Real-Time Analysis of Neuronal Cell Cultures for CNS Drug Discovery
Abstract
1. Introduction
1.1. CNS Drug Discovery Challenges
1.2. Neuronal Cell Cultures
1.3. Live-Cell Imaging Systems
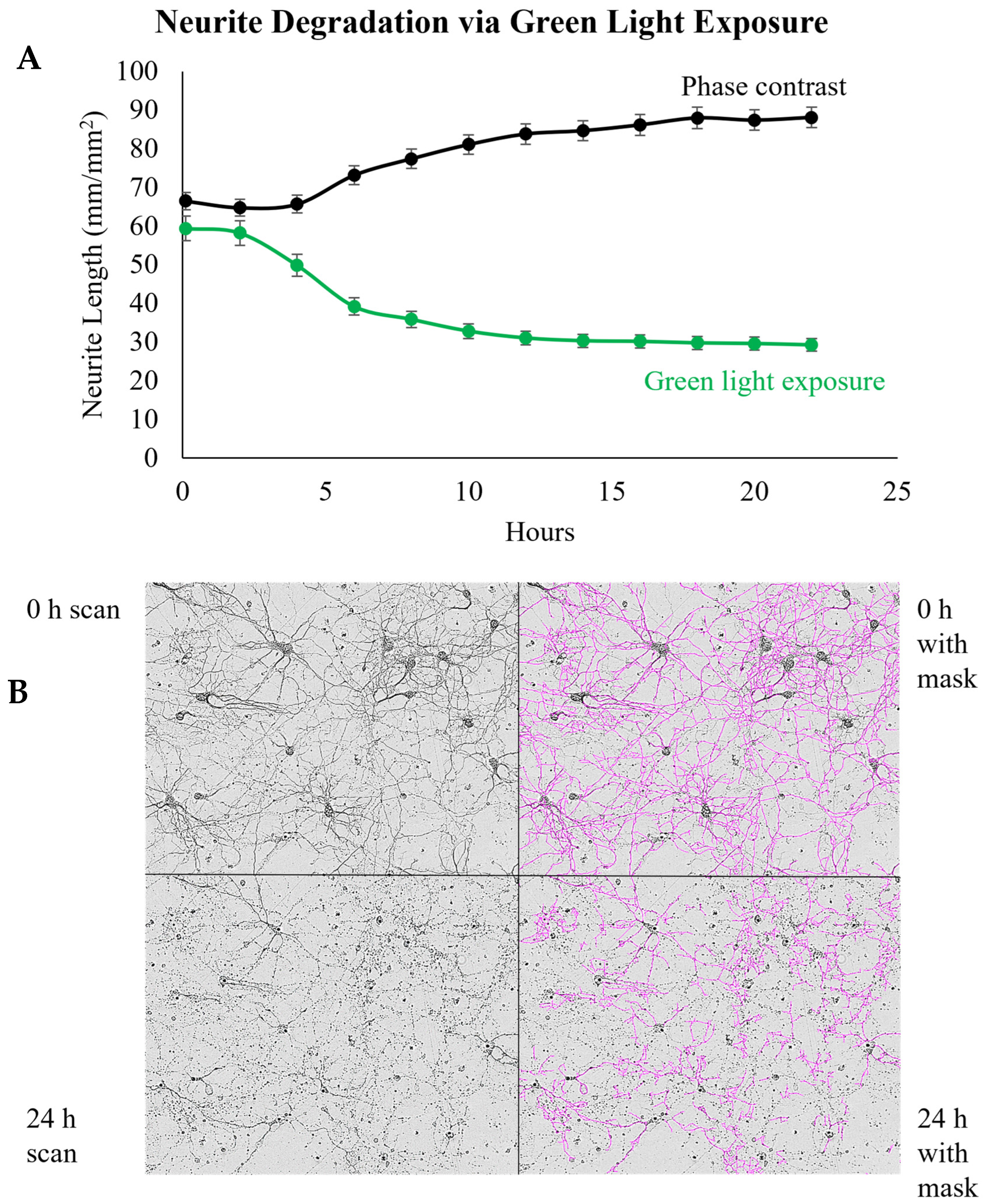
2. Key Features and Components of Live-Cell Imaging
3. In-Depth Analysis of IncuCyte Systems: A Common Live-Cell Imaging System
3.1. Specifications and Features
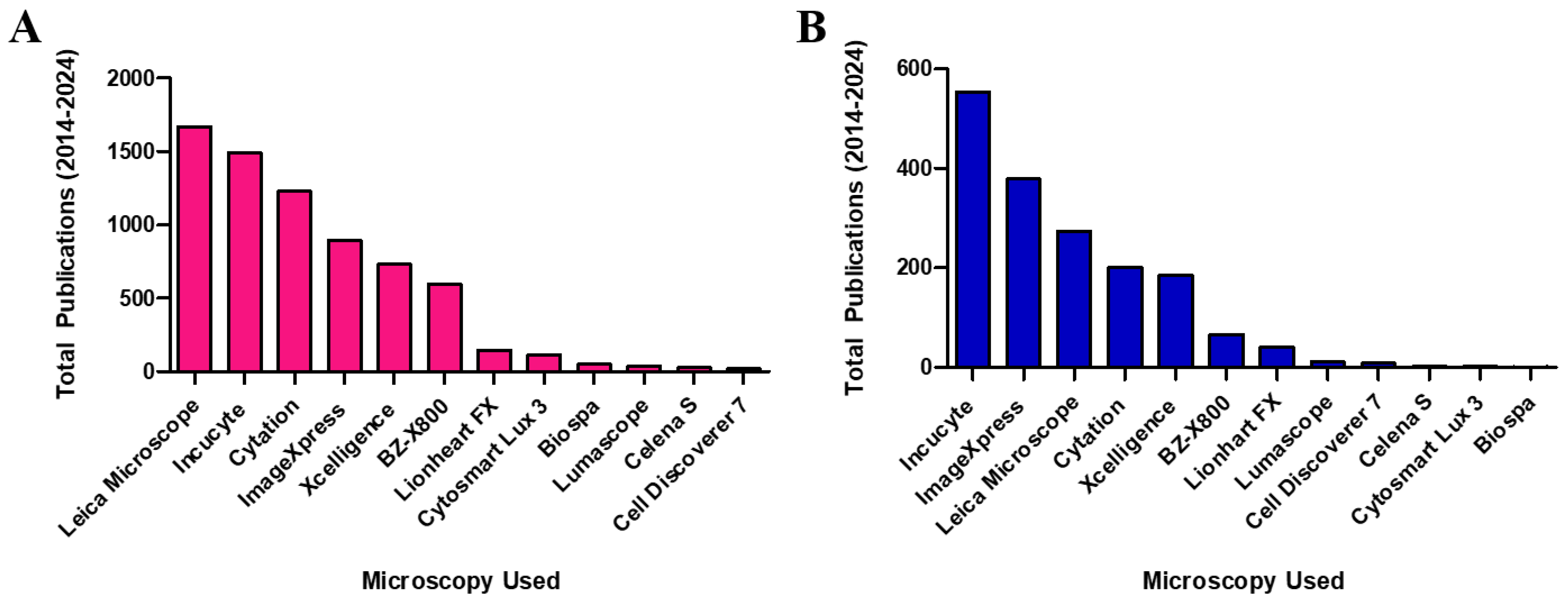
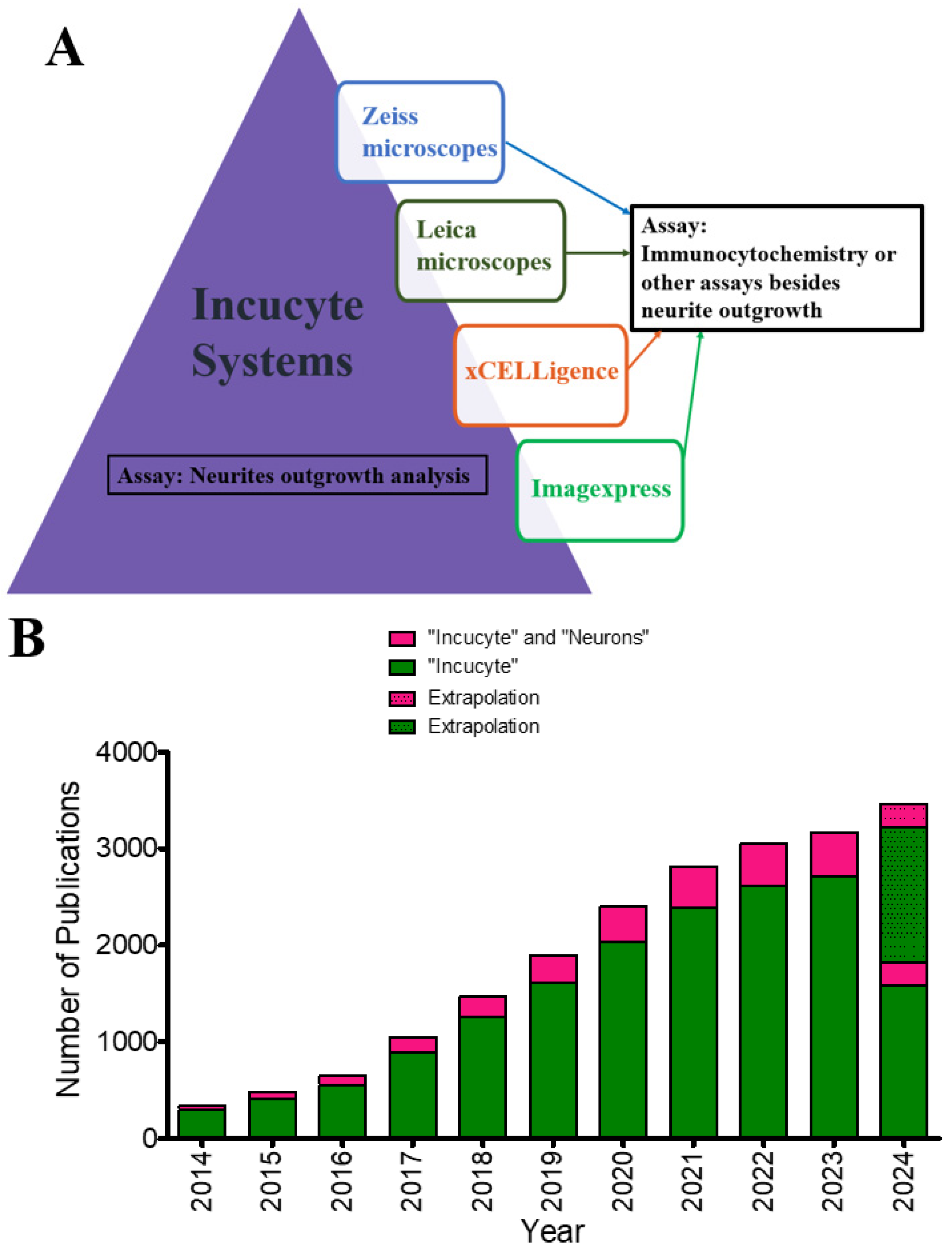
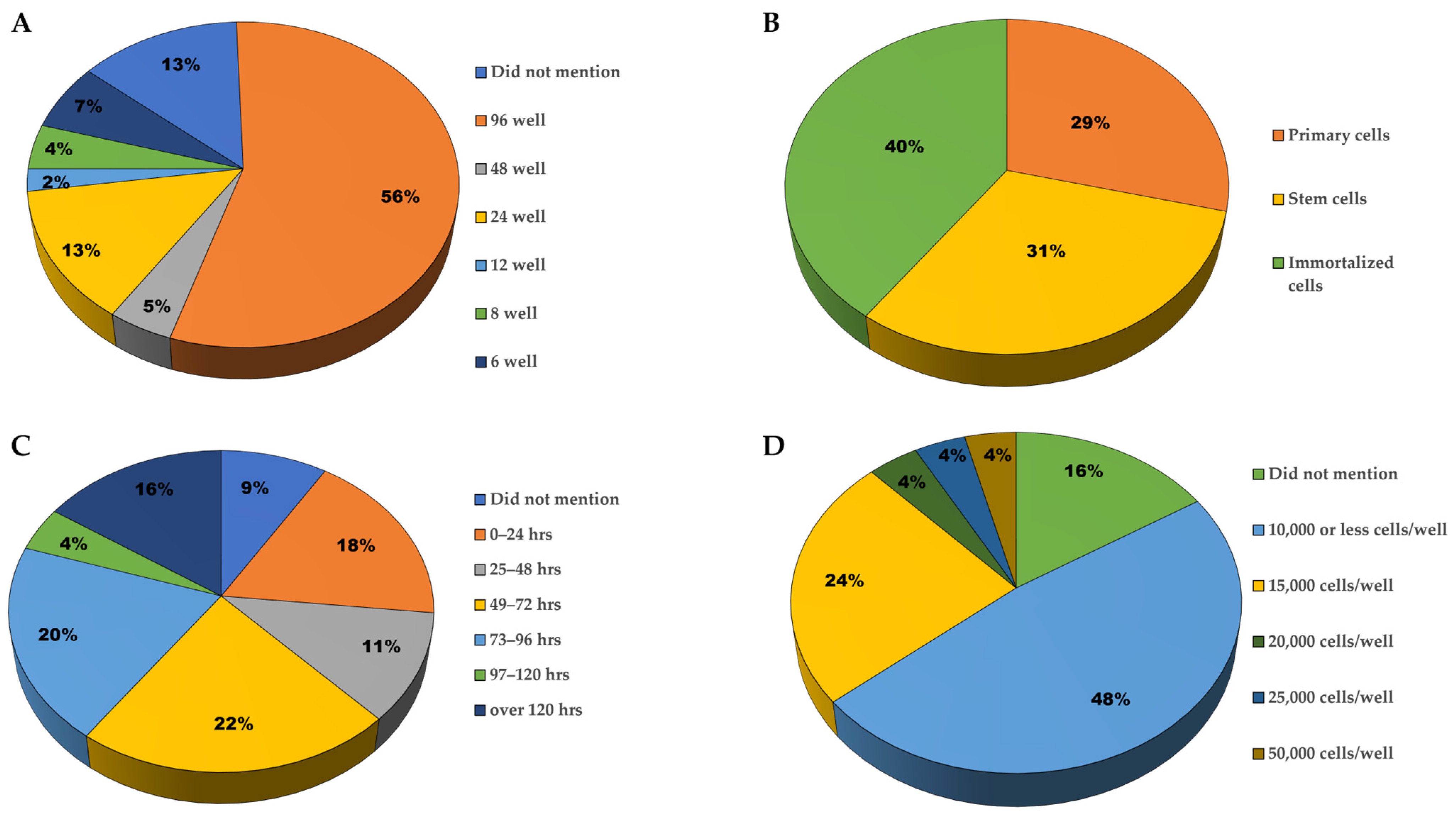
3.2. Analysis of Publications Utilizing IncuCyte in Their Neurite Quantification Methods
3.2.1. Plating Characteristics
3.2.2. Type of Neurons Used
3.2.3. Secondary Readouts and Confirmatory Experiments
3.2.4. Types of Microscopy Employed
3.3. Imaging Duration
4. Discussion and Future Directions
5. Conclusions
6. Experimental Methods
6.1. Reagents
6.2. Neuron Cultures
6.3. Live-Cell Imaging
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nagappan, P.G.; Chen, H.; Wang, D.-Y. Neuroregeneration and plasticity: A review of the physiological mechanisms for achieving functional recovery postinjury. Mil. Med. Res. 2020, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Haq, E.U.; Huang, J.; Kang, L.; Haq, H.U.; Zhan, T. Image-based state-of-the-art techniques for the identification and classification of brain diseases: A review. Med. Biol. Eng. Comput. 2020, 58, 2603–2620. [Google Scholar] [CrossRef] [PubMed]
- Gribkoff, V.K.; Kaczmarek, L.K. The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 2017, 120, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Checkoway, H.; Lundin, J.I.; Kelada, S.N. Neurodegenerative diseases. IARC Sci. Publ. 2011, 163, 407–419. [Google Scholar]
- Zhang, X.; Hu, D.; Shang, Y.; Qi, X. Using induced pluripotent stem cell neuronal models to study neurodegenerative diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165431. [Google Scholar] [CrossRef] [PubMed]
- Butlen-Ducuing, F.; Pétavy, F.; Guizzaro, L.; Zienowicz, M.; Salmonson, T.; Haas, M.; Corruble, E.; Alteri, E. Challenges in drug development for central nervous system disorders: A European Medicines Agency perspective. Nat. Rev. Drug Discov. 2016, 15, 813–814. [Google Scholar] [CrossRef]
- Kesselheim, A.S.; Hwang, T.J.; Franklin, J.M. Two decades of new drug development for central nervous system disorders. Nat. Rev. Drug Discov. 2015, 14, 815–816. [Google Scholar] [CrossRef]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef]
- Danon, J.J.; Reekie, T.A.; Kassiou, M. Challenges and Opportunities in Central Nervous System Drug Discovery. Trends Chem. 2019, 1, 612–624. [Google Scholar] [CrossRef]
- Gordon, J.; Amini, S.; White, M.K. General overview of neuronal cell culture. Methods Mol. Biol. 2013, 1078, 1–8. [Google Scholar] [CrossRef]
- Wharton, S.B.; Minett, T.; Drew, D.; Forster, G.; Matthews, F.; Brayne, C.; Ince, P.G.; MRC Cognitive Function and Ageing Neuropathology Study Group. Epidemiological pathology of Tau in the ageing brain: Application of staging for neuropil threads (BrainNet Europe protocol) to the MRC cognitive function and ageing brain study. Acta Neuropathol. Commun. 2016, 4, 11. [Google Scholar] [CrossRef]
- Wishart, T.M.; Parson, S.H.; Gillingwater, T.H. Synaptic vulnerability in neurodegenerative disease. J. Neuropathol. Exp. Neurol. 2006, 65, 733–739. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Ayaki, T.; Li, F.; Tsujimura, A.; Kamada, M.; Ito, H.; Maki, T.; Sawamoto, N.; Urushitani, M.; Takahashi, R.J. Phosphorylated NF-κB subunit p65 aggregates in granulovacuolar degeneration and neurites in neurodegenerative diseases with tauopathy. Neurosci. Lett. 2019, 704, 229–235. [Google Scholar] [CrossRef]
- Olson, D.E. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1179069518800508. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Vargas, M.V.; Duim, W.C.; Grodzki, A.C.G.; Lein, P.J.; Olson, D.E. Transient Stimulation with Psychoplastogens Is Sufficient to Initiate Neuronal Growth. ACS Pharmacol. Transl. Sci. 2021, 4, 452–460. [Google Scholar] [CrossRef]
- Meijering, E. Neuron tracing in perspective. Cytom. Part A 2010, 77, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Yeyeodu, S.T.; Witherspoon, S.M.; Gilyazova, N.; Ibeanu, G.C. A rapid, inexpensive high throughput screen method for neurite outgrowth. Curr. Chem. Genom. 2010, 4, 74–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smit, M.; Leng, J.; Klemke, R.L. Assay for neurite outgrowth quantification. Biotechniques 2003, 35, 254–256. [Google Scholar] [CrossRef]
- Rønn, L.C.; Ralets, I.; Hartz, B.P.; Bech, M.; Berezin, A.; Berezin, V.; Møller, A.; Bock, E. A simple procedure for quantification of neurite outgrowth based on stereological principles. J. Neurosci. Methods 2000, 100, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Isherwood, B.; Timpson, P.; McGhee, E.J.; Anderson, K.I.; Canel, M.; Serrels, A.; Brunton, V.G.; Carragher, N.O. Live cell in vitro and in vivo imaging applications: Accelerating drug discovery. Pharmaceutics 2011, 3, 141–170. [Google Scholar] [CrossRef]
- Schnell, U.; Dijk, F.; Sjollema, K.A.; Giepmans, B.N. Immunolabeling artifacts and the need for live-cell imaging. Nat. Methods 2012, 9, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Zhang, F.; Li, M.; Wo, X.; Su, Y.-W.; Wang, W. Influence of Fixation and Permeabilization on the Mass Density of Single Cells: A Surface Plasmon Resonance Imaging Study. Front. Chem. 2019, 7, 588. [Google Scholar] [CrossRef] [PubMed]
- Leica Microsystems. Mica The World’s First Microhub. Available online: https://www.leica-microsystems.com/products/p/mica/ (accessed on 9 February 2024).
- Sartorius. Live-Cell Analysis Instrument Features. Available online: https://www.sartorius.com/en/products/live-cell-imaging-analysis/live-cell-analysis-instruments (accessed on 9 February 2024).
- Agilent BioTek. Agilent BioTek Imaging and Microscopy. Available online: https://explore.agilent.com/imaging-microscopy?Product_Interest_Source=Marketing%20Campaign&source=Marketing%20Campaign&Lead_Campaign_Source=7011O000002TCTe&Campaign_Source=7011O000002TCTe&utm_source=biotek&utm_medium=cpc&utm_campaign=7011O000002TCTe&gad_source=1&gclid=EAIaIQobChMIhr_fy6GDhAMVQAStBh0iGAmVEAAYAiAAEgKS2fD_BwE&gclsrc=aw.ds (accessed on 1 July 2024).
- Agilent. xCELLigence RTCA eSight—Imaging & Impedance. Available online: https://www.agilent.com/en/product/cell-analysis/real-time-cell-analysis/rtca-analyzers/xcelligence-rtca-esight-imaging-impedance-741228#howitworks (accessed on 9 February 2024).
- Agilent. Cell Imaging Multimode Readers:BioTek BioSpa Live Cell Analysis System. Available online: https://www.agilent.com/en/product/cell-analysis/cell-imaging-microscopy/cell-imaging-multimode-readers/biotek-biospa-live-cell-analysis-system-1623215 (accessed on 9 February 2024).
- Molecular Devices. ImageXpress Micro Confocal High-Content Imaging System. Available online: https://www.moleculardevices.com/products/cellular-imaging-systems/high-content-imaging/imagexpress-micro-confocal (accessed on 9 February 2024).
- Etaluma. Our Ls Microscopes: Live Cell Imaging in Your Incubator. Available online: https://etaluma.com/live-cell/?gad_source=1&gclid=EAIaIQobChMIoP3i_a-DhAMVQgOtBh23lwB8EAAYAiAAEgKW3vD_BwE (accessed on 9 February 2024).
- Logos Biosystems. Cell Imaging: CELENA® S Digital Imaging System. Available online: https://logosbio.com/celena-s/ (accessed on 9 February 2024).
- Biocompare. Lux3 FL|Fluorescence Live Cell Imager from Axion BioSystems. Available online: https://www.biocompare.com/25608-Microscopes-and-Cell-Imaging-Systems/16062465-CytoSMART-Lux3-FL-Fluorescence-Live-Cell-Imager/?pda=25608|16062465_0_0|2254335|17| (accessed on 1 July 2024).
- Zeiss. ZEISS Celldiscoverer 7: Your Automated Microscope for Live Cell Imaging. Available online: https://www.zeiss.com/microscopy/en/products/imaging-systems/celldiscoverer-7.html (accessed on 9 February 2024).
- Keyence. All-in-One Fluorescence Microscope. Available online: https://www.keyence.com/landing/lpc/all-in-one-fluorescence-microscope.jsp?aw=google-kaenBZ658206pp-br&gad_source=1&gclid=EAIaIQobChMIjbiNvMWDhAMVSC-tBh0Mlga4EAAYASAAEgI8cPD_BwE (accessed on 9 February 2024).
- Ettinger, A.; Wittmann, T. Fluorescence live cell imaging. Methods Cell Biol. 2014, 123, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C. Overview of Live-Cell Imaging: Requirements and Methods Used. Anat. Rec. 2013, 296, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lemon, W.C.; McDole, K. Live-cell imaging in the era of too many microscopes. Curr. Opin. Cell Biol. 2020, 66, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Nketia, T.A.; Sailem, H.; Rohde, G.; Machiraju, R.; Rittscher, J. Analysis of live cell images: Methods, tools and opportunities. Methods 2017, 115, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.J.; Allan, V.J. Light Microscopy Techniques for Live Cell Imaging. Science 2003, 300, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Terrien, E.; Delhommel, F.; Lefebvre-Omar, C.; Bohl, D.; Vitry, S.; Bernard, C.; Ramirez, J.; Chaffotte, A.; Ricquier, K. Structure-based optimization of a PDZ-binding motif within a viral peptide stimulates neurite outgrowth. J. Biol. Chem. 2019, 294, 13755–13768. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, M.; Nakaoki, Y.; Yamaguchi, A.; Hashimoto, K.; Miyamoto, Y. Vitronectin is involved in the morphological transition of neurites in retinoic acid-induced neurogenesis of neuroblastoma cell line neuro2a. Neurochem. Res. 2019, 44, 1621–1635. [Google Scholar] [CrossRef]
- Salazar, K.; Espinoza, F.; Cerda-Gallardo, G.; Ferrada, L.; Magdalena, R.; Ramírez, E.; Ulloa, V.; Saldivia, N.; Troncoso, N.; Oviedo, M.J. SVCT2 overexpression and ascorbic acid uptake increase cortical neuron differentiation, which is dependent on vitamin c recycling between neurons and astrocytes. Antioxidants 2021, 10, 1413. [Google Scholar] [CrossRef]
- Srikanth, P.; Lagomarsino, V.N.; Pearse, R.V.; Liao, M.; Ghosh, S.; Nehme, R.; Seyfried, N.; Eggan, K.; Young-Pearse, T.L. Convergence of independent DISC1 mutations on impaired neurite growth via decreased UNC5D expression. Transl. Psychiatry 2018, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Zosen, D.; Kondratskaya, E.; Kaplan-Arabaci, O.; Haugen, F.; Paulsen, R.E. Antidepressants escitalopram and venlafaxine up-regulate BDNF promoter IV but down-regulate neurite outgrowth in differentiating SH-SY5Y neurons. Neurochem. Int. 2023, 169, 105571. [Google Scholar] [CrossRef] [PubMed]
- Efthymiou, A.; Shaltouki, A.; Steiner, J.P.; Jha, B.; Heman-Ackah, S.M.; Swistowski, A.; Zeng, X.; Rao, M.S.; Malik, N. Functional screening assays with neurons generated from pluripotent stem cell-derived neural stem cells. J. Biomol. Screen. 2014, 19, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Lichvarova, L.; Blum, W.; Schwaller, B.; Szabolcsi, V. Parvalbumin expression in oligodendrocyte-like CG4 cells causes a reduction in mitochondrial volume, attenuation in reactive oxygen species production and a decrease in cell processes’ length and branching. Sci. Rep. 2019, 9, 10603. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.K.; Alecu, J.E.; Ziegler, M.; Vasilopoulou, C.G.; Merciai, F.; Jumo, H.; Afshar-Saber, W.; Sahin, M.; Ebrahimi-Fakhari, D.; Borner, G.H.H. AP-4-mediated axonal transport controls endocannabinoid production in neurons. Nat. Commun. 2022, 13, 1058. [Google Scholar] [CrossRef] [PubMed]
- Dincã, D.M.; Lallemant, L.; González-Barriga, A.; Cresto, N.; Braz, S.O.; Sicot, G.; Pillet, L.-E.; Polvèche, H.; Magneron, P.; Huguet-Lachon, A.; et al. Myotonic dystrophy RNA toxicity alters morphology, adhesion and migration of mouse and human astrocytes. Nat. Commun. 2022, 13, 3841. [Google Scholar] [CrossRef] [PubMed]
- Soppa, U.; Schumacher, J.; Florencio Ortiz, V.; Pasqualon, T.; Tejedor, F.J.; Becker, W. The Down syndrome-related protein kinase DYRK1A phosphorylates p27(Kip1) and Cyclin D1 and induces cell cycle exit and neuronal differentiation. Cell Cycle 2014, 13, 2084–2100. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C. The Role of the δ-Opioid Receptor in Skin Homeostasis and Wound Healing. Ph.D. Thesis, Université de Lausanne, Faculté de Biologie et Médecine, Lausanne, Switzerland, 2013. [Google Scholar]
- Delsing, L.; Kallur, T.; Zetterberg, H.; Hicks, R.; Synnergren, J. Enhanced xeno-free differentiation of hiPSC-derived astroglia applied in a blood–brain barrier model. Fluids Barriers CNS 2019, 16, 27. [Google Scholar] [CrossRef]
- Nguyen, L.D.; Wei, Z.; Silva, M.C.; Barberán-Soler, S.; Zhang, J.; Rabinovsky, R.; Muratore, C.R.; Stricker, J.M.S.; Hortman, C.; Young-Pearse, T.L.; et al. Small molecule regulators of microRNAs identified by high-throughput screen coupled with high-throughput sequencing. Nat. Commun. 2023, 14, 7575. [Google Scholar] [CrossRef]
- Behne, R.; Teinert, J.; Wimmer, M.; D’Amore, A.; Davies, A.K.; Scarrott, J.M.; Eberhardt, K.; Brechmann, B.; Chen, I.P.-F.; Buttermore, E.D.; et al. Adaptor protein complex 4 deficiency: A paradigm of childhood-onset hereditary spastic paraplegia caused by defective protein trafficking. Human Mol. Genet. 2020, 29, 320–334. [Google Scholar] [CrossRef]
- Camarena, V.; Cao, L.; Abad, C.; Abrams, A.; Toledo, Y.; Araki, K.; Araki, M.; Walz, K.; Young, J.I. Disruption of Mbd5 in mice causes neuronal functional deficits and neurobehavioral abnormalities consistent with 2q23.1 microdeletion syndrome. EMBO Mol. Med. 2014, 6, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; Cerf, L.; Dehay, B.; Ramos-Gonzalez, P.; De Giorgi, F.; Bourdenx, M.; Bessede, A.; Obeso, J.A.; Matute, C.; Ichas, F.; et al. In vitro α-synuclein neurotoxicity and spreading among neurons and astrocytes using Lewy body extracts from Parkinson disease brains. Neurobiol. Dis. 2017, 103, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Wang, Z.; Liu, W.; O’Malley, T.T.; Jin, M.; Willem, M.; Haass, C.; Frosch, M.P.; Walsh, D.M. Diffusible, highly bioactive oligomers represent a critical minority of soluble Aβ in Alzheimer’s disease brain. Acta Neuropathol. 2018, 136, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; O’Nuallain, B.; Hong, W.; Boyd, J.; Lagomarsino, V.N.; O’Malley, T.T.; Liu, W.; Vanderburg, C.R.; Frosch, M.P.; Young-Pearse, T.; et al. An in vitro paradigm to assess potential anti-Aβ antibodies for Alzheimer’s disease. Nat. Commun. 2018, 9, 2676. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, W.; Onishi, A.; Tu, H.Y.; Takihara, Y.; Matsumura, M.; Tsujimoto, K.; Inatani, M.; Nakazawa, T.; Takahashi, M. Culture Systems of Dissociated Mouse and Human Pluripotent Stem Cell-Derived Retinal Ganglion Cells Purified by Two-Step Immunopanning. Investig. Ophthalmol. Vis. Sci. 2018, 59, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Korecka, J.A.; Talbot, S.; Osborn, T.M.; de Leeuw, S.M.; Levy, S.A.; Ferrari, E.J.; Moskites, A.; Atkinson, E.; Jodelka, F.M.; Hinrich, A.J.; et al. Neurite Collapse and Altered ER Ca(2+) Control in Human Parkinson Disease Patient iPSC-Derived Neurons with LRRK2 G2019S Mutation. Stem Cell Rep. 2019, 12, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Laferrière, F.; Maniecka, Z.; Pérez-Berlanga, M.; Hruska-Plochan, M.; Gilhespy, L.; Hock, E.M.; Wagner, U.; Afroz, T.; Boersema, P.J.; Barmettler, G.; et al. TDP-43 extracted from frontotemporal lobar degeneration subject brains displays distinct aggregate assemblies and neurotoxic effects reflecting disease progression rates. Nat. Neurosci. 2019, 22, 65–77. [Google Scholar] [CrossRef]
- Li, S.; Jin, M.; Liu, L.; Dang, Y.; Ostaszewski, B.L.; Selkoe, D.J. Decoding the synaptic dysfunction of bioactive human AD brain soluble Aβ to inspire novel therapeutic avenues for Alzheimer’s disease. Acta Neuropathol. Commun. 2018, 6, 121. [Google Scholar] [CrossRef]
- Li, W.Y.; Wang, Y.; Zhai, F.G.; Sun, P.; Cheng, Y.X.; Deng, L.X.; Wang, Z.Y. AAV-KLF7 Promotes Descending Propriospinal Neuron Axonal Plasticity after Spinal Cord Injury. Neural Plast. 2017, 2017, 1621629. [Google Scholar] [CrossRef] [PubMed]
- Medina-Cano, D.; Ucuncu, E.; Nguyen, L.S.; Nicouleau, M.; Lipecka, J.; Bizot, J.C.; Thiel, C.; Foulquier, F.; Lefort, N.; Faivre-Sarrailh, C.; et al. High N-glycan multiplicity is critical for neuronal adhesion and sensitizes the developing cerebellum to N-glycosylation defect. eLife 2018, 7, e38309. [Google Scholar] [CrossRef]
- Mengel, D.; Hong, W.; Corbett, G.T.; Liu, W.; DeSousa, A.; Solforosi, L.; Fang, C.; Frosch, M.P.; Collinge, J.; Harris, D.A.; et al. PrP-grafted antibodies bind certain amyloid β-protein aggregates, but do not prevent toxicity. Brain Res. 2019, 1710, 125–135. [Google Scholar] [CrossRef]
- Mohtaram, N.K.; Ko, J.; Agbay, A.; Rattray, D.; Neill, P.O.; Rajwani, A.; Vasandani, R.; Thu, H.L.; Jun, M.B.G.; Willerth, S.M. Development of a glial cell-derived neurotrophic factor-releasing artificial dura for neural tissue engineering applications. J. Mater. Chem. B 2015, 3, 7974–7985. [Google Scholar] [CrossRef] [PubMed]
- Park, N.I.; Guilhamon, P.; Desai, K.; McAdam, R.F.; Langille, E.; O’Connor, M.; Lan, X.; Whetstone, H.; Coutinho, F.J.; Vanner, R.J.; et al. ASCL1 Reorganizes Chromatin to Direct Neuronal Fate and Suppress Tumorigenicity of Glioblastoma Stem Cells. Cell Stem Cell 2017, 21, 209–224.e7. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Chanthaphavong, R.S.; Sun, S.; Trigilio, J.A.; Phasouk, K.; Jin, L.; Layton, E.D.; Li, A.Z.; Correnti, C.E.; De van der Schueren, W.; et al. Keratinocytes produce IL-17c to protect peripheral nervous systems during human HSV-2 reactivation. J. Exp. Med. 2017, 214, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Douglas, S.; Michelle Willerth, S. Mechanically stable fibrin scaffolds promote viability and induce neurite outgrowth in neural aggregates derived from human induced pluripotent stem cells. Sci. Rep. 2017, 7, 6250. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.; Yau, S.-y.; Sun, L.; Gabers, N.; Bibault, E.; Christie, B.R.; Willerth, S.M. Optimizing Differentiation Protocols for Producing Dopaminergic Neurons from Human Induced Pluripotent Stem Cells for Tissue Engineering Applications: Supplementary Issue: Stem Cell Biology. Biomark. Insights 2015, 10 (Suppl. S1), 61–70. [Google Scholar] [CrossRef] [PubMed]
- Schwaid, A.G.; Krasowka-Zoladek, A.; Chi, A.; Cornella-Taracido, I. Comparison of the Rat and Human Dorsal Root Ganglion Proteome. Sci. Rep. 2018, 8, 13469. [Google Scholar] [CrossRef] [PubMed]
- Snyder, C.; Yu, L.; Ngo, T.; Sheinson, D.M.; Zhu, Y.; Tseng, M.; Misner, D.L.; Staflin, K. In vitro assessment of chemotherapy-induced neuronal toxicity. Toxicol. Vitr. 2018, 50, 109–123. [Google Scholar] [CrossRef]
- Song, J.J.; Oh, S.M.; Kwon, O.C.; Wulansari, N.; Lee, H.S.; Chang, M.Y.; Lee, E.; Sun, W.; Lee, S.E.; Chang, S.; et al. Cografting astrocytes improves cell therapeutic outcomes in a Parkinson’s disease model. J. Clin. Investig. 2018, 128, 463–482. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P.; Do, M.H.; Lee, T.H.; Kim, S.Y. Anti-neuroinflammatory and neuroprotective effects of the Lindera neesiana fruit in vitro. Phytomedicine 2016, 23, 872–881. [Google Scholar] [CrossRef]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Tortoriello, G.; Morris, C.V.; Alpar, A.; Fuzik, J.; Shirran, S.L.; Calvigioni, D.; Keimpema, E.; Botting, C.H.; Reinecke, K.; Herdegen, T.; et al. Miswiring the brain: Δ9-tetrahydrocannabinol disrupts cortical development by inducing an SCG10/stathmin-2 degradation pathway. Embo J. 2014, 33, 668–685. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, R.; Subedi, L.; Yeo, E.J.; Kim, S.Y. Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway. Neurochem. Int. 2016, 99, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Jin, M.; Wang, Z.; Liu, W.; Lagomarsino, V.; Weeden, T.; Reczek, D.; Young-Pearse, T.; Pradier, L.; Selkoe, D. A Head-to-Head Comparison of Lead Clinical Anti-Aβ Antibodies. Alzheimer’s Dement. 2018, 14, P312. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, W.; Wu, X.; Sun, Y.; Zhang, Y.P.; Deng, L.-X.; Walker, M.J.; Qu, W.; Chen, C.; Liu, N.-K. Remodeling of lumbar motor circuitry remote to a thoracic spinal cord injury promotes locomotor recovery. eLife 2018, 7, e39016. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.W.; Kwon, O.W.; Kim, S.Y.; Choi, S.Z.; Son, M.W.; Kim, K.H.; Lee, K.R. Phenolic derivatives from the rhizomes of Dioscorea nipponica and their anti-neuroinflammatory and neuroprotective activities. J. Ethnopharmacol. 2014, 155, 1164–1170. [Google Scholar] [CrossRef]
- Yagi, H.; Ohkawara, B.; Nakashima, H.; Ito, K.; Tsushima, M.; Ishii, H.; Noto, K.; Ohta, K.; Masuda, A.; Imagama, S.; et al. Zonisamide Enhances Neurite Elongation of Primary Motor Neurons and Facilitates Peripheral Nerve Regeneration In Vitro and in a Mouse Model. PLoS ONE 2015, 10, e0142786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zheng, H.; Zhang, X.; Tian, X.; Xu, S.; Liu, Y.; Jiang, S.; Liu, X.; Shi, R.; Gong, K.; et al. Involvement of nerve growth factor in mouse hippocampal neuronal cell line (HT22) differentiation and underlying role of DNA methyltransferases. J. Toxicol. Environ. Health Part A 2018, 81, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, X.; Hsiao, T.-H.; Lin, G.; Kosti, A.; Yu, X.; Suresh, U.; Chen, Y.; Tomlinson, G.E.; Pertsemlidis, A.; et al. A high-content morphological screen identifies novel microRNAs that regulate neuroblastoma cell differentiation. Oncotarget 2014, 5, 2499–2512. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Shelton, S.D.; Sung, D.C.; Li, M.; Hernandez, D.; Zhang, M.; Losiewicz, M.D.; Chen, Y.; Pertsemlidis, A.; et al. A combined gene expression and functional study reveals the crosstalk between N-Myc and differentiation-inducing microRNAs in neuroblastoma cells. Oncotarget 2016, 7, 79372–79387. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Sung, D.; Li, M.; Kosti, A.; Lin, G.; Chen, Y.; Pertsemlidis, A.; Hsiao, T.H.; Du, L. microRNA-449a functions as a tumor suppressor in neuroblastoma through inducing cell differentiation and cell cycle arrest. RNA Biol. 2015, 12, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Partridge, V.; Sousares, M.; Shelton, S.D.; Holland, C.L.; Pertsemlidis, A.; Du, L. microRNA-2110 functions as an onco-suppressor in neuroblastoma by directly targeting Tsukushi. PLoS ONE 2018, 13, e0208777. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Alnakhala, H.; Terry-Kantor, E.; Newman, A.; Liu, L.; Imberdis, T.; Fanning, S.; Nuber, S.; Ramalingam, N.; Selkoe, D.; et al. Pathogenic Mechanisms of Cytosolic and Membrane-Enriched α-Synuclein Converge on Fatty Acid Homeostasis. J. Neurosci. 2022, 42, 2116–2130. [Google Scholar] [CrossRef]
- Bartlett, R.; Ly, D.; Cashman, N.R.; Sluyter, R.; Yerbury, J.J. P2X7 receptor activation mediates superoxide dismutase 1 (SOD1) release from murine NSC-34 motor neurons. Purinergic Signal. 2022, 18, 451–467. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Mojica-Perez, S.P.; Azaria, R.D.; Schultz, M.; Parent, J.M.; Niu, W. Loss of POGZ alters neural differentiation of human embryonic stem cells. Mol. Cell. Neurosci. 2022, 120, 103727. [Google Scholar] [CrossRef] [PubMed]
- Baytas, O.; Davidson, S.M.; DeBerardinis, R.J.; Morrow, E.M. Mitochondrial enzyme GPT2 regulates metabolic mechanisms required for neuron growth and motor function in vivo. Human Mol. Genet. 2021, 31, 587–603. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Hersh, S.W.; Aslebagh, R.; Shaffer, S.A.; Ikezu, S.; Mez, J.; Lunetta, K.L.; Logue, M.W.; Farrer, L.A.; Ikezu, T. Alzheimer’s disease associated AKAP9 I2558M mutation alters posttranslational modification and interactome of tau and cellular functions in CRISPR-edited human neuronal cells. Aging Cell 2022, 21, e13617. [Google Scholar] [CrossRef]
- Smith, P.O.; Powell, R.; Gregory, H.; Phillips, J.; Bohnhorst, P.; Rayner, M. Exploring the Effect of Vitamins B1, B6 and B12 on Neurite Regeneration using a 3D Co-Culture Model of Neurodegeneration. Int. J. Phys. Med. Rehabil. 2023, 11, 667. [Google Scholar]
- Sum, W.C.; Ebada, S.S.; Kirchenwitz, M.; Wanga, L.; Decock, C.; Stradal, T.E.; Matasyoh, J.C.; Mándi, A.; Kurtán, T.; Stadler, M. Neurite outgrowth-inducing Drimane-type Sesquiterpenoids Isolated from Cultures of the Polypore Abundisporus violaceus MUCL 56355. J. Nat. Prod. 2023, 86, 2457–2467. [Google Scholar] [CrossRef]
- Sum, W.C.; Ebada, S.S.; Kirchenwitz, M.; Kellner, H.; Ibrahim, M.A.; Stradal, T.E.; Matasyoh, J.C.; Stadler, M. Hericioic Acids A–G and Hericiofuranoic Acid; Neurotrophic Agents from Cultures of the European Mushroom Hericium flagellum. J. Agric. Food Chem. 2023, 71, 11094–11103. [Google Scholar] [CrossRef]
- Al-Ali, H.; Blackmore, M.; Bixby, J.L.; Lemmon, V.P. High Content Screening with Primary Neurons. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company: Indianapolis, IN, USA; National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Wagener, J.; Plennevaux, C. Eppendorf 96-Well Cell Culture Plate–A simple method of minimizing the edge effect in cell-based assays. Eppendorf Appl. Note 2014, 326, 1–6. [Google Scholar]
- Akum, B.F.; Chen, M.; Gunderson, S.I.; Riefler, G.M.; Scerri-Hansen, M.M.; Firestein, B.L. Cypin regulates dendrite patterning in hippocampal neurons by promoting microtubule assembly. Nat. Neurosci. 2004, 7, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.K.; Gilroy, M.E.; Irons, H.R.; Laplaca, M.C. Synapse-to-neuron ratio is inversely related to neuronal density in mature neuronal cultures. Brain Res. 2010, 1359, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Previtera, M.L.; Langhammer, C.G.; Firestein, B.L. Effects of substrate stiffness and cell density on primary hippocampal cultures. J. Biosci. Bioeng. 2010, 110, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Segeritz, C.-P.; Vallier, L. Cell Culture: Growing Cells as Model Systems In Vitro. In Basic Science Methods for Clinical Researchers; Academic Press: Cambridge, MA, USA, 2017; pp. 151–172. [Google Scholar] [CrossRef]
- McKinney, C.E. Using induced pluripotent stem cells derived neurons to model brain diseases. Neural Regen. Res. 2017, 12, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Masters, J.R.W. Human cancer cell lines: Fact and fantasy. Nat. Rev. Mol. Cell Biol. 2000, 1, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.-P.; Tong, F. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Costa, L.G. Primary neurons in culture and neuronal cell lines for in vitro neurotoxicological studies. In In Vitro Neurotoxicology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 13–27. [Google Scholar]
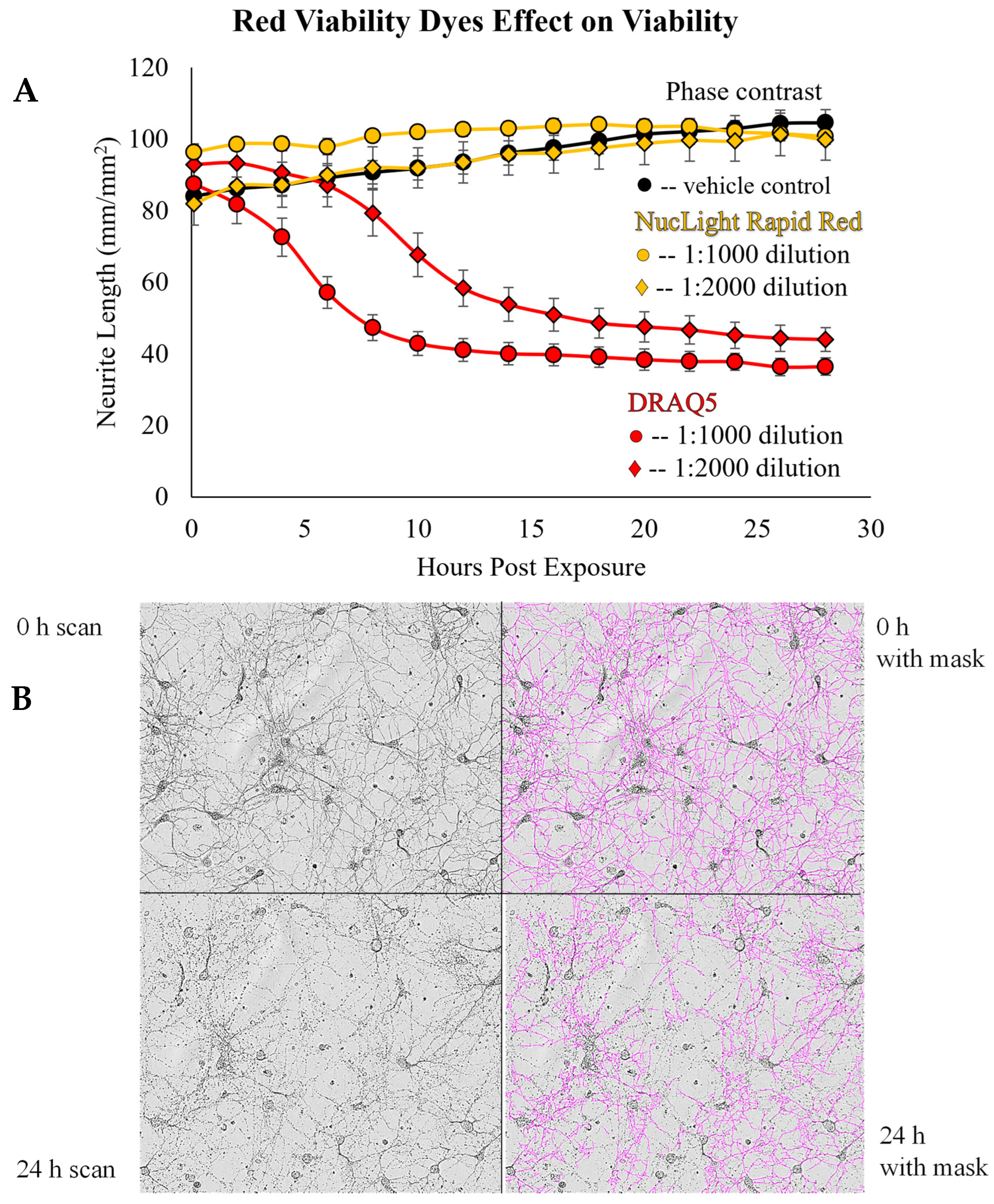
- Burch, C.R.; Stock, J.P.P. Phase-Contrast Microscopy. J. Sci. Instrum. 1942, 19, 71–75. [Google Scholar] [CrossRef]
- Icha, J.; Weber, M.; Waters, J.C.; Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. BioEssays 2017, 39, 1700003. [Google Scholar] [CrossRef]
- Smith, P.J.; Blunt, N.; Wiltshire, M.; Hoy, T.; Teesdale-Spittle, P.; Craven, M.R.; Watson, J.V.; Amos, W.B.; Errington, R.J.; Patterson, L.H. Characteristics of a novel deep red/infrared fluorescent cell-permeant DNA probe, DRAQ5, in intact human cells analyzed by flow cytometry, confocal and multiphoton microscopy. Cytom. J. Int. Soc. Anal. Cytol. 2000, 40, 280–291. [Google Scholar] [CrossRef]
- Sung, M.-H. A Checklist for Successful Quantitative Live Cell Imaging in Systems Biology. Cells 2013, 2, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Bellon, A.; Hasoglu, T.; Peterson, M.; Gao, K.; Chen, M.; Blandin, E.; Cortez-Resendiz, A.; Clawson, G.A.; Hong, L.E. Optimization of Neurite Tracing and Further Characterization of Human Monocyte-Derived-Neuronal-like Cells. Brain Sci. 2021, 11, 1372. [Google Scholar] [CrossRef] [PubMed]
| Manufacturer | Instrument | Features |
|---|---|---|
| Leica Microscope Systems | Mica microhub [23] | 4-color widefield fluorescence imaging Confocal microscopy Automated microscope Incubator for cell viability |
| Sartorius | IncuCyte [24] | 5-color Fluorescence imaging channels. Phase-contrast microscopy. Automated microscope. Incubation up to 42 °C. |
| Agilent | Cytation 10 [25] | Widefield and Spinning disk confocal fluorescence microscopy. Brightfield and phase-contrast microscopy. Automated live-cell incubation. |
| Lionheart FX [25] | Widefield fluorescence imaging. Brightfield and phase-contrast microscopy. Automated live-cell incubation. | |
| xCELLigence RTCA eSight [26] | Brightfield microscopy. Three fluorescence imaging channels. | |
| Biospa [27] | Fluorescence brightfield, color brightfield, and phase-contrast microscopy. Incubation up to 45 °C. Multi-component environmental control. | |
| Molecular Devices | ImageXpress pico [28] | Imaging modes: phase contrast and brightfield, fluorescence, widefield, colorimetric, and confocal imaging. |
| Etaluma | Lumascope [29] (LS720) | 3-fluorescence imaging channels. Brightfield and phase-contrast microscopy. External incubation is required. |
| Logos Biosystems | Celena S [30] | 3-fluorescence imaging channels. Brightfield and phase-contrast microscopy. Onstage incubation System. |
| Axion Biosystems | Cytosmart Lux 3 [31] | 2-fluorescence imaging channels. Brightfield microscopy. Incubation up to 40 °C. |
| Zeiss | Cell Discoverer 7 [32] | Brightfield, confocal, and widefield fluorescence microscopy. Temperature and atmospheric control features. |
| Keyence | BZ-X800 [33] | Fluorescent, brightfield, and phase-contrast microscopy. Built-in dark room. Time-lapse incubation. |
| Articles | Types of Plates | Density Plated | Type of Cells Used | Correlative Experiments Used | Disease State/Aim | Microscopy | Duration of Exposure | Other Imaging Instruments Used |
|---|---|---|---|---|---|---|---|---|
| Camarena, 2014 [53] | 6-well | 750,000 cells/well | Mouse Cortical neurons (Primary) | Immunocytochemistry | 2q23.1 microdeletion syndrome | Phase-contrast | Q 3 h/48 h | LSM710 Zeiss confocal microscope |
| Cavaliere, 2017 [54] | 96-well | 10,000 cells/cm2 | Rat Cortical neurons (Primary) | Immunocytochemistry | Parkinson’s Disease | Phase-contrast | Did not mention | IncuCyte |
| Efthymiou, 2014 [44] | 96-well | 10,000 cells/well | Stem cells (iC23-GFP NSCs) | Immunocytochemistry | Deriving neurons from pluripotent stem cell | Phase-contrast and Fluorescence | Q 2 h/14 days | Leica fluorescence microscope |
| Hong, 2018 [55] | 96-well | 5000 cells/well | induced Neurons from Stem cells (hIPSCs) | Immunocytochemistry | Alzheimer’s | Phase-contrast | Q 2 h/84 h (Baseline 2 h/6 h) | Zeiss LSM710 confocal microscope |
| Jin, 2018 [56] | 96-well | not mentioned | hIPSCs | ELISA | Alzheimer’s | Phase-contrast | Q 2 h/3 days (Baseline 2 h/6 h) | Not applicable |
| Kobayashi, 2018 [57] | not mentioned | not mentioned | Retinal Ganglion cells from Embryonic Stem cells | Immunocytochemistry | Glaucoma/Blindness | Phase-contrast | Q 6 h/450 h | Confocal microscope (LSM700; Carl Zeiss |
| Korecka, 2019 [58] | 96-well | 15,000 cells/well | Stem cells (hIPSCs) | Parkinson’s Disease | Phase-contrast (implied) | 24 h | IncuCyte | |
| Laferrière, 2019 [59] | 96-well | 20,000 calls/well | Mouse Cortical neurons (Primary) | Immunocytochemistry | ALS/FTLD | Phase-contrast and Fluorescence | 6 days | Life Technologies EVOS FL Auto imaging system |
| Li, 2018 [60] | 96-well | 50,000 cells/well | hIPSCs | Western Blot | Alzheimer’s (antibodies) | Phase-contrast | Q 2 h/3 days | Not Applicable |
| Li, 2017 [61] | 48-well | 200,000 cells/well | Rat Spinal Cord neurons (Primary) | Immunocytochemistry/Western Blot | Spinal Cord Injury | Phase-contrast and Fluorescence | Q 4 h/4 days | Olympus BX-60 epifluorescent microscope |
| Medina-Cano, 2018 [62] | 24-well | not mentioned | Mouse Cerebellar granule cells (Primary) | Western Blot | N-glycosylation defect in brain development | Phase-contrast (implied) | Q 3 h/21 h | Not applicable |
| Mengel, 2019 [63] | 96-well | 5000 cells/well | hIPSCs | Western Blot | Alzheimer’s | Phase-contrast | Q 2 h/3 days) | Not applicable |
| Mohtaram, 2015 [64] | 24-well | not mentioned | Cancer cells (PC-12) | Bioactivity assay | Delivery of GD neurotrophic factor for the treatment of CNS disorders during injury | Phase-contrast | Q 12 h/10 days | IncuCyte |
| Park, 2017 [65] | 24-well | not mentioned | Glioblastoma Stem cells (Cancer Stem Cells) | Immunocytochemistry | ASCL1 suppresses tumorigenicity of glioblastoma | Phase-contrast | Q 4 h/10 days | Leica STP-6000 microscope and Leica SP8 Confocal microscope |
| Peng, 2017 [66] | 8-well chamber slides | not mentioned | Human Sensory neuron | Immunocytochemistry | Protection of the PNS by a cytokine | Phase-contrast | Q 1 h/16 h 74 h after plating and treatment | Not mentioned |
| Robinson, 2017 [67] | not mentioned | not mentioned | Stem cells (hIPSCs) | Immunocytochemistry/Cell viability assay MTT | Deriving neurons from pluripotent stem cell | Phase-contrast | Not Mentioned | Leica DMI 30000B |
| Robinson, 2015 [68] | not mentioned | not mentioned | Stem cells (hIPSCs) | Immunocytochemistry | Parkinson’s Disease | Phase-contrast | Not clear | Leica DMI 30000B |
| Schwaid, 2018 [69] | 96-well | 2000 cells/well | Rat Dorsal Root Ganglion Neurons (Primary) | Mass Spectrometry (proteomics) | Comparison of rat and human proteome | Phase-contrast (implied) | 7 days | Not applicable |
| Snyder, 2018 [70] | 96-well | not mentioned | Stem cells (hIPSCs) | Immunocytochemistry | Neurotoxicity during drug development | Phase-contrast and Fluorescence | Q 12 h/3 days | LSM 710 confocal microscope (Zeiss) |
| Song, 2018 [71] | not mentioned | not mentioned | Mouse Ventral Mid-brain Neural Progenitor cells (Primary cell) | Immunocytochemistry | Parkinson’s Disease | Fluorescence | 42 h | confocal microscope (Leica PCS SP5) |
| Soppa, 2014 [48] | 24-well | 90,000 cells/well | Cancer cells (SH-SY5Y) | Immunocytochemistry | Down Syndrome | Phase-contrast and Fluorescence | 4 days | Axiovert 200 M inverted microscope (Zeiss) |
| Srikanth, 2018 [42] | 96-well | 15,000 cells/well | hIPSCs | Immunocytochemistry | Neuropsychiatric diseases | Phase-contrast | 3 days | Zeiss LSM710 |
| Subedi, 2016 [72] | 6-well | 10,000 cells/well | N2a (Mouse neuroblastoma cell line) Cancer cells | Cell viability assay MTT | Neuroprotective abilities of Lindera neesiana | Phase-contrast | 24 h | Not applicable |
| Subedi, 2017 [73] | 12-well | 600,000 cells/well | N2a (Mouse neuroblastoma cell line) Cancer cells | Cell viability assay MTT/Western Blot | Neuroprotection due to Equol | Phase-contrast (implied) | Q 2 h/24 h | Not applicable |
| Tortoriello, 2014 [74] | not mentioned | 25,000 cells/well | Mouse Primary cortical neurons | Western Blot | Substance abuse’s effect on children | Phase-contrast (implied) | Q 2 h/62 h | Not applicable |
| Venkatesan, 2016 [75] | 24-well | 50,000 cells/well | N2a (Mouse neuroblastoma cell line) Cancer cells | Cell viability and NGF assay | Lactucopicrin neuroprotective effect against scopolamine | Phase-contrast | 24 h | Not applicable |
| Walsh, 2018 [76] | 96-well | 5000 cells/well | hIPSCs | Not applicable | Alzheimer’s | Phase-contrast (implied) | Q 2 h/4 days | Not applicable |
| Wang, 2018 [77] | 48-well | 200,000 cells/well | Rat Spinal Cord neurons (Primary) | Immunocytochemistry and ELISA | Spinal Cord Injury | Phase-contrast and Fluorescence | Q 4 h/5 days | Zeiss confocal microscopy |
| Woo, 2014 [78] | 6-well | 10,000 cells/well | N2a (Mouse neuroblastoma cell line) Cancer cells | Cell viability and NGF assay | Neuroprotective effect of Dioscorea nipponica | Phase-contrast | 72 h | Not applicable |
| Yagi, 2015 [79] | 96-well | 6000 cells/well | Mouse Primary motor neurons | Cell viability assay MTS/Western Blot | Nerve regeneration due to Zonisamide | Phase-contrast | Q 8 h/3 days | Not applicable |
| Zhang, 2018 [80] | not mentioned | not mentioned | HT22 (mouse hippocampal cell line) immortalized cells | Western Blot | Differentiation of an immortalized cell line | Phase-contrast (implied) | 24 h | Not applicable |
| Zhao, 2014 [81] | 96-well | 2500 cells/well | BE(2)-C Human Neuroblastoma cell line Cancer cells | Cell viability/Western blots | Neuroblastoma | Phase-contrast | Q 6 h/5 days | Not applicable |
| Zhao, 2016 [82] | 96-well | not mentioned (but can be implied) | BE(2)-C Human Neuroblastoma cell line Cancer cells | Cell viability/Western blots | Neuroblastoma | Phase-contrast (implied) | 4 days | Not applicable |
| Zhao, 2015 [83] | 96-well | 2500 cells/well | BE(2)-C Human Neuroblastoma cell line Cancer cells | Cell viability/Western blots | Neuroblastoma | Phase-contrast | Q 6 h/4 days | Not applicable |
| Zhao, 2018 [84] | 96-well | 2500 cells/well | BE(2)-C Human Neuroblastoma cell line Cancer cells | Cell viability/Western blots | Neuroblastoma | Phase-contrast | Q 12 h/4 days | Not applicable |
| Davies, 2022 [46] | 96-well (day 6 neurons) | 10,000 cells/well | iPSC-derived cortical neurons (stem) | Mass spec-based lipidomics, Western blot | AP-4 deficiency | Phase-contrast | Q 3 h/21 h | Not applicable |
| Tripathi, 2022 [85] | 96-well | 15,000 cells/well | Rat Cortical Neuron transfected with pLVX-IRES-mCherry plasmids (primary) | Immunocytochemistry | Synucleinopathies, e.g., Parkinson disease | Fluorescence | 72 h after transfection | Imaging-based toxicity assay “mCherry coexpression” |
| Bartlett, 2022 [86] | 24-well | 50,000 cells/well | NSC-34 spinal cord × Neuroblastoma hybrid cell line | ER Stress Assay | ALS | Fluorescence | not mentioned | Leica DM IBRE |
| Deng, 2022 [87] | 8-well | not mentioned | Neural stem cell | Immunocytochemistry | Autism | Phase-contrast | 4 days | Leica SP5 confocal microscope |
| Baytas, 2021 [88] | 96-well | 25,000 cells/well | Mouse Hippocampal neurons (primary) | Immunocytochemistry | Postnatal growth defects | Phase-contrast | 16 days | Olympus FV3000 confocal laser scanning microscope |
| You, 2022 [89] | 96-well | not mentioned | SH-SY5Y P301L cells (cancer) | Western blotting | Alzheimer’s | Fluorescence | Q 15 min/1.5 h | Not applicable |
| Smith, 2023 [90] | 96-well | 5000 cells/well | NG108-15 (Neuroblastoma and glioma hybrid | Immunocytochemistry | Peripheral Neuropathy | Phase-contrast and Fluorescence | Q 4 h for 72 h | IncuCyte S3 |
| Zosen, 2023 [43] | 96-well | 15,000 cells/well | Cancer cells (SH-SY5Y) | Western blotting/Immunocytochemistry | Depression | Phase-contrast | Q 4 h for 48 h | IncuCyte ZOOM |
| Sum, 2023 [91] | 96-well | 15,000 cells/well | Cancer cells (PC-12) | Cell viability assay MTT | neurotrophic activities of the drimane-type molecules | Phase-contrast | 48 h | Not applicable |
| Sum, 2023 [92] | 96-well | 15,000 cells/well | Cancer cells (PC-12) | Cytotoxicity assay | neurotrophic activities of the Hericioic acids | Phase-contrast | 48 h | Not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akere, M.T.; Zajac, K.K.; Bretz, J.D.; Madhavaram, A.R.; Horton, A.C.; Schiefer, I.T. Real-Time Analysis of Neuronal Cell Cultures for CNS Drug Discovery. Brain Sci. 2024, 14, 770. https://doi.org/10.3390/brainsci14080770
Akere MT, Zajac KK, Bretz JD, Madhavaram AR, Horton AC, Schiefer IT. Real-Time Analysis of Neuronal Cell Cultures for CNS Drug Discovery. Brain Sciences. 2024; 14(8):770. https://doi.org/10.3390/brainsci14080770
Chicago/Turabian StyleAkere, Millicent T., Kelsee K. Zajac, James D. Bretz, Anvitha R. Madhavaram, Austin C. Horton, and Isaac T. Schiefer. 2024. "Real-Time Analysis of Neuronal Cell Cultures for CNS Drug Discovery" Brain Sciences 14, no. 8: 770. https://doi.org/10.3390/brainsci14080770
APA StyleAkere, M. T., Zajac, K. K., Bretz, J. D., Madhavaram, A. R., Horton, A. C., & Schiefer, I. T. (2024). Real-Time Analysis of Neuronal Cell Cultures for CNS Drug Discovery. Brain Sciences, 14(8), 770. https://doi.org/10.3390/brainsci14080770






