Prospects of Electrocorticography in Neuropharmacological Studies in Small Laboratory Animals
Abstract
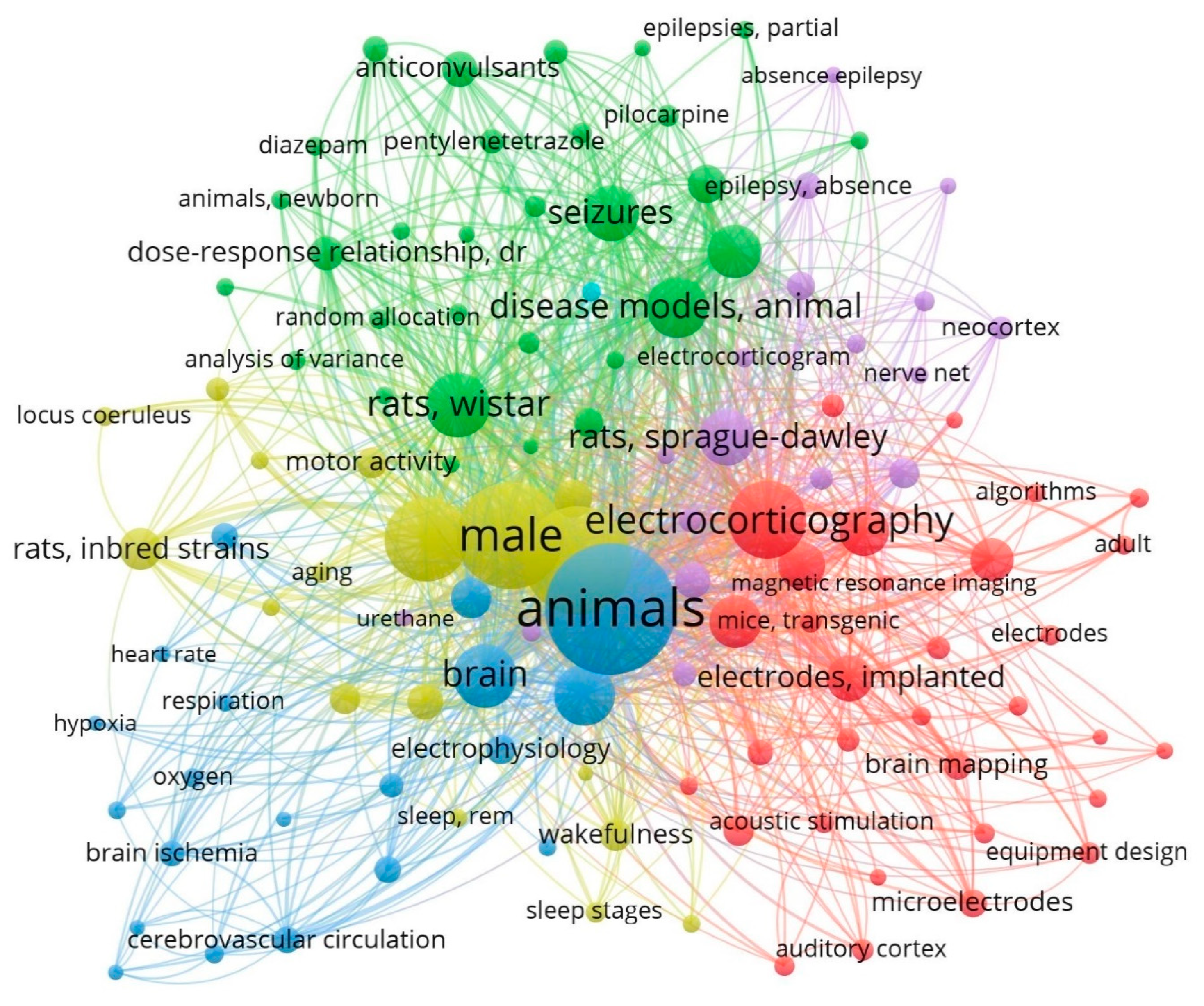
:1. Introduction
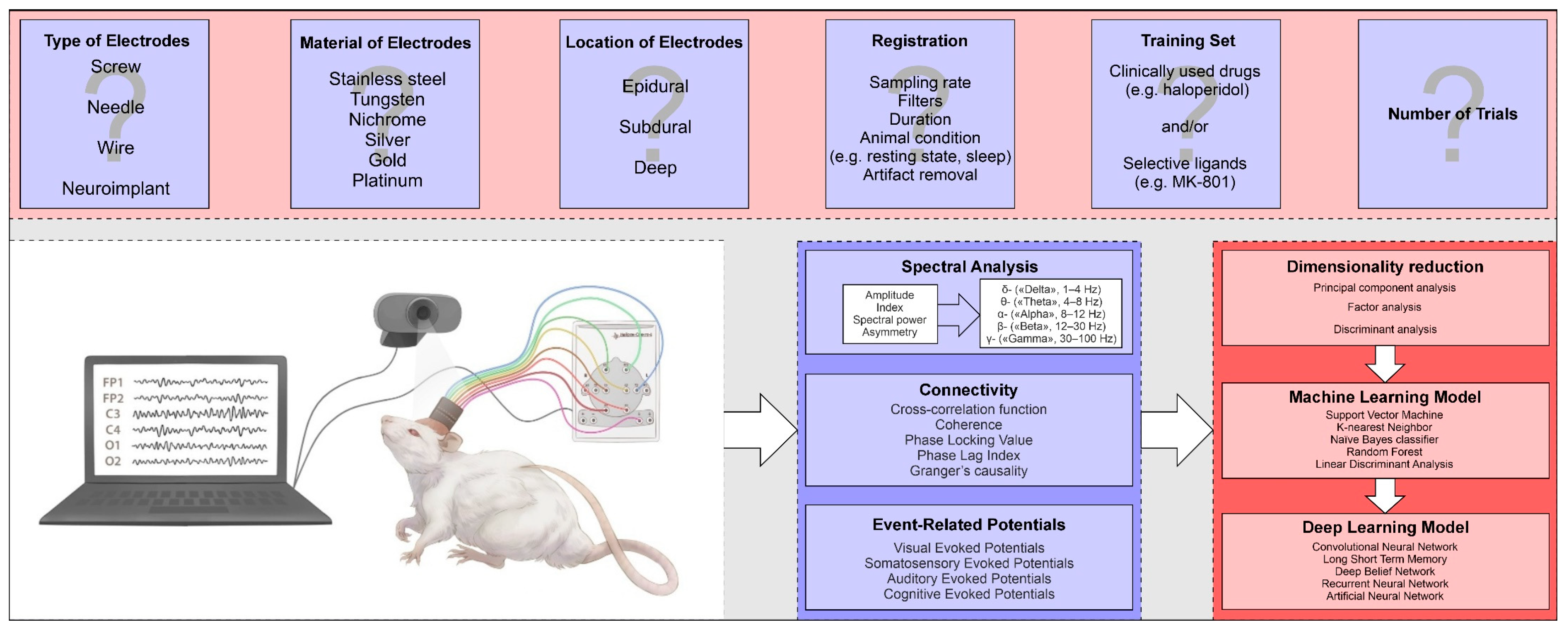
2. Electrodes for Recording of Brain Bioelectrical Activity
| Electrode Type | Materials Used | Insulation Type | Experimental Tasks |
|---|---|---|---|
| Screw electrodes | Stainless steel | None | Studying the rhythms wakeful rest, slow-wave, and REM sleep at different brain areas in rats [21]. Detection of interhemispheric symmetry and asymmetry of absence-type spike-wave discharges caused by systemic administration of pentylenetetrazole [25]. |
| Needle electrodes | Stainless steel, silver | None | Study of the motion of epileptiform activity wave on a model of cortical epilepsy [26]. Assessment of age-related dynamics of ECoG and seizure activity of Wistar rats in a cortical model of focal epilepsy [19,30]. |
| Wire electrodes | Nichrome | Shrink tube | Analysis of changes in amplitude spectral characteristics, coherence, VEP, and SSEP in rats after traumatic brain injury [31,32,33,34]. |
| Platinum/iridium (80%/20%) | Evaluation of the effect of clozapine and the 5-HT2A-antagonist RU-31 on the power spectral density of EEG rhythms in rats using a model of schizophrenia with neonatal destruction of the ventral hippocampus [8]. | ||
| Electrode arrays | Aluminum (2.5%), chromium (20%), copper (2.5%), and nickel (75%) | Parafilm | Registration of activity of motor and somatosensory cortex during locomotion on a treadmill [24]. |
| Platinum | Silicone | Registration and analysis of spectral power during standing and walking, MEP and SSEP [22]. |
3. Basic Methods of Analyzing Bioelectrical Activity of the Brain
3.1. Spectral Analysis
3.2. Connectivity Analysis
3.3. Evoked Potentials
4. Application of ECoG in Neuropharmacological Research
4.1. Study of Antiepileptic Activity
4.2. The Use of ECoG in the Valuation of Neuroprotecive Activity
4.3. Pharmacoencephalography as a Classification and Prediction Tool
4.4. Other Applications
4.4.1. Evaluation of Hypnotic Effects
4.4.2. Evaluation of Analgesic Activity
4.4.3. Evaluation of Antidepressant Effects
4.4.4. Evaluation of Antipsychotic Activity
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berger, H. Über Das Elektrenkephalogramm Des Menschen. Arch. Psychiatr. Nervenkr. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Löscher, W. Animal Models of Seizures and Epilepsy: Past, Present, and Future Role for the Discovery of Antiseizure Drugs. Neurochem. Res. 2017, 42, 1873–1888. [Google Scholar] [CrossRef]
- Kim, S.; Jo, K.; Hong, K.-B.; Han, S.H.; Suh, H.J. GABA and I-Theanine Mixture Decreases Sleep Latency and Improves NREM Sleep. Pharm. Biol. 2019, 57, 64–72. [Google Scholar] [CrossRef]
- Morairty, S.R.; Sun, Y.; Toll, L.; Bruchas, M.R.; Kilduff, T.S. Activation of the Nociceptin/Orphanin-FQ Receptor Promotes NREM Sleep and EEG Slow Wave Activity. Proc. Natl. Acad. Sci. USA 2023, 120, e2214171120. [Google Scholar] [CrossRef] [PubMed]
- Dimpfel, W. Pharmacological Classification of Herbal Extracts by Means of Comparison to Spectral EEG Signatures Induced by Synthetic Drugs in the Freely Moving Rat. J. Ethnopharmacol. 2013, 149, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Murai, N.; Sekizawa, T.; Gotoh, T.; Watabik, T.; Takahashi, M.; Kakimoto, S.; Takahashi, Y.; Iino, M.; Nagakura, Y. Spontaneous and Evoked Pain-Associated Behaviors in a Rat Model of Neuropathic Pain Respond Differently to Drugs with Different Mechanisms of Action. Pharmacol. Biochem. Behav. 2016, 141, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kudelina, O.M.; Safronenko, A.V.; Maklyakov, Y.S.; Gantsgorn, E.V.; Morozov, N.M.; Jabr, M.A. Analytical Evaluation of Treating Depression in Animal Models by Quantitative Pharmaco-EEG. J. Biomed. 2020, 16, 43–47. (In Russian) [Google Scholar] [CrossRef]
- Kalitin, K.Y.; Pridvorov, G.V.; Spasov, A.A.; Mukha, O.Y. Effect of Clozapine and 5-NT2A-Antagonist RU-31 on Electroencephalography and Motor Activity of Rats in a Model of Schizophrenia with Neonatal Destruction of the Ventral Hippocampus. Kuban Sci. Med. Bull. 2022, 29, 108–122. (In Russian) [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Shits, D.D.; Puchik, M.M.; Gutiy, T.A.; Fedorova, E.V.; Prikhodko, V.A.; Titovich, I.A.; Melekhova, A.S.; Bespalov, A.Y.; Knyazeva, I.S.; et al. Pharmacological Screening of a New Valproic Acid Derivative Using the Pharmacoencephalography Method in Rats. Drug Dev. Regist. 2024, 2, 120–136. [Google Scholar] [CrossRef]
- Jobert, M.; Wilson, F.J.; Ruigt, G.S.F.; Brunovsky, M.; Prichep, L.S.; Drinkenburg, W.H.I.M. Guidelines for the Recording and Evaluation of Pharmaco-EEG Data in Man: The International Pharmaco-EEG Society (IPEG). Neuropsychobiology 2012, 66, 201–220. [Google Scholar] [CrossRef]
- Yoshimura, M.; Koenig, T.; Irisawa, S.; Isotani, T.; Yamada, K.; Kikuchi, M.; Okugawa, G.; Yagyu, T.; Kinoshita, T.; Strik, W.; et al. A Pharmaco-EEG Study on Antipsychotic Drugs in Healthy Volunteers. Psychopharmacology 2007, 191, 995–1004. [Google Scholar] [CrossRef]
- Ricci, L.; Assenza, G.; Pulitano, P.; Simonelli, V.; Vollero, L.; Lanzone, J.; Mecarelli, O.; Di Lazzaro, V.; Tombini, M. Measuring the Effects of First Antiepileptic Medication in Temporal Lobe Epilepsy: Predictive Value of Quantitative-EEG Analysis. Clin. Neurophysiol. 2021, 132, 25–35. [Google Scholar] [CrossRef] [PubMed]
- De Pieri, M.; Rochas, V.; Sabe, M.; Michel, C.; Kaiser, S. Pharmaco-EEG of Antipsychotic Treatment Response: A Systematic Review. Schizophrenia 2023, 9, 85. [Google Scholar] [CrossRef]
- Drinkenburg, W.H.I.M.; Ruigt, G.S.F.; Ahnaou, A. Pharmaco-EEG Studies in Animals: An Overview of Contemporary Translational Applications. Neuropsychobiology 2015, 72, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Drinkenburg, W.H.I.M.; Ahnaou, A.; Ruigt, G.S.F. Pharmaco-EEG Studies in Animals: A History-Based Introduction to Contemporary Translational Applications. Neuropsychobiology 2015, 72, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, E.; Elger, C. Introduction to the Neurophysiological Basis of the EEG and DC Potentials. In Electroencephalography: Basic Principles, Clinical Applications and Related Fields; Niedermeyer, E.L.d.S.F., Ed.; Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 15–27. [Google Scholar]
- Green, J.D.; Arduini, A.A. Hippocampal Electrical Activity in Arousal. J. Neurophysiol. 1954, 17, 533–557. [Google Scholar] [CrossRef]
- Whishaw, I.Q.; Vanderwolf, C.H. Hippocampal EEG and Behavior: Change in Amplitude and Frequency of RSA (Theta Rhythm) Associated with Spontaneous and Learned Movement Patterns in Rats and Cats. Behav. Biol. 1973, 8, 461–484. [Google Scholar] [CrossRef]
- Kalinina, D.S.; Lenkov, D.N.; Zhuravin, I.A.; Volnova, A.B. Age Dynamics of ECoG and Epileptiform Activity in Wistar Rat Cortical Model of Focal Epilepsy. Zhurnal Vyss. Nervn. Deyatelnosti Im. IP Pavlov. 2017, 67, 322–333. (In Russian) [Google Scholar]
- Sysoev, Y.I.; Prikhodko, V.A.; Idiyatullin, R.D.; Chernyakov, R.T.; Karev, V.E.; Okovityi, S.V. A Method for Chronic Registration of Brain Cortical Electrical Activity in Rats. J. Evol. Biochem. Physiol. 2022, 58, 292–301. [Google Scholar] [CrossRef]
- Jing, W.; Wang, Y.; Fang, G.; Chen, M.; Xue, M.; Guo, D.; Yao, D.; Xia, Y. EEG Bands of Wakeful Rest, Slow-Wave and Rapid-Eye-Movement Sleep at Different Brain Areas in Rats. Front. Comput. Neurosci. 2016, 10, 79. [Google Scholar] [CrossRef]
- Afanasenkau, D.; Kalinina, D.; Lyakhovetskii, V.; Tondera, C.; Gorsky, O.; Moosavi, S.; Pavlova, N.; Merkulyeva, N.; Kalueff, A.V.; Minev, I.R.; et al. Rapid Prototyping of Soft Bioelectronic Implants for Use as Neuromuscular Interfaces. Nat. Biomed. Eng. 2020, 4, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Čvančara, P.; Boretius, T.; López-Álvarez, V.M.; Maciejasz, P.; Andreu, D.; Raspopovic, S.; Petrini, F.; Micera, S.; Granata, G.; Fernandez, E.; et al. Stability of Flexible Thin-Film Metallization Stimulation Electrodes: Analysis of Explants after First-in-Human Study Improvement of in Vivo Performance. J. Neural. Eng. 2020, 17, 046006. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, N.; Ajam, A.; Ramos, R.; Koripalli, M.; Chennamsetti, M.; Choi, Y. Handcrafted Electrocorticography Electrodes for a Rodent Behavioral Model. Technologies 2016, 4, 23. [Google Scholar] [CrossRef]
- Ershova, A.S.; Suleymanova, E.M.; Grishchenko, A.A.; Vinogradova, L.V.; Sysoev, I.V. Interhemispheric Symmetry and Asymmetry of Absence Type Spike-Wave Discharges Caused by Systemic Administration of Pentylenetetrazole. J. Evol. Biochem. Physiol. 2023, 59, 293–301. [Google Scholar] [CrossRef]
- Belov, D.R.; Volnova, A.V.; Ahmediev, D.O. Travelling Wave of ECoG Epileptic Activity in Local Cortical Seizure Modeling in Awake Rats. Shurnal Vyss. Nervn. Deyatelnosti Im. IP Pavlov. 2016, 66, 751–762. (In Russian) [Google Scholar]
- Meeren, H.K.M.; van Cappellen van Walsum, A.M.; van Luijtelaar, E.L.J.M.; Coenen, A.M.L. Auditory Evoked Potentials from Auditory Cortex, Medial Geniculate Nucleus, and Inferior Colliculus during Sleep–Wake States and Spike-Wave Discharges in the WAG/Rij Rat. Brain Res. 2001, 898, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Akman, O.; Raol, Y.H.; Auvin, S.; Cortez, M.A.; Kubova, H.; de Curtis, M.; Ikeda, A.; Dudek, F.E.; Galanopoulou, A.S. Methodologic Recommendations and Possible Interpretations of Video-EEG-Recordings in Immature Rodents Used as Experimental Controls: A TASK1-WG2 Report of the ILAE/AES Joint Translational Task Force. Epilepsia Open 2018, 3, 437–459. [Google Scholar] [CrossRef] [PubMed]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L.; et al. Electronic Dura Mater for Long-Term Multimodal Neural Interfaces. Science 2015, 347, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, D.S.; Vasilev, D.S.; Volnova, A.B.; Nalivaeva, N.N.; Zhuravin, I.A. Age-Dependent Electrocorticogram Dynamics and Epileptogenic Responsiveness in Rats Subjected to Prenatal Hypoxia. Dev. Neurosci. 2019, 41, 56–66. [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Pyankova, V.A.; Kroshkina, K.A.; Karev, V.E.; Okovityi, S.V. Cross-Correlation and Coherence Analysis of Electrocortigrams in Rats Subjected to Craniocerebral Trauma. Neurosci. Behav. Physiol. 2020, 50, 1216–1223. [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Kroshkina, K.A.; P’yankova, V.A.; Karev, V.E.; Okovitiy, S.V. Amplitude and Spectral Analysis of Electrocorticograms in Rats with Traumatic Brain Injury. J. Biomed. 2019, 107–120. (In Russian) [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Chernyakov, R.T.; Idiyatullin, R.D.; Kroshkina, K.A.; Piankova, V.A.; Prikhodko, V.A.; Okovitiy, S.V. Changes of Visually Evoked Potentials in Rats after Brain Trauma. J. Biomed. 2020, 68–77. (In Russian) [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Prikhodko, V.A.; Chernyakov, R.T.; Idiyatullin, R.D.; Musienko, P.E.; Okovityi, S.V. Effects of Alpha-2 Adrenergic Agonist Mafedine on Brain Electrical Activity in Rats after Traumatic Brain Injury. Brain Sci. 2021, 11, 981. [Google Scholar] [CrossRef]
- Nuwer, M.; Lehmann, D.; da Silva, F.; Matsuoka, S.; Sutherling, W.; Vibert, J. IFCN Guidelines for Topographic and Frequency Analysis of EEGs and EPs.The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999, 52, 15–20. [Google Scholar]
- Mecarelli, O. (Ed.) Clinical Electroencephalography; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Buzsáki, G. Theta Oscillations in the Hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Rayan, A.; Agarwal, A.; Samanta, A.; Severijnen, E.; van der Meij, J.; Genzel, L. Sleep Scoring in Rodents: Criteria, Automatic Approaches and Outstanding Issues. Eur. J. Neurosci. 2024, 59, 526–553. [Google Scholar] [CrossRef]
- Hernan, A.E.; Schevon, C.A.; Worrell, G.A.; Galanopoulou, A.S.; Kahane, P.; de Curtis, M.; Ikeda, A.; Quilichini, P.; Williamson, A.; Garcia-Cairasco, N.; et al. Methodological Standards Functional Correlates of Depth in Vivo Electrophysiological Recordings in Control Rodents. ATASK1-WG3 report of the AES/ILAETranslational Task Force of the ILAEA. Epilepsia 2017, 58, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Im, C.-H. (Ed.) Computational EEG Analysis; Springer Singapore: Singapore, 2018. [Google Scholar]
- Maheshwari, A. Rodent EEG: Expanding the Spectrum of Analysis. Epilepsy Curr. 2020, 20, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Chiarion, G.; Sparacino, L.; Antonacci, Y.; Faes, L.; Mesin, L. Connectivity Analysis in EEG Data: A Tutorial Review of the State of the Art and Emerging Trends. Bioengineering 2023, 10, 372. [Google Scholar] [CrossRef]
- Bowyer, S.M. Coherence a Measure of the Brain Networks: Past and Present. Neuropsychiatr. Electrophysiol. 2016, 2, 1. [Google Scholar] [CrossRef]
- Shim, M.; Kim, D.W.; Lee, S.H.; Im, C.H. Disruptions in Small-World Cortical Functional Connectivity Network during an Auditory Oddball Paradigm Task in Patients with Schizophrenia. Schizophr. Res. 2014, 156, 197–203. [Google Scholar] [CrossRef]
- Cea-Cañas, B.; Gomez-Pilar, J.; Núñez, P.; Rodríguez-Vázquez, E.; de Uribe, N.; Díez, Á.; Pérez-Escudero, A.; Molina, V. Connectivity Strength of the EEG Functional Network in Schizophrenia and Bipolar Disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 98, 109801. [Google Scholar] [CrossRef]
- Cozac, V.V.; Gschwandtner, U.; Hatz, F.; Hardmeier, M.; Rüegg, S.; Fuhr, P. Quantitative EEG and Cognitive Decline in Parkinson’s Disease. Parkinsons Dis. 2016, 2016, 9060649. [Google Scholar] [CrossRef]
- Imperatori, C.; Farina, B.; Quintiliani, M.I.; Onofri, A.; Castelli Gattinara, P.; Lepore, M.; Gnoni, V.; Mazzucchi, E.; Contardi, A.; Della Marca, G. Aberrant EEG Functional Connectivity and EEG Power Spectra in Resting State Post-Traumatic Stress Disorder: A SLORETA Study. Biol. Psychol. 2014, 102, 10–17. [Google Scholar] [CrossRef]
- Zandvakili, A.; Swearingen, H.R.; Philip, N.S. Changes in Functional Connectivity after Theta-Burst Transcranial Magnetic Stimulation for Post-Traumatic Stress Disorder: A Machine-Learning Study. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shi, C.; Ma, X.; Zhao, B.; Chen, X.; Tao, L. Cognitive Deficits and Rehabilitation Mechanisms in Mild Traumatic Brain Injury Patients Revealed by EEG Connectivity Markers. Clin. Neurophysiol. 2021, 132, 554–567. [Google Scholar] [CrossRef]
- van Mierlo, P.; Papadopoulou, M.; Carrette, E.; Boon, P.; Vandenberghe, S.; Vonck, K.; Marinazzo, D. Functional Brain Connectivity from EEG in Epilepsy: Seizure Prediction and Epileptogenic Focus Localization. Prog. Neurobiol. 2014, 121, 19–35. [Google Scholar] [CrossRef]
- Lascano, A.M.; Lalive, P.H.; Hardmeier, M.; Fuhr, P.; Seeck, M. Clinical Evoked Potentials in Neurology: A Review of Techniques and Indications. J. Neurol. Neurosurg. Psychiatry 2017, 88, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Chirapapaisan, N.; Laotaweerungsawat, S.; Chuenkongkaew, W.; Samsen, P.; Ruangvaravate, N.; Thuangtong, A.; Chanvarapha, N. Diagnostic Value of Visual Evoked Potentials for Clinical Diagnosis of Multiple Sclerosis. Doc. Ophthalmol. 2015, 130, 25–30. [Google Scholar] [CrossRef]
- Niazy, H.M.A.; Lundervold, A. Correlation of Evoked Potentials (SEP and VEP), EEG and CT in the Diagnosis of Brain Tumors and Cerebrovascular Diseases. Clin. Electroencephalogr. 1982, 13, 71–81. [Google Scholar] [CrossRef]
- Papathanasiou, E.S.; Cronin, T.; Seemungal, B.; Sandhu, J. Electrophysiological Testing in Concussion: A Guide to Clinical Applications. J. Concussion 2018, 2, 205970021881263. [Google Scholar] [CrossRef]
- Oeur, A.; Torp, W.H.; Arbogast, K.B.; Master, C.L.; Margulies, S.S. Altered Auditory and Visual Evoked Potentials Following Single and Repeated Low-Velocity Head Rotations in 4-Week-Old Swine. Biomedicines 2023, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Falco-Walter, J. Epilepsy—Definition, Classification, Pathophysiology, and Epidemiology. Semin. Neurol. 2020, 40, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Höller, Y.; Helmstaedter, C.; Lehnertz, K. Quantitative Pharmaco-Electroencephalography in Antiepileptic Drug Research. CNS Drugs 2018, 32, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xia, L.; Zhao, F.L.; Li, S.C. Clinical Efficacy and Safety of the Newer Antiepileptic Drugs as Adjunctive Treatment in Adults with Refractory Partial-Onset Epilepsy: A Meta-Analysis of Randomized Placebo-Controlled Trials. Epilepsy Res. 2013, 103, 31–44. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, P.; Yan, F.; Luo, Y.; Zhao, G. Animal Models of Epilepsy: A Phenotype-Oriented Review. Aging Dis. 2022, 13, 215. [Google Scholar] [CrossRef]
- Song, H.; Tufa, U.; Chow, J.; Sivanenthiran, N.; Cheng, C.; Lim, S.; Wu, C.; Feng, J.; Eubanks, J.H.; Zhang, L. Effects of Antiepileptic Drugs on Spontaneous Recurrent Seizures in a Novel Model of Extended Hippocampal Kindling in Mice. Front. Pharmacol. 2018, 9, 451. [Google Scholar] [CrossRef]
- van Luijtelaar, G.; van Oijen, G. Establishing Drug Effects on Electrocorticographic Activity in a Genetic Absence Epilepsy Model: Advances and Pitfalls. Front. Pharmacol. 2020, 11, 395. [Google Scholar] [CrossRef]
- Löscher, W.; White, H.S. Animal Models of Drug-Resistant Epilepsy as Tools for Deciphering the Cellular and Molecular Mechanisms of Pharmacoresistance and Discovering More Effective Treatments. Cells 2023, 12, 1233. [Google Scholar] [CrossRef]
- Rusina, E.; Bernard, C.; Williamson, A. The Kainic Acid Models of Temporal Lobe Epilepsy. eNeuro 2021, 8. [Google Scholar] [CrossRef]
- Cambiaghi, M.; Magri, L.; Cursi, M. Importance of EEG in Validating the Chronic Effects of Drugs: Suggestions from Animal Models of Epilepsy Treated with Rapamycin. Seizure 2015, 27, 30–39. [Google Scholar] [CrossRef]
- Mironov, A.N. Guidelines for Preclinical Studies of Drugs. Part I; Grif i K: Moscow, Russia, 2012. (In Russian) [Google Scholar]
- Li, Q.; Gao, J.; Zhang, Z.; Huang, Q.; Wu, Y.; Xu, B. Distinguishing Epileptiform Discharges From Normal Electroencephalograms Using Adaptive Fractal and Network Analysis: A Clinical Perspective. Front. Physiol. 2020, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Boutouil, H.R.; Gerbatin, R.; Mamad, O.; Heiland, M.; Reschke, C.R.; Del Gallo, F.; Fabene, P.; Henshall, D.C.; Lowery, M.; et al. Detection of Spontaneous Seizures in EEGs in Multiple Experimental Mouse Models of Epilepsy. J. Neural. Eng. 2021, 18, 056060. [Google Scholar] [CrossRef]
- Kotloski, R.J. A Machine Learning Approach to Seizure Detection in a Rat Model of Post-Traumatic Epilepsy. Sci. Rep. 2023, 13, 15807. [Google Scholar] [CrossRef] [PubMed]
- Ogren, J.A.; Bragin, A.; Wilson, C.L.; Hoftman, G.D.; Lin, J.J.; Dutton, R.A.; Fields, T.A.; Toga, A.W.; Thompson, P.M.; Engel Jr, J.; et al. Three-dimensional Hippocampal Atrophy Maps Distinguish Two Common Temporal Lobe Seizure–Onset Patterns. Epilepsia 2009, 50, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Behr, C.; Lévesque, M.; Stroh, T.; Avoli, M. Time-Dependent Evolution of Seizures in a Model of Mesial Temporal Lobe Epilepsy. Neurobiol. Dis. 2017, 106, 205–213. [Google Scholar] [CrossRef]
- Kadam, S.D.; D’Ambrosio, R.; Duveau, V.; Roucard, C.; Garcia-Cairasco, N.; Ikeda, A.; de Curtis, M.; Galanopoulou, A.S.; Kelly, K.M. Methodological Standards and Interpretation of Video-electroencephalography in Adult Control Rodents. A TASK1-WG1 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia 2017, 58, 10–27. [Google Scholar] [CrossRef]
- Moyer, J.T.; Gnatkovsky, V.; Ono, T.; Otáhal, J.; Wagenaar, J.; Stacey, W.C.; Noebels, J.; Ikeda, A.; Staley, K.; de Curtis, M.; et al. Standards for Data Acquisition Software-based Analysis of in Vivo Electroencephalography Recordings from Animals ATASK1-WG5 report of the AES/ILAETranslational Task Force of the, I.L.A.E. Epilepsia 2017, 58, 53–67. [Google Scholar] [CrossRef]
- Olanow, C.W.; Kieburtz, K.; Schapira, A.H.V. Why Have We Failed to Achieve Neuroprotection in Parkinson’s Disease? Ann. Neurol. 2009, 64, S101–S110. [Google Scholar] [CrossRef]
- Xu, S.; Pan, S. Clinical Translational Failure in Neuroprotective Agents: The Steps from Animal Experiments to Clinical Trials. Med. Sci. Monit. Basic Res. 2013, 19, 37–45. [Google Scholar] [CrossRef]
- Huntemann, N.; Rolfes, L.; Pawlitzki, M.; Ruck, T.; Pfeuffer, S.; Wiendl, H.; Meuth, S.G. Failed, Interrupted, or Inconclusive Trials on Neuroprotective and Neuroregenerative Treatment Strategies in Multiple Sclerosis: Update 2015–2020. Drugs 2021, 81, 1031–1063. [Google Scholar] [CrossRef] [PubMed]
- Sysoev, Y.; Kroshkina, K.; Okovityi, S.V. Characteristic of Somatosensory Evoked Potentials in Rats after Traumatic Brain Injury. Russ. J. Physiol. 2019, 105, 749–760. (In Russian) [Google Scholar]
- Zhang, S.; Ke, Z.; Li, L.; Yip, S.; Tong, K. EEG Patterns from Acute to Chronic Stroke Phases in Focal Cerebral Ischemic Rats: Correlations with Functional Recovery. Physiol. Meas. 2013, 34, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Gantsgorn, E.V.; Khloponin, D.P.; Maklyakov, Y.S. Survival Analysis and Spatial-Frequency Distribution of EEG-Indices in Rats Global Cerebral Ischemia. Kuban. Nauchnyj Med. Vestn. 2017, 1, 43–49. (In Russian) [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Prikhodko, V.A.; Titovich, I.A.; Karev, V.E.; Okovityy, S.V. Changes in Somatosensory Evoked Potentials in Rats Following Transient Cerebral Ischemia. Acta Biomed. Sci. 2022, 7, 190–200. (In Russian) [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Prikhodko, V.A.; Kan, A.V.; Titovich, I.A.; Karev, V.E.; Okovityi, S.V. Changes in Brain Electrical Activity after Transient Middle Cerebral Artery Occlusion in Rats. Neurol. Int. 2022, 14, 547–560. [Google Scholar] [CrossRef] [PubMed]
- Chamuleau, R.A.F.M.; Deutz, N.E.P.; de Haan, J.G.; van Gool, J. Correlation between Electroencephalographic and Biochemical Indices in Acute Hepatic Encephalopathy in Rats. J. Hepatol. 1987, 4, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.A.F.; Zhang, W.X.; Cherian, P.V.; Chakrabarti, S. Impaired Visual Evoked Potential and Primary Axonopathy of the Optic Nerve in the Diabetic BB/W-Rat. Diabetologia 1992, 35, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, M.; Cherian, P.V.; Sima, A.A.F. The Preventive Effect of Aldose Reductase Inhibition on Diabetic Optic Neuropathy in the BB/W-Rat. Diabetologia 1993, 36, 893–898. [Google Scholar] [CrossRef]
- Aksoy, D.; Solmaz, V.; Taşkıran, D.; Erbaş, O. The Association between Seizure Predisposition and Inflammation in a Rat Model of Fatty Liver Disease. Neurol. Sci. 2014, 35, 1441–1446. [Google Scholar] [CrossRef]
- Kapitsa, I.; Nerobkova, L.; Valdman, E.; Voronina, T.; Sharkova, L. Electrophysiological Analysis of the Himantane Effects on the Model of Parkinsonian Syndrome Induced by MPP+. Psychopharmacol. Biol. Narcol. 2005, 5, 822–826. (In Russian) [Google Scholar]
- Moyanova, S.G.; Dijkhuizen, R.M. Present Status and Future Challenges of Electroencephalography- and Magnetic Resonance Imaging-Based Monitoring in Preclinical Models of Focal Cerebral Ischemia. Brain Res. Bull. 2014, 102, 22–36. [Google Scholar] [CrossRef]
- Pu, J.; Xu, H.; Wang, Y.; Cui, H.; Hu, Y. Combined Nonlinear Metrics to Evaluate Spontaneous EEG Recordings from Chronic Spinal Cord Injury in a Rat Model: A Pilot Study. Cogn. Neurodyn. 2016, 10, 367–373. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, M.; Lu, Y.; Cao, Y.; Chang, Y.; Dai, Z. The Protective Effects of Dexmedetomidine on Ischemic Brain Injury: A Meta-Analysis. J. Clin. Anesth. 2017, 40, 25–32. [Google Scholar] [CrossRef]
- Sysoev, Y.; Dagaev, S.; Kubarskaja, L.; Gaikova, O.; Uzuegbunam, B.; Modise, K.; Makwana, T.; Okovitiy, S. Study of the Neuroprotective Activity of Mafedine, an Alpha-2 Adrenergic Receptor Agfonist, by Modelling a Traumatic Brain Injury in Rats. Biomedicine 2019, 15, 62–77. (In Russian) [Google Scholar]
- Kamiya, S. Comparative Studies on the EEG Effects of Imipramine and Chlorpromazine in the Normal and Reserpinized Rabbits. Jpn. J. Pharmacol. 1967, 17, 351–363. [Google Scholar] [CrossRef]
- Wallach, M.B.; Winters, W.D.; Mandell, A.J.; Spooner, C.E. A Correlation of EEG, Reticular Multiple Unit Activity and Gross Behavior Following Various Antidepressant Agents in the Cat. IV. Electroencephalogr. Clin. Neurophysiol. 1969, 27, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Suzuki, J. Effects of Psychotropic Drugs on Behavior and EEG, Following Direct Administration to Dog’s Brain BRAIN. Psychiatry Clin. Neurosci. 1966, 20, 151–165. [Google Scholar] [CrossRef]
- Das, N.; Dasgupta, S.; Werner, G. Changes of Behaviour and Electroencephalogram in Rhesus Monkeys Caused by Chlorpromazine. Arch. Int. Pharmacodyn. Ther. 1954, 99, 451–457. [Google Scholar]
- Itil, T.; Polvan, N.; Hsu, W. Clinical and EEG Effects of GB-94, a “Tetracyclic” Antidepressant (EEG Model in Discovery of a New Psychotropic Drug). Curr. Ther. Res. Clin. Exp. 1972, 14, 395–413. [Google Scholar]
- Krijzer, F.; van der Molen, R. Classification of Psychotropic Drugs by Rat EEG Analysis: Learning Set Development. Neuropsychobiology 1986, 16, 205–214. [Google Scholar] [CrossRef]
- Krijzer, F.N.C.M.; van der Molen, R. Classification of Psychotropic Drugs by Rat EEG Analysis: The Anxiolytic Profile in Comparison to the Antidepressant and Neuroleptic Profile. Neuropsychobiology 1987, 18, 51–56. [Google Scholar] [CrossRef]
- Dimpfel, W.; Decker, H. Classification of Sulpiride, Clozapine and Haloperidol by Toposelective Recording from Different Brain Structures in the Immobilized Rat (Stereo-EEG). Neuropsychobiology 1985, 14, 157–164. [Google Scholar] [CrossRef]
- Ruigt, G.S.F.; Engelen, S.; Gerrits, A.; Verbon, F. Computer-Based Prediction of Psychotropic Drug Classes Based on a Discriminant Analysis of Drug Effects on Rat Sleep. Neuropsychobiology 1993, 28, 138–153. [Google Scholar] [CrossRef]
- Dimpfel, W. Preclinical Data Base of Pharmaco-Specific Rat EEG Fingerprints (Tele-Stereo-EEG). Eur. J. Med. Res. 2003, 8, 199–207. [Google Scholar]
- Dimpfel, W. Rat Electropharmacograms of the Flavonoids Rutin and Quercetin in Comparison to Those of Moclobemide and Clinically Used Reference Drugs Suggest Antidepressive and/or Neuroprotective Action. Phytomedicine 2009, 16, 287–294. [Google Scholar] [CrossRef]
- Krijzer, F.; Koopman, P.; Olivier, B. Classification of Psychotropic Drugs Based on Pharmaco-Electrocorticographic Studies in Vigilance-Controlled Rats. Neuropsychobiology 1993, 28, 122–137. [Google Scholar] [CrossRef]
- Nordin, C.; Krijzer, F. Antidepressant and Anxiolytic Profiles of E-10-Hydroxynortriptyline on Electrocorticograms of Rats. Neuropsychobiology 1996, 34, 44–48. [Google Scholar] [CrossRef]
- Herrmann, W.; Schärer, E.; Wendt, G.; Delini-Stula, A. Pharmaco-EEG Profile of Maroxepine. Pharmacopsychiatry 1991, 24, 214–224. [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Shits, D.D.; Puchik, M.M.; Knyazeva, I.S.; Korelov, M.S.; Prikhodko, V.A.; Titovich, I.A.; Selizarova, N.O.; Okovityi, S.V. Pharmacoencephalographic Assessment of Antiphyschotic Agents’ Effect Dose-Dependency in Rats. J. Evol. Biochem. Physiol. 2023, 59, 2153–2167. [Google Scholar] [CrossRef]
- Sysoev, Y.I.; Shits, D.D.; Puchik, M.M.; Prikhodko, V.A.; Idiyatullin, R.D.; Kotelnikova, A.A.; Okovityi, S.V. Use of Naïve Bayes Classifier to Assess the Effects of Antipsychotic Agents on Brain Electrical Activity Parameters in Rats. J. Evol. Biochem. Physiol. 2022, 58, 1130–1141. [Google Scholar] [CrossRef]
- Guo, B.; Zheng, Q. Using Naïve Bayes Algorithm to Estimate the Response to Drug in Lung Cancer Patients. Comb. Chem. High Throughput Screen. 2019, 21, 734–748. [Google Scholar] [CrossRef]
- Ning, Q.; Wang, D.; Cheng, F.; Zhong, Y.; Ding, Q.; You, J. Predicting Rifampicin Resistance Mutations in Bacterial RNA Polymerase Subunit Beta Based on Majority Consensus. BMC Bioinform. 2021, 22, 210. [Google Scholar] [CrossRef]
- Muchira, J.M.; Gona, P.N.; Leveille, S.; Hayman, L.L. A Review of Methodological Approaches for Developing Diagnostic Algorithms for Diabetes Screening. J. Nurs. Meas. 2019, 27, 433–457. [Google Scholar] [CrossRef]
- Nagavelli, U.; Samanta, D.; Chakraborty, P. Machine Learning Technology-Based Heart Disease Detection Models. J. Healthc. Eng. 2022, 2022, 7351061. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, L.; Zou, Y.; Hu, S.Q.; Huang, H.G.; Kong, W.B.; Zhang, J. Predicting Drug-Induced Liver Injury in Human with Naïve Bayes Classifier Approach. J. Comput. Aided. Mol. Des. 2016, 30, 889–898. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.T.; Mao, J.; Shen, C.; Xie, R.L.; Mu, B. Development of Novel in Silico Prediction Model for Drug-Induced Ototoxicity by Using Naïve Bayes Classifier Approach. Toxicol. Vitr. 2020, 65, 104812. [Google Scholar] [CrossRef]
- Hansen, I.H.; Agerskov, C.; Arvastson, L.; Bastlund, J.F.; Sørensen, H.B.D.; Herrik, K.F. Pharmaco-electroencephalographic Responses in the Rat Differ between Active and Inactive Locomotor States. Eur. J. Neurosci. 2019, 50, 1948–1971. [Google Scholar] [CrossRef]
- Ringnér, M. What Is Principal Component Analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Melekhova, A.S.; Shustov, E.B.; Zorina, V.N.; Bespalov, A.Y.; Mel’nikova, M.V.; Belskaya, A.V.; Bondarenko, A.A. Study of the Anticonvulsant Activity of the Original Valproic Acid Amino Ester in Various Models of Convulsive Syndrome. Toxicol. Rev. 2023, 31, 288–296. [Google Scholar] [CrossRef]
- Cai, H.; Cao, T.; Zhou, X.; Yao, J.K. Neurosteroids in Schizophrenia: Pathogenic and Therapeutic Implications. Front. Psychiatry 2018, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.A. Brief Review of the Pharmacology of Amitriptyline and Clinical Outcomes in Treating Fibromyalgia. Biomedicines 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.M. Pattern Recognition and Machine Learning; Springer: New York, NY, USA, 2006. [Google Scholar]
- Sotnikova, T.D.; Beaulieu, J.M.; Barak, L.S.; Wetsel, W.C.; Caron, M.G.; Gainetdinov, R.R. Dopamine-Independent Locomotor Actions of Amphetamines in a Novel Acute Mouse Model of Parkinson Disease. PLoS Biol. 2005, 3, e271. [Google Scholar] [CrossRef] [PubMed]
- Musienko, P.; van den Brand, R.; Marzendorfer, O.; Roy, R.R.; Gerasimenko, Y.; Edgerton, V.R.; Courtine, G. Controlling Specific Locomotor Behaviors through Multidimensional Monoaminergic Modulation of Spinal Circuitries. J. Neurosci. 2011, 31, 9264–9278. [Google Scholar] [CrossRef] [PubMed]
- Cavelli, M.; Castro, S.; Schwarzkopf, N.; Chase, M.H.; Falconi, A.; Torterolo, P. Coherent Neocortical Gamma Oscillations Decrease during REM Sleep in the Rat. Behav. Brain Res. 2015, 281, 318–325. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Mateos, D.; Cavelli, M.; Mondino, A.; Pascovich, C.; Torterolo, P.; Rubido, N. Low Frequency Oscillations Drive EEG’s Complexity Changes during Wakefulness and Sleep. Neuroscience 2022, 494, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.d.; Lopes Aguiar, C.; Moraes, M.F.D.; Fisone, G. Sleep Disorders in Rodent Models of Parkinson’s Disease. Front. Pharmacol. 2019, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Winrow, C.J.; Gotter, A.L.; Cox, C.D.; Doran, S.M.; Tannenbaum, P.L.; Breslin, M.J.; Garson, S.L.; Fox, S.V.; Harrell, C.M.; Stevens, J.; et al. Promotion of Sleep by Suvorexant—ANovel Dual Orexin Receptor Antagonist. J. Neurogenet. 2011, 25, 52–61. [Google Scholar] [CrossRef]
- Hanley, N.; Paulissen, J.; Eastwood, B.J.; Gilmour, G.; Loomis, S.; Wafford, K.A.; McCarthy, A. Pharmacological Modulation of Sleep Homeostasis in Rat: Novel Effects of an MGluR2/3 Antagonist. Sleep 2019, 42, zsz123. [Google Scholar] [CrossRef]
- Benington, J.H.; Kodali, S.K.; Heller, H.C. Scoring Transitions to REM Sleep in Rats Based on the EEG Phenomena of Pre-REM Sleep: An Improved Analysis of Sleep Structure. Sleep 1994, 17, 28–36. [Google Scholar] [CrossRef]
- Lampert, T.; Plano, A.; Austin, J.; Platt, B. On the Identification of Sleep Stages in Mouse Electroencephalography Time-Series. J. Neurosci. Methods 2015, 246, 52–64. [Google Scholar] [CrossRef]
- Geuther, B.; Chen, M.; Galante, R.J.; Han, O.; Lian, J.; George, J.; Pack, A.I.; Kumar, V. High-Throughput Visual Assessment of Sleep Stages in Mice Using Machine Learning. Sleep 2022, 45, zsab260. [Google Scholar] [CrossRef]
- Silva-Pérez, M.; Sánchez-López, A.; Pompa-del-Toro, N.; Escudero, M. Identification of the Sleep–Wake States in Rats Using the High-frequency Activity of the Electroencephalogram. J. Sleep Res. 2021, 30, e13233. [Google Scholar] [CrossRef] [PubMed]
- Pernold, K.; Rullman, E.; Ulfhake, B. Bouts of Rest and Physical Activity in C57BL/6J Mice. PLoS ONE 2023, 18, e0280416. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, B.W.; Bowary, P.M.; Chao, Y.C.; Lii, T.R.; Saab, C.Y. Electroencephalographic Signatures of Pain and Analgesia in Rats. Pain 2016, 157, 2330–2340. [Google Scholar] [CrossRef]
- Sarbadhikari, S.N. A Neural Network Confirms That Physical Exercise Reverses EEG Changes in Depressed Rats. Med. Eng. Phys. 1995, 17, 579–582. [Google Scholar] [CrossRef]
- Ahnaou, A.; Drinkenburg, W.H.I.M. Simultaneous Changes in Sleep, QEEG, Physiology, Behaviour and Neurochemistry in Rats Exposed to Repeated Social Defeat Stress. Neuropsychobiology 2016, 73, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Kantor, S.; Lanigan, M.; Giggins, L.; Lione, L.; Magomedova, L.; de Lannoy, I.; Upton, N.; Duxon, M. Ketamine Supresses REM Sleep and Markedly Increases EEG Gamma Oscillations in the Wistar Kyoto Rat Model of Treatment-Resistant Depression. Behav. Brain Res. 2023, 449, 114473. [Google Scholar] [CrossRef]
- Khanna, A.; Pascual-Leone, A.; Michel, C.M.; Farzan, F. Microstates in Resting-State EEG: Current Status and Future Directions. Neurosci. Biobehav. Rev. 2015, 49, 105–113. [Google Scholar] [CrossRef]
- Sharma, R.; Meena, H.K. Emerging Trends in EEG Signal Processing: A Systematic Review. SN Comput. Sci. 2024, 5, 415. [Google Scholar] [CrossRef]


| Publication | Questions Discussed | Reference |
|---|---|---|
| Methodological standards and interpretation of video-electroencephalography in adult control rodents. A TASK1-WG1 report of the AES/ILAE Translational Task Force of the ILAE. |
| [71] |
| Methodologic recommendations and possible interpretations of video-EEG recordings in immature rodents used as experimental controls: A TASK1-WG2 report of the ILAE/AES Joint Translational Task Force. |
| [28] |
| Methodological standards and functional correlates of depth in vivo electrophysiological recordings in control rodents. A TASK1-WG3 report of the AES/ILAE Translational Task Force of the ILAE |
| [39] |
| Standards for data acquisition and software-based analysis of in vivo electroencephalography recordings from animals. A TASK1-WG5 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia. |
| [72] |
| Model | Phenomena | Time | References |
|---|---|---|---|
| Traumatic brain injury | |||
| Controlled cortical impact injury (sensorimotor cortex area and underlying structures) | ↑ mean amplitude and index of δ-rhythm; ↓ mean amplitude and index of θ-, α-, and β-rhythms. | Days 3 and 7 after the injury | [32] |
| ↓ CCR of interhemispheric and intrahemispheric connections, as well as average coherence powers of δ-, θ-, α-, and β-rhythms. | [31] | ||
| ↑ latency of N1 and N3 peaks of VEP in the area of trauma on day 3 after the trauma with subsequent normalization by day 7; ↑ amplitude of P2 peak on day 3; ↓ P2 peak amplitude on day 7. | [33] | ||
| Decreased amplitudes of early (N1 and P2) and late (N2, P3, and N3) SSEP components, increased latency of early and shortened latency of late waves. | Day 7 after the injury | [76] | |
| Ischemic stroke | |||
| Bilateral ligation of the common carotid arteries | ↑ RSP of δ-rhythm and ↓ RSP of θ-, α-, and β-rhythms in somatosensory cortex. | 24 h after the ligation | [78] |
| Temporary occlusion of MCA (30 and 45 min) | ↑ index of δ-rhythm and ↓ index of θ-, α-, and β-rhythms in the ischemic hemisphere on day 3 after 45 min MCA occlusion; ↑ mean amplitude, index, and mean power of θ-rhythm on day 7; ↓ CCR of interhemispheric and intrahemispheric connections. | Days 3 and 7 after the occlusion | [80] |
| ↓ amplitude of P2 and N3 peaks and P3–N3 interpeak interval of ipsilateral SSEPs; ↓ amplitude of P3 peak and duration of N2–P3 interpeak interval of contralateral SSEPs. | Day 7 after the occlusion | [79] | |
| Temporary occlusion of MCA (90 min) | ↑ power of δ-rhythm in sensorimotor cortex in the acute phase; ↓ power of α-rhythm in sensorimotor cortex in the subacute phase. | Acute phase (3 h and 6 h); subacute phase (12, 24, 48, and 72 h); and chronic period (96, 120, 144, and 168 h). | [77] |
| Neurometabolic disorders | |||
| Hepatic encephalopathy caused by hepatic artery ligation or administration of 1000 mg/kg galactosamine | “Leftward shift” of the spectral power. | 4–5 h (hepatic artery ligation); and 30 h (administration of galactosamine) | [81] |
| Diabetes: BB/Wor rats | ↑ VEP latency. | Month 6 and 12 | [82,83] |
| Non-alcoholic fatty liver disease: 35% fructose solution for 8 weeks | ↑ the number of spikes after pentylenetetrazole administration. | 8 weeks | [84] |
| Neurodegenerative diseases | |||
| A model of parkinsonism induced by intranigral administration of the neurotoxin 1-methyl-4-phenylpyridinium ion (MFP+) | Registration of synchronous discharges of high-amplitude slow waves in the range of δ- and θ-frequencies with amplitude of 150–200 μV in SMC, SN, and CN (24 h); ↑ δ-activity in CN (24 h); ↑ θ-activity in SMC and SN (24 h); ↑ power of θ-rhythms in SMC, SN, and CN (day 7); ↑ β-activity in the SMC of the right hemisphere (day 7). | 24 h and day 7 | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sysoev, Y.I.; Okovityi, S.V. Prospects of Electrocorticography in Neuropharmacological Studies in Small Laboratory Animals. Brain Sci. 2024, 14, 772. https://doi.org/10.3390/brainsci14080772
Sysoev YI, Okovityi SV. Prospects of Electrocorticography in Neuropharmacological Studies in Small Laboratory Animals. Brain Sciences. 2024; 14(8):772. https://doi.org/10.3390/brainsci14080772
Chicago/Turabian StyleSysoev, Yuriy I., and Sergey V. Okovityi. 2024. "Prospects of Electrocorticography in Neuropharmacological Studies in Small Laboratory Animals" Brain Sciences 14, no. 8: 772. https://doi.org/10.3390/brainsci14080772
APA StyleSysoev, Y. I., & Okovityi, S. V. (2024). Prospects of Electrocorticography in Neuropharmacological Studies in Small Laboratory Animals. Brain Sciences, 14(8), 772. https://doi.org/10.3390/brainsci14080772






