An Exploratory Study of Large-Scale Brain Networks during Gambling Using SEEG
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Electrophysiological Recordings
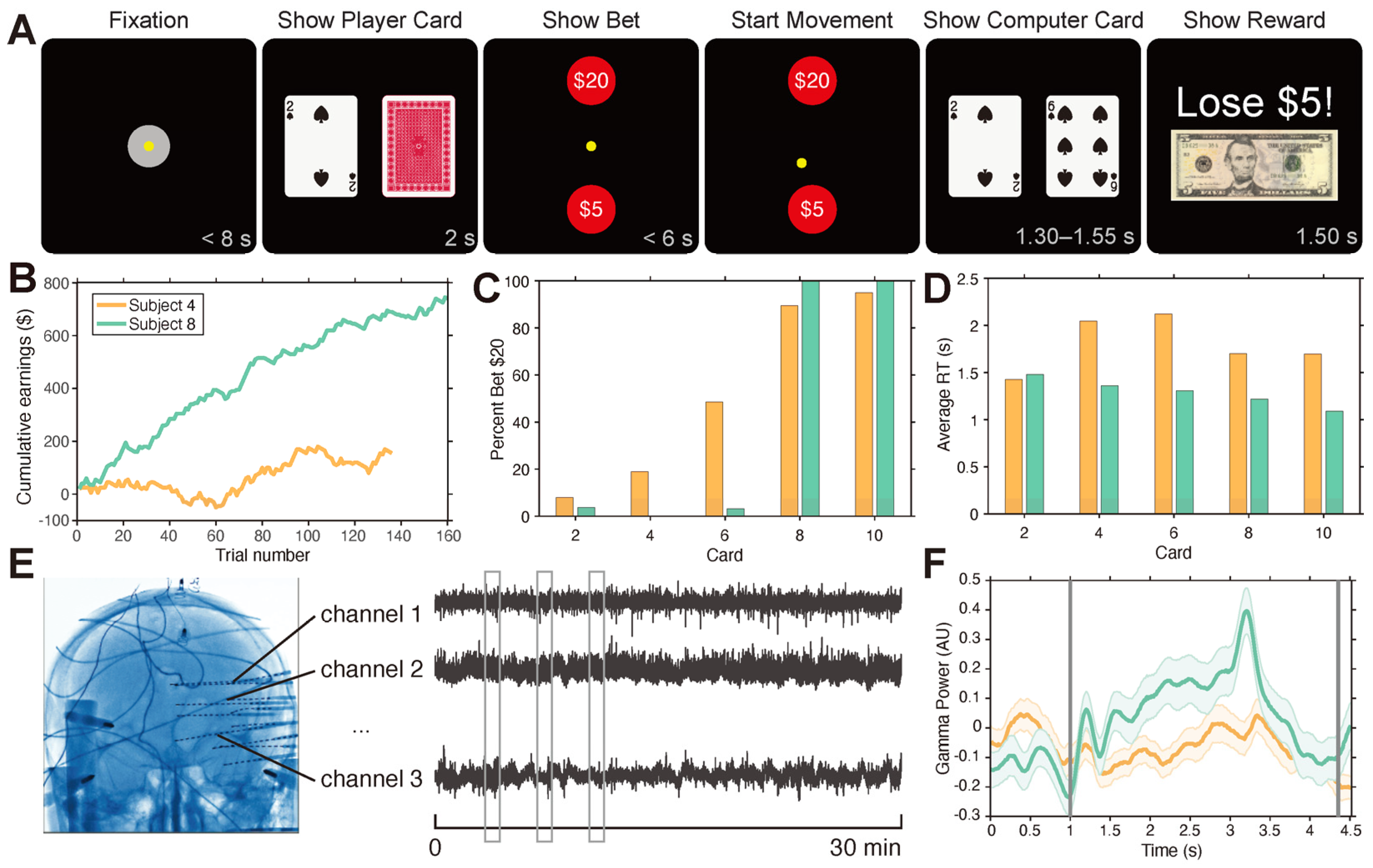
2.3. Gambling Task
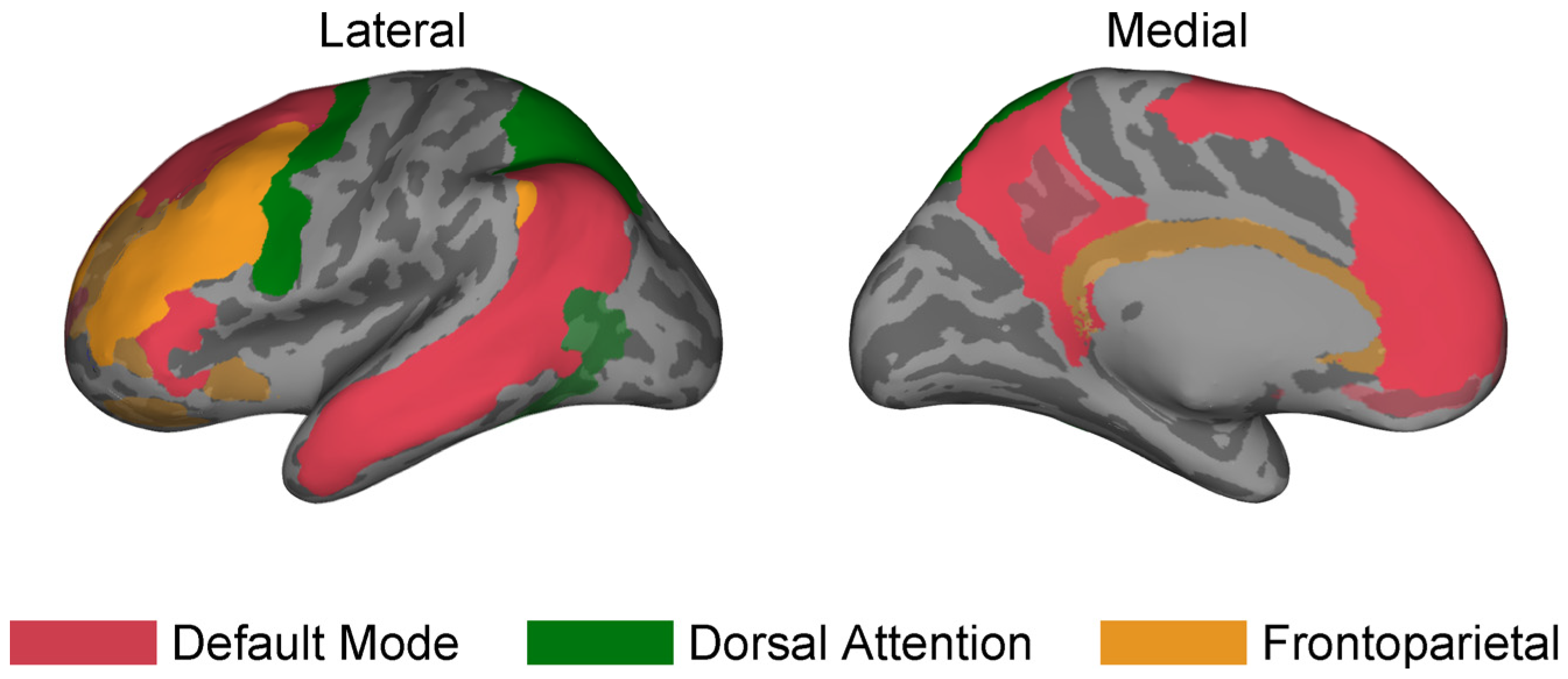
2.4. Large-Scale Brain Networks
2.5. Electrophysiological Preprocessing
2.6. Compute Neural Activity within Networks during Decision-Making
2.7. Compute Connectivity within and between Networks during Decision-Making
3. Results
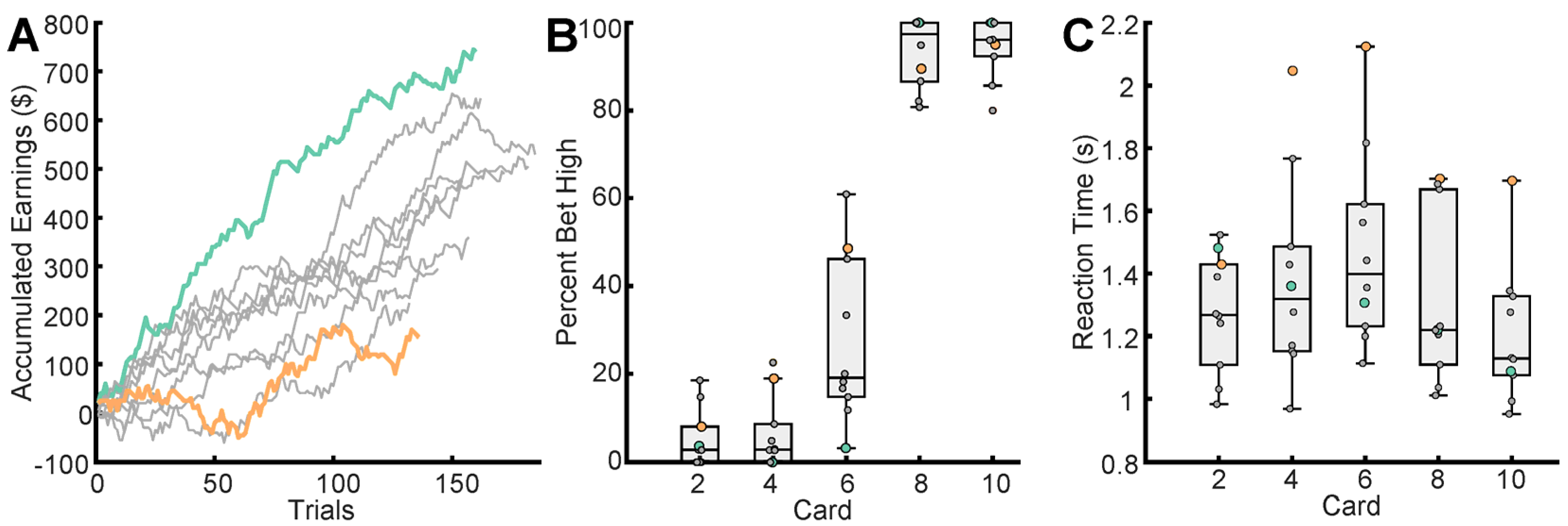
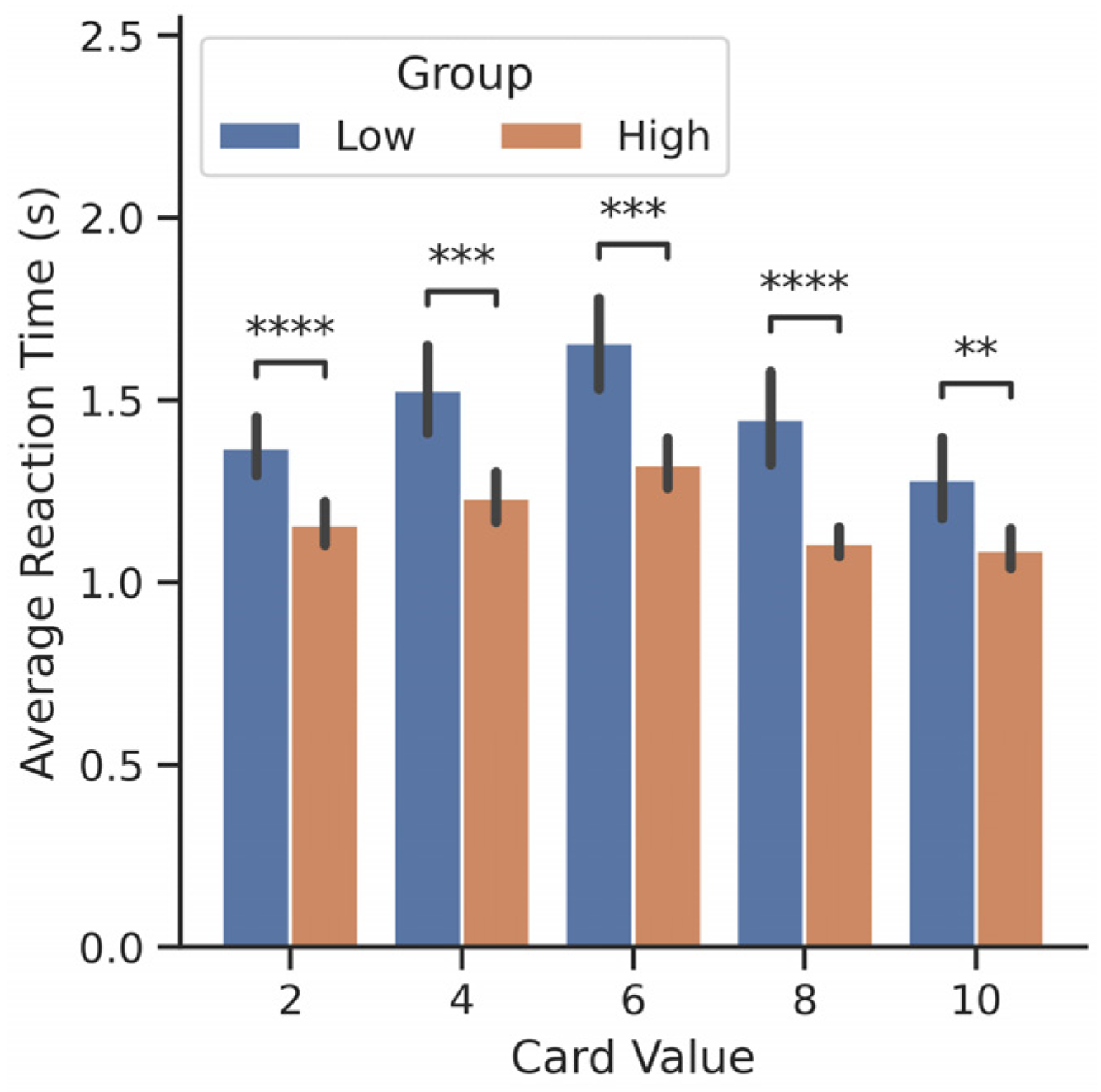
3.1. Betting Behavior and Performance Varies across Participants
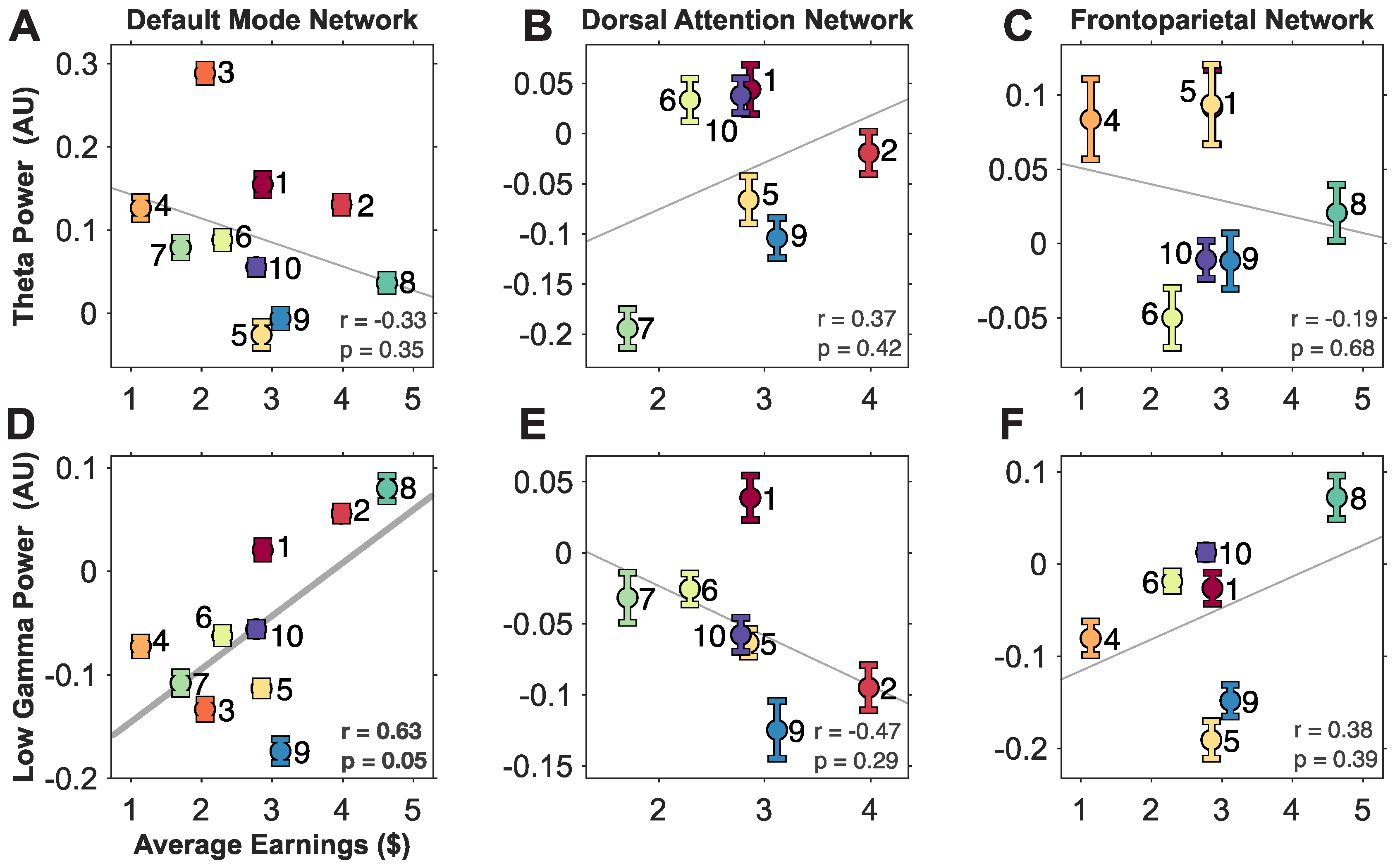
3.2. A Trend between Theta and Gamma Activity in Large-Scale Brain Networks and Gambling Performance
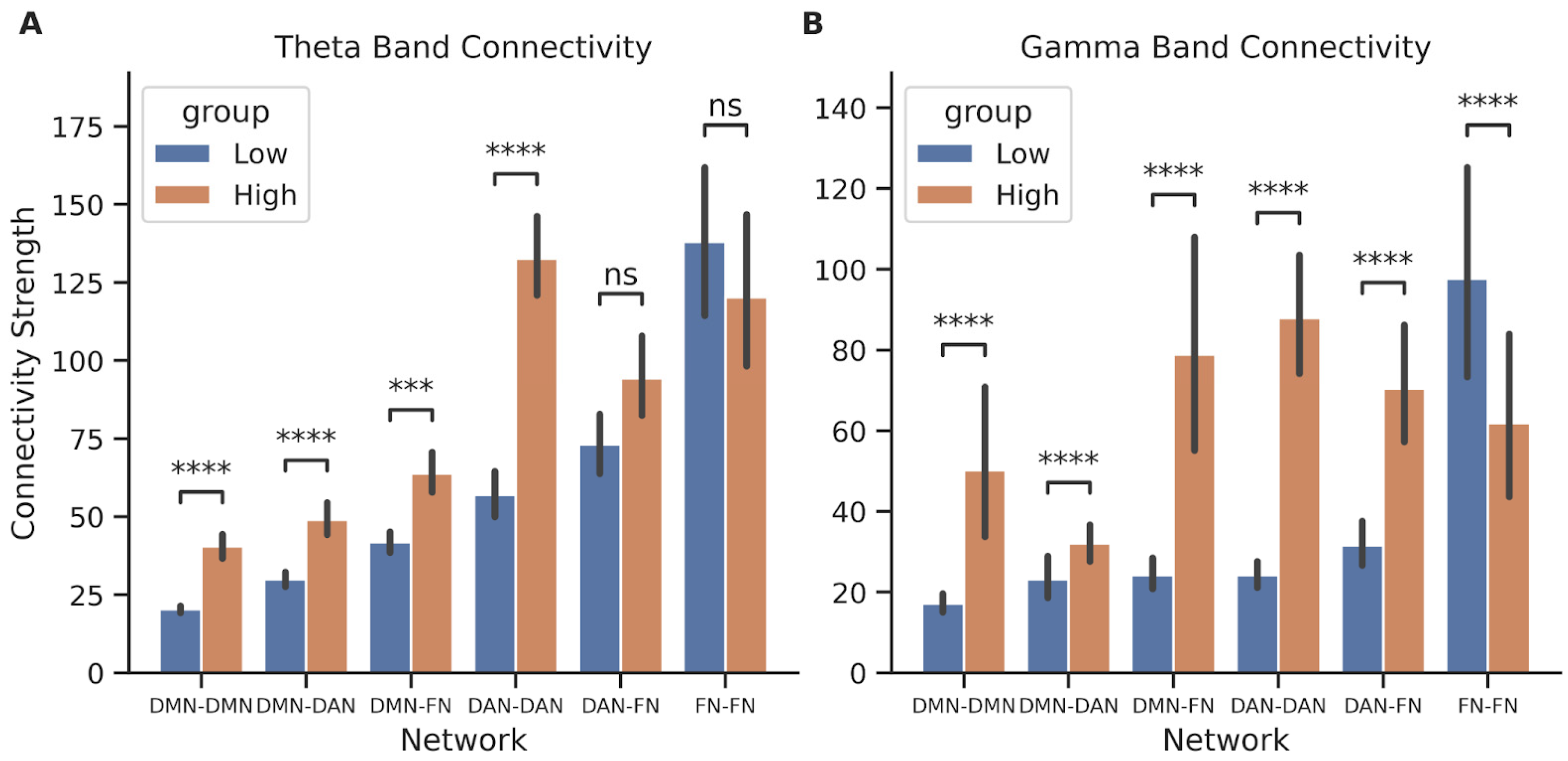
3.3. Connectivity Strengths within and between Large-Scale Brain Networks Correlate to Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzalez, C. Decision-Making: A Cognitive Science Perspective. In The Oxford Handbook of Cognitive Science; Oxford University Press: New York City, NY, USA, 2017; pp. 249–263. [Google Scholar]
- De Neys, W.; Novitskiy, N.; Geeraerts, L.; Ramautar, J.; Wagemans, J. Cognitive Control and Individual Differences in Economic Ultimatum Decision-Making. PLoS ONE 2011, 6, e27107. [Google Scholar] [CrossRef]
- Spreng, R.N.; Stevens, W.D.; Chamberlain, J.P.; Gilmore, A.W.; Schacter, D.L. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage 2010, 53, 303–317. [Google Scholar] [CrossRef]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Lanssens, A.; Pizzamiglio, G.; Mantini, D.; Gillebert, C.R. Role of the dorsal attention network in distracter suppression based on features. Cogn. Neurosci. 2020, 11, 37–46. [Google Scholar] [CrossRef]
- Ptak, R.; Schnider, A. The Dorsal Attention Network Mediates Orienting toward Behaviorally Relevant Stimuli in Spatial Neglect. J. Neurosci. 2010, 30, 12557–12565. [Google Scholar] [CrossRef]
- Fellrath, J.; Mottaz, A.; Schnider, A.; Guggisberg, A.G.; Ptak, R. Theta-band functional connectivity in the dorsal fronto-parietal network predicts goal-directed attention. Neuropsychologia 2016, 92, 20–30. [Google Scholar] [CrossRef]
- Ossandon, T.; Vidal, J.R.; Ciumas, C.; Jerbi, K.; Hamamé, C.M.; Dalal, S.S.; Bertrand, O.; Minotti, L.; Kahane, P.; Lachaux, J.-P. Efficient ‘Pop-Out’ Visual Search Elicits Sustained Broadband Gamma Activity in the Dorsal Attention Network. J. Neurosci. 2012, 32, 3414–3421. [Google Scholar] [CrossRef]
- Krawczyk, D.C. The cognition and neuroscience of relational reasoning. Brain Res. 2010, 1428, 13–23. [Google Scholar] [CrossRef]
- Eldaif, M.; Deckersbach, T.; Carlson, L.; Beucke, J.; Dougherty, D. Emotional and cognitive stimuli differentially engage the default network during inductive reasoning. Soc. Cogn. Affect. Neurosci. 2012, 7, 380–392. [Google Scholar] [CrossRef]
- Thomas Ye, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Konishi, M.; McLaren, D.G.; Engen, H.; Smallwood, J. Shaped by the Past: The Default Mode Network Supports Cognition that Is Independent of Immediate Perceptual Input. PLoS ONE 2015, 10, e0132209. [Google Scholar] [CrossRef] [PubMed]
- Jerbi, K.; Vidal, J.R.; Bayle, D.J.; Henaff, M.-A.; Jung, J.; Minotti, L.; Bertrand, O.; Kahane, P.; Lachaux, J.-P. Transient Suppression of Broadband Gamma Power in the Default-Mode Network Is Correlated with Task Complexity and Subject Performance. J. Neurosci. 2011, 31, 14521–14530. [Google Scholar] [CrossRef]
- Marek, S.; Dosenbach, N.U.F. The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues Clin. Neurosci. 2018, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.W.; Laurent, P.; Stocco, A. Rapid instructed task learning: A new window into the human brain’s unique capacity for flexible cognitive control. Cogn. Affect. Behav. Neurosci. 2013, 13, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Harding, I.H.; Yücel, M.; Harrison, B.J.; Pantelis, C.; Breakspear, M. Effective connectivity within the frontoparietal control network differentiates cognitive control and working memory. NeuroImage 2015, 106, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.; Uhlhaas, P.J. Working memory and neural oscillations: Alpha–gamma versus theta–gamma codes for distinct WM information? Trends Cogn. Sci. 2014, 18, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Vendetti, M.S.; Bunge, S.A. Evolutionary and Developmental Changes in the Lateral Frontoparietal Network: A Little Goes a Long Way for Higher-Level Cognition. Neuron 2014, 84, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Gulbinaite, R.; Van Rijn, H.; Cohen, M.X. Fronto-parietal network oscillations reveal relationship between working memory capacity and cognitive control. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Coull, J.T.; Frith, C.D.; Frackowiak, R.S.J.; Grasby, P.M. A fronto-parietal network for rapid visual information processing: A PET study of sustained attention and working memory. Neuropsychologia 1996, 34, 1085–1095. [Google Scholar] [CrossRef]
- Breault, M.S.; Sacré, P.; Fitzgerald, Z.B.; Gale, J.T.; Cullen, K.E.; González-Martínez, J.A.; Sarma, S.V. Internal states as a source of subject-dependent movement variability are represented by large-scale brain networks. Nat. Commun. 2023, 14, 7837. [Google Scholar] [CrossRef]
- Araujo, Y.I.D.; Nebe, S.; Neukam, P.T.; Pooseh, S.; Sebold, M.; Garbusow, M.; Heinz, A.; Smolka, M.N. Risk seeking for losses modulates the functional connectivity of the default mode and left frontoparietal networks in young males. Cogn. Affect. Behav. Neurosci. 2018, 18, 536–549. [Google Scholar] [CrossRef]
- Han, K.; Chapman, S.B.; Krawczyk, D.C. Disrupted Intrinsic Connectivity among Default, Dorsal Attention, and Frontoparietal Control Networks in Individuals with Chronic Traumatic Brain Injury. J. Int. Neuropsychol. Soc. 2016, 22, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K. What we can do and what we cannot do with fMRI. Nature 2008, 453, 869–878. [Google Scholar] [CrossRef]
- Shih, W.Y.; Yu, H.Y.; Lee, C.C.; Chou, C.C.; Chen, C.; Glimcher, P.W.; Wu, S.W. Electrophysiological population dynamics reveal context dependencies during decision making in human frontal cortex. Nat. Commun. 2023, 14, 7821. [Google Scholar] [CrossRef] [PubMed]
- Sacré, P.; Kerr, M.S.D.; Subramanian, S.; Fitzgerald, Z.; Kahn, K.; Johnson, M.A.; Niebur, E.; Eden, U.T.; González-Martínez, J.A.; Gale, J.T.; et al. Risk-taking bias in human decision-making is encoded via a right–left brain push–pull system. Proc. Natl. Acad. Sci. USA 2019, 116, 1404–1413. [Google Scholar] [CrossRef]
- Sacre, P.; Kerr, M.S.D.; Kahn, K.; Gonzalez-Martinez, J.; Bulacio, J.; Park, H.-J.; Johnson, M.A.; Thompson, S.; Jones, J.; Chib, V.S.; et al. Lucky rhythms in orbitofrontal cortex bias gambling decisions in humans. Sci. Rep. 2016, 6, 36206. [Google Scholar] [CrossRef] [PubMed]
- Sacre, P.; Subramanian, S.; Kerr, M.S.D.; Kahn, K.; Johnson, M.A.; Bulacio, J.; González-Martínez, J.A.; Sarma, S.V.; Gale, J.T. The influences and neural correlates of past and present during gambling in humans. Sci. Rep. 2017, 7, 17111. [Google Scholar] [CrossRef]
- Gunaratnam, S.; Talluri, D.; Greene, P.; Sacre, P.; Gonzalez-Martinez, J.; Sarma, S. High Frequency Activity in the Orbital Frontal Cortex Modulates with Mismatched Expectations During Gambling in Humans. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020. [Google Scholar]
- Gonzalez-Martinez, J.; Bulacio, J.; Thompson, S.; Gale, J.; Smithason, S.; Najm, I.; Bingaman, W. Technique, results, and complications related to robot-assisted stereoelectroencephalography. Neurosurgery 2016, 28, 169–180. [Google Scholar] [CrossRef]
- Destrieux, C.; Fischl, B.; Dale, A.; Halgren, E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 2010, 53, 1–15. [Google Scholar] [CrossRef]
- Asaad, W.; Eskandar, E. A flexible software tool for temporally-precise behavioral control in Matlab. J. Neurosci. Methods 2008, 174, 245–258. [Google Scholar] [CrossRef]
- Johnson, M.; Thompson, S.; Gonzalez-Martinez, J.; Park, H.-J.; Bulacio, J.; Najm, I.; Kahn, K.; Kerr, M.; Sarma, S.V.; Gale, J.T. Performing Behavioral Tasks in Subjects with Intracranial Electrodes. J. Vis. Exp. 2014, 92, 51947. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. NeuroImage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Browner, W.; Newman, T.B.; Cummings, S.R.; Grady, D.G. Designing Clinical Research, 5th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2023. [Google Scholar]
- Smallwood, J.; Bernhardt, B.C.; Leech, R.; Bzdok, D.; Jefferies, E.; Margulies, D.S. The default mode network in cognition: A topographical perspective. Nat. Rev. Neurosci. 2021, 22, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Ramnani, N.; Mackay, C.; Wilson, J.L.; Jezzard, P.; Carter, C.S.; Smith, S.M. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol. Psychiatry 2004, 55, 594–602. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Guise, K.; Knight, R.; Ghajar, J.; Fan, J. Effective Connectivity of the Fronto-parietal Network during Attentional Control. J. Cogn. Neurosci. 2010, 22, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The Brain’s Default Network: Anatomy, Function, and Relevance to Disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.J.; Bird, G.; Frith, C.D.; Burgess, P.W. Does ‘Task Difficulty’ Explain ‘Task-Induced Deactivation?’. Front. Psychol. 2012, 3, 125. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, D.; Menon, D.K.; Stamatakis, E.A. Default mode contributions to automated information processing. Proc. Natl. Acad. Sci. USA 2017, 114, 12821–12826. [Google Scholar] [CrossRef] [PubMed]
- Jordan, T.; Dhamala, M. Enhanced Dorsal Attention Network to Salience Network Interaction in Video Gamers During Sensorimotor Decision-Making Tasks. Brain Connect. 2023, 13, 97–106. [Google Scholar] [CrossRef]
- Li, N.; Ma, N.; Liu, Y.; He, X.-S.; Sun, D.-L.; Fu, X.-M.; Zhang, X.; Han, S.; Zhang, D.-R. Resting-State Functional Connectivity Predicts Impulsivity in Economic Decision-Making. J. Neurosci. 2013, 33, 4886–4895. [Google Scholar] [CrossRef]
- Fornito, A.; Harrison, B.; Zalesky, A.; Simons, J. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proc. Natl. Acad. Sci. USA 2012, 109, 12788–12793. [Google Scholar] [CrossRef]
- Borst, J.P.; Anderson, J.R. Using model-based functional MRI to locate working memory updates and declarative memory retrievals in the fronto-parietal network. Proc. Natl. Acad. Sci. USA 2013, 110, 1628–1633. [Google Scholar] [CrossRef]
- Fox, M.; Snyder, A.; Vincent, J.; Corbetta, M.; Van Essen, D.; Raichle, M. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Spreng, N. The Fallacy of a ‘Task-Negative’ Network. Front. Psychol. 2012, 3, 145. [Google Scholar] [CrossRef]
- Piccoli, T.; Valente, G.; Linden, D.E.J.; Re, M.; Esposito, F.; Sack, A.T.; Di Salle, F. The Default Mode Network and the Working Memory Network Are Not Anti-Correlated during All Phases of a Working Memory Task. PLoS ONE 2015, 10, e0123354. [Google Scholar] [CrossRef]

| Participant | Sex | Age | Handedness | EZ | DMN | DAN | FN |
|---|---|---|---|---|---|---|---|
| 1 | M | 26 | L | Hippo L, STG L | PCC (2), MTG (9), PC (2), SFG (1), STS (3) | IPS (1), SPL (2) | IPL (3), MFG (4) |
| 2 | F | 41 | R | Hippo R, EC R, TP R, PCC R, STG R | AG (4), PCC (3), F3o (2), ITS (2), MTG (6), PC (2), STS (4) | IPS (2), SPL (3) | |
| 3 | F | 31 | R | Hippo R | AG (4), PCC (1), F3o (6), F3t (4), ITS (1), MTG (8), PC (2), STS (3) | ||
| 4 | F | 53 | R | INSa R | ACC (5), PCC (3), F3o (1), F3t (1), ITS (1), MTG (9), mPFC (2), STS (1) | IFS (1), MFG (3) | |
| 5 | F | 60 | R | TP L | AG (7), PCC (3), MTG (11), PC (4) | IPS (3) | IPL (1) |
| 6 | F | 36 | R | PC R | AG (3), ACC (3), PCC (3), MTG (1), PC (7), SFS (1) | IPS (5), PRG (2), SPL (2) | IPL (5) |
| 7 | F | 23 | R | TP R, Hippo L/R | AG (5), PCC (4), ITS (1), MTG (14), PC (3), STS (1) | IPS (2) | |
| 8 | M | 32 | R | Hippo L | PCC (2), MTG (9) | IPL (2) | |
| 9 | F | 32 | R | PCC L | AG (4), F3t (2), ITS (2), MTG (21), PC (8), STS (3) | IPS (1), SPL (2) | MFG (7) |
| 10 | M | 28 | R | Hippo L | ACC (3), PCC (3), F3o (5), F3t (5), MTG (5), PC (5), SFG (5) | IPS (1) | MFG (7) |
| Participant | Total Trials | Won (%) | Lost (%) | Draw (%) | Cumulative Earnings ($) | Average Earnings ($) | Reaction Time (s) |
|---|---|---|---|---|---|---|---|
| 1 | 185 | 40.00 | 42.70 | 17.30 | 530 | 2.86 | 1.01 ± 0.21 |
| 2 | 162 | 45.06 | 35.80 | 19.14 | 645 | 3.98 | 1.22 ± 0.38 |
| 3 | 144 | 36.81 | 39.58 | 23.61 | 295 | 2.05 | 1.63 ± 0.81 |
| 4 | 136 | 37.50 | 45.59 | 16.91 | 155 | 1.14 | 1.85 ± 1.05 |
| 5 | 172 | 37.79 | 48.84 | 13.37 | 490 | 2.85 | 1.10 ± 0.33 |
| 6 | 157 | 35.67 | 41.4 | 22.93 | 360 | 2.29 | 1.48 ± 0.63 |
| 7 | 132 | 37.88 | 37.8 | 24.24 | 225 | 1.70 | 1.19 ± 0.35 |
| 8 | 160 | 42.50 | 34.38 | 23.13 | 740 | 4.63 | 1.28 ± 0.40 |
| 9 | 154 | 40.26 | 36.36 | 23.38 | 480 | 3.12 | 1.37 ± 0.56 |
| 10 | 182 | 35.71 | 44.51 | 19.78 | 505 | 2.77 | 1.22 ± 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, C.; Breault, M.S.; Dorman, D.; Greene, P.; Sacré, P.; Sampson, A.; Niebur, E.; Stuphorn, V.; González-Martínez, J.; Sarma, S. An Exploratory Study of Large-Scale Brain Networks during Gambling Using SEEG. Brain Sci. 2024, 14, 773. https://doi.org/10.3390/brainsci14080773
Taylor C, Breault MS, Dorman D, Greene P, Sacré P, Sampson A, Niebur E, Stuphorn V, González-Martínez J, Sarma S. An Exploratory Study of Large-Scale Brain Networks during Gambling Using SEEG. Brain Sciences. 2024; 14(8):773. https://doi.org/10.3390/brainsci14080773
Chicago/Turabian StyleTaylor, Christopher, Macauley Smith Breault, Daniel Dorman, Patrick Greene, Pierre Sacré, Aaron Sampson, Ernst Niebur, Veit Stuphorn, Jorge González-Martínez, and Sridevi Sarma. 2024. "An Exploratory Study of Large-Scale Brain Networks during Gambling Using SEEG" Brain Sciences 14, no. 8: 773. https://doi.org/10.3390/brainsci14080773





