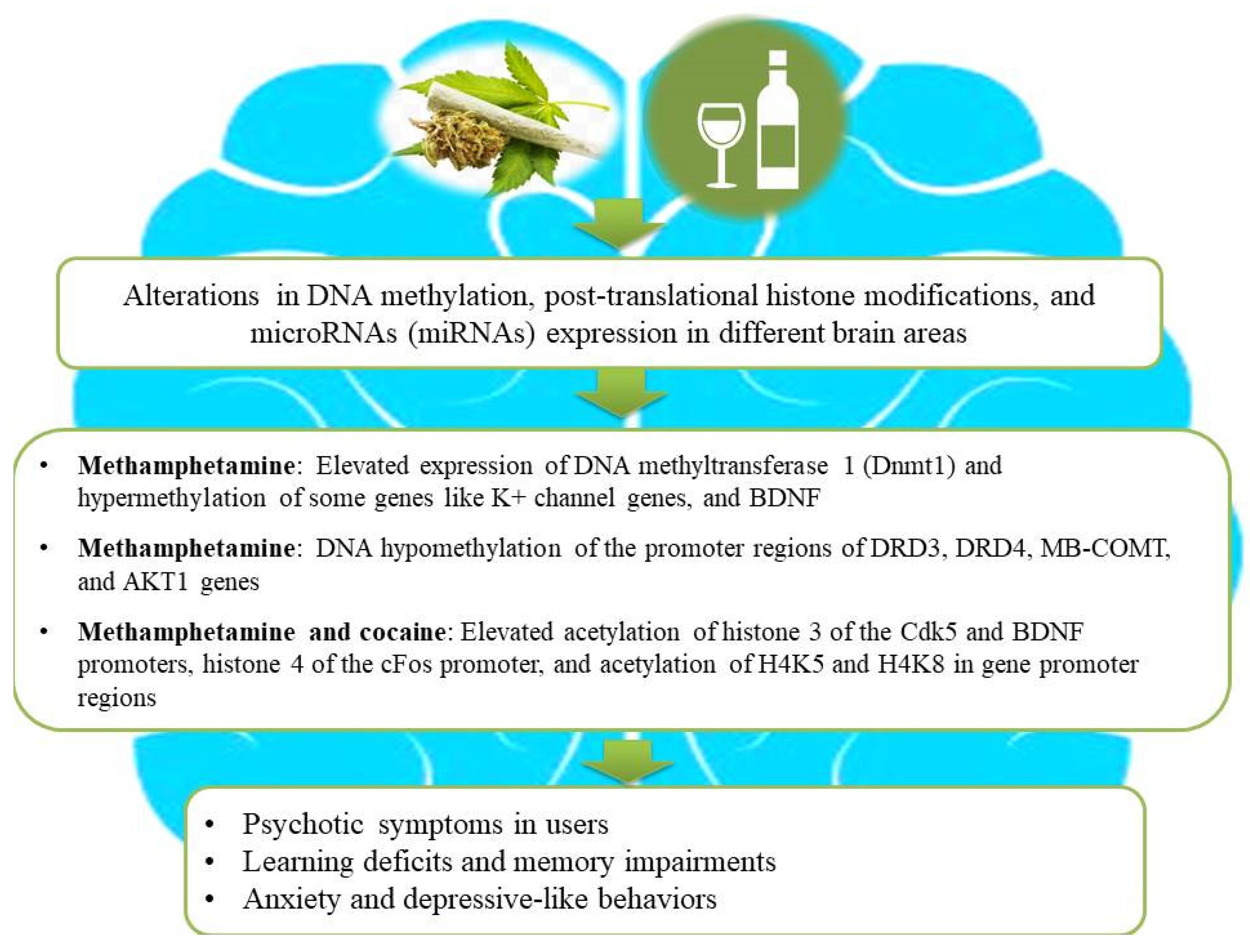
Figure 2.
An illustration of association among substance use, epigenetic aberrations, and the development of neurological impairments in users. Substance use causes aberrant alterations in DNA methylation, post-translational histone modifications, and microRNA (miRNA) expression in different brain areas of substance users and subsequently gives rise to the development of psychosis, depressive-like behaviors, and learning or memory deficits. Ethanol intake and illicit drugs (for example, cannabis, methamphetamine, and cocaine) lead to changes in gene expression by combinatorial epigenetic events and, hence, increase the risk of developing psychosis and other neurological impairments.
Other lines of evidence linking substance use to epigenetic alterations, including DNA methylation and histone modifications in the different brain areas, are summarized in Table 1 and Table 2, respectively. In addition to DNA methylation and histone modifications, substance use epigenetically alters gene expression by changing the levels of endogenous non-coding RNAs, like miRNA and circular RNA (circRNA), in the brain tissue [43,44,45]. circRNAs are capable of influencing substance behavioral effects through interplay with miRNAs [44]. For instance, striatal miRNAs play powerful roles in neuroplasticity, learning and memory, and reward circuit function and regulation [46,47]. As another example, a study by Chavoshi et al. indicated that astrocyte over-activation and striatal atrophy following treatment with methamphetamine is connected to 167 differentially expressed miRNAs in the striatum region [48]. Gu et al. reported that heroin users could exhibit elevated serum levels of miR-486-5p, miR-206, and let-7b-5p, whereas methamphetamine users had increased serum levels of miR-9-3p [49]. Another study found that elevated levels of let-7b-3p in the nucleus accumbens and the ventral tegmental area of methamphetamine users may be considered a potential biomarker for the diagnosis of addiction in these patients [50]. Furthermore, miRNA–mRNA network analysis of postmortem brains and blood samples of subjects with opioid use disorder revealed a potent overlap between their differentially expressed target genes, despite the distinct profiles of the altered brain and blood miRNAs [51].

Table 1.
Substance-induced DNA methylation alterations in brain tissue.

Table 2.
Substance-induced histone modifications in brain tissue.
More studies linking substance use to the changes in the expression of miRNAs in the brain tissue are summarized in Table 3.

Table 3.
Association between substance use and alterations in miRNAs in various brain regions.
Generally, aberrant changes in DNA methylation, post-translational histone modifications, and miRNA expression in various brain regions of substance users heavily affect neuronal functions relevant to synaptic plasticity, learning, and memory, which may accelerate the development of psychiatric disorders. However, it is crucial to recognize that while these epigenetic alterations are associated with substance use, the exact causal mechanisms and their implications for psychiatric disorders require further investigation. Understanding these complex interactions will necessitate extensive research to differentiate between correlation and causation and develop targeted therapeutic interventions.
4. Paternal and Maternal Substance Use and Epigenetic Alterations in the Brains of the Offspring
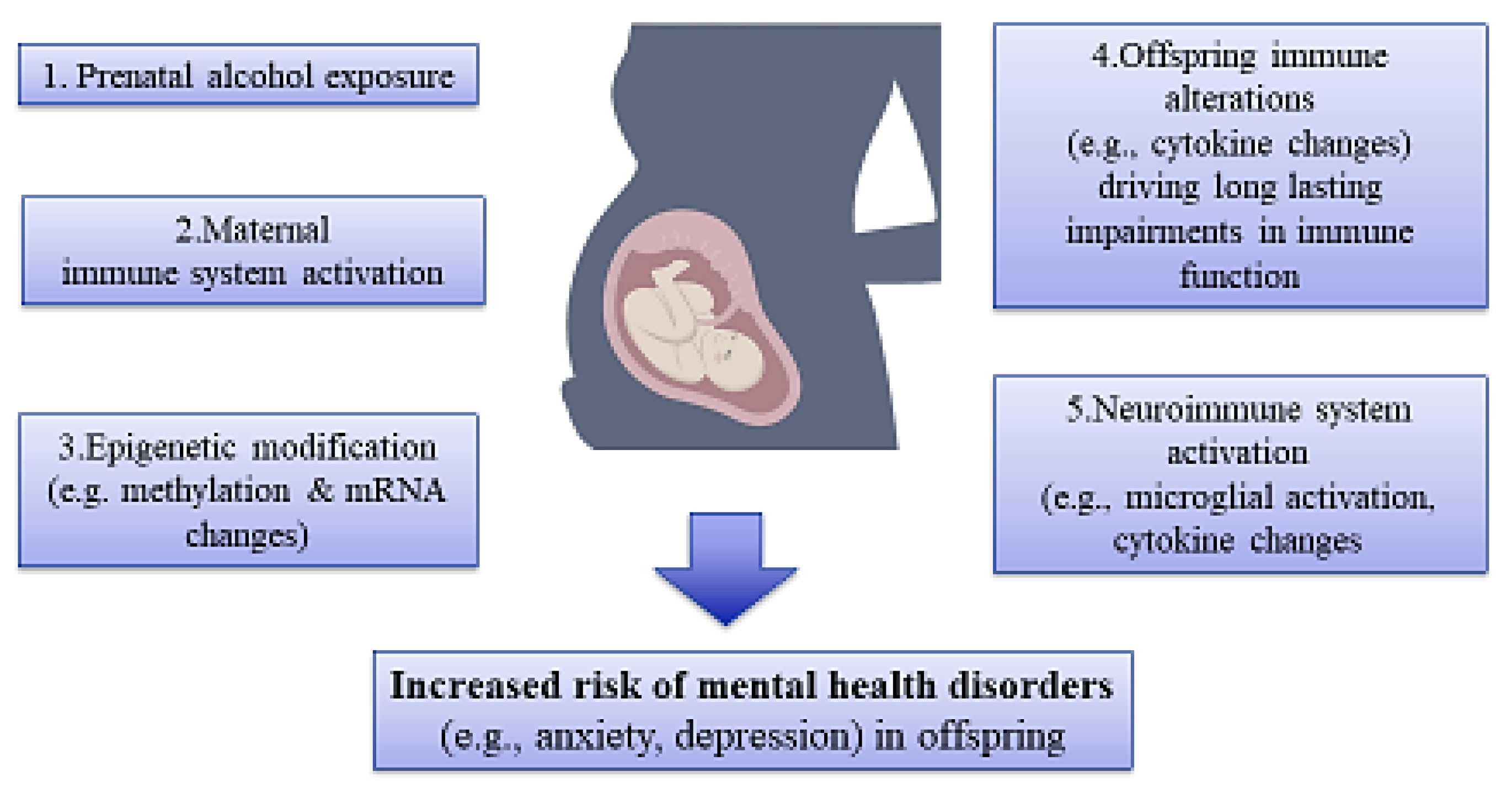
It has been reported that illicit drugs and other substances are capable of passing via the placenta, activation of the immune system, and disrupting the development of offspring by changing gene expression and/or causing epigenetic aberrations in various body organs, especially the brain tissue, and subsequently increasing the risk of mental disorders (Figure 3) [110,111].
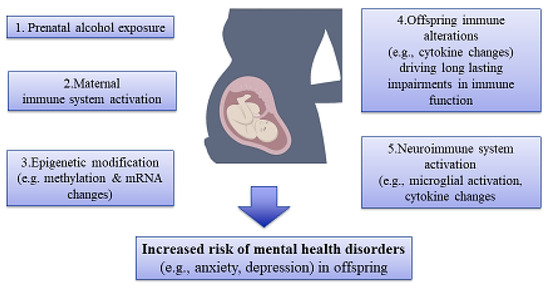
Figure 3.
An illustration of association among maternal substance use, epigenetic alterations, and risk of mental disorders in offspring. Prenatal exposure to substances like alcohol affects the immune system, epigenetic mechanisms, and, hence, the development of mental disorders in offspring.
For example, methamphetamine has been found to be the most common illicit drug taken by pregnant mothers, which gives rise to changes in the expression of neurodevelopment-related genes and thereby cognitive deficits and neuropsychiatric diseases in offspring [112,113]. Other lines of evidence linking paternal and maternal substance use during pregnancy to epigenetic changes with respect to DNA methylation and histone modifications in various brain regions are summarized in Table 4. It has been found that maternal substance use during pregnancy is connected to an elevated risk of psychotic symptoms in offspring as well [114]. For example, both paternal and maternal cannabis use is linked to the higher number of psychotic-like experiences in the offspring in childhood (at age ten years) [115]. The development of psychotic symptoms and other neurological impairments in offspring owing to substance use can be mediated by epigenetic changes. For instance, a recent study by Wendt Viola et al. indicated that prenatal cocaine exposure in humans can increase the risk for psychosis in offspring, which is connected to epigenetic alterations [116]. In another study by Hollins et al., it was shown that a combination of prenatal treatment with poly I:C and cannabinoid exposure caused strong differences (98%) in the miRNA expression of the brain hemispheric region within the Dlk1-Dio3-imprinted domain on 6q32 that is related to the syntenic human locus in schizophrenia [117]. A more recent genome-wide human study showed that subjects with cannabis use disorder had differential DNA methylation at four CpG sites, remarkably at the AHRR cg0557592 site, found to be an important mediator linking cannabis use to mental disorders, particularly mood disorders [118]. Moreover, maternal exposure to e-cigarette aerosols with nicotine could impair short-term memory, which was connected to the elevated levels of global DNA methylation in the brains of offspring [119].
There is evidence showing that such epigenetic changes in offspring can occur due to malabsorption of nutrients in pregnant women with SUDs. In this line, it has been shown that drug use and alcohol consumption result in derangements in the absorption of micronutrients such as folic acid, choline, and omega 3 during pregnancy, and hence supplementation with such diets in pregnant women with SUD may prevent neurological deficits in the offspring through normalizing epigenetic aberrations [120,121,122,123]. As another interesting example, it was shown that prenatal alcohol exposure caused perturbances in hippocampal miRNA expression, and choline supplementation could reverse an ethanol-dependent increase in hippocampal miR-200c expression [124]. Another animal study demonstrated that alcohol consumption increases DNA methylation in the PFC and hippocampus of rat pups during the neonatal period, and supplementation with choline could reduce DNA hypermethylation in both of these brain regions [125]. Collectively, these findings show that drugs and other substances like alcohol can pass through the placenta, activate the immune system, impair the development of offspring, and elevate the risk of mental illnesses by altering gene expression and/or causing epigenetic aberrations in brain tissue. However, while these studies provide significant insights, further research is needed to fully understand the mechanisms involved and determine the short- and long-term effects of these substances on human development and mental health.
Table 4 shows more examples of the associations between paternal and maternal substance use and epigenetic alterations in various brain regions in offspring.

Table 4.
Paternal and maternal substance use and epigenetic changes in various brain regions of offspring.
Table 4.
Paternal and maternal substance use and epigenetic changes in various brain regions of offspring.
| Type of Substance/Type of Study | Brain Area | Epigenetic Changes | Effects on the Brains of Offspring | Ref. |
|---|---|---|---|---|
| Long-term parental methamphetamine exposure in mice | Hippocampus | DNA methylation | Presence of DMSs in the brains of offspring exposed to methamphetamine during embryonic development compared to control | [126] |
| Maternal methamphetamine exposure in mice | NA | DNA methylation | DMSs of some genes involved in neurodevelopmental process | [127] |
| Maternal cocaine exposure in mice | Hippocampus | DNA methylation | Reducing global DNA methylation at 3 and elevation of global DNA methylation at 30 days postpartum | [128] |
| Prenatal cocaine exposure in mice | Hippocampus | DNA methylation | Cognitive deficits in offspring due to overexpression of DNMT 1 and L-methionine and subsequently elevating DNA methylation of IGF-2 | [129] |
| Maternal alcohol consumption | Hippocampus | DNA methylation | Alterations in DNA methylation, gene expression, and brain function in offspring | [130] |
| Maternal alcohol consumption | Hippocampus | DNA methylation | Elevated levels of dnmt1, dnmt3a, and hdac2 in offspring | [131] |
| Paternal cocaine exposure in rats | Hippocampus | Histone acetylation/methylation | Elevated levels of a single methylated lysine 4 on histone H3 (H3K4me1) and acetylated histone H3 (H3Ac) near the Dao1 gene responsible for the oxidative deamination of D-serine (an amino acid with antipsychotic activity) | [132] |
| Prenatal cocaine exposure in mice | Frontal cortex | Histone acetylation | Hyperacetylation of histone H3 at the BDNF promoter and thereby elevating the mRNA and protein levels of BDNF at adult postnatal day 60 | [133] |
| Parental morphine exposure in rats | PFC and hippocampus. | Histone acetylation | Decrease in histone H3 acetylation and ΔFosB in the offspring of morphine-withdrawn parents during postnatal days 5, 21, 30, and 60 | [134] |
| Prenatal morphine exposure in rats | VTA | Histone acetylation | Overexpression of HDAC5 | [135] |
| Maternal cannabis exposure | NA | Histone lysine methylation | Reduced 3meH3K4 and elevated 2meH3K9 repressive marks at the DRD2 gene locus in the cannabis-exposed offspring | [136] |
| Perinatal alcohol and nicotine–alcohol exposure in rats | VTA | miRNA | Alterations in the expression of miRNAs in dopaminergic neurons | [137] |
| Prenatal alcohol exposure in mice | Brain cortex and microvascular endothelium at embryonic day 18 | miRNA | Elevated levels of MicroRNA-150-5p and suppressing the angiogenic factor Vezf1 | [138] |
5. Therapeutic Approaches Using Diet Modification or Epigenetic Drugs to Improve Psychotic Symptoms, Learning Deficits, and Memory Impairments in Animal Models and Patients with SUDs
Several lines of evidence have indicated that psychotic symptoms in patients with SUDs are associated with changes in the nutrients that influence methylation machinery for post-transcriptional gene regulation. For example, lower levels of folate, a cofactor for methylation reactions involved in gene transcription regulation levels [139], have been reported in psychotic methamphetamine users versus non-psychotic methamphetamine users. In fact, every 1-unit decrease in serum folate level may increase the risk of psychosis by 27% [140]. Therefore, the use of a methyl-rich diet is considered a promising strategy for alleviating psychotic symptoms in patients with methamphetamine use. As another remarkable example, one study showed DNA hypomethylation of the promoter regions of the DRD3, DRD4, MB-COMT, and AKT1 genes in patients with methamphetamine psychosis [141]. It was concluded that the use of a methyl-rich diet may help improve psychotic symptoms in these patients [141]. Tian et al. also reported that repeated treatment with methionine (the main methyl donor amino acid in mammals) for 25 days before and during conditioned place preference training could suppress the establishment of cocaine rewarding effects by reversing global DNA hypomethylation in the PFCs of mice [142].
In another study, Wright et al. found that global hypomethylation and decreased methylation at CpG dinucleotides in the c-Fos gene promoter are connected to cocaine-induced c-Fos expression in the nucleus accumbens cores of rats [143]. Their results revealed that prolonged methyl supplementation by L-methionine could alleviate drug-seeking attitudes and behavioral sensitization to the locomotor-activating effects of cocaine by enhancing DNA methylation of the c-Fos promoter region [143]. Likewise, treatment with choline (the main source of methyl groups in mammals) is capable of reversing detrimental effects of alcohol on brain function [121]. As other examples, in the adult offspring of rats who consumed alcohol during pregnancy, elevated levels of MeCP2 (methyl-CpG-binding protein), the Dnmt1 enzyme (DNA-methylating enzyme 1), and several repressive histone marks (Setdb1, H3K9me2, and G9a), along with reduced levels of histone activation marks (H3K9ac, H3K4me3, and H3S10 phosphorylation), were reported in the thalamic β-EP-producing proopiomelanocortin neurons, which were normalized by gestational choline supplementation [144]. Gitik et al. also identified 462 genes with altered promoter DNA methylation in adult mice dorsal hippocampus after nicotine exposure associated with learning deficits. They found that dietary choline supplementation was capable of reducing learning deficits in mice exposed to nicotine by normalizing DNA methylation of the hippocampus [145]. In addition to methyl-rich diets, a ketogenic diet is a promising candidate for reducing neurotoxicity in substance users. During alcohol detoxification, a paradoxical energy-deficit state occurs in the human brain owing to reduced plasma levels of acetate and beta-hydroxybutyrate as epigenetic modifiers, which further contributes to withdrawal symptoms and neurotoxicity in patients with alcohol use disorder [146]. Wiers et al. found that a shift in energy substrates during withdrawal in patients with alcohol use disorder may be a major reason for withdrawal severity and neurotoxicity, and a ketogenic diet could contribute to reducing withdrawal symptoms by elevating ketone bodies (beta-hydroxybutyrate, acetoacetate, and acetone) and decreasing levels of neuroinflammatory markers [147]. In another study, the same group reported that three weeks of treatment with a ketogenic diet is capable of reducing a neurobiological craving signature in patients with alcohol use disorder [148].
Likewise, some drugs are capable of maintaining neuronal function in substance users by reversing epigenetic aberrations. For instance, Wang et al. reported that acute treatment of mice with phencyclidine resulted in a drastic decrease in miRNA-143 expression in astrocytes of the PFC region and hence the development of psychotic symptoms resembling schizophrenia. Their results revealed that while a D2 receptor-specific agonist (quinpirole) also decreased miRNA-143 expression, antipsychotic drugs like clozapine or haloperidol could prevent phencyclidine-induced hyperactivity by restoring miR-143 expression and suppress D2 receptors induced expression of Neuregulin-1, a target of miRNA-143 [149]. In another study, it was found that cocaine could impair DNMT activity in astrocytes, which, in turn, accelerates neurodegeneration [150]. However, Piracetam, a drug for the treatment of cognitive disorders, could hamper cocaine induced-impairments of DNMT activity and reduce cell death [150]. In sum, psychotic symptoms, learning deficits, and memory impairments in patients with SUDs are linked to altering the nutrients capable of modulating methylation machinery for post-transcriptional gene regulation, and, hence, methyl donor micronutrients or epigenetic drugs may be considered as potential candidates to prevent or treat such abnormalities in patients with SUDs. However, it is important to note that the results from animal studies presented in this work cannot be directly translated to humans; clinical trials are necessary to validate the efficacy of these approaches and their potential side effects in human subjects.
6. Substance Use-Induced Gut Microbiome Alterations May Intensify Psychopathology via Epigenetic Aberrations
Accumulating evidence has demonstrated that substance use contributes to the disturbance of gut barrier integrity, elevation of intestinal permeability, and changing the gut microbiota composition, which further result in derangements in brain function and the patient’s mental status. Alterations in the gut microbiota and their metabolites by substance use heavily affect brain function by causing unfavorable shifts in the immune and inflammatory pathways and the release of specific neurotransmitters [151,152]. Numerous bacteria have demonstrated their ability to produce different types of neurotransmitters, like serotonin, dopamine, and GABA [153]. While the link between gut dysbiosis and the pathogenesis of various psychiatric diseases are reviewed elsewhere [154], the impacts of substance use on the gut microbiome and corresponding neurochemical changes in the brain tissue may also contribute to the development of psychiatric disorders [155]. For instance, as patients with psychiatric disorders exhibit reduced abundance of the butyrate-producing Faecalibacterium and increased abundance of pathogenic and pro-inflammatory bacteria such as Eggerthella and Streptococcus, methamphetamine use can lead to similar bacterial dysbiosis as well [154,156,157].
A growing body of evidence has demonstrated a close relationship between SUDs and gut microbiome dysbiosis [158,159,160]. Opioid-induced bowel dysfunction is one of the adverse effects of chronic opioid use [161]. A clinical study showed that methadone-treated individuals exhibited lower fecal bacterial α-diversity and composition compared to non-opioid users [162]. In addition, patients with heroin use disorder exhibited drastic changes in gut microbiome diversity, composition, and functions, and the abundance levels of Turicibacter, Actinomyces, and Weissella bacteria could be considered biomarkers for predicting heroin-induced depression symptoms [163]. In methamphetamine users, it has been shown that an altered gut microbiome is associated with cognitive decline, psychotic syndrome, and the pathogenesis of methamphetamine-induced psychosis [164]. Furthermore, elevated abundance levels of Lachnospiraceae, Xanthomonadale, Romboutsia, and Sphingomonadales, as well as decreased abundance levels of Bacteroidaceae and Deltaproteobacteria, were connected to the development of psychotic symptoms in methamphetamine users [164]. Interestingly, fecal microbiota transplantation from methamphetamine-administered mice was also capable of creating methamphetamine-induced anxiety- and depressive-like behaviors and elevating neuroinflammation in the hippocampus region of recipient mice [165].
In another study, Panee et al. found that the lower Prevotella–Bacteroides ratio of the fecal microbiome in marijuana users was associated with cognitive deficits [166].
Remarkably, alerted gut microbiome composition in subjects with SUDs can heavily influence the production of gut microbiome-derived metabolites as well, which further affect mental health. For example, a reduction in the abundance of Akkermansia muciniphila, a species responsible for the production of some metabolites involved in modulating the expression of tight junction proteins and maintenance of intestinal barrier integrity, was seen in methadone-treated individuals in comparison with non-opioid users [162]. It has been reported that substances like cannabis, nicotine, and methamphetamine have a great impact on the regulation of bacterially derived products like neuroactive metabolites, epigenetic modifiers, neurotransmitters, and anti-inflammatory metabolites, which play critical roles in the cross-talk between the gut and the central nervous system (CNS) [167,168,169,170]. For instance, a reduced concentration of butyric acid, an epigenetic modifier and anti-inflammatory metabolite involved in preventing the development and progression of neuropsychiatric diseases, has been reported as the result of oral and fecal bacteria alterations in patients with cocaine use disorder [171]. Overall, it appears that systemic inflammation in methamphetamine use disorder is due to the decreased abundance levels of butyrate-producing bacteria like Faecalibacterium, Dorea, and Blautia as well as increased abundance of pro-inflammatory bacteria [157,172,173].
Therefore, dietary sodium butyrate supplementation and the microbiome-derived short-chain fatty acids (SCFAs) may act as potent epigenetic modifiers and anti-inflammatory agents to treat drug-induced toxicity and substance-induced psychosis [174,175,176,177]. Some supporting evidence comes from the recent Zhang et al. studies showing that gut microbiome-derived SCFAs could exhibit great potential for reducing methamphetamine-induced anxiety- and depressive-like behaviors by suppressing colonic inflammation and improving gut homeostasis [178]. In another study, it has been shown that sodium butyrate supplementation is capable of alleviating detrimental effects of alcohol use disorder on the CNS by inhibiting neuroinflammation [179]. Some therapeutic agents are also capable of improving mental disorders induced by methamphetamine use through increasing the abundance of butyrate-producing and hydrogen-producing bacteria. For example, Wang et al. reported altered gut microbial composition (decreased abundance of butyrate-producing bacteria like Bacteroides and Roseburia), reduced alpha diversity, and elevated self-rating scales of depression (SDS) and anxiety (SAS) in methamphetamine users compared to their age-matched healthy subjects [180]. They found that inhaling hydrogen could improve neuropsychiatric impairments induced by methamphetamine use through changing gut microbiota profiles and increasing the abundance of Bacteroides and Roseburia [180]. Considering current data indicating that SCFAs are affected in SUDs and microbial or other therapeutic interventions improve substance-induced psychiatric symptoms by increasing butyrate or other SCFAs levels, it is conceivable to suggest that epigenetic alterations mediate mental health impacts of substance-induced gut dysbiosis in SUDs. Taken together, these findings indicate that substance use is capable of disturbing the gut barrier’s integrity, increasing intestinal permeability, and alterations in the gut microbiota composition, which further lead to disturbances in brain function and the patient’s mental status. As an example, since gut microbiome dysbiosis in patients with SUDs is related to a reduced abundance of butyrate-producing bacteria and increased abundance of pathogenic and pro-inflammatory bacteria, dietary sodium butyrate supplementation and/or SCFA-producing probiotics may serve as epigenetic remedies to reduce the risk of mental illnesses in substance users pending confirmation in human clinical studies.
7. Challenges and Potentials for Clinical Translation
In order to accelerate translational relevance, consistency in substance exposure paradigms and the establishment of human-relevant dosage regimens or change to volitional models of substance exposure are essential. In addition, epigenetic alterations in the brain tissue should be assessed in both short-term and long-term use/exposure or withdrawal to precisely estimate the stability of substance-induced epigenetic aberrations [181]. Moreover, in order to obtain greater insights on shared substance-induced neuroplasticity changes and neurological impairments and promote clinical translation based on these findings, systematic comparisons of different substances and their epigenetic consequences are required. Some studies have shown that there are sex-dependent differences in substance-induced epigenetic modifications in the brain; hence, future studies should have more of a focus on unresolved issues to explore how sex differences affect substance-induced epigenetic alterations [182].
In order to achieve more effective diagnostic and therapeutic strategies for substance-induced neurological impairments, single-cell next-generation sequencing technologies and approaches should be utilized more extensively in future research relevant to substance-induced epigenetic alterations in the brain tissue. Single-cell RNA sequencing can also be applied for the detection of new gene targets regulated by opioids and other substances. Investigation of the genome for areas of opened or closed chromatin after short or long-term exposures to substance can be conducted by ATAC-seq. ChIP-seq is capable of connecting this type of epigenetic alteration with the affected gene loci. Moreover, locus-specific epigenetic editing tools provide an opportunity for researchers to detect the functional consequences of substance-induced epigenetic alterations via manipulating such targets in a cell type-specific manner [183,184].
Owing to the complexity of experimental variables like the host’s genotype and diet and the difficulty of controlling them, investigating substance use disorder–gut microbiome interactions in humans and their roles in the development and progression of mental disorders is still a great challenge [185]. Similarly, it might be difficult to reproduce microbiome research data using animal models since many factors, such as co-housing with other animals, vendors, facility conditions, and other environmental conditions, give rise to differences in the composition and structure of the gut microbiome [186].
8. Conclusions
This literature review supports the idea that drug use is capable of influencing neuroplasticity, learning and memory, and reward circuit functions by targeting DNA methylation, post-translational histone modifications, and miRNA in different regions of the brain tissue. Moreover, these findings show that substance-induced psychosis can be associated with epigenetic alterations, and, hence, epigenetic-based therapies can be considered interesting approaches for alleviating psychotic symptoms in patients with SUDs. Dietary nutrients such as methyl donors (folic acid, vitamins B6 and B12, methionine, betaine, and choline) can serve as therapeutic agents for alleviating psychotic symptoms and depressive-like behaviors in patients with SUDs by reversing the epigenetic aberrations caused by substance use or modulation of the gut microbiome. Therefore, it is strongly reasonable to investigate new connections between them, since alterations in the human diet may be considered the easiest and first stage of treatment of substance-induced psychosis or may actualize a neuroprotective role in neuropsychiatric diseases.
Author Contributions
Conceptualization, S.T. and H.M.A.; writing—original draft preparation S.N. and H.M.A.; editing and reorganization of the article H.M.A. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We acknowledge Faria Ashrafi for her assistance with the illustrations in this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Smith, M.J.; Thirthalli, J.; Abdallah, A.B.; Murray, R.M.; Cottler, L.B. Prevalence of psychotic symptoms in substance users: A comparison across substances. Compr. Psychiatry 2009, 50, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Arseneault, L.; Cannon, M.; Poulton, R.; Murray, R.; Caspi, A.; Moffitt, T.E. Cannabis use in adolescence and risk for adult psychosis: Longitudinal prospective study. Bmj 2002, 325, 1212–1213. [Google Scholar] [CrossRef]
- Huang, C.-L.; Tsai, I.-J.; Lee, C.W.-S. Risk of psychosis in illicit amphetamine users: A 10 year retrospective cohort study. Evid.-Based Ment. Health 2022, 25, 163. [Google Scholar] [CrossRef] [PubMed]
- McKetin, R. Methamphetamine psychosis: Insights from the past. Addiction 2018, 113, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Capuzzi, E.; Caldiroli, A.; Besana, F.; Cova, F.; Buoli, M.; Clerici, M. Factors associated with psychotic symptoms among a sample of male prisoners with substance use disorder: A cross-sectional study. J. Subst. Abus. Treat. 2020, 118, 108104. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Gonzalez-Giraldo, Y.; Wegman-Ostrosky, T.; Forero, D.A. Molecular genetics of substance use disorders: An umbrella review. Neurosci. Biobehav. Rev. 2021, 124, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.F.; O’Dell, S.J. Methamphetamine influences on brain and behavior: Unsafe at any speed? Trends Neurosci. 2012, 35, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.H.; Stein, D.J.; Howells, F.M. The neurobiology of methamphetamine induced psychosis. Front. Hum. Neurosci. 2014, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Hartz, S.M.; Horton, A.C.; Oehlert, M.; Carey, C.E.; Agrawal, A.; Bogdan, R.; Chen, L.-S.; Hancock, D.B.; Johnson, E.O.; Pato, C.N. Association between substance use disorder and polygenic liability to schizophrenia. Biol. Psychiatry 2017, 82, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Deak, J.D.; Johnson, E.C. Genetics of substance use disorders: A review. Psychol. Med. 2021, 51, 2189–2200. [Google Scholar] [CrossRef] [PubMed]
- Gelernter, J.; Polimanti, R. Genetics of substance use disorders in the era of big data. Nat. Rev. Genet. 2021, 22, 712–729. [Google Scholar] [CrossRef]
- Silva, G.M.; Hamilton, P.J. Epigenetics and substance use disorders: Translational aspects. In Neuropsychiatric Disorders and Epigenetics; Elsevier: Amsterdam, The Netherlands, 2024; pp. 353–378. [Google Scholar]
- Renthal, W.; Carle, T.L.; Maze, I.; Covington, H.E.; Truong, H.-T.; Alibhai, I.; Kumar, A.; Montgomery, R.L.; Olson, E.N.; Nestler, E.J. ΔFosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J. Neurosci. 2008, 28, 7344–7349. [Google Scholar] [CrossRef] [PubMed]
- McCowan, T.J.; Dhasarathy, A.; Carvelli, L. The epigenetic mechanisms of amphetamine. J. Addict. Prev. 2015, 2015. [Google Scholar] [CrossRef]
- Guerrini, I.; Thomson, A.D.; Gurling, H.D. The importance of alcohol misuse, malnutrition and genetic susceptibility on brain growth and plasticity. Neurosci. Biobehav. Rev. 2007, 31, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Abdolmaleky, H.M.; Zhou, J.-R.; Thiagalingam, S. An update on the epigenetics of psychotic diseases and autism. Epigenomics 2015, 7, 427–449. [Google Scholar] [CrossRef]
- Degenhardt, L.; Coffey, C.; Hearps, S.; Kinner, S.A.; Borschmann, R.; Moran, P.; Patton, G. Associations between psychotic symptoms and substance use in young offenders. Drug Alcohol Rev. 2015, 34, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Keshavan, M.S.; Kaneko, Y. Secondary psychoses: An update. World Psychiatry 2013, 12, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ying, L.; Liu, Y.; Liu, N.; Tu, G.; Zhu, M.; Wu, Y.; Xiao, B.; Ye, L.; Li, J. Different roles of Rac1 in the acquisition and extinction of methamphetamine-associated contextual memory in the nucleus accumbens. Theranostics 2019, 9, 7051. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Tag, S.H.; Nam, E.; Ham, S.; Ahn, S.; Kim, J.; Cho, D.-W.; Lee, S.; Yang, Y.-S.; Lee, S.E. SYNCRIP controls miR-137 and striatal learning in animal models of methamphetamine abstinence. Acta Pharm. Sin. B 2022, 12, 3281–3297. [Google Scholar] [CrossRef] [PubMed]
- Kalayasiri, R.; Dadwat, K.; Thika, S.; Sirivichayakul, S.; Maes, M. Methamphetamine (MA) use and MA-induced psychosis are associated with increasing aberrations in the compensatory immunoregulatory system, interleukin-1α, and CCL5 levels. Transl. Psychiatry 2023, 13, 361. [Google Scholar] [CrossRef]
- Al-Hakeim, H.K.; Altufaili, M.F.; Almulla, A.F.; Moustafa, S.R.; Maes, M. Increased lipid peroxidation and lowered antioxidant defenses predict methamphetamine induced psychosis. Cells 2022, 11, 3694. [Google Scholar] [CrossRef] [PubMed]
- Niemi-Pynttäri, J.A.; Sund, R.; Putkonen, H.; Vorma, H.; Wahlbeck, K.; Pirkola, S.P. Substance-induced psychoses converting into schizophrenia: A register-based study of 18,478 Finnish inpatient cases. J. Clin. Psychiatry 2013, 74, 20155. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.; Lombardi, D.; Du, J.; Makrum, U.; Sitthichai, R.; Harrington, A.; Shukair, N.; Zhao, M.; Fan, X. Methamphetamine-associated psychosis: Clinical presentation, biological basis, and treatment options. Hum. Psychopharmacol. Clin. Exp. 2019, 34, e2710. [Google Scholar] [CrossRef] [PubMed]
- McKetin, R.; McLaren, J.; Lubman, D.I.; Hides, L. The prevalence of psychotic symptoms among methamphetamine users. Addiction 2006, 101, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.; Liu, M.N.; Liu, M.Q.; Tian, X.Y.; Zhang, X.J.; Liu, F.; Hao, W.; Wu, N.; Li, H.; Li, J. A preliminary study on the association of single nucleotide polymorphisms and methylation of dopamine system-related genes with psychotic symptoms in patients with methamphetamine use disorder. Eur. J. Neurosci. 2023, 59, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Veerasakul, S.; Watiktinkorn, P.; Thanoi, S.; Dalton, C.F.; Fachim, H.A.; Nudmamud-Thanoi, S.; Reynolds, G.P. Increased DNA methylation in the parvalbumin gene promoter is associated with methamphetamine dependence. Pharmacogenomics 2017, 18, 1317–1322. [Google Scholar] [CrossRef]
- Kalayasiri, R.; Kraijak, K.; Maes, M.; Mutirangura, A. Methamphetamine (MA) use induces specific changes in LINE-1 partial methylation patterns, which are associated with MA-induced paranoia: A multivariate and neuronal network study. Mol. Neurobiol. 2019, 56, 4258–4272. [Google Scholar] [CrossRef]
- Sun, Q.; Zhao, Y.; Zhang, K.; Su, H.; Chen, T.; Jiang, H.; Du, J.; Zhong, N.; Yu, S.; Zhao, M. An association study between methamphetamine use disorder with psychosis and polymorphisms in MiRNA. Neurosci. Lett. 2020, 717, 134725. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xu, Y.; Shi, K.; Zhang, Z.; Xie, Z.; Wu, H.; Ma, Y.; Zhou, Y.; Chen, C.; Yang, J. Multi-omics study reveals associations among neurotransmitter, extracellular vesicle-derived microRNA and psychiatric comorbidities during heroin and methamphetamine withdrawal. Biomed. Pharmacother. 2022, 155, 113685. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Jayanthi, S. Epigenetics of addiction. Neurochem. Int. 2021, 147, 105069. [Google Scholar] [CrossRef] [PubMed]
- Lax, E.; Szyf, M. The role of DNA methylation in drug addiction: Implications for diagnostic and therapeutics. Prog. Mol. Biol. Transl. Sci. 2018, 157, 93–104. [Google Scholar] [PubMed]
- Barbier, E.; Tapocik, J.D.; Juergens, N.; Pitcairn, C.; Borich, A.; Schank, J.R.; Sun, H.; Schuebel, K.; Zhou, Z.; Yuan, Q. DNA methylation in the medial prefrontal cortex regulates alcohol-induced behavior and plasticity. J. Neurosci. 2015, 35, 6153–6164. [Google Scholar] [CrossRef] [PubMed]
- Sen, N. Epigenetic regulation of memory by acetylation and methylation of chromatin: Implications in neurological disorders, aging, and addiction. Neuromolecular Med. 2015, 17, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-G.; Wang, Y.-J.; Chen, R.-S.; Geng, F.; Gan, C.-L.; Wang, W.-S.; Liu, X.; Zhou, H.; He, L.; Hu, G. Ube2b-dependent degradation of DNMT3a relieves a transcriptional brake on opiate-induced synaptic and behavioral plasticity. Mol. Psychiatry 2021, 26, 1162–1177. [Google Scholar] [CrossRef]
- Chmielowiec, K.; Chmielowiec, J.; Masiak, J.; Strońska-Pluta, A.; Lachowicz, M.; Boroń, A.; Larysz, D.; Dzitkowska-Zabielska, M.; Cięszczyk, P.; Grzywacz, A. DNA Methylation of the Dopamine Transporter DAT1 Gene—Bliss Seekers in the Light of Epigenetics. Int. J. Mol. Sci. 2023, 24, 5265. [Google Scholar] [CrossRef] [PubMed]
- Quaioto, B.R.; Borçoi, A.R.; Mendes, S.O.; Doblas, P.C.; dos Santos Vieira, T.; Moreno, I.A.A.; Dos Santos, J.G.; Hollais, A.W.; Olinda, A.S.; de Souza, M.L.M. Tobacco use modify exon IV BDNF gene methylation levels in depression. J. Psychiatr. Res. 2023, 159, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, K.; Doke, M.; Khan, M.A.; Samikkannu, T. Influence of psychostimulants and opioids on epigenetic modification of class III histone deacetylase (HDAC)-sirtuins in glial cells. Sci. Rep. 2021, 11, 21335. [Google Scholar] [CrossRef] [PubMed]
- Doke, M.; Pendyala, G.; Samikkannu, T. Psychostimulants and opioids differentially influence the epigenetic modification of histone acetyltransferase and histone deacetylase in astrocytes. PLoS ONE 2021, 16, e0252895. [Google Scholar] [CrossRef] [PubMed]
- Verhoeff, N.J.; Dhillon, S.K. The Relationship between Using Cannabis, Consuming Isothiocyanate Rich Broccoli, and HDAC Expression in Schizophrenia: A Research Protocol. Undergrad. Res. Nat. Clin. Sci. Technol. J. 2020, 4, 1–9. [Google Scholar] [CrossRef][Green Version]
- Cheng, J.; Wang, B.; Hu, H.; Lin, X.; Liu, Y.; Lin, J.; Zhang, J.; Niu, S.; Yan, J. Regulation of histone acetylation by garcinol blocks the reconsolidation of heroin-associated memory. Biomed. Pharmacother. 2024, 173, 116414. [Google Scholar] [CrossRef] [PubMed]
- Sakharkar, A.J.; Vetreno, R.P.; Zhang, H.; Kokare, D.M.; Crews, F.T.; Pandey, S.C. A role for histone acetylation mechanisms in adolescent alcohol exposure-induced deficits in hippocampal brain-derived neurotrophic factor expression and neurogenesis markers in adulthood. Brain Struct. Funct. 2016, 221, 4691–4703. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, S.; Kenny, P.J. Molecular, cellular, and structural mechanisms of cocaine addiction: A key role for microRNAs. Neuropsychopharmacology 2013, 38, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Long, H.; Shao, X.; Gu, H.; Kong, J.; Luo, L.; Liu, B.; Guo, W.; Wang, H.; Tian, J. Cocaine induces differential circular RNA expression in striatum. Transl. Psychiatry 2019, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Floris, G.; Gillespie, A.; Zanda, M.T.; Dabrowski, K.R.; Sillivan, S.E. Heroin regulates orbitofrontal circular RNAs. Int. J. Mol. Sci. 2022, 23, 1453. [Google Scholar] [CrossRef] [PubMed]
- Eipper-Mains, J.E.; Kiraly, D.D.; Palakodeti, D.; Mains, R.E.; Eipper, B.A.; Graveley, B.R. microRNA-Seq reveals cocaine-regulated expression of striatal microRNAs. Rna 2011, 17, 1529–1543. [Google Scholar] [CrossRef]
- Bu, Q.; Hu, Z.; Chen, F.; Zhu, R.; Deng, Y.; Shao, X.; Li, Y.; Zhao, J.; Li, H.; Zhang, B. Transcriptome analysis of long non-coding RNA s of the nucleus accumbens in cocaine-conditioned mice. J. Neurochem. 2012, 123, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Chavoshi, H.; Boroujeni, M.E.; Abdollahifar, M.-A.; Amini, A.; Tehrani, A.M.; Moghaddam, M.H.; Norozian, M.; Farahani, R.M.; Aliaghaei, A. From dysregulated microRNAs to structural alterations in the striatal region of METH-injected rats. J. Chem. Neuroanat. 2020, 109, 101854. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.-J.; Zhang, C.; Zhong, Y.; Luo, J.; Zhang, C.-Y.; Zhang, C.; Wang, C. Altered serum microRNA expression profile in subjects with heroin and methamphetamine use disorder. Biomed. Pharmacother. 2020, 125, 109918. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Tanoglu, E.G.; Aslıyuksek, H. Evaluation of microRNA let-7b-3p expression levels in methamphetamine abuse. Rev. Assoc. Médica Bras. 2023, 69, e20221391. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.L.; Mendez, E.F.; Stertz, L.; Fries, G.R.; Kanchi, R.; Selvaraj, S.; Teixeira, A.L.; Kosten, T.R.; Coarfa, C.; Walss-Bass, C. MicroRNA–mRNA networks are dysregulated in opioid use disorder postmortem brain: Further evidence for opioid-induced neurovascular alterations. Front. Psychiatry 2023, 13, 1025346. [Google Scholar] [CrossRef] [PubMed]
- Numachi, Y.; Shen, H.; Yoshida, S.; Fujiyama, K.; Toda, S.; Matsuoka, H.; Sora, I.; Sato, M. Methamphetamine alters expression of DNA methyltransferase 1 mRNA in rat brain. Neurosci. Lett. 2007, 414, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Hsu, S.-H.; Chen, C.-H. Chronic methamphetamine treatment reduces the expression of synaptic plasticity genes and changes their DNA methylation status in the mouse brain. Brain Res. 2015, 1629, 126–134. [Google Scholar] [CrossRef] [PubMed]
- González, B.; Jayanthi, S.; Gomez, N.; Torres, O.V.; Sosa, M.H.; Bernardi, A.; Urbano, F.J.; García-Rill, E.; Cadet, J.-L.; Bisagno, V. Repeated methamphetamine and modafinil induce differential cognitive effects and specific histone acetylation and DNA methylation profiles in the mouse medial prefrontal cortex. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, F.; Ferese, R.; Limanaqi, F.; Madonna, M.; Lenzi, P.; Gambardella, S.; Fornai, F. Methamphetamine persistently increases alpha-synuclein and suppresses gene promoter methylation within striatal neurons. Brain Res. 2019, 1719, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, S.; Torres, O.V.; Ladenheim, B.; Cadet, J.L. A single prior injection of methamphetamine enhances methamphetamine self-administration (SA) and blocks SA-induced changes in DNA methylation and mRNA expression of potassium channels in the rat nucleus accumbens. Mol. Neurobiol. 2020, 57, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Salehzadeh, S.A.; Mohammadian, A.; Salimi, F. Effect of chronic methamphetamine injection on levels of BDNF mRNA and its CpG island methylation in prefrontal cortex of rats. Asian J. Psychiatry 2020, 48, 101884. [Google Scholar] [CrossRef] [PubMed]
- Iamjan, S.-a.; Thanoi, S.; Watiktinkorn, P.; Fachim, H.; Dalton, C.F.; Nudmamud-Thanoi, S.; Reynolds, G.P. Changes of BDNF exon IV DNA methylation are associated with methamphetamine dependence. Epigenomics 2021, 13, 953–965. [Google Scholar] [CrossRef] [PubMed]
- Anier, K.; Malinovskaja, K.; Aonurm-Helm, A.; Zharkovsky, A.; Kalda, A. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology 2010, 35, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Bodetto, S.P.; Carouge, D.; Fonteneau, M.; Dietrich, J.-B.; Zwiller, J.; Anglard, P. Cocaine represses protein phosphatase-1Cβ through DNA methylation and Methyl-CpG Binding Protein-2 recruitment in adult rat brain. Neuropharmacology 2013, 73, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Søvik, E.; Berthier, P.; Klare, W.P.; Helliwell, P.; Buckle, E.L.; Plath, J.A.; Barron, A.B.; Maleszka, R. Cocaine directly impairs memory extinction and alters brain DNA methylation dynamics in honey bees. Front. Physiol. 2018, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Vaher, K.; Anier, K.; Jürgenson, M.; Harro, J.; Kalda, A. Cocaine-induced changes in behaviour and DNA methylation in rats are influenced by inter-individual differences in spontaneous exploratory activity. J. Psychopharmacol. 2020, 34, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, K.; Yang, J.; Chen, G.G.; Yerko, V.; Théroux, J.-F.; Aouabed, Z.; Lopez, A.; Thibeault, K.C.; Calipari, E.S.; Labonté, B. Cocaine-related DNA methylation in caudate neurons alters 3D chromatin structure of the IRXA gene cluster. Mol. Psychiatry 2021, 26, 3134–3151. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, K.; Chen, G.G.; Fiori, L.; Maussion, G.; Yerko, V.; Théroux, J.-F.; Ernst, C.; Labonté, B.; Calipari, E.; Nestler, E.J. Methylation of the tyrosine hydroxylase gene is dysregulated by cocaine dependence in the human striatum. Iscience 2021, 24, 103169. [Google Scholar] [CrossRef] [PubMed]
- Poisel, E.; Zillich, L.; Streit, F.; Frank, J.; Friske, M.M.; Foo, J.C.; Mechawar, N.; Turecki, G.; Hansson, A.C.; Nöthen, M.M. DNA methylation in cocaine use disorder–An epigenome-wide approach in the human prefrontal cortex. Front. Psychiatry 2023, 14, 1075250. [Google Scholar] [CrossRef] [PubMed]
- Mychasiuk, R.; Muhammad, A.; Ilnytskyy, S.; Kolb, B. Persistent gene expression changes in NAc, mPFC, and OFC associated with previous nicotine or amphetamine exposure. Behav. Brain Res. 2013, 256, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, F.; Zhao, N.; Mu, X.; Li, P.; Wang, W.; Liu, J.; Ma, X. Methylation of glucocorticoid receptor gene promoter modulates morphine dependence and accompanied hypothalamus–pituitary–adrenal axis dysfunction. J. Neurosci. Res. 2017, 95, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Barrow, T.M.; Byun, H.-M.; Li, X.; Smart, C.; Wang, Y.-X.; Zhang, Y.; Baccarelli, A.A.; Guo, L. The effect of morphine upon DNA methylation in ten regions of the rat brain. Epigenetics 2017, 12, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liang, J.; Jiang, F.; Cai, W.; Shen, F.; Liang, J.; Zhang, J.; Sun, Z.; Sui, N. Gnas promoter hypermethylation in the basolateral amygdala regulates reconsolidation of morphine reward memory in rats. Genes 2022, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- Kozlenkov, A.; Jaffe, A.E.; Timashpolsky, A.; Apontes, P.; Rudchenko, S.; Barbu, M.; Byne, W.; Hurd, Y.L.; Horvath, S.; Dracheva, S. DNA methylation profiling of human prefrontal cortex neurons in heroin users shows significant difference between genomic contexts of hyper-and hypomethylation and a younger epigenetic age. Genes 2017, 8, 152. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Xu, W.; Lin, Z.; Liu, J.; Chen, W.; Zhu, H.; Lai, M.; Zhuang, D.; Xu, Z.; Fu, D. Role of GABRD gene methylation in the nucleus accumbens in heroin-seeking behavior in rats. Front. Pharmacol. 2021, 11, 612200. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.-Y.; Shi, G.; Zhao, P. Reversal of oxycodone conditioned place preference by oxytocin: Promoting global DNA methylation in the hippocampus. Neuropharmacology 2019, 160, 107778. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Choi, K.-H.; Renthal, W.; Tsankova, N.M.; Theobald, D.E.; Truong, H.-T.; Russo, S.J.; LaPlant, Q.; Sasaki, T.S.; Whistler, K.N. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron 2005, 48, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lv, Z.; Hu, Z.; Sheng, J.; Hui, B.; Sun, J.; Ma, L. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIα in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology 2010, 35, 913–928. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.D.; Sangrey, G.R.; Darnell, S.B.; Schassburger, R.L.; Cha, J.H.J.; Pierce, R.C.; Sadri-Vakili, G. Increased brain-derived neurotrophic factor (BDNF) expression in the ventral tegmental area during cocaine abstinence is associated with increased histone acetylation at BDNF exon I-containing promoters. J. Neurochem. 2012, 120, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Perrine, S.A.; Alsharif, W.F.; Harutyunyan, A.; Kamal, S.; Viola, N.T.; Gelovani, J.G. Low-and high-cocaine intake affects the spatial and temporal dynamics of class IIa HDAC expression-activity in the nucleus accumbens and hippocampus of male rats as measured by [18F] TFAHA PET/CT neuroimaging. Addict. Neurosci. 2022, 4, 100046. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-Y.; Kalda, A.; Yu, L.; Ferrara, J.; Zhu, J.; Chen, J.-F. Additive effects of histone deacetylase inhibitors and amphetamine on histone H4 acetylation, cAMP responsive element binding protein phosphorylation and ΔFosB expression in the striatum and locomotor sensitization in mice. Neuroscience 2008, 157, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Jayanthi, S.; McCoy, M.T.; Brannock, C.; Ladenheim, B.; Garrett, T.; Lehrmann, E.; Becker, K.G.; Cadet, J.L. Methamphetamine causes differential alterations in gene expression and patterns of histone acetylation/hypoacetylation in the rat nucleus accumbens. PLoS ONE 2012, 7, e34236. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.L.; Jayanthi, S.; McCoy, M.T.; Ladenheim, B.; Saint-Preux, F.; Lehrmann, E.; De, S.; Becker, K.G.; Brannock, C. Genome-wide profiling identifies a subset of methamphetamine (METH)-induced genes associated with METH-induced increased H4K5Ac binding in the rat striatum. BMC Genom. 2013, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Omonijo, O.; Wongprayoon, P.; Ladenheim, B.; McCoy, M.T.; Govitrapong, P.; Jayanthi, S.; Cadet, J.L. Differential effects of binge methamphetamine injections on the mRNA expression of histone deacetylases (HDACs) in the rat striatum. Neurotoxicology 2014, 45, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.V.; Ladenheim, B.; Jayanthi, S.; McCoy, M.T.; Krasnova, I.N.; Vautier, F.A.; Cadet, J.L. An acute methamphetamine injection downregulates the expression of several histone deacetylases (HDACs) in the mouse nucleus accumbens: Potential regulatory role of HDAC2 expression. Neurotox. Res. 2016, 30, 32–40. [Google Scholar] [CrossRef]
- González, B.; Bernardi, A.; Torres, O.V.; Jayanthi, S.; Gomez, N.; Sosa, M.H.; García-Rill, E.; Urbano, F.J.; Cadet, J.L.; Bisagno, V. HDAC superfamily promoters acetylation is differentially regulated by modafinil and methamphetamine in the mouse medial prefrontal cortex. Addict. Biol. 2020, 25, e12737. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, J.-A.; Ding, Q.-Z.; Lu, G.-Y.; Wu, N.; Su, R.-B.; Li, F.; Li, J. Behavioral sensitization induced by methamphetamine causes differential alterations in gene expression and histone acetylation of the prefrontal cortex in rats. BMC Neurosci. 2021, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Egervari, G.; Landry, J.; Callens, J.; Fullard, J.F.; Roussos, P.; Keller, E.; Hurd, Y.L. Striatal H3K27 acetylation linked to glutamatergic gene dysregulation in human heroin abusers holds promise as therapeutic target. Biol. Psychiatry 2017, 81, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Liu, J.; Lin, Z.; Zhuang, D.; Xu, W.; Xu, Z.; Lai, M.; Zhu, H.; Zhou, W.; Liu, H. Histone 3 lysine 9 acetylation of BRG1 in the medial prefrontal cortex is associated with heroin self-administration in rats. Mol. Med. Rep. 2020, 21, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-X.; Wei, J.-Y.; Liu, M.; Shi, W.-J.; Song, L.-H.; Li, S.; Zhu, S.-Q.; Xin, W.-J.; Zhang, X.-Q. Epigenetic upregulation of hippocampal CXCL12 contributes to context spatial memory-associated morphine conditioning. Brain Behav. Immun. 2020, 84, 72–79. [Google Scholar] [CrossRef]
- Liu, S.X.; Gades, M.S.; Swain, Y.; Ramakrishnan, A.; Harris, A.C.; Tran, P.V.; Gewirtz, J.C. Repeated morphine exposure activates synaptogenesis and other neuroplasticity-related gene networks in the dorsomedial prefrontal cortex of male and female rats. Drug Alcohol Depend. 2021, 221, 108598. [Google Scholar] [CrossRef] [PubMed]
- Franco-García, A.; Gómez-Murcia, V.; Fernández-Gómez, F.J.; González-Andreu, R.; Hidalgo, J.M.; Milanés, M.V.; Núñez, C. Morphine-withdrawal aversive memories and their extinction modulate H4K5 acetylation and Brd4 activation in the rat hippocampus and basolateral amygdala. Biomed. Pharmacother. 2023, 165, 115055. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, C.A.; McCoy, M.T.; Ladenheim, B.; Cadet, J.L. Oxycodone self-administration activates the mitogen-activated protein kinase/mitogen-and stress-activated protein kinase (MAPK-MSK) signaling pathway in the rat dorsal striatum. Sci. Rep. 2021, 11, 2567. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, J.; Liu, Y.; Chen, Y.; Li, Y.; Chen, S.; Li, T.; Dang, Y.; Chen, T. Chronic methamphetamine regulates the expression of MicroRNAs and putative target genes in the nucleus accumbens of mice. J. Neurosci. Res. 2015, 93, 1600–1610. [Google Scholar] [CrossRef] [PubMed]
- Bosch, P.; Benton, M.; Macartney-Coxson, D.; Kivell, B. mRNA and microRNA analysis reveals modulation of biochemical pathways related to addiction in the ventral tegmental area of methamphetamine self-administering rats. BMC Neurosci. 2015, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Sim, M.S.; Soga, T.; Pandy, V.; Wu, Y.S.; Parhar, I.S.; Mohamed, Z. MicroRNA expression signature of methamphetamine use and addiction in the rat nucleus accumbens. Metab. Brain Dis. 2017, 32, 1767–1783. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, L.; Hong, S.; Zhang, D.; Zhou, Y. Methamphetamine leads to the alterations of microRNA profiles in the nucleus accumbens of rats. Pharm. Biol. 2020, 58, 797–805. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Su, H.; Liu, D.; Yan, Z.; Ni, T.; Wei, H.; Goh, E.L.; Chen, T. Regulation of miR-128 in the nucleus accumbens affects methamphetamine-induced behavioral sensitization by modulating proteins involved in neuroplasticity. Addict. Biol. 2021, 26, e12881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, T.; Zhao, W.; Ren, Z.; Wang, Y.; Zhou, Y.; Song, X.; Zhou, R.; Zhang, X.; Jiao, D. MicroRNA-181a is involved in methamphetamine addiction through the ERAD pathway. Front. Mol. Neurosci. 2021, 14, 667725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wu, F.; Yan, Z.; He, L.; Wang, S.; Hu, H.; Goh, E.L.; Zhu, Y.; Guan, F.; Chen, T. A novel microRNA, novel-m009C, regulates methamphetamine rewarding effects. Mol. Psychiatry 2022, 27, 3885–3897. [Google Scholar] [CrossRef] [PubMed]
- Hollander, J.A.; Im, H.-I.; Amelio, A.L.; Kocerha, J.; Bali, P.; Lu, Q.; Willoughby, D.; Wahlestedt, C.; Conkright, M.D.; Kenny, P.J. Striatal microRNA controls cocaine intake through CREB signalling. Nature 2010, 466, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Im, H.-I.; Hollander, J.A.; Bali, P.; Kenny, P.J. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat. Neurosci. 2010, 13, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.K.; James, M.H.; Hawkins, G.E.; Brown, A.L.; Heathcote, A.; Smith, D.W.; Cairns, M.J.; Dayas, C.V. Temporally specific miRNA expression patterns in the dorsal and ventral striatum of addiction-prone rats. Addict. Biol. 2018, 23, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, P.; Liao, K.; Kook, Y.H.; Niu, F.; Callen, S.E.; Guo, M.-L.; Buch, S. Cocaine-mediated downregulation of miR-124 activates microglia by targeting KLF4 and TLR4 signaling. Mol. Neurobiol. 2018, 55, 3196–3210. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Balasubramaniam, M.; Martínez-Rivera, F.J.; Godino, A.; Peck, E.G.; Patnaik, S.; Suar, M.; Calipari, E.S.; Nestler, E.J.; Villalta, F. Cocaine-regulated microRNA miR-124 controls poly (ADP-ribose) polymerase-1 expression in neuronal cells. Sci. Rep. 2020, 10, 11197. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Rodriguez, L.; Cabana-Domínguez, J.; Fernàndez-Castillo, N.; Cormand, B.; Martín-García, E.; Maldonado, R. Differential expression of miR-1249-3p and miR-34b-5p between vulnerable and resilient phenotypes of cocaine addiction. Addict. Biol. 2022, 27, e13201. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Hong, Q.; Lin, Z.; Ma, H.; Chen, W.; Zhuang, D.; Zhu, H.; Lai, M.; Fu, D.; Zhou, W. Role of nucleus accumbens microRNA-181a and MeCP2 in incubation of heroin craving in male rats. Psychopharmacology 2021, 238, 2313–2324. [Google Scholar] [CrossRef] [PubMed]
- Zanda, M.T.; Floris, G.; Sillivan, S.E. Orbitofrontal cortex microRNAs support long-lasting heroin seeking behavior in male rats. Transl. Psychiatry 2023, 13, 117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Im, H.-I.; Moon, C. Intravenous morphine self-administration alters accumbal microRNA profiles in the mouse brain. Neural Regen. Res. 2018, 13, 77. [Google Scholar] [PubMed]
- Jia, M.; Wang, X.; Zhang, H.; Wang, X.; Ma, H.; Yang, M.; Li, Y.; Cui, C. MicroRNA-132 is involved in morphine dependence via modifying the structural plasticity of the dentate gyrus neurons in rats. Addict. Biol. 2022, 27, e13086. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Han, J.S.; Jin, Y.-B.; Lee, S.-R.; Choi, I.Y.; Lee, H.; Cho, H.; Kim, D.-J. Differential expression of microRNAs in the hippocampi of male and female rodents after chronic alcohol administration. Biol. Sex Differ. 2020, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Santos-Bezerra, D.P.; Cavaleiro, A.M.; Santos, A.S.; Suemoto, C.K.; Pasqualucci, C.A.; Jacob-Filho, W.; Leite, R.E.P.; Passarelli, M.; Marie, S.K.N.; Machado, U.F. Alcohol Use Disorder is Associated with Upregulation of MicroRNA-34a and MicroRNA-34c in Hippocampal Postmortem Tissue. Alcohol. Clin. Exp. Res. 2021, 45, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.; Beane-Ebel, J.E.; Tanaka, Y.; Ning, B.; Husted, C.R.; Henderson, D.C.; Xiang, Y.; Park, I.-H.; Farrer, L.A.; Zhang, H. Exploration of alcohol use disorder-associated brain miRNA–mRNA regulatory networks. Transl. Psychiatry 2021, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Zoubková, H.; Tomášková, A.; Nohejlová, K.; Černá, M.; Šlamberová, R. Prenatal exposure to methamphetamine: Up-regulation of brain receptor genes. Front. Neurosci. 2019, 13, 771. [Google Scholar] [CrossRef] [PubMed]
- Oni-Orisan, O.O.; Dansereau, L.M.; Marsit, C.J.; Smith, L.M.; Neal, C.R.; Della Grotta, S.A.; Padbury, J.F.; Lester, B.M. DNA methylation in children with prenatal methamphetamine exposure and environmental adversity. Pediatr. Res. 2021, 89, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Marwick, C. NIDA seeking data on effect of fetal exposure to methamphetamine. JAMA 2000, 283, 2225–2226. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Zhu, J.; Han, W.; Wang, S.; Yan, Z.; Ma, D.; Goh, E.L.; Chen, T. Maternal methamphetamine exposure causes cognitive impairment and alteration of neurodevelopment-related genes in adult offspring mice. Neuropharmacology 2018, 140, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Zammit, S.; Thomas, K.; Thompson, A.; Horwood, J.; Menezes, P.; Gunnell, D.; Hollis, C.; Wolke, D.; Lewis, G.; Harrison, G. Maternal tobacco, cannabis and alcohol use during pregnancy and risk of adolescent psychotic symptoms in offspring. Br. J. Psychiatry 2009, 195, 294–300. [Google Scholar] [CrossRef]
- Bolhuis, K.; Kushner, S.A.; Yalniz, S.; Hillegers, M.H.; Jaddoe, V.W.; Tiemeier, H.; El Marroun, H. Maternal and paternal cannabis use during pregnancy and the risk of psychotic-like experiences in the offspring. Schizophr. Res. 2018, 202, 322–327. [Google Scholar] [CrossRef]
- Viola, T.W.; Danzer, C.; Mardini, V.; Szobot, C.; Chrusciel, J.H.; Stertz, L.; Schmitz, J.M.; Walss-Bass, C.; Fries, G.R.; Grassi-Oliveira, R. Prenatal cocaine exposure and its influence on pediatric epigenetic clocks and epigenetic scores in humans. Sci. Rep. 2024, 14, 1946. [Google Scholar] [CrossRef]
- Hollins, S.; Zavitsanou, K.; Walker, F.; Cairns, M. Alteration of imprinted Dlk1-Dio3 miRNA cluster expression in the entorhinal cortex induced by maternal immune activation and adolescent cannabinoid exposure. Transl. Psychiatry 2014, 4, e452. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.E.; Dennis, M.F.; Bourassa, K.J.; Workgroup, V.M.-A.M.; Hauser, M.A.; Kimbrel, N.A.; Beckham, J.C.; Ashley-Koch, A.E. Genome-wide DNA methylation analysis of cannabis use disorder in a veteran cohort enriched for posttraumatic stress disorder. Psychiatry research 2024, 333, 115757. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Li, G.E.; Chen, H.; Cranfield, C.G.; McGrath, K.C.; Gorrie, C.A. Maternal e-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem. Res. Toxicol. 2018, 31, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H. Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: A randomized, double-blind, placebo-controlled clinical trial. Alcohol. Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef]
- Warton, F.L.; Molteno, C.D.; Warton, C.M.; Wintermark, P.; Lindinger, N.M.; Dodge, N.C.; Zöllei, L.; van Der Kouwe, A.J.; Carter, R.C.; Jacobson, J.L. Maternal choline supplementation mitigates alcohol exposure effects on neonatal brain volumes. Alcohol. Clin. Exp. Res. 2021, 45, 1762–1774. [Google Scholar] [CrossRef] [PubMed]
- Sogut, I.; Uysal, O.; Oglakci, A.; Yucel, F.; Kartkaya, K.; Kanbak, G. Prenatal alcohol–induced neuroapoptosis in rat brain cerebral cortex: Protective effect of folic acid and betaine. Child’s Nerv. Syst. 2017, 33, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Borrás-Novell, C.; Alsina Casanova, M.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The effects of alcohol and drugs of abuse on maternal nutritional profile during pregnancy. Nutrients 2018, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Balaraman, S.; Idrus, N.M.; Miranda, R.C.; Thomas, J.D. Postnatal choline supplementation selectively attenuates hippocampal microRNA alterations associated with developmental alcohol exposure. Alcohol 2017, 60, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Otero, N.K.; Thomas, J.D.; Saski, C.A.; Xia, X.; Kelly, S.J. Choline supplementation and DNA methylation in the hippocampus and prefrontal cortex of rats exposed to alcohol during development. Alcohol. Clin. Exp. Res. 2012, 36, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Itzhak, Y.; Ergui, I.; Young, J. Long-term parental methamphetamine exposure of mice influences behavior and hippocampal DNA methylation of the offspring. Mol. Psychiatry 2015, 20, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Zhu, J.; Wang, R.; Wang, S.; Chen, Y.; Wang, C.; Goh, E.L.; Chen, T. Maternal methamphetamine exposure influences behavioral sensitization and nucleus accumbens DNA methylation in subsequent generation. Front. Pharmacol. 2022, 13, 940798. [Google Scholar] [CrossRef] [PubMed]
- Novikova, S.I.; He, F.; Bai, J.; Cutrufello, N.J.; Lidow, M.S.; Undieh, A.S. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS ONE 2008, 3, e1919. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hou, J.; Chen, B.; Shao, X.; Zhu, R.; Bu, Q.; Gu, H.; Li, Y.; Zhang, B.; Du, C. Prenatal cocaine exposure impairs cognitive function of progeny via insulin growth factor II epigenetic regulation. Neurobiol. Dis. 2015, 82, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Marjonen, H.; Sierra, A.; Nyman, A.; Rogojin, V.; Gröhn, O.; Linden, A.-M.; Hautaniemi, S.; Kaminen-Ahola, N. Early maternal alcohol consumption alters hippocampal DNA methylation, gene expression and volume in a mouse model. PLoS ONE 2015, 10, e0124931. [Google Scholar] [CrossRef] [PubMed]
- Lucia, D.; Burgess, D.; Cullen, C.; Dorey, E.; Rawashdeh, O.; Moritz, K. Periconceptional maternal alcohol consumption leads to behavioural changes in adult and aged offspring and alters the expression of hippocampal genes associated with learning and memory and regulators of the epigenome. Behav. Brain Res. 2019, 362, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, M.; Briand, L.; Fant, B.; Guercio, L.; Arreola, A.; Schmidt, H.; Sidoli, S.; Han, Y.; Garcia, B.; Pierce, R. Paternal cocaine taking elicits epigenetic remodeling and memory deficits in male progeny. Mol. Psychiatry 2017, 22, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.M.; Mueller, K.A.; Cannon, E.N.; Huizenga, M.N.; Darnell, S.B.; Bhide, P.G.; Sadri-Vakili, G. Prenatal cocaine exposure alters BDNF-TrkB signaling in the embryonic and adult brain. Dev. Neurosci. 2017, 38, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shirazi, M.-S.; Asgari, P.; Mahboubi, S.; Zadeh-Tehrani, S.N.; Ashabi, G.; Rohbani, K.; Sabzevari, S.; Soltani, H.; Khalifeh, S.; Zarrindast, M.-R. Effect of morphine exposure on novel object memory of the offspring: The role of histone H3 and ΔFosB. Brain Res. Bull. 2020, 156, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Xiao, M.; Kang, N.; Zeng, W.; Zhang, J. Prenatal morphine exposure increases gamma oscillation and theta coherence in the rat reward system. Neurotoxicology 2022, 90, 246–255. [Google Scholar] [CrossRef] [PubMed]
- DiNieri, J.A.; Wang, X.; Szutorisz, H.; Spano, S.M.; Kaur, J.; Casaccia, P.; Dow-Edwards, D.; Hurd, Y.L. Maternal cannabis use alters ventral striatal dopamine D2 gene regulation in the offspring. Biol. Psychiatry 2011, 70, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, T.; Huang, S.; Avci, N.G.; Waits, C.M.K.; Akay, Y.M.; Akay, M. Investigating the influence of perinatal nicotine and alcohol exposure on the genetic profiles of dopaminergic neurons in the VTA using miRNA–mRNA analysis. Sci. Rep. 2020, 10, 15016. [Google Scholar] [CrossRef] [PubMed]
- Perales, G.; Westenskow, M.; Gutierrez, R.; Caldwell, K.K.; Allan, A.M.; Gardiner, A.S. MicroRNA-150-5p is upregulated in the brain microvasculature during prenatal alcohol exposure and inhibits the angiogenic factor Vezf1. Alcohol. Clin. Exp. Res. 2022, 46, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Salbaum, J.M.; Kappen, C. Genetic and epigenomic footprints of folate. Prog. Mol. Biol. Transl. Sci. 2012, 108, 129–158. [Google Scholar] [PubMed]
- Hadizadeh, H.; Salehi, M.; Bozorgnia, A.R.; Ahmadkhaniha, H.R. Lower folate levels in methamphetamine-induced psychosis: A cross-sectional study. Drug Alcohol Depend. 2020, 207, 107682. [Google Scholar] [CrossRef] [PubMed]
- Nohesara, S.; Ghadirivasfi, M.; Barati, M.; Ghasemzadeh, M.R.; Narimani, S.; Mousavi-Behbahani, Z.; Joghataei, M.; Soleimani, M.; Taban, M.; Mehrabi, S. Methamphetamine-induced psychosis is associated with DNA hypomethylation and increased expression of AKT1 and key dopaminergic genes. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhao, M.; Li, M.; Song, T.; Zhang, M.; Quan, L.; Li, S.; Sun, Z.S. Reversal of cocaine-conditioned place preference through methyl supplementation in mice: Altering global DNA methylation in the prefrontal cortex. PLoS ONE 2012, 7, e33435. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.N.; Hollis, F.; Duclot, F.; Dossat, A.M.; Strong, C.E.; Francis, T.C.; Mercer, R.; Feng, J.; Dietz, D.M.; Lobo, M.K. Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J. Neurosci. 2015, 35, 8948–8958. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in β-endorphin-producing POMC neurons of the hypothalamus. Alcohol. Clin. Exp. Res. 2013, 37, 1133–1142. [Google Scholar] [CrossRef]
- Gitik, M.; Holliday, E.D.; Leung, M.; Yuan, Q.; Logue, S.F.; Tikkanen, R.; Goldman, D.; Gould, T.J. Choline ameliorates adult learning deficits and reverses epigenetic modification of chromatin remodeling factors related to adolescent nicotine exposure. Neurobiol. Learn. Mem. 2018, 155, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wiers, C.E.; Shokri-Kojori, E.; Tomasi, D.; Wang, G.-J.; Baler, R. Neurochemical and metabolic effects of acute and chronic alcohol in the human brain: Studies with positron emission tomography. Neuropharmacology 2017, 122, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Wiers, C.; Vendruscolo, L.; van der Veen, J.; Manza, P.; Shokri-Kojori, E.; Kroll, D.; Feldman, D.; McPherson, K.; Biesecker, C.; Zhang, R. Ketogenic diet reduces alcohol withdrawal symptoms in humans and alcohol intake in rodents. Sci Adv 2021, 7, eabf6780. [Google Scholar] [CrossRef]
- Wiers, C.E.; Manza, P.; Wang, G.-J.; Volkow, N.D. Ketogenic diet reduces a neurobiological craving signature in alcohol use disorder. medRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Cao, T.; Chen, J.; Jiang, Y.; Wang, C.; Waddington, J.L.; Zhen, X. D2 receptor-mediated miRNA-143 expression is associated with the effects of antipsychotic drugs on phencyclidine-induced schizophrenia-related locomotor hyperactivity and with Neuregulin-1 expression in mice. Neuropharmacology 2019, 157, 107675. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, K.; Samikkannu, T. Neuroprotective effect of piracetam against cocaine-induced neuro epigenetic modification of DNA methylation in astrocytes. Brain Sci. 2020, 10, 611. [Google Scholar] [CrossRef]
- Chen, G.; Shi, F.; Yin, W.; Guo, Y.; Liu, A.; Shuai, J.; Sun, J. Gut microbiota dysbiosis: The potential mechanisms by which alcohol disrupts gut and brain functions. Front. Microbiol. 2022, 13, 916765. [Google Scholar] [CrossRef]
- Chivero, E.T.; Ahmad, R.; Thangaraj, A.; Periyasamy, P.; Kumar, B.; Kroeger, E.; Feng, D.; Guo, M.-L.; Roy, S.; Dhawan, P. Cocaine induces inflammatory gut milieu by compromising the mucosal barrier integrity and altering the gut microbiota colonization. Sci. Rep. 2019, 9, 12187. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, V.L.; Smith, M.R.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Chivero, E.T.; Sil, S.; Kumar, M.; Buch, S. Substance use, microbiome and psychiatric disorders. Pharmacol. Biochem. Behav. 2022, 219, 173432. [Google Scholar] [CrossRef]
- Borkent, J.; Ioannou, M.; Laman, J.D.; Haarman, B.C.; Sommer, I.E. Role of the gut microbiome in three major psychiatric disorders. Psychol. Med. 2022, 52, 1222–1242. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.R.; Fulcher, J.A.; Tobin, N.H.; Li, F.; Lee, D.J.; Woodward, C.; Javanbakht, M.; Brookmeyer, R.; Shoptaw, S.; Bolan, R. Alterations to the gastrointestinal microbiome associated with methamphetamine use among young men who have sex with men. Sci. Rep. 2019, 9, 14840. [Google Scholar] [CrossRef]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy lifestyle and gut dysbiosis: A better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Yang, C.; Fu, X.; Hao, W.; Xiang, X.; Liu, T.; Yang, B.Z.; Zhang, X. Gut dysbiosis associated with the rats’ responses in methamphetamine-induced conditioned place preference. Addict. Biol. 2021, 26, e12975. [Google Scholar] [CrossRef]
- Chen, L.-J.; Zhi, X.; Zhang, K.-K.; Wang, L.-B.; Li, J.-H.; Liu, J.-L.; Xu, L.-L.; Yoshida, J.S.; Xie, X.-L.; Wang, Q. Escalating dose-multiple binge methamphetamine treatment elicits neurotoxicity, altering gut microbiota and fecal metabolites in mice. Food Chem. Toxicol. 2021, 148, 111946. [Google Scholar] [CrossRef] [PubMed]
- Allais, L.; Kerckhof, F.M.; Verschuere, S.; Bracke, K.R.; De Smet, R.; Laukens, D.; Van den Abbeele, P.; De Vos, M.; Boon, N.; Brusselle, G.G. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ. Microbiol. 2016, 18, 1352–1363. [Google Scholar] [CrossRef]
- Cruz-Lebrón, A.; Johnson, R.; Mazahery, C.; Troyer, Z.; Joussef-Piña, S.; Quiñones-Mateu, M.E.; Strauch, C.M.; Hazen, S.L.; Levine, A.D. Chronic opioid use modulates human enteric microbiota and intestinal barrier integrity. Gut Microbes 2021, 13, 1946368. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Ma, H.; Tian, W.; Liu, J.; Yan, X.; Ma, L.; Wei, S.; Zhu, J.; Zhu, Y.; Lai, J. Methadone maintenance treatment is more effective than compulsory detoxification in addressing gut microbiota dysbiosis caused by heroin abuse. Front. Microbiol. 2023, 14, 1283276. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, X.; Liu, X.; Liu, G.; Zeng, K.; Wang, G. Altered fecal microbiota composition in individuals who abuse methamphetamine. Sci. Rep. 2021, 11, 18178. [Google Scholar] [CrossRef]
- Yang, J.-Z.; Zhang, K.-K.; He, J.-T.; Chen, L.-J.; Ding, J.-F.; Liu, J.-L.; Li, J.-H.; Liu, Y.; Li, X.-W.; Zhao, D. Obeticholic acid protects against methamphetamine-induced anxiety-like behavior by ameliorating microbiota-mediated intestinal barrier impairment. Toxicology 2023, 486, 153447. [Google Scholar] [CrossRef] [PubMed]
- Panee, J.; Gerschenson, M.; Chang, L. Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J. Neuroimmune Pharmacol. 2018, 13, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Mahbub, R.; Gao, B.; Bian, X.; Tu, P.; Ru, H.; Lu, K. Nicotine alters the gut microbiome and metabolites of gut–brain interactions in a sex-specific manner. Chem. Res. Toxicol. 2017, 30, 2110–2119. [Google Scholar] [CrossRef]
- Angoa-Perez, M.; Kuhn, D.M. Evidence for modulation of substance use disorders by the gut microbiome: Hidden in plain sight. Pharmacol. Rev. 2021, 73, 571–596. [Google Scholar] [CrossRef]
- Ning, T.; Gong, X.; Xie, L.; Ma, B. Gut microbiota analysis in rats with methamphetamine-induced conditioned place preference. Front. Microbiol. 2017, 8, 1620. [Google Scholar] [CrossRef]
- Wan, X.; Eguchi, A.; Qu, Y.; Yang, Y.; Chang, L.; Shan, J.; Mori, C.; Hashimoto, K. Gut–microbiota–brain axis in the vulnerability to psychosis in adulthood after repeated cannabis exposure during adolescence. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 1297–1309. [Google Scholar] [CrossRef]
- Gerace, E.; Baldi, S.; Salimova, M.; Di Gloria, L.; Curini, L.; Cimino, V.; Nannini, G.; Russo, E.; Pallecchi, M.; Ramazzotti, M. Oral and fecal microbiota perturbance in cocaine users: Can rTMS-induced cocaine abstinence support eubiosis restoration? Iscience 2023, 26, 106627. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Su, H.; Song, Y.; Chen, T.; Sun, Q.; Jiang, H.; Zhao, M. Altered fecal microbiota correlated with systemic inflammation in Male subjects with methamphetamine use disorder. Front. Cell. Infect. Microbiol. 2021, 11, 783917. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Wang, J.; Wang, B.; Wang, R.; Li, G.; Jia, Y.; Chen, T.; Chen, Y. Alterations in gut microbiota affect behavioral and inflammatory responses to methamphetamine in mice. Psychopharmacology 2022, 239, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, L.; Jiang, B.; Hui, B.; Lv, Z.; Ma, L. The effects of sodium butyrate, an inhibitor of histone deacetylase, on the cocaine-and sucrose-maintained self-administration in rats. Neurosci. Lett. 2008, 441, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Harkness, J.H.; Hitzemann, R.J.; Edmunds, S.; Phillips, T.J. Effects of sodium butyrate on methamphetamine-sensitized locomotor activity. Behav. Brain Res. 2013, 239, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-J.; Li, X.-W.; Liu, Y.; Liu, J.-L.; Yang, J.-Z.; Li, J.-H.; Hsu, C.; Chen, L.; Zeng, J.-H.; Xie, X.-L. Propionate, rather than acetate or butyrate, ameliorates methamphetamine-induced hepatotoxicity and enterotoxicity in mice by downregulating the TLR4/NF-κB pathway. J. Funct. Foods 2023, 109, 105796. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, N.; Chen, Y.; Zhu, L.; Zhong, Q.; Liu, J.; Chen, T. Sodium butyrate modulates a methamphetamine-induced conditioned place preference. J. Neurosci. Res. 2017, 95, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, L.; Yang, J.; Liu, J.; Li, J.; Liu, Y.; Li, X.; Chen, L.; Hsu, C.; Zeng, J. Gut microbiota-derived short-chain fatty acids ameliorate methamphetamine-induced depression-and anxiety-like behaviors in a Sigmar-1 receptor-dependent manner. Acta Pharm. Sin. B 2023, 13, 4801–4822. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Davies, D.L.; Asatryan, L. Sodium Butyrate Supplementation Modulates Neuroinflammatory Response Aggravated by Antibiotic Treatment in a Mouse Model of Binge-like Ethanol Drinking. Int. J. Mol. Sci. 2022, 23, 15688. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, M.; Xie, B.; Wen, D.; Li, W.; Zhou, M.; Wang, X.; Lu, Y.; Cong, B.; Ni, Z. Effects of molecular hydrogen intervention on the gut microbiome in methamphetamine abusers with mental disorder. Brain Res. Bull. 2023, 193, 47–58. [Google Scholar] [CrossRef]
- Browne, C.J.; Godino, A.; Salery, M.; Nestler, E.J. Epigenetic mechanisms of opioid addiction. Biol. Psychiatry 2020, 87, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Mavrikaki, M.; Pravetoni, M.; Page, S.; Potter, D.; Chartoff, E. Oxycodone self-administration in male and female rats. Psychopharmacology 2017, 234, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, P.J.; Lim, C.J.; Nestler, E.J.; Heller, E.A. Neuroepigenetic editing. Epigenome Ed. Methods Protoc. 2018, 1767, 113–136. [Google Scholar]
- Lobo, M.K.; Covington III, H.E.; Chaudhury, D.; Friedman, A.K.; Sun, H.; Damez-Werno, D.; Dietz, D.M.; Zaman, S.; Koo, J.W.; Kennedy, P.J. Cell type–specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 2010, 330, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.T.; Zhou, Y.; Weinstock, G.M.; Bubier, J.A. The gut microbiome and substance use disorder. Front. Neurosci. 2021, 15, 1134. [Google Scholar] [CrossRef] [PubMed]
- Justice, M.J.; Dhillon, P. Using the Mouse to Model Human Disease: Increasing Validity and Reproducibility; The Company of Biologists Ltd.: Cambridge, UK, 2016; Volume 9, pp. 101–103. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).