Checkpoint for Considering Interleukin-6 as a Potential Target to Mitigate Secondary Brain Injury after Cardiac Arrest
Abstract
:1. Introduction
2. Materials and Methods
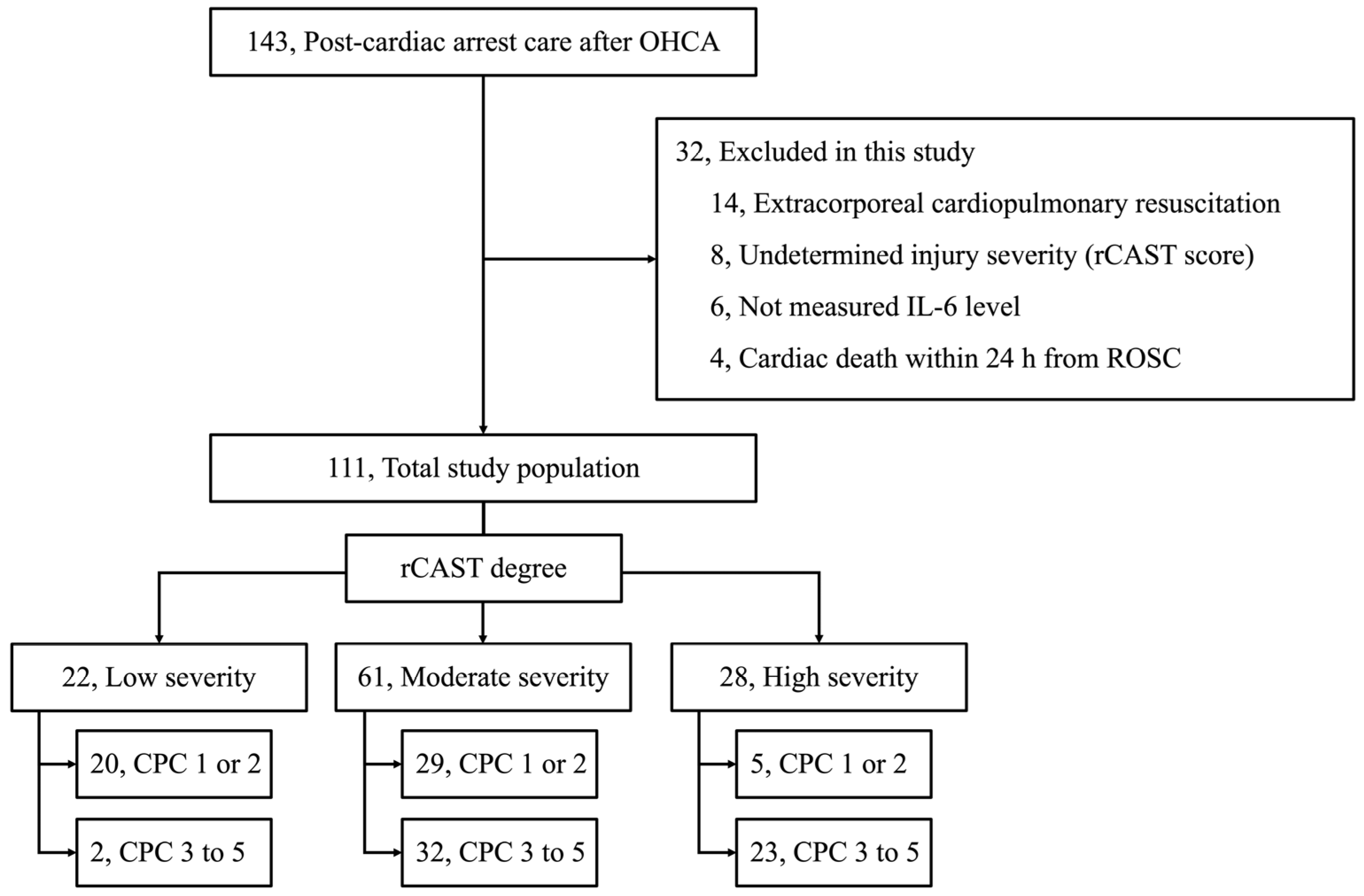
2.1. Study Design and Population
2.2. Post-Cardiac Arrest Care
2.3. Data Acquisition
2.3.1. Baseline Characteristics
2.3.2. Measurement of IL-6, Systemic Inflammatory, Myocardial Injury, and Neuronal Injury Markers
2.3.3. Assessment for Injury Severity of the Cardiac Arrest
2.4. Assessment for the Injury Severity of the Cardiac Arrest
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Cohort
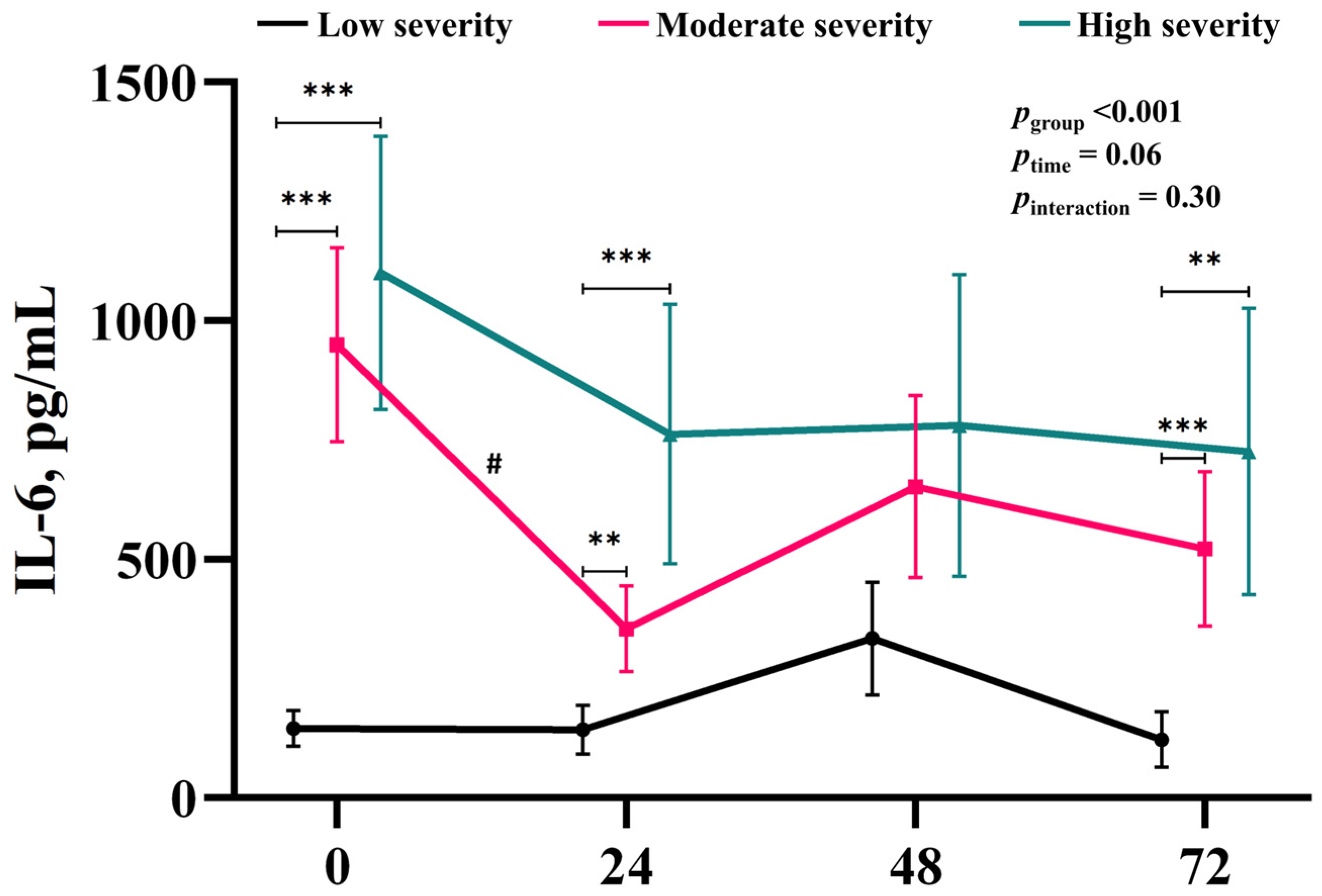
3.2. Time-Course of the Changes in IL-6 Associated with Injury Severity
3.3. Time-Course of the Changes in the Other Biomarkers Associated with Injury Severity
| Biomarker | Time | Injury Severity | pgroup a | ptime a | ||
|---|---|---|---|---|---|---|
| Low, 22 | Moderate, 61 | High, 28 | ||||
| NSE, ng/mL | baseline | 24.4 ± 1.4, 22 | 48.7 ± 6.7, 61 * | 63.2 ± 13.7, 28 * | <0.001 | 0.05 |
| 24 h | 28.1 ± 3.2, 21 | 68.7 ± 9.7, 60 * | 96.9 ± 18.4, 28 * | |||
| 48 h | 24.7 ± 3.1, 21 | 76.1 ± 11.8, 59 * | 114.0 ± 19.7, 27 * | |||
| 72 h | 27.4 ± 6.4, 21 | 68.5 ± 13.8, 58 * | 121.9 ± 18.2, 26 * | |||
| CRP, mg/dL | baseline | 1.5 ± 0.7, 21 | 1.2 ± 0.3, 59 | 1.4 ± 0.6, 26 | 0.18 | <0.001 |
| 24 h | 2.8 ± 0.5, 21 | 4.1 ± 0.5, 57 * | 4.7 ± 0.9, 26 * | |||
| 48 h | 5.2 ± 0.7, 19 | 8.5 ± 0.8, 55 * | 8.0 ± 1.1, 23 | |||
| 72 h | 5.5 ± 1.0, 19 | 8.2 ± 0.8, 54 | 8.4 ± 1.2, 24 | |||
| PCT, ng/mL | baseline | 0.4 ± 0.1, 12 | 2.0 ± 0.6, 34 * | 3.3 ± 1.2, 22 * | <0.001 | 0.01 |
| 24 h | 0.5 ± 0.1, 20 | 12.9 ± 4.7, 46 * | 20.8 ± 8.0, 24 * | |||
| 48 h | 0.4 ± 0.1, 20 | 8.8 ± 2.1, 46 * | 15.2 ± 6.3, 26 * | |||
| 72 h | 0.5 ± 0.1, 13 | 7.4 ± 2.1, 37 * | 12.5 ± 5.5, 22 * | |||
| CK-MB, ng/mL | baseline | 11.5 ± 3.3, 22 | 20.7 ± 4.4, 60 | 11.2 ± 2.0, 28 | 0.006 | <0.001 |
| 24 h | 48.7 ± 17.2, 19 | 82.2 ± 14.1, 40 | 66.5 ± 12.3, 24 | |||
| 48 h | 31.1 ± 16.5, 18 | 61.6 ± 12.4, 41 * | 60.2 ± 16.2, 21 * | |||
| 72 h | 9.6 ± 3.7, 18 | 15.6 ± 3.1, 40 | 20.9 ± 9.2, 21 * | |||
| TnI, pg/mL | baseline | 613.7 ± 410.9, 22 | 1310.2 ± 473.7, 60 | 330.7 ± 68.1, 28 | 0.06 | 0.97 |
| 24 h | 2528.9 ± 1355.3, 18 | 7557.2 ± 1668.8, 37 | 2811.0 ± 737.4, 22 | |||
| 48 h | 2007.9 ± 1534.7, 17 | 4890.9 ± 1247.9, 39 | 2022.5 ± 780.1, 21 | |||
| 72 h | 1169.8 ± 895.3, 18 | 3745.4 ± 1136.0, 39 | 2045.5 ± 1008.6, 21 | |||
3.4. Independently Associated Biomarkers for IL-6 Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adrie, C.; Laurent, I.; Monchi, M.; Cariou, A.; Dhainaou, J.F.; Spaulding, C. Postresuscitation disease after cardiac arrest: A sepsis-like syndrome? Curr. Opin. Crit. Care 2004, 10, 208–212. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef]
- Adrie, C.; Adib-Conquy, M.; Laurent, I.; Monchi, M.; Vinsonneau, C.; Fitting, C.; Fraisse, F.; Dinh-Xuan, A.T.; Carli, P.; Spaulding, C.; et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 2002, 106, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Bro-Jeppesen, J.; Kjaergaard, J.; Wanscher, M.; Nielsen, N.; Friberg, H.; Bjerre, M.; Hassager, C. Systemic inflammatory response and potential prognostic implications after out-of-hospital cardiac arrest: A substudy of the target temperature management trial. Crit. Care Med. 2015, 43, 1223–1232. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Kjaergaard, J.; Stammet, P.; Wise, M.P.; Hovdenes, J.; Aneman, A.; Horn, J.; Devaux, Y.; Erlinge, D.; Gasche, Y.; et al. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 °C or 36 °C. Resuscitation 2016, 98, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.A.S.; Bjerre, M.; Wiberg, S.; Grand, J.; Obling, L.E.R.; Meyer, A.S.P.; Josiassen, J.; Frydland, M.; Thomsen, J.H.; Frikke-Schmidt, R.; et al. Modulation of inflammation by treatment with tocilizumab after out-of-hospital cardiac arrest and associations with clinical status, myocardial- and brain injury. Resuscitation 2023, 184, 109676. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.A.S.; Wiberg, S.; Grand, J.; Meyer, A.S.P.; Obling, L.E.R.; Frydland, M.; Thomsen, J.H.; Josiassen, J.; Møller, J.E.; Kjaergaard, J.; et al. Treatment effects of interleukin-6 receptor antibodies for modulating the systemic inflammatory response after out-of-hospital cardiac arrest (the IMICA trial): A double-blinded, placebo-controlled, single-center, randomized, clinical trial. Circulation 2021, 143, 1841–1851. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.; et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: Post-resuscitation care. Resuscitation 2021, 161, 220–269. [Google Scholar] [CrossRef]
- Jeong, E.; Baik, S.; Park, H.; Oh, J.; Lee, Y.; Lee, J.M. First organ donation after circulatory death following withdrawal of life-sustaining treatment in Korea: A case report. J. Korean Med. Sci. 2021, 36, e171. [Google Scholar] [CrossRef]
- Cho, W.H. Organ donation in Korea in 2018 and an introduction of the Korea national organ donation system. Korean J. Transplant. 2019, 33, 83–97. [Google Scholar] [CrossRef]
- Nishikimi, M.; Ogura, T.; Nishida, K.; Takahashi, K.; Nakamura, M.; Matsui, S.; Matsuda, N.; Iwami, T. External validation of a risk classification at the emergency department of post-cardiac arrest syndrome patients undergoing targeted temperature management. Resuscitation 2019, 140, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Matsuda, N.; Matsui, K.; Takahashi, K.; Ejima, T.; Liu, K.; Ogura, T.; Higashi, M.; Umino, H.; Makishi, G.; et al. CAST: A new score for early prediction of neurological outcomes after cardiac arrest before therapeutic hypothermia with high accuracy. Intensive Care Med. 2016, 42, 2106–2107. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Matsuda, N.; Matsui, K.; Takahashi, K.; Ejima, T.; Liu, K.; Ogura, T.; Higashi, M.; Umino, H.; Makishi, G.; et al. A novel scoring system for predicting the neurologic prognosis prior to the initiation of induced hypothermia in cases of post-cardiac arrest syndrome: The CAST score. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 49. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mazumdar, M.; Memtsoudis, S.G. Beyond repeated-measures analysis of variance: Advanced statistical methods for the analysis of longitudinal data in anesthesia research. Reg. Anesth. Pain Med. 2012, 37, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Kochanek, P.M.; Simon, D.W.; Wagner, A.K. Targeting interleukin-6 after cardiac arrest-Let us not forget the brain. Resuscitation 2023, 184, 109715. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.P.; Sandroni, C.; Andersen, L.W.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Lilja, G.; Morley, P.T.; et al. ERC-ESICM guidelines on temperature control after cardiac arrest in adults. Resuscitation 2022, 172, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Lascarrou, J.-B.; Merdji, H.; Le Gouge, A.L.; Colin, G.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.-F.; Cariou, A.; Boulain, T.; et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N. Engl. J. Med. 2019, 381, 2327–2337. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kitlen, E.; Garcia, G.; Khosla, A.; Miller, P.E.; Johnson, J.; Wira, C.; Greer, D.M.; Gilmore, E.J.; Beekman, R. Validation of the rCAST score and comparison to the PCAC and FOUR scores for prognostication after out-of-hospital cardiac arrest. Resuscitation 2023, 188, 109832. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Ogura, T.; Nishida, K.; Hayashida, K.; Emoto, R.; Matsui, S.; Matsuda, N.; Iwami, T. Outcome related to level of targeted temperature management in postcardiac arrest syndrome of low, moderate, and high severities: A nationwide multicenter prospective registry. Crit. Care Med. 2021, 49, e741–e750. [Google Scholar] [CrossRef]
- Moseby-Knappe, M.; Mattsson-Carlgren, N.; Stammet, P.; Backman, S.; Blennow, K.; Dankiewicz, J.; Friberg, H.; Hassager, C.; Horn, J.; Kjaergaard, J.; et al. Serum markers of brain injury can predict good neurological outcome after out-of-hospital cardiac arrest. Intensive Care Med. 2021, 47, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Annborn, M.; Dankiewicz, J.; Erlinge, D.; Hertel, S.; Rundgren, M.; Smith, J.G.; Struck, J.; Friberg, H. Procalcitonin after cardiac arrest—An indicator of severity of illness, ischemia-reperfusion injury and outcome. Resuscitation 2013, 84, 782–787. [Google Scholar] [CrossRef]
- Nellan, A.; McCully, C.M.L.; Cruz Garcia, R.; Jayaprakash, N.; Widemann, B.C.; Lee, D.W.; Warren, K.E. Improved CNS exposure to tocilizumab after cerebrospinal fluid compared to intravenous administration in rhesus macaques. Blood 2018, 132, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Park, J.S.; Min, J.H.; Jeong, W.; Ahn, H.J.; In, Y.N.; Jeon, S.Y.; Lee, J.K.; Kang, C. Blood–brain barrier permeability for the first 24 hours in hypoxic-ischemic brain injury following cardiac arrest. Resuscitation 2024, 198, 110150. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.R.; Hernandez, Y.; Miyasaki, K.F.; Kwon, E.J. Engineered nanomaterials that exploit blood–brain barrier dysfunction for delivery to the brain. Adv. Drug Deliv. Rev. 2023, 197, 114820. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; He, X.; Qiu, Z.; Zhang, H.; Xie, R.; Liu, Z.; Gu, Y.; Zhao, N.; Xiang, Q.; Cui, Y. Targeting integrin pathways: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2023, 8, 1. [Google Scholar] [CrossRef]
- Barton, D.J.; Elmer, J. Open for business: The blood–brain barrier after cardiac arrest. Resuscitation 2024, 198, 110187. [Google Scholar] [CrossRef]
| Variables | Total Cohort, 111 | Injury Severity | p | ||
|---|---|---|---|---|---|
| Low, 22 | Moderate, 61 | High, 28 | |||
| Age, years | 58 (41–68) | 60 (46–71) | 60 (49–68) | 50 (35–62) | 0.06 |
| Sex, male | 85 (76.6) | 19 (86.4) | 44 (72.1) | 22 (78.6) | 0.39 |
| Comorbidities | |||||
| Myocardial infarction | 15 (13.5) | 3 (13.6) | 10 (16.4) | 2 (7.1) | 0.50 |
| Peripheral vascular disease | 4 (3.6) | 1 (4.5) | 2 (3.3) | 1 (3.6) | 0.96 |
| Congestive heart failure | 9 (8.1) | 2 (9.1) | 6 (9.8) | 1 (3.6) | 0.59 |
| COPD | 5 (4.5) | 1 (4.5) | 1 (1.6) | 3 (10.7) | 0.16 |
| Chronic kidney disease | 16 (14.4) | 5 (22.7) | 8 (13.1) | 3 (10.7) | 0.44 |
| Previous cardiac arrest | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Charlson comorbidity index | 2 (1–4) | 2 (1–6) | 3 (1–5) | 1 (0–3) | 0.22 |
| Cardiac arrest characteristics | |||||
| Witnessed | 73 (65.8) | 22 (100.0) | 40 (65.6) ** | 11 (39.3) *** | <0.001 |
| Bystander CPR | 79 (71.2) | 20 (90.9) | 37 (60.7) * | 22 (78.6) | 0.02 |
| Shockable rhythm | 42 (37.8) | 14 (63.6) | 22 (36.1) | 6 (21.4) * | 0.009 |
| Cardiac etiology | 55 (49.5) | 16 (72.7) | 31 (50.8) | 8 (28.6) * | 0.008 |
| Anoxic time a, min | 20 (9–30) | 1 (0–1) | 1 (0–12) | 5 (1–24) ** | 0.003 |
| GCS score after the ROSC | 3 (3–4) | 4 (3–6) | 3 (3–4) ** | 3 (3–3) *** | <0.001 |
| SOFA score at admission | 10 (7–12) | 8 (7–11) | 10 (8–12) | 11 (9–13) | 0.67 |
| Cause of cardiac arrest | |||||
| Cardiogenic | 54 (48.6) | 17 (77.3) | 29 (47.5) | 8 (28.6) ** | 0.02 |
| Hypoxia | 43 (38.7) | 3 (13.6) | 26 (42.6) | 14 (50.0) ** | |
| Nephrogenic | 5 (4.5) | 1 (4.5) | 3 (4.9) | 1 (3.6) ** | |
| Others | 9 (8.1) | 1 (4.5) | 3 (4.9) | 5 (17.9) ** | |
| Echocardiography after the ROSC | |||||
| Left ventricle contractility | |||||
| Normal | 59 (53.2) | 9 (40.9) | 33 (54.1) | 17 (60.7) | 0.43 |
| Mild dysfunction | 7 (6.3) | 3 (13.6) | 4 (6.6) | 0 (0) | |
| Moderate dysfunction | 33 (29.7) | 8 (36.4) | 16 (26.2) | 9 (32.1) | |
| Severe dysfunction | 12 (10.8) | 2 (9.1) | 8 (13.1) | 2 (7.1) | |
| Right ventricle dysfunction | 5 (4.5) | 1 (4.5) | 3 (4.9) | 1 (3.6) | 0.96 |
| Regional wall motion abnormality | |||||
| LAD territory | 12 (10.8) | 6 (27.3) | 3 (4.9) | 3 (10.7) | 0.05 |
| LCx territory | 6 (5.4) | 2 (9.1) | 4 (6.6) | 0 (0) | |
| RCA territory | 2 (1.8) | 0 (0) | 2 (3.3) | 0 (0) | |
| CAG performed b | |||||
| 1VD | 5 (4.5) | 2 (9.1) | 2 (3.3) | 1 (3.6) | 0.52 |
| 2VD | 3 (2.7) | 1 (4.5) | 2 (3.3) | 0 (0) | |
| 3VD | 3 (2.7) | 0 (0.0) | 3 (4.9) | 0 (0) | |
| PCI performed before TTM c | 7 (6.3) | 2 (9.1) | 4 (6.6) | 1 (3.6) | 0.72 |
| Presented infection | |||||
| Pulmonology | 7 (6.3) | 1 (4.5) | 4 (6.6) | 2 (7.1) | 0.93 |
| Genitourinary | 1 (0.9) | 0 (0) | 1 (1.6) | 0 (0) | |
| Neck | 1 (0.9) | 0 (0) | 1 (1.6) | 0 (0) | |
| Administration of steroid, mg/kg | 160 (61–536) | 91 (31–371) d | 60 (11–381) e | 240 (80–720) f | 0.11 |
| Outcome | |||||
| Good neurological outcome | 54 (48.6) | 20 (90.9) | 29 (47.5) *** | 5 (17.9) ***, † | <0.001 |
| Survival at discharge | 64 (57.7) | 20 (90.9) | 36 (59.0) * | 8 (28.6) ***, † | <0.001 |
| Severity | NSE | CRP | PCT | CK-MB | TnI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| Low | 6.1 (−5.3; 17.5) | 0.29 | 9.6 (−23.5; 42.6) | 0.56 | 184.4 (−56.3; 425.1) | 0.13 | −1.5 (−3.7; 0.8) | 0.21 | −0.1 (−0.4; 0.1) | 0.50 |
| Moderate | 4.3 a (0.1; 8.6) | 0.04 | 1.2 (−47.4; 49.8) | 0.96 | 17.0 a (5.9; 28.2) | 0.003 | 1.2 (−3.6; 6.1) | 0.62 | −0.05 (−0.08; 0.02) | 0.32 |
| High | b 7.9 (3.4; 12.5) | 0.001 | −24.8 (−92.9; 43.3) | 0.47 | −11.8 (−23.8; 0.2) | 0.05 | 2.5 (−3.5; 8.5) | 0.40 | −0.1 (−0.2; 0.1) | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.A.; You, Y.; Park, J.S.; Min, J.H.; Jeong, W.; Ahn, H.J.; Jeon, S.Y.; Kim, D.; Kang, C. Checkpoint for Considering Interleukin-6 as a Potential Target to Mitigate Secondary Brain Injury after Cardiac Arrest. Brain Sci. 2024, 14, 779. https://doi.org/10.3390/brainsci14080779
Yoon JA, You Y, Park JS, Min JH, Jeong W, Ahn HJ, Jeon SY, Kim D, Kang C. Checkpoint for Considering Interleukin-6 as a Potential Target to Mitigate Secondary Brain Injury after Cardiac Arrest. Brain Sciences. 2024; 14(8):779. https://doi.org/10.3390/brainsci14080779
Chicago/Turabian StyleYoon, Jung A, Yeonho You, Jung Soo Park, Jin Hong Min, Wonjoon Jeong, Hong Joon Ahn, So Young Jeon, Dongha Kim, and Changshin Kang. 2024. "Checkpoint for Considering Interleukin-6 as a Potential Target to Mitigate Secondary Brain Injury after Cardiac Arrest" Brain Sciences 14, no. 8: 779. https://doi.org/10.3390/brainsci14080779





