Effect of Aerobic Exercise versus Non-Invasive Brain Stimulation on Cognitive Function in Multiple Sclerosis: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
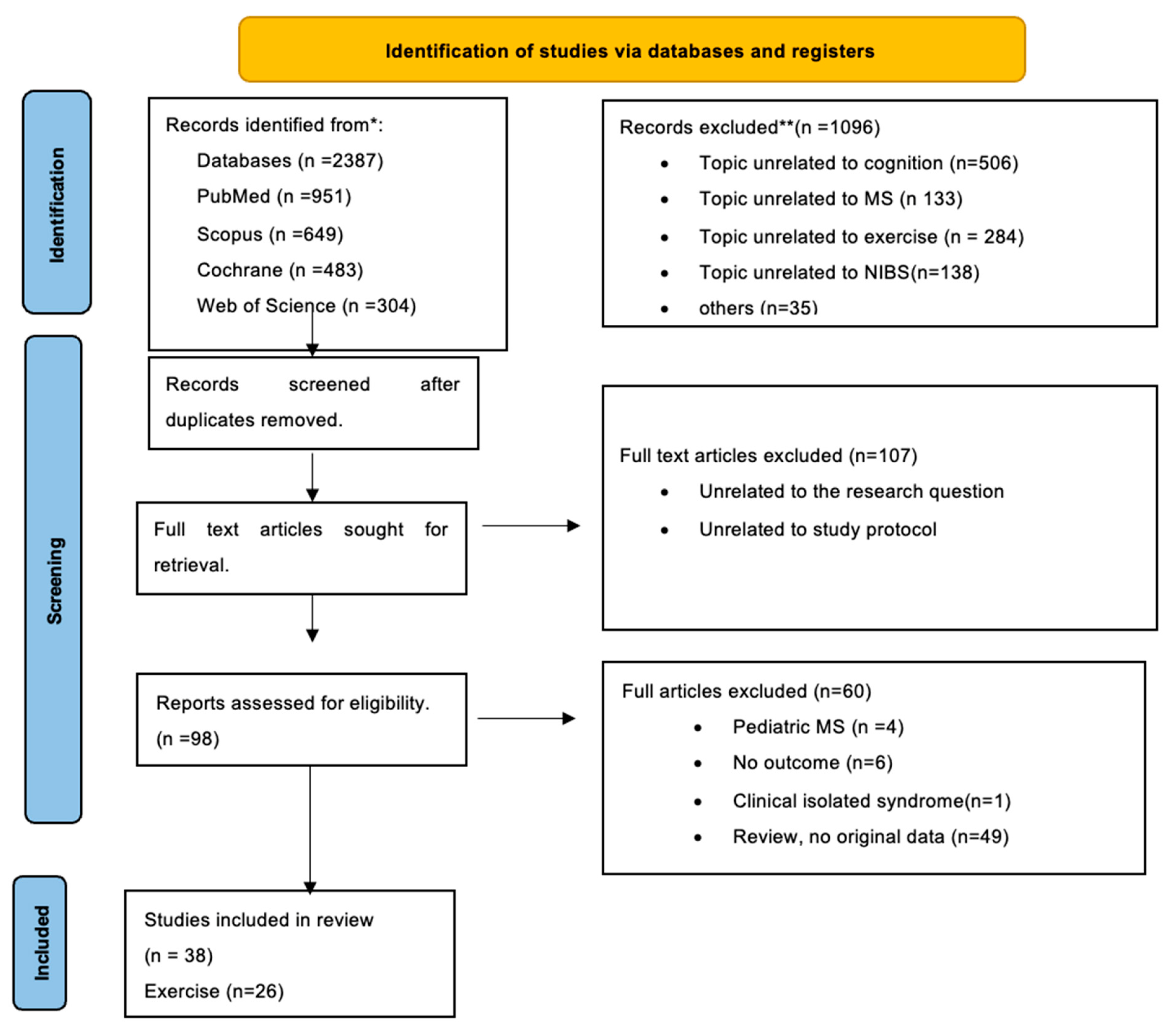
2. Methods
Data Extraction and Statistical Analyses
3. Results
3.1. Effect of NIBS on Cognition among pwMS
3.2. Effect of Exercise on Cognition among pwMS
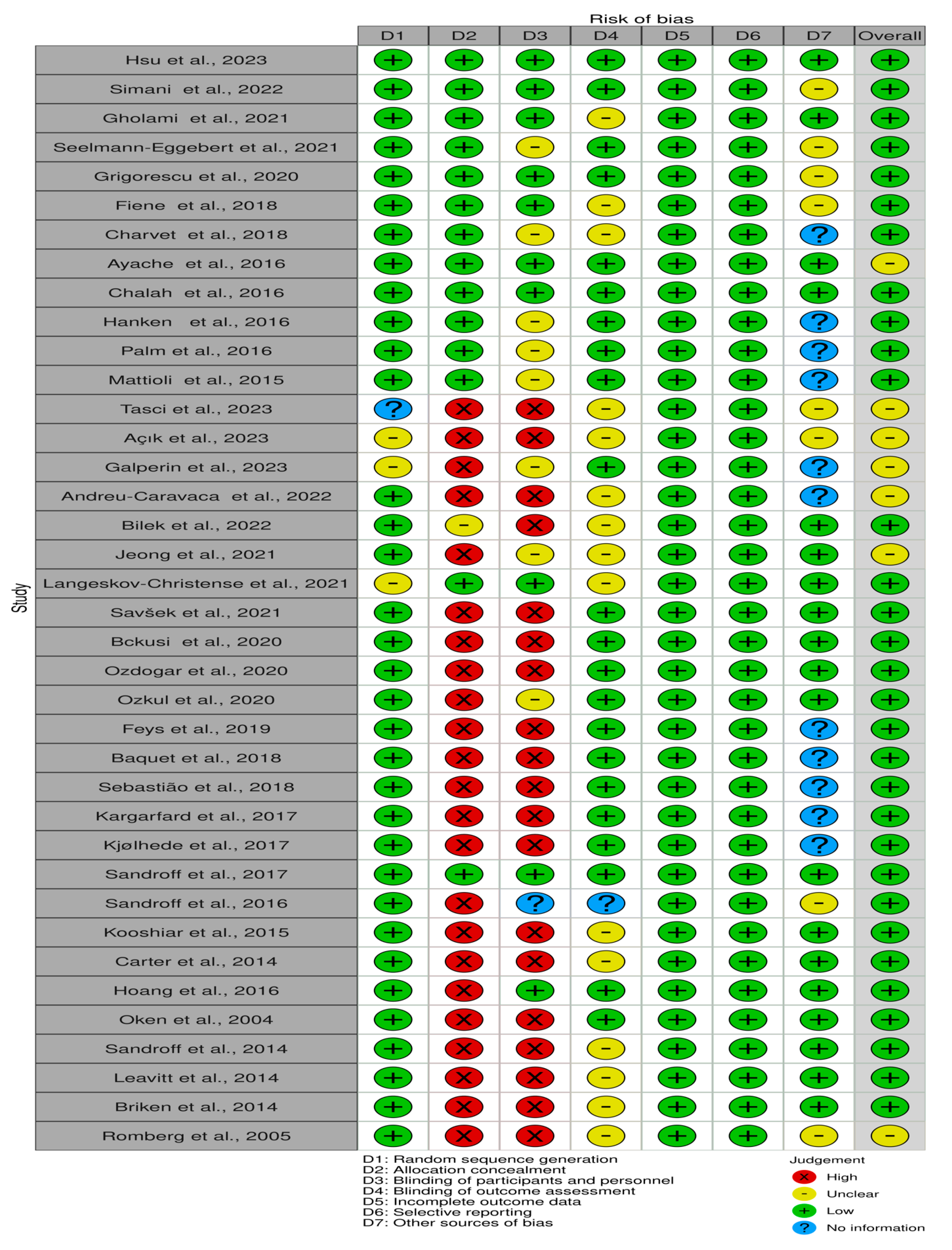
3.3. Risk of Bias Assessment
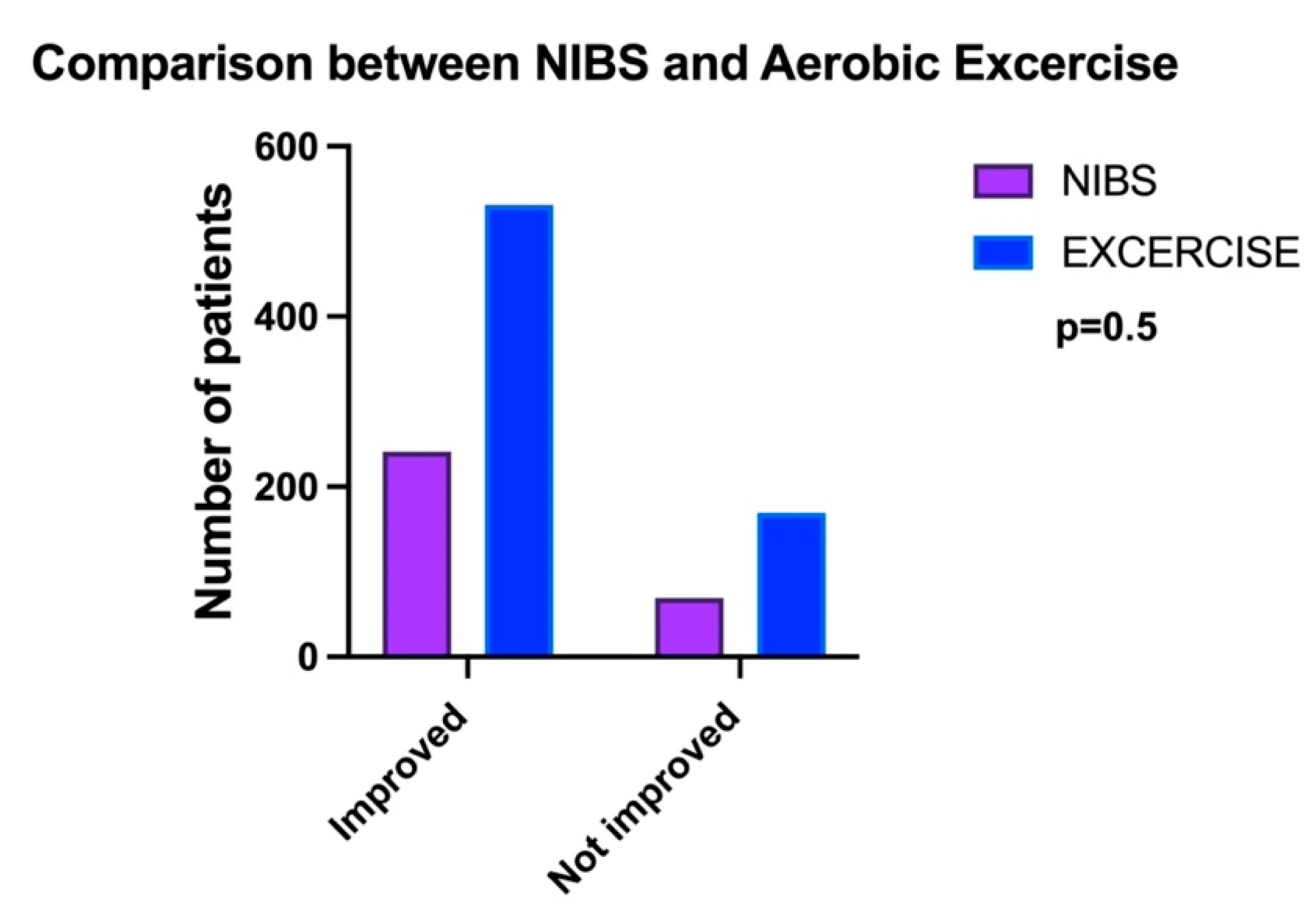
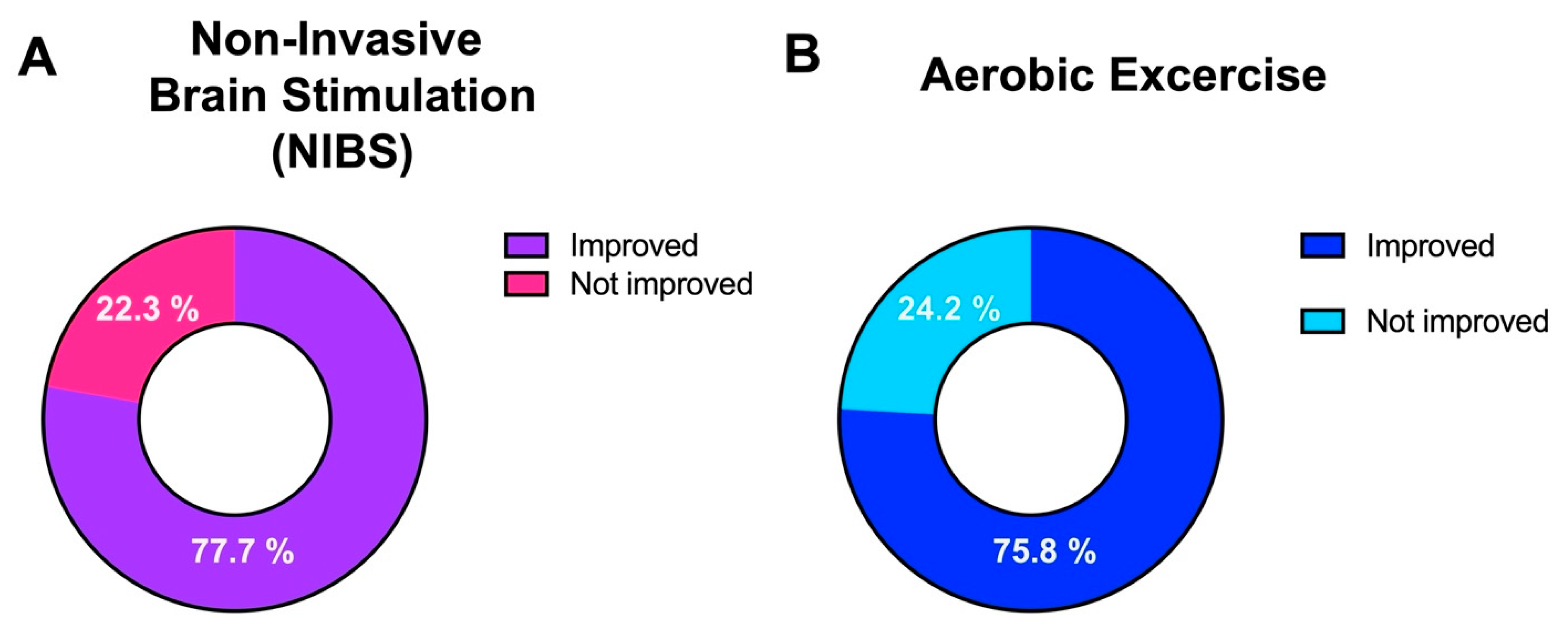
Comparison between NIBS and Exercise on Cognition
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Planche, V.; Gibelin, M.; Cregut, D.; Pereira, B.; Clavelou, P. Cognitive impairment in a population-based study of patients with multiple sclerosis: Differences between late relapsing−remitting, secondary progressive and primary progressive multiple sclerosis. Eur. J. Neurol. 2015, 23, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Koch-Henriksen, N.; Magyari, M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat. Rev. Neurol. 2021, 17, 676–688. [Google Scholar] [CrossRef]
- Oh, J.; Vidal-Jordana, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.G.; Russo, M.; Cuzzola, M.F.; Destro, M.; La Rosa, G.; Molonia, F.; Bramanti, P.; Lombardo, G.; De Luca, R.; Calabrò, R.S. Virtual reality in multiple sclerosis rehabilitation: A review on cognitive and motor outcomes. J. Clin. Neurosci. 2019, 65, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Q.; Zheng, S.; Li, G.; Li, S.; He, L.; Zeng, Y.; Chen, L.; Chen, S.; Zheng, X.; et al. Efficacy of non-invasive brain stimulation on cognitive and motor functions in multiple sclerosis: A systematic review and meta-analysis. Front. Neurol. 2023, 14, 1091252. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, X.; Liu, Q.; Zhao, P.; Zhong, J.; Pan, P.; Wang, G.; Yi, Z. Social cognition in multiple sclerosis and its subtypes: A meta-analysis. Mult. Scler. Relat. Disord. 2021, 52, 102973. [Google Scholar] [CrossRef] [PubMed]
- Elkhooly, M.; Bao, F.; Raghib, M.; Millis, S.; Bernitsas, E. Role of white matter in cognitive impairment among relapsing remitting multiple sclerosis patients. Mult. Scler. Relat. Disord. 2023, 79, 105030. [Google Scholar] [CrossRef] [PubMed]
- Bross, M.; Hackett, M.; Bernitsas, M.M.; Bao, F.; Carla Santiago, M.; Bernitsas, E. Cortical surface thickness, subcortical volumes and disability between races in relapsing-remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 53, 103025. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.P.; Langdon, D.; Montalban, X.; Benedict, R.H.B.; DeLuca, J.; Krupp, L.B.; Thompson, A.J.; Comi, G. Treatment of cognitive impairment in multiple sclerosis: Position paper. J. Neurol. 2012, 260, 1452–1468. [Google Scholar] [CrossRef] [PubMed]
- Elkhooly, M.; Bao, F.; Bernitsas, E. Impact of Disease Modifying Therapy on MS-Related Fatigue: A Narrative Review. Brain Sci. 2023, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Bross, M.; Hackett, M.; Bernitsas, E. Approved and emerging disease modifying therapies on neurodegeneration in multiple sclerosis. Int. J. Mol. Sci. 2020, 21, 4312. [Google Scholar] [CrossRef] [PubMed]
- Leocani, L.; Chieffo, R.; Gentile, A.; Centonze, D. Beyond rehabilitation in MS: Insights from non-invasive brain stimulation. Mult. Scler. 2019, 25, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.G.; Rapport, L.J.; Darling, R.; Waldron-Perrine, B.; Lumley, M.A.; Whitfield, K.E.; Bernitsas, E. Cognitive enrichment and education quality moderate cognitive dysfunction in black and white adults with multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 78, 104916. [Google Scholar] [CrossRef] [PubMed]
- Begemann, M.J.; Brand, B.A.; Ćurčić-Blake, B.; Aleman, A.; Sommer, I.E. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: A meta-analysis. Psychol. Med. 2020, 50, 2465–2486. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.-C.; Guo, N.-W.; Su, B.-Y.; Chen, S.-J.; Tsai, H.-F.; Lee, K.-Y. Frontal Beta Activity in the Meta-Intention of Children With Attention Deficit Hyperactivity Disorder. Clin. EEG Neurosci. 2020, 52, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Boes, A.D.; Kelly, M.S.; Trapp, N.T.; Stern, A.P.; Press, D.Z.; Pascual-Leone, A. Noninvasive Brain Stimulation: Challenges and Opportunities for a New Clinical Specialty. J. Neuropsychiatry Clin. Neurosci. 2018, 30, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Di Lazzaro, V.; Rothwell, J.; Capogna, M. Noninvasive Stimulation of the Human Brain: Activation of Multiple Cortical Circuits. Neuroscientist 2017, 24, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, C.; Guclu-Gunduz, A.; Eldemir, K.; Apaydin, Y.; Yazici, G.; Irkec, C. Combined exercise training improves cognitive functions in multiple sclerosis patients with cognitive impairment: A single-blinded randomized controlled trial. Mult. Scler. Relat. Disord. 2020, 45, 102419. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Wylie, G.R.; Sutton, B.P.; Johnson, C.L.; DeLuca, J.; Motl, R.W. Treadmill walking exercise training and brain function in multiple sclerosis: Preliminary evidence setting the stage for a network-based approach to rehabilitation. Mult. Scler. J. Exp. Transl. Clin. 2018, 4, 2055217318760641. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Johnson, C.L.; Motl, R.W. Exercise training effects on memory and hippocampal viscoelasticity in multiple sclerosis: A novel application of magnetic resonance elastography. Neuroradiology 2016, 59, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Klaren, R.E.; Pilutti, L.A.; Dlugonski, D.; Benedict, R.H.B.; Motl, R.W. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. J. Neurol. 2013, 261, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Langeskov-Christensen, M.; Grondahl Hvid, L.; Boye Jensen, H.; Hvilsted Nielsen, H.; Petersen, T.; Stenager, E.; Hamalainen, P.; Dalgas, U. High-intensity aerobic exercise does not improve cognitive performance in people with multiple sclerosis: A randomised controlled trial. Mult. Scler. J. 2019, 25, 900–901. [Google Scholar] [CrossRef]
- Kooshiar, H.; Moshtagh, M.; Sardar, M.A.; Foroughipour, M.; Shakeri, M.T.; Vahdatinia, B. Fatigue and quality of life of women with multiple sclerosis: A randomized controlled clinical trial. J. Sports Med. Phys. Fit. 2015, 55, 668–674. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Centre for Reviews and Dissemination (CRD). Encyclopedia of Public Health; Springer: Dordrecht, The Netherlands, 2008; p. 105. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Cobb, W.; London, G.B.; Gastaut, H.; Hess, R., Jr.; Jung, R.; Magnus, O.; Terzian, H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1958, 10, 370–375. [Google Scholar]
- Hartwigsen, G.; Silvanto, J. Noninvasive brain stimulation: Multiple effects on cognition. Neuroscientist 2023, 29, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.R.; Pogosyan, A.; Brown, P.; Brittain, J.-S. Montage matters: The influence of transcranial alternating current stimulation on human physiological tremor. Brain Stimul. 2015, 8, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Veniero, D.; Strüber, D.; Thut, G.; Herrmann, C.S. Noninvasive brain stimulation techniques can modulate cognitive processing. Organ. Res. Methods 2019, 22, 116–147. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Zanto, T.; Park, J.E.; Gazzaley, A.; Bove, R.M. Effects of transcranial alternating current stimulation on cognitive function in people with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2023, 80, 105090. [Google Scholar] [CrossRef] [PubMed]
- Simani, L.; Roozbeh, M.; Shojaei, M.; Ramezani, M.; Roozbeh, M.; Gharehgozli, K.; Rostami, M. The effectiveness of anodal tDCS and cognitive training on cognitive functions in multiple sclerosis; a randomized, double-blind, parallel-group study. Mult. Scler. Relat. Disord. 2022, 68, 104392. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Nami, M.; Shamsi, F.; Jaberi, K.R.; Kateb, B.; Rahimi Jaberi, A. Effects of transcranial direct current stimulation on cognitive dysfunction in multiple sclerosis. Neurophysiol. Clin. 2021, 51, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Seelmann-Eggebert, H.; Stoppe, M.; Then Bergh, F.; Classen, J.; Rumpf, J.-J. Motor Sequence Learning across Multiple Sessions Is Not Facilitated by Targeting Consolidation with Posttraining tDCS in Patients with Progressive Multiple Sclerosis. Neural Plast. 2021, 2021, 6696341. [Google Scholar] [CrossRef] [PubMed]
- Grigorescu, C.; Chalah, M.A.; Lefaucheur, J.-P.; Kümpfel, T.; Padberg, F.; Ayache, S.S.; Palm, U. Effects of transcranial direct current stimulation on information processing speed, working memory, attention, and social cognition in multiple sclerosis. Front. Neurol. 2020, 11, 545377. [Google Scholar] [CrossRef] [PubMed]
- Fiene, M.; Rufener, K.S.; Kuehne, M.; Matzke, M.; Heinze, H.J.; Zaehle, T. Electrophysiological and behavioral effects of frontal transcranial direct current stimulation on cognitive fatigue in multiple sclerosis. J. Neurol. 2018, 265, 607–617. [Google Scholar] [CrossRef]
- Charvet, L.; Shaw, M.; Dobbs, B.; Frontario, A.; Sherman, K.; Bikson, M.; Datta, A.; Krupp, L.; Zeinapour, E.; Kasschau, M. Remotely supervised transcranial direct current stimulation increases the benefit of at-home cognitive training in multiple sclerosis. Neuromodulation Technol. Neural Interface 2018, 21, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Ayache, S.S.; Palm, U.; Chalah, M.A.; Al-Ani, T.; Brignol, A.; Abdellaoui, M.; Dimitri, D.; Sorel, M.; Créange, A.; Lefaucheur, J.-P. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Front. Neurosci. 2016, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Chalah, M.A.; Riachi, N.; Ahdab, R.; Mhalla, A.; Abdellaoui, M.; Créange, A.; Lefaucheur, J.-P.; Ayache, S.S. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J. Neurol. Sci. 2017, 372, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Hanken, K.; Bosse, M.; Möhrke, K.; Eling, P.; Kastrup, A.; Antal, A.; Hildebrandt, H. Counteracting Fatigue in Multiple Sclerosis with Right Parietal Anodal Transcranial Direct Current Stimulation. Front. Neurol. 2016, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Palm, U.; Chalah, M.A.; Padberg, F.; Al-Ani, T.; Abdellaoui, M.; Sorel, M.; Dimitri, D.; Créange, A.; Lefaucheur, J.-P.; Ayache, S.S. Effects of transcranial random noise stimulation (tRNS) on affect, pain and attention in multiple sclerosis. Restor. Neurol. Neurosci. 2016, 34, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, F.; Bellomi, F.; Stampatori, C.; Capra, R.; Miniussi, C. Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Mult. Scler. 2016, 22, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Tasci, I.; Demir, C.F.; Bilek, F.; Albayrak, S. Physical exercise may improve problem-solving skills and emotional intelligence in patients with relapsing-remitting multiple sclerosis: A cross-sectional study. Mult. Scler. Relat. Disord. 2022, 59, 103641. [Google Scholar] [CrossRef]
- Açık, M.; Şenışık, S.; Taşkıran, D.; Akşit, T.; Aydınoğlu, R.; Yüceyar, A.N. Exercise Improves Physical Capacity, Cognition, Quality of Life and Promotes Neurotrophic Factors in Patients with Multiple Sclerosis. Noro Psikiyatr. Ars. 2023, 60, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Galperin, I.; Mirelman, A.; Schmitz-Hubsch, T.; Hsieh, K.L.; Regev, K.; Karni, A.; Brozgol, M.; Cornejo Thumm, P.; Lynch, S.G.; Paul, F.; et al. Treadmill training with virtual reality to enhance gait and cognitive function among people with multiple sclerosis: A randomized controlled trial. J. Neurol. 2023, 270, 1388–1401. [Google Scholar] [CrossRef] [PubMed]
- Andreu-Caravaca, L.; Ramos-Campo, D.J.; Chung, L.H.; Manonelles, P.; Abellán-Aynés, O.; Rubio-Arias, J. Effects of fast-velocity concentric resistance training in people with multiple sclerosis: A randomized controlled trial. Acta Neurol. Scand. 2022, 146, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Bilek, F.; Cetisli-Korkmaz, N.; Ercan, Z.; Deniz, G.; Demir, C.F. Aerobic exercise increases irisin serum levels and improves depression and fatigue in patients with relapsing remitting multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2022, 61, 103742. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.c.; Karpatkin, H.; Finkelstein, J. Physical Telerehabilitation Improves Quality of Life in Patients with Multiple Sclerosis. In Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Langeskov-Christensen, M.; Hvid, L.G.; Jensen, H.B.; Nielsen, H.H.; Petersen, T.; Stenager, E.; Hämäläinen, P.; Dalgas, U. Efficacy of high-intensity aerobic exercise on cognitive performance in people with multiple sclerosis: A randomized controlled trial. Mult. Scler. J. 2020, 27, 1585–1596. [Google Scholar] [CrossRef] [PubMed]
- Savšek, L.; Stergar, T.; Strojnik, V.; Ihan, A.; Koren, A.; Špiclin, Ž.; Šega Jazbec, S. Impact of aerobic exercise on clinical and magnetic resonance imaging biomarkers in persons with multiple sclerosis: An exploratory randomized controlled trial. J. Rehabil. Med. 2021, 53, jrm00178. [Google Scholar] [CrossRef] [PubMed]
- Backus, D.; Moldavskiy, M.; Sweatman, W.M. Effects of functional electrical stimulation cycling on fatigue and quality of life in people with multiple sclerosis who are nonambulatory. Int. J. MS Care 2020, 22, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ozdogar, A.T.; Ertekin, O.; Kahraman, T.; Yigit, P.; Ozakbas, S. Effect of video-based exergaming on arm and cognitive function in persons with multiple sclerosis: A randomized controlled trial. Mult. Scler. Relat. Disord. 2020, 40, 101966. [Google Scholar] [CrossRef] [PubMed]
- Feys, P.; Moumdjian, L.; Van Halewyck, F.; Wens, I.; Eijnde, B.O.; Van Wijmeersch, B.; Popescu, V.; Van Asch, P. Effects of an individual 12-week community-located “start-to-run” program on physical capacity, walking, fatigue, cognitive function, brain volumes, and structures in persons with multiple sclerosis. Mult. Scler. J. 2019, 25, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Baquet, L.; Hasselmann, H.; Patra, S.; Stellmann, J.-P.; Vettorazzi, E.; Engel, A.K.; Rosenkranz, S.C.; Poettgen, J.; Gold, S.M.; Schulz, K.-H.; et al. Short-term interval aerobic exercise training does not improve memory functioning in relapsing-remitting multiple sclerosis-a randomized controlled trial. PeerJ 2018, 6, e6037. [Google Scholar] [CrossRef] [PubMed]
- Sebastião, E.; McAuley, E.; Shigematsu, R.; Adamson, B.C.; Bollaert, R.E.; Motl, R.W. Home-based, square-stepping exercise program among older adults with multiple sclerosis: Results of a feasibility randomized controlled study. Contemp. Clin. Trials 2018, 73, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Kara, B.; Küçük, F.; Poyraz, E.C.; Tomruk, M.S.; İdıman, E. Different types of exercise in Multiple Sclerosis: Aerobic exercise or Pilates, a single-blind clinical study. J. Back. Musculoskelet. Rehabil. 2017, 30, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Kjølhede, T.; Siemonsen, S.; Wenzel, D.; Stellmann, J.-P.; Ringgaard, S.; Pedersen, B.G.; Stenager, E.; Petersen, T.; Vissing, K.; Heesen, C. Can resistance training impact MRI outcomes in relapsing-remitting multiple sclerosis? Mult. Scler. J. 2018, 24, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Bollaert, R.E.; Pilutti, L.A.; Peterson, M.L.; Baynard, T.; Fernhall, B.; McAuley, E.; Motl, R.W. Multimodal exercise training in multiple sclerosis: A randomized controlled trial in persons with substantial mobility disability. Contemp. Clin. Trials 2017, 61, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Daley, A.; Humphreys, L.; Snowdon, N.; Woodroofe, N.; Petty, J.; Roalfe, A.; Tosh, J.; Sharrack, B.; Saxton, J. Pragmatic intervention for increasing self-directed exercise behaviour and improving important health outcomes in people with multiple sclerosis: A randomised controlled trial. Mult. Scler. J. 2014, 20, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Hoang, P.; Schoene, D.; Gandevia, S.; Smith, S.; Lord, S.R. Effects of a home-based step training programme on balance, stepping, cognition and functional performance in people with multiple sclerosis–a randomized controlled trial. Mult. Scler. J. 2016, 22, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Oken, B.S.; Kishiyama, S.; Zajdel, D.; Bourdette, D.; Carlsen, J.; Haas, M.; Hugos, C.; Kraemer, D.F.; Lawrence, J.; Mass, M. Randomized controlled trial of yoga and exercise in multiple sclerosis. Neurology 2004, 62, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Dlugonski, D.; Pilutti, L.A.; Pula, J.H.; Benedict, R.H.B.; Motl, R.W. Physical activity is associated with cognitive processing speed in persons with multiple sclerosis. Mult. Scler. Relat. Disord. 2014, 3, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, V.; Cohen, A.; Farag, A.; Cirnigliaro, C.; Chiaravalloti, N.; Sumowski, J.; DeLuca, J. Aerobic exercise increases hippocampal volume and improves memory in persons with multiple sclerosis: Pilot findings from a randomized controlled trial. Neurology 2013, 80, 695–697. [Google Scholar] [CrossRef]
- Briken, S.; Gold, S.M.; Patra, S.; Vettorazzi, E.; Harbs, D.; Tallner, A.; Ketels, G.; Schulz, K.H.; Heesen, C. Effects of exercise on fitness and cognition in progressive MS: A randomized, controlled pilot trial. Mult. Scler. 2014, 20, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Romberg, A.; Virtanen, A.; Ruutiainen, J. Long–term exercise improves functional impairment but not quality of life in multiple sclerosis. J. Neurol. 2005, 252, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Baione, V.; Belvisi, D.; Cortese, A.; Cetta, I.; Tartaglia, M.; Millefiorini, E.; Berardelli, A.; Conte, A. Cortical M1 plasticity and metaplasticity in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 38, 101494. [Google Scholar] [CrossRef]
- Rocca, M.A.; Filippi, M. Modulation of cortical excitability to normalise brain function and improve cognition in multiple sclerosis. J. Neurol. Neurosurg. Amp Psychiatry 2016, 88, 373. [Google Scholar] [CrossRef] [PubMed]
- Miniussi, C.; Harris, J.A.; Ruzzoli, M. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci. Amp Biobehav. Rev. 2013, 37, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; You, Q.; Hou, X.; Zhang, S.; Du, L.; Lv, Y.; Yu, L. The effect of exercise on cognitive function in people with multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2023, 270, 2908–2923. [Google Scholar] [CrossRef] [PubMed]
- Gharakhanlou, R.; Wesselmann, L.; Rademacher, A.; Lampit, A.; Negaresh, R.; Kaviani, M.; Oberste, M.; Motl, R.W.; Sandroff, B.M.; Bansi, J. Exercise training and cognitive performance in persons with multiple sclerosis: A systematic review and multilevel meta-analysis of clinical trials. Mult. Scler. J. 2021, 27, 1977–1993. [Google Scholar] [CrossRef] [PubMed]
- Van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [PubMed]
- Kong, L.; Miu, L.; Yao, W.; Shi, Z. Effect of regular aerobic exercise on cognitive function, depression level and regulative role of neurotrophic factor: A prospective cohort study in the young and the middle-aged sample. Risk Manag. Healthc. Policy 2024, 17, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Proschinger, S.; Joisten, N.; Rademacher, A.; Schlagheck, M.L.; Walzik, D.; Metcalfe, A.J.; Oberste, M.; Warnke, C.; Bloch, W.; Schenk, A.; et al. Influence of combined functional resistance and endurance exercise over 12 weeks on matrix metalloproteinase-2 serum concentration in persons with relapsing-remitting multiple sclerosis—A community-based randomized controlled trial. BMC Neurol. 2019, 19, 314. [Google Scholar] [CrossRef]
- Laurin, D.; Verreault, R.; Lindsay, J.; MacPherson, K.; Rockwood, K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch. Neurol. 2001, 58, 498–504. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, A.; Solana, E.; Corpas, R.; Bartrés-Faz, D.; Pallàs, M.; Vina, J.; Sanfeliu, C.; Gomez-Cabrera, M.C. Long-term exercise training improves memory in middle-aged men and modulates peripheral levels of BDNF and Cathepsin B. Sci. Rep. 2019, 9, 3337. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, M.; Banitalebi, E.; Raisi, Z.; Samieyan, M.; Saberi, Z.; Ghahfarrokhi, M.M.; Negaresh, R.; Motl, R.W. Effect of combined exercise training on pentraxins and pro-inflammatory cytokines in people with multiple sclerosis as a function of disability status. Cytokine 2020, 134, 155196. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Gilio, L.; Buttari, F.; Maffei, P.; Marfia, G.A.; Restivo, D.A.; Centonze, D.; Iezzi, E. Remodeling functional connectivity in multiple sclerosis: A challenging therapeutic approach. Front. Neurosci. 2017, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chen, C.; Zhu, T.; Sun, Z.; Li, S.; Gong, L.; Dong, X.; Shen, W.; Zeng, L.; Xie, Y. TRPM2 contributes to neuroinflammation and cognitive deficits in a cuprizone-induced multiple sclerosis model via NLRP3 inflammasome. Neurobiol. Dis. 2021, 160, 105534. [Google Scholar] [CrossRef] [PubMed]
| Study/Year/Country | Design | Participant | Intervention | Location | Intensity | Duration | Tool/Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | Control | Experimental | Control | |||||||
| Hsu/2023/USA [36] | parallel | 19 | 18 | 20 | tACS | Sham | NA | 2 mA, 1 mA | 20 min | SDMT improved with both 1 and 2 mA. |
| Simani et al./2022/Iran [37] | parallel | 20 | 20 | tDCS, CT | CT | A: F3 C: right shoulder | 2 mA | 30 min | IVA-2 Improved attention and inhibitory control | |
| Gholami et al. (2021)/Iran/2021 [38] | parallel | 12 | 12 | tDCS | sham tDCS | A: F3 C: FP2 | 2 mA | 20 min | CBS-CP improved reasoning and executive function | |
| Seelmann-Eggebert/Germany/2021 [39] | Crossover | 16 | tDCS and sham 4 weeks washout | A: C3 C: supra orbital | 1 mA | 15 min | SDMT: no improvement | |||
| Grigorescu et al./Germany/2020 [40] | Crossover | 11 | tDCS or sham with 3-week washout interval | A: F3 C: F4 | 2 mA | 20 | SDMT: not improved | |||
| Fiene er al./Germany/2018 [41] | Crossover | 15 | tDCS with one-week washout interval | A; F3 C: right shoulder | 1.5 mA | 30 | RT: cognitive fatigue improves | |||
| Charvet et al./USA/2018 [42] | parallel | 25 | 20 | RS tDCS, CT | CT | A: F3 C: F4 | 1.5 mA | 20 min | BICAMS, ANT, IV Improve complex attention and response variability | |
| Ayache et al./France/2016 [43] | Crossover | 16 | block of tDCS active or sham with 3 w washout | A: F3 C: Supraorbital | 2 mA | 20 min | SDMT not improved | |||
| Chalah et al./France/2016 [44] | Crossover | 10 | block tDCS active or sham with 3 w washout | A: F3 or P4 C: CZ | 2 mA | 20 min | ANT: not improved | |||
| Hanken et al./Germany/2016 [45] | parallel | 20 | 20 | tDCS, Vigilant task | Sham tDCS Vigilant | A: P4 C: forehead | 1.5 mA | 20 | RT: improved | |
| Palm/Germnay/2016 [46] | Crossover | 16 | blocks of tRANS followed by 3 w washout | A: F3 C: AF4 | 2 | NA | ANT not improved | |||
| Mattioli et al./Italy/2015 [47] | parallel | 10 | 10 | tDCS CT | Sham tDCS CT | A:F3 C: Right shoulder | 2 | 20 | PASAT, WCST and SDMT improve attention and executive function | |
| Study/Year/Country | Design | Participant | Intervention | Frequency | Duration | Duration Per Session | Tool/Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | Control | Experimental | Control | |||||||
| Tasci/2023/Turkey [48] | RCT | 18 | 18 | Aerobic, strength | No intervention | 3 | 12 w | NA | PBI, MFIS improved | |
| Açık/2023/Turkey [49] | RCT | Aerobic 16 | Strength 11 | 16 | Aerobic, strength | No intervention | 3 | 12 w | NA | BICAMS improved |
| Galperin//2023/USA [50] | RCT, parallel | 62 | 62 | Treadmill training with virtual reality | treadmill training | 3 | 6 w | NA | SDMT improved | |
| Andreu-Caravaca et al./2022/Germany [51] | RCT, parallel | 18 | 12 | Resistance | No intervention | 3/W | 10 w | NA | MSQOL-54 cognition improved | |
| Bilek/2022/Turkey [52] | RCT, parallel | 34 | 34 | Aerobic and Frenkel coordination exercise | Frenkel coordination only | 3 w | 6 w | 30 min | PASAT improved | |
| Jeong/2021/USA [53] | RCT, parallel | 29 | 16 | Telerehabilitation system plus home-based exercise | Home-based exercise only | 7/w | 12 W | NA | MSQOL cognitive improved | |
| Langeskov-Christense/2021/Denmark [54] | RCT, parallel | 43 | 43 | Supervised progressive aerobic exercise followed by self-guided physical exercise | Habitual lifestyle then supervised progressive aerobic exercise | 2/w | 24 w | 60 min | SRT-LT SRT-C SRT-D SPART-D WLG PASAST, SDMT only improved; the rest, no effect | |
| Savšek/2021/Slovenia [55] | RCT, parallel | 14 | 14 | Aerobic | No intervention | 2 | 12 w | 60 min | MFIS SDMT BVMT-R CVLT-II No improvement | |
| Bckusi et al. (2020)/USA [56] | RCT, parallel | 6 | 6 | Aerobic/Functional electrical stimulation | No intervention | 3/w | 12 W | 30 min | MSQOL-54 cognition improved | |
| Ozdogar/2020/Turkey [57] | RCT, Parallel | Video-based, 21; conventional, 19 | 20 | Video-based core stabilization and vs. conventional rehab vs. control group | 1/w | 8 W | 45 min | CVLT, BVMT-R, SDMT: all are improved | ||
| Ozk §0/Turkey [19] | RCT, parallel | 17 | 17 | Aerobic and Pilates | Relaxation exercise | 3/w | 8 w | 90 min | SRT-ST SRT-LT SDMT, SPART-T, SPART-D, PASAST, WLG Improved verbal memory, visuospatial, verbal fluency in the | |
| Feys/2019/Belgium [58] | RCT, parallel | 21 | 21 | Aerobic | No intervention | 3 | 12 w | NA | PASAT, WLG, SPART, SRT, DSST all improved | |
| Baquet/2018/Germany [59] | RCT, parallel | 34 | 34 | Aerobic | No intervention | 2 | 12 w | 54.5 | SDMT, PASAT, TAP, BVMT, RWT no improvement | |
| Sebastião,/2018/USA [60] | RCT, parallel | 15 | 10 | Aerobic | No intervention | 2 | 12 w | 20 min | SDMT, CVLT, BVMT, PASAT no improvement due to small sample size | |
| Kargarfard/2017/Iran [61] | RCT, parallel | 17 | 15 | Aerobic functional exercise and balance | No intervention | 3 | 8 w | 60 min | MFIS improved | |
| Kjølhede/2017/Denmark [62] | RCT, parallel | 17 | 12 | Resistance | No intervention | 2 | 24 w | 55 min | MSFC improved, TUG | |
| Sandroff/2017/USA [63] | RCT, parallel | 32 | 30 | Aerobic, balance and resistance | Stretching | 3 | 24 w | 60 min | SDMT not improved, PASAT improved | |
| Sandroff/2016/USA [21] | RCT, parallel | 4 | 4 | Aerobic, treadmill | NA | 3 | 12 W | 20 min | CVLT-II improved, MFT, DKFFS, | |
| Kooshiar/2015/Iran [24] | RCT, parallel | 18 | 19 | Stretching | No intervention | 3 | 8 w | 45 min | MFIS, PASAT cognitive no improved | |
| Carter/2014/UK [64] | RCT, parallel | 60 | 60 | Aerobic | No intervention | 3 | 6 w | 60 min | MSQOL improved. PASAT. | |
| Hoang/2016/Australia [65] | RCT, parallel | 28 | 22 | Aerobic | Regular physical therapy | 2 | 12 w | 30 min | SDMT, TMT, TUG all are improved | |
| Oken/2004/turkey [66] | RCT, parallel | 15 | 20 | Aerobic | No intervention | 1 | 24 w | 90 min | SCWT no improvement | |
| Oken/2004/turkey [66] | RCT, parallel | 22 | 20 | Yoga | No intervention | 1 | 24 w | 90 min | SCWT no improvement | |
| Sandroff/2014/USA [67] | RCT | 37 | 39 | Aerobic | No intervention | NA | 24 w | NA | SDMT improved | |
| Leavitt/2014/USA [68] | RCT, parallel | 1 | 1 | Aerobic | No intervention | NA | 12 W | NA | CVLT-II BVMT-R SDMT All were improved. | |
| Briken/2014/Germany [69] | RCT progressive MS | Total: 32 Arm:10 Rowing:11 Bicycle:11 | 10 | Arm, rowing, and bicycle | No intervention | 2–3/ | 8–10 W | 20 min | SDMT no effect LPS no improvement VLMT improved. TAP improved | |
| Romberg/2005/Finland [70] | RCT, parallel, RRMS | 47 | 48 | Resistance | No intervention | 3 | 24 w | NA | PASAT MSQOL-cognitive Both improved | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkhooly, M.; Di Stadio, A.; Bernitsas, E. Effect of Aerobic Exercise versus Non-Invasive Brain Stimulation on Cognitive Function in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Brain Sci. 2024, 14, 771. https://doi.org/10.3390/brainsci14080771
Elkhooly M, Di Stadio A, Bernitsas E. Effect of Aerobic Exercise versus Non-Invasive Brain Stimulation on Cognitive Function in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Brain Sciences. 2024; 14(8):771. https://doi.org/10.3390/brainsci14080771
Chicago/Turabian StyleElkhooly, Mahmoud, Arianna Di Stadio, and Evanthia Bernitsas. 2024. "Effect of Aerobic Exercise versus Non-Invasive Brain Stimulation on Cognitive Function in Multiple Sclerosis: A Systematic Review and Meta-Analysis" Brain Sciences 14, no. 8: 771. https://doi.org/10.3390/brainsci14080771
APA StyleElkhooly, M., Di Stadio, A., & Bernitsas, E. (2024). Effect of Aerobic Exercise versus Non-Invasive Brain Stimulation on Cognitive Function in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Brain Sciences, 14(8), 771. https://doi.org/10.3390/brainsci14080771









