A Combination of Astragaloside IV and Hydroxysafflor Yellow A Attenuates Cerebral Ischemia-Reperfusion Injury via NF-κB/NLRP3/Caspase-1/GSDMD Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experiment Animals
2.3. Establishment of Ischemia-Reperfusion Injury Model by MCAO and Animal Grouping
2.4. Evaluation of Neurological Deficits
2.5. Analysis of Infarct Volume in the Brain
2.6. Histopathological Examination
2.7. Water Content of Brain Tissue
2.8. Immunohistochemical Staining of Brain Tissue
2.9. Assessment by ELISA
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. AS-IV+HSYA Improves Neurological Deficits and Reduces Cerebral Infarction after Cerebral Ischemia-Reperfusion
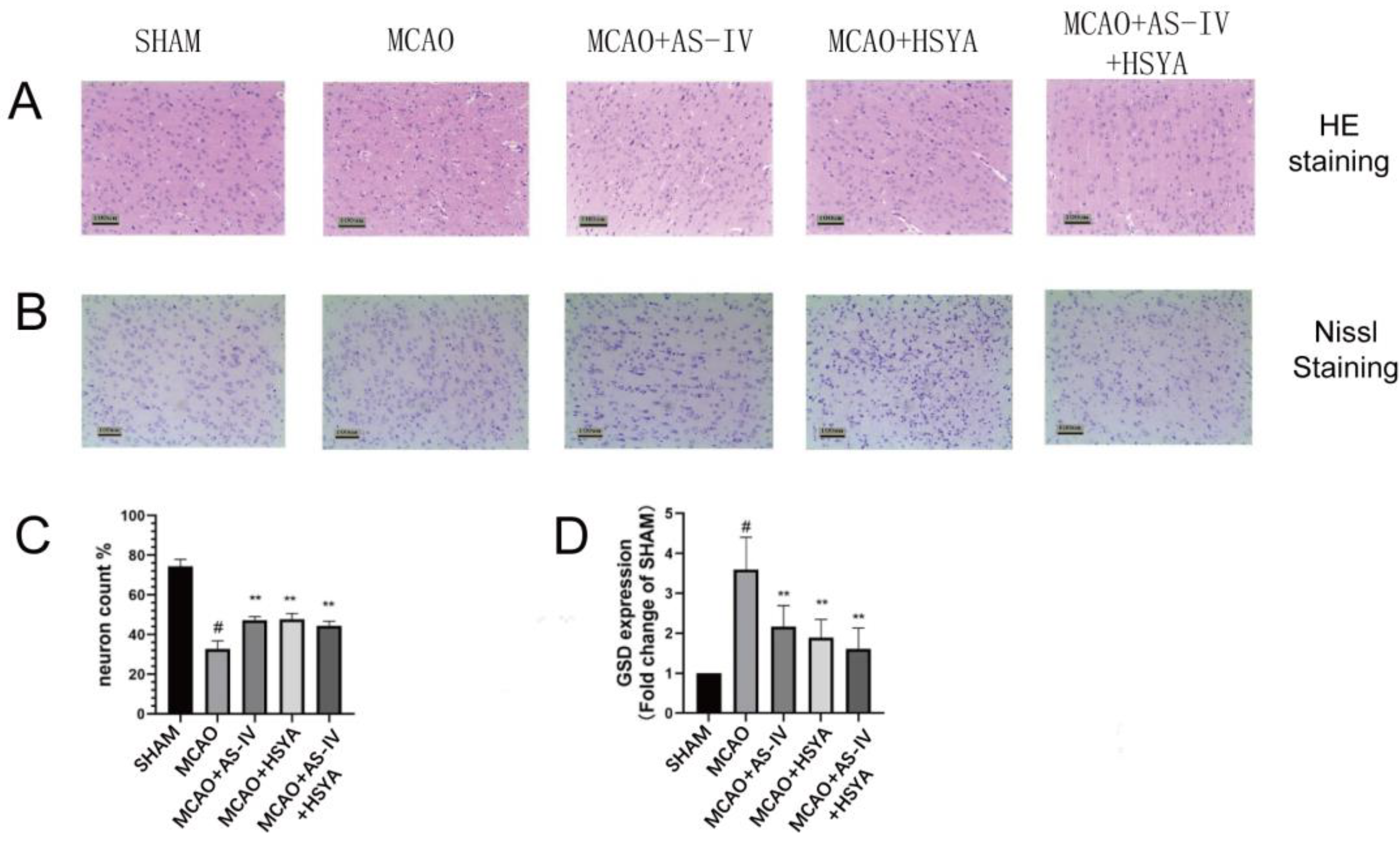
3.2. AS-IV+HSYA Alleviates Brain Tissue and Cell Damage in Rats after Cerebral Ischemia-Reperfusion Injury
3.3. The Reduction in Inflammatory Factors in the Cerebral Cortex and Blood by AS-IV+HSYA after Cerebral Ischemia-Reperfusion Injury
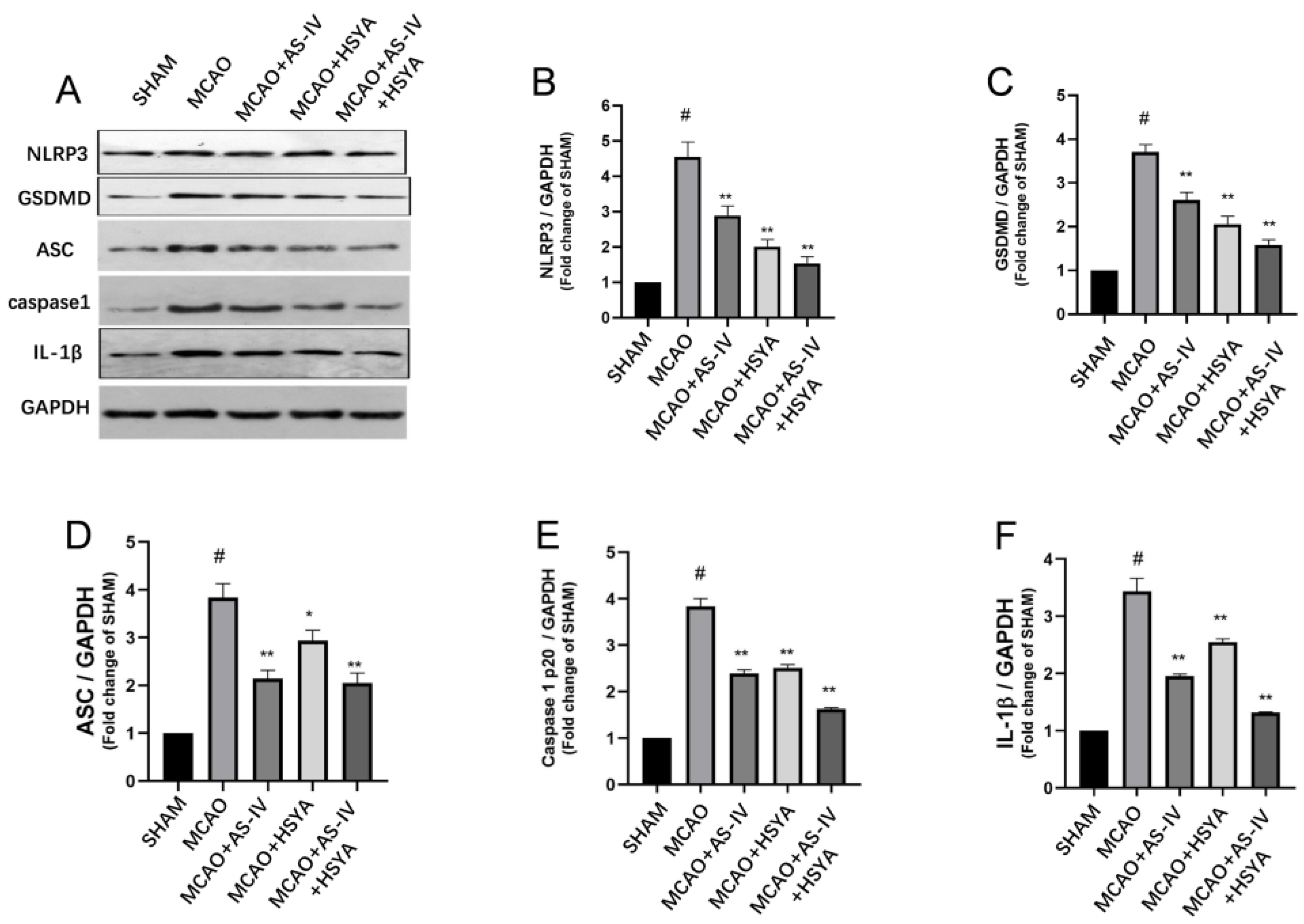
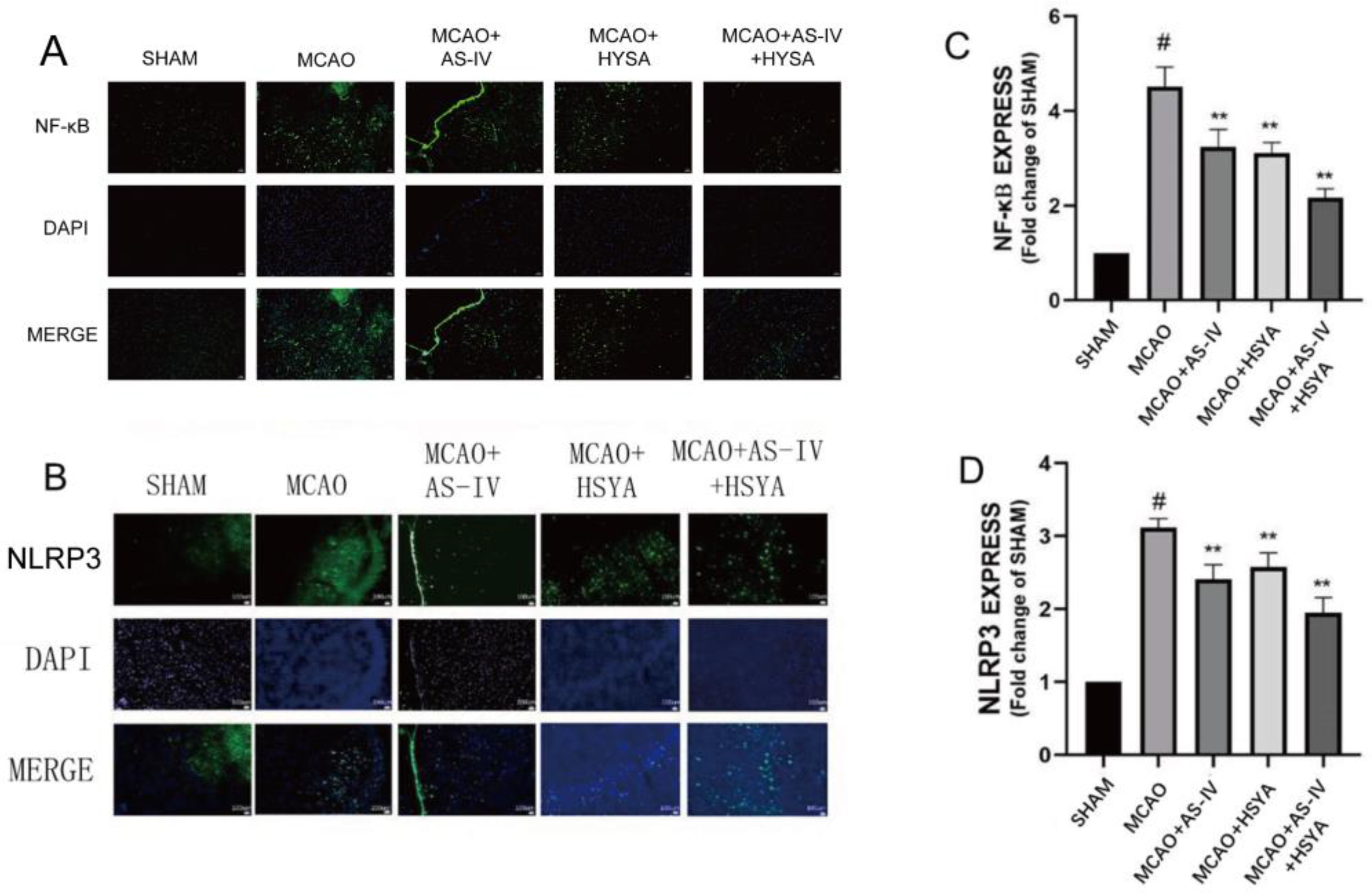
3.4. The Effects of AS-IV+HSYA on NLRP3/Caspase-1/GSDMD Signaling and Inflammasome-Related Molecules
3.5. The Effect of AS-IV+HSYA on the Contents of Inflammation-Related Protein Molecules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.C.; Baumann, J.; Huang, S.F.; Kindler, D.; Schroeter, A.; Kachappilly, N.; Gassmann, M.; Rudin, M.; Ogunshola, O.O. Pericyte hypoxia-inducible factor-1 (HIF-1) drives blood-brain barrier disruption and impacts acute ischemic stroke outcome. Angiogenesis 2021, 24, 823–842. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, Y.; Wang, H.; Zhang, Q. Protective effect of melatonin against chronic cadmium-induced hepatotoxicity by suppressing oxidative stress, inflammation, and apoptosis in mice. Ecotoxicol. Environ. Saf. 2021, 228, 112947. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.L.; Huang, J.; Chen, X.Y.; Xie, J.; Yang, Q.; Wang, J.F.; Deng, X.M. Senkyunolide I alleviates renal Ischemia-Reperfusion injury by inhibiting oxidative stress, endoplasmic reticulum stress and apoptosis. Int. Immunopharmacol. 2021, 102, 108393. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xu, D.; Hou, K.; Gou, X.; Lv, N.; Fang, W.; Li, Y. Pretreatment of Indobufen and Aspirin and their Combinations with Clopidogrel or Ticagrelor Alleviates Inflammasome Mediated Pyroptosis via Inhibiting NF-κB/NLRP3 Pathway in Ischemic Stroke. J. Neuroimmune Pharmacol. 2021, 16, 835–853. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Yao, N.; Lin, Z. Immunoproteasome modulates NLRP3 inflammasome-mediated neuroinflammation under cerebral ischaemia and reperfusion conditions. J. Cell. Mol. Med. 2022, 26, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhao, B.; Ye, Y.; Li, Y.; Zhang, Y.; Xiong, X.; Gu, L. Relevant mediators involved in and therapies targeting the inflammatory response induced by activation of the NLRP3 inflammasome in ischemic stroke. J. Neuroinflamm. 2021, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, L.; Gao, X.; Chen, S.; Wu, P.; Wang, C.; Liu, Z. Covalent modification of Keap1 at Cys77 and Cys434 by pubescenoside a suppresses oxidative stress-induced NLRP3 inflammasome activation in myocardial ischemia-reperfusion injury. Theranostics 2021, 11, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Fangma, Y.; Zhou, H.; Shao, C.; Yu, L.; Yang, J.; Wan, H.; He, Y. Hydroxysafflor Yellow A and Anhydrosafflor Yellow B Protect Against Cerebral Ischemia/Reperfusion Injury by Attenuating Oxidative Stress and Apoptosis via the Silent Information Regulator 1 Signaling Pathway. Front. Pharmacol. 2021, 12, 739864. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Jin, Z.; Li, M.; Liu, H.; Tao, J.; Xu, C.; Wang, L.; Zhang, Q. Protective potential of hydroxysafflor yellow A in cerebral ischemia and reperfusion injury: An overview of evidence from experimental studies. Front. Pharmacol. 2022, 13, 1063035. [Google Scholar] [CrossRef]
- Du, S.J.; Zhang, Y.; Zhao, Y.M.; Dong, Y.J.; Tang, J.L.; Zhou, X.H.; Gao, W.J. Astragaloside IV attenuates cerebral ischemia-reperfusion injury in rats through the inhibition of calcium-sensing receptor-mediated apoptosis. Int. J. Mol. Med. 2021, 47, 302–314. [Google Scholar] [CrossRef]
- Wang, H.W.; Liou, K.T.; Wang, Y.H.; Lu, C.K.; Lin, Y.L.; Lee, I.J.; Huang, S.T.; Tsai, Y.H.; Cheng, Y.C.; Lin, H.J.; et al. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J. Ethnopharmacol. 2011, 138, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Funamoto, K.; Ito, T.; Funamoto, K.; Velayo, C.L.; Kimura, Y. Ultrasound Imaging of Mouse Fetal Intracranial Hemorrhage Due to Ischemia/Reperfusion. Front. Physiol. 2017, 8, 340. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.G.; Tan, L.J.; Wang, J.F.; Li, X.L.; Huang, W.F.; Zhou, H.J. Fermented Chinese formula Shuan-Tong-Ling attenuates ischemic stroke by inhibiting inflammation and apoptosis. Neural Regen. Res. 2017, 12, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Lin, N.; Shan, G.; Zuo, P.; Cui, L. Safflower yellow for acute ischemic stroke: A systematic review of randomized controlled trials. Complement. Ther. Med. 2014, 22, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lavayen, B.P.; Liu, L.; Sanz, B.D.; DeMars, K.M.; Larochelle, J.; Pompilus, M.; Febo, M.; Sun, Y.Y.; Kuo, Y.M.; et al. Neurovascular protection by adropin in experimental ischemic stroke through an endothelial nitric oxide synthase-dependent mechanism. Redox Biol. 2021, 48, 102197. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Qin, Z.; Hong, Z.; Zhang, X.; Ding, D.; Fu, J.H.; Zhang, W.D.; Chen, J. Astragaloside IV protects against ischemic brain injury in a murine model of transient focal ischemia. Neurosci. Lett. 2004, 363, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.X.; Liang, C.; Wang, J.; Duan, C.Q.; Wang, P.; Wang, Y.L. Astragaloside IV improves neurobehavior and promotes hippocampal neurogenesis in MCAO rats though BDNF-TrkB signaling pathway. Biomed. Pharmacother. 2020, 130, 110353. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Huang, F.; Jin, J.; Wu, H.; Zhang, B.; Wang, Z.; Shi, H.; Wu, X. Astragaloside IV protects blood-brain barrier integrity from LPS-induced disruption via activating Nrf2 antioxidant signaling pathway in mice. Toxicol. Appl. Pharmacol. 2018, 340, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Huang, J.; Ma, B.; Yang, B.; Chang, D.; Liu, J. Astragaloside IV Protects Primary Cerebral Cortical Neurons from Oxygen and Glucose Deprivation/Reoxygenation by Activating the PKA/CREB Pathway. Neuroscience 2019, 404, 326–337. [Google Scholar] [CrossRef]
- Cao, J.; Wang, K.; Lei, L.; Bai, L.; Liang, R.; Qiao, Y.; Duan, J.; Gao, K.; Cao, S.; Zhao, C.; et al. Astragaloside and/or Hydroxysafflor Yellow A Attenuates Oxygen-Glucose Deprivation-Induced Cultured Brain Microvessel Endothelial Cell Death through Downregulation of PHLPP-1. Evid.-Based Complement. Alternat. Med. 2020, 2020, 3597527. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Lu, M.; Chen, Z.; Chen, C.; Han, L.; Zhang, M.; Xu, Y. Hydroxysafflor yellow A suppresses inflammatory responses of BV2 microglia after oxygen-glucose deprivation. Neurosci. Lett. 2013, 535, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wan, H.; Wang, Q.; Sun, H.; Sun, Y.; Wang, K.; Zhang, C. Safflor Yellow B Attenuates Ischemic Brain Injury via Downregulation of Long Noncoding AK046177 and Inhibition of MicroRNA-134 Expression in Rats. Oxid. Med. Cell. Longev. 2020, 2020, 4586839. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Joh, T. Oxidative stress and ischemia-reperfusion injury in gastrointestinal tract and antioxidant, protective agents. J. Clin. Biochem. Nutr. 2007, 40, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, Y.; Jiang, Y.; Wang, R.; Zu, J.; Tan, R. Hydroxysafflor Yellow A Together with Blood-Brain Barrier Regulator Lexiscan for Cerebral Ischemia Reperfusion Injury Treatment. ACS Omega 2020, 5, 19151–19164. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, A.R.; Muster, M.; Reverdin, A.; de Tribolet, N.; Ruefenacht, D.A. Preoperative silk suture embolization of cerebral and dural arteriovenous malformations. Neurosurg. Focus 2001, 11, e6. [Google Scholar] [CrossRef] [PubMed]
- Pantoni, L.; Fierini, F.; Poggesi, A. Thrombolysis in acute stroke patients with cerebral small vessel disease. Cerebrovasc. Dis. 2014, 37, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, Y.; Liu, C. Autophagy and Ischemic Stroke. Adv. Exp. Med. Biol. 2020, 1207, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Simic, T.; Leonard, C.; Laird, L.; Stewart, S.; Rochon, E. The effects of intensity on a phonological treatment for anomia in post-stroke aphasia. J. Commun. Disord. 2021, 93, 106125. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.F.; Wang, Z.J.; Li, K.; Jin, R.M. Huai Qi Huang Potentiates Dexamethasone-Mediated Lethality in Acute Lymphoblastic Leukemia Cells by Upregulating Glucocorticoid Receptor Alpha. Med. Sci. Monitor 2020, 26, e921649-1. [Google Scholar]
- Nallathamby, N.; Phan, C.W.; Sova, M.; Saso, L.; Sabaratnam, V. Synthesized 2-Trifluoromethylquinazolines and Quinazolinones Protect BV2 and N2a Cells against LPS- and H2O2-Induced Cytotoxicity. Med. Chem. 2019, 17, 623–629. [Google Scholar] [CrossRef]
- Guo, Y.R.; Jin, H.; Kim, M.; Shin, M.B.; Lee, J.H.; Maeng, S.; Cha, S.Y.; Lee, J.; Koh, Y.H.; Kim, K.Y.; et al. Synergistic Neuroprotective Effects of Mature Silkworm and Angelica gigas against Scopolamine-Induced Mild Cognitive Impairment in Mice and H2O2-Induced Cell Death in HT22 Mouse Hippocampal Neuronal Cells. J. Med. Food 2021, 24, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, Z.; Ru, J.; Wang, S.; Huang, L.; Ruan, L.; Lin, X.; Jin, K.; Zhuge, Q.; Yang, S. Ablation of GSDMD Improves Outcome of Ischemic Stroke Through Blocking Canonical and Non-canonical Inflammasomes Dependent Pyroptosis in Microglia. Front. Neurol. 2020, 11, 577927. [Google Scholar] [CrossRef] [PubMed]
- Bejot, Y.; Duloquin, G.; Thomas, Q.; Mohr, S.; Garnier, L.; Graber, M.; Giroud, M. Temporal Trends in the Incidence of Ischemic Stroke in Young Adults: Dijon Stroke Registry. Neuroepidemiology 2021, 55, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhou, Q.H.; Xu, M.B.; Zhou, X.L.; Zheng, G.Q. Astragaloside IV for Experimental Focal Cerebral Ischemia: Preclinical Evidence and Possible Mechanisms. Oxid. Med. Cell. Longev. 2017, 2017, 8424326. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Su, S.; Hong, W.; Geng, W.; Tang, H. Research Progress on the Ability of Astragaloside IV to Protect the Brain against Ischemia-Reperfusion Injury. Front. Neurosci. 2021, 15, 755902. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.H.; Wu, H.C.; Lin, N.W.; Hsieh, J.J.; Yeh, J.W.; Chiu, H.P.; Wu, M.C.; Tsai, T.Y.; Yeh, C.C.; Li, T.M. Long-term use of combined conventional medicine and Chinese herbal medicine decreases the mortality risk of patients with lung cancer. Complement. Ther. Med. 2020, 52, 102427. [Google Scholar] [CrossRef] [PubMed]
- Cichon, N.; Wlodarczyk, L.; Saluk-Bijak, J.; Bijak, M.; Redlicka, J.; Gorniak, L.; Miller, E. Novel Advances to Post-Stroke Aphasia Pharmacology and Rehabilitation. J. Clin. Med. 2021, 10, 3778. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, S.; Song, L.; Liu, M.; Sun, Z.; Liu, J. Astragaloside IV antagonizes M2 phenotype macrophage polarization-evoked ovarian cancer cell malignant progression by suppressing the HMGB1-TLR4 axis. Mol. Immunol. 2021, 130, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.H.; You, S.; Kim, G.H.; Park, H.J. Neuroprotective effect of Allium hookeri against H2O2-induced PC12 cell cytotoxicity by reducing oxidative stress. Food Sci. Biotechnol. 2020, 29, 1519–1530. [Google Scholar] [CrossRef]
- Lee, N.T.; Ong, L.K.; Gyawali, P.; Nassir, C.; Mustapha, M.; Nandurkar, H.H.; Sashindranath, M. Role of Purinergic Signalling in Endothelial Dysfunction and Thrombo-Inflammation in Ischaemic Stroke and Cerebral Small Vessel Disease. Biomolecules 2021, 11, 994. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, J.; Bi, H.; Yang, X.; Chen, W.; Jiang, K.; Yao, Y.; Ni, W. Bioactive Ingredients from Nitraria tangutorun Bobr. Protect against Cerebral Ischemia/Reperfusion Injury through Attenuation of Oxidative Stress and the Inflammatory Response. J. Med. Food 2021, 24, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.Z.; Lee, K.Y.; Qi, Z.X.; Wang, Z.; Xu, Z.Y.; Wu, X.H.; Mao, Y. Neuroinflammation Following Traumatic Brain Injury: Take It Seriously or Not. Front. Immunol. 2022, 13, 855701. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Li, C.; Ma, H.; Hua, S.; Liu, Z.; Huang, S.; Liu, K.; Li, J.; Feng, Z.; Cai, Y.; et al. Hydroxysafflor yellow a confers neuroprotection against acute traumatic brain injury by modulating neuronal autophagy to inhibit NLRP3 inflammasomes. J. Ethnopharmacol. 2023, 308, 116268. [Google Scholar] [CrossRef]
- Diamantis, D.A.; Oblukova, M.; Chatziathanasiadou, M.V.; Gemenetzi, A.; Papaemmanouil, C.; Gerogianni, P.S.; Syed, N.; Crook, T.; Galaris, D.; Deligiannakis, Y.; et al. Bioinspired tailoring of fluorogenic thiol responsive antioxidant precursors to protect cells against H2O2-induced DNA damage. Free Radic. Biol. Med. 2020, 160, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shergis, J.L.; Yang, L.H.; Zhang, A.L.; Guo, X.F.; Zhang, L.; Zhou, S.Z.; Zeng, L.; Mao, W.; Xue, C.C. Astragalus membranaceus (Huang Qi) as adjunctive therapy for diabetic kidney disease: An updated systematic review and meta-analysis. J. Ethnopharmacol. 2019, 239, 111921. [Google Scholar] [CrossRef]
- Li, L.; Sha, Z.; Wang, Y.; Yang, D.; Li, J.; Duan, Z.; Wang, H.; Li, Y. Pre-treatment with a combination of Shenmai and Danshen injection protects cardiomyocytes against hypoxia/reoxygenation-and H2O2-induced injury by inhibiting mitochondrial permeability transition pore opening. Exp. Ther. Med. 2019, 17, 4643–4652. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Xu, T.; Yu, X.; Zhang, S.; Liu, S.; Gao, Y.; Fan, S.; Li, C.; Zhai, C.; et al. Qingkailing injection ameliorates cerebral ischemia-reperfusion injury and modulates the AMPK/NLRP3 inflammasome signalling pathway. BMC Complement. Altern. Med. 2019, 19, 320. [Google Scholar] [CrossRef]
- Wang, X.; Li, R.; Wang, X.; Fu, Q.; Ma, S. Umbelliferone ameliorates cerebral ischemia-reperfusion injury via upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3 inflammasome. Neurosci. Lett. 2015, 600, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhong, D.; Chen, H.; Jin, J.; Liu, Q.; Li, G. NLRP3 inflammasome activates interleukin-23/interleukin-17 axis during ischaemia-reperfusion injury in cerebral ischaemia in mice. Life Sci. 2019, 227, 101–113. [Google Scholar] [CrossRef]
- Ward, R.; Li, W.; Abdul, Y.; Jackson, L.; Dong, G.; Jamil, S.; Filosa, J.; Fagan, S.C.; Ergul, A. NLRP3 inflammasome inhibition with MCC950 improves diabetes-mediated cognitive impairment and vasoneuronal remodeling after ischemia. Pharmacol. Res. 2019, 142, 237–250. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, J.; Zhang, P.; Li, H.; Shen, H.; Li, X.; Chen, G. Gasdermin D serves as a key executioner of pyroptosis in experimental cerebral ischemia and reperfusion model both in vivo and in vitro. J. Neurosci. Res. 2019, 97, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Li, L.X.; Wang, Q.; Chen, Z.J.; You, Y.T.; Gao, P.P.; Zhao, X.S.; Luo, R. Qi-dan-di-huang decoction alleviates diabetic nephropathy by inhibiting the NF-kappaB pathway. Front. Biosci.-Landmark 2019, 24, 1477–1486. [Google Scholar]
- Di, S.; Han, L.; Wang, Q.; Liu, X.K.; Yang, Y.Y.; Li, F.; Zhao, L.H.; Tong, X.L. A Network Pharmacology Approach to Uncover the Mechanisms of Shen-Qi-Di-Huang Decoction against Diabetic Nephropathy. Evid.-Based Compl. Alt. 2018, 2018, 7043402. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, S.; Chen, H.; Zheng, Y.; Lin, L.; Yao, H.; Lin, X. Protective effects of five compounds from Livistona chinensis R. Brown leaves against hypoxia/reoxygenation, H2O2, or adriamycin-induced injury in H9c2 cells. Drug Des. Devel. Ther. 2019, 13, 1555–1566. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tian, C.; Zhang, Z.; Qin, Y.; Meng, R.; Dai, X.; Zhong, Y.; Wei, X.; Zhang, J.; Shen, C. Astragaloside IV Inhibits NLRP3 Inflammasome-Mediated Pyroptosis via Activation of Nrf-2/HO-1 Signaling Pathway and Protects against Doxorubicin-Induced Cardiac Dysfunction. Front. Biosci. (Landmark Ed.) 2023, 28, 45. [Google Scholar] [CrossRef]
- Xu, X.; Guo, Y.; Zhao, J.; Wang, N.; Ding, J.; Liu, Q. Hydroxysafflor Yellow A Inhibits LPS-Induced NLRP3 Inflammasome Activation via Binding to Xanthine Oxidase in Mouse RAW264.7 Macrophages. Mediators Inflamm. 2016, 2016, 8172706. [Google Scholar] [CrossRef]

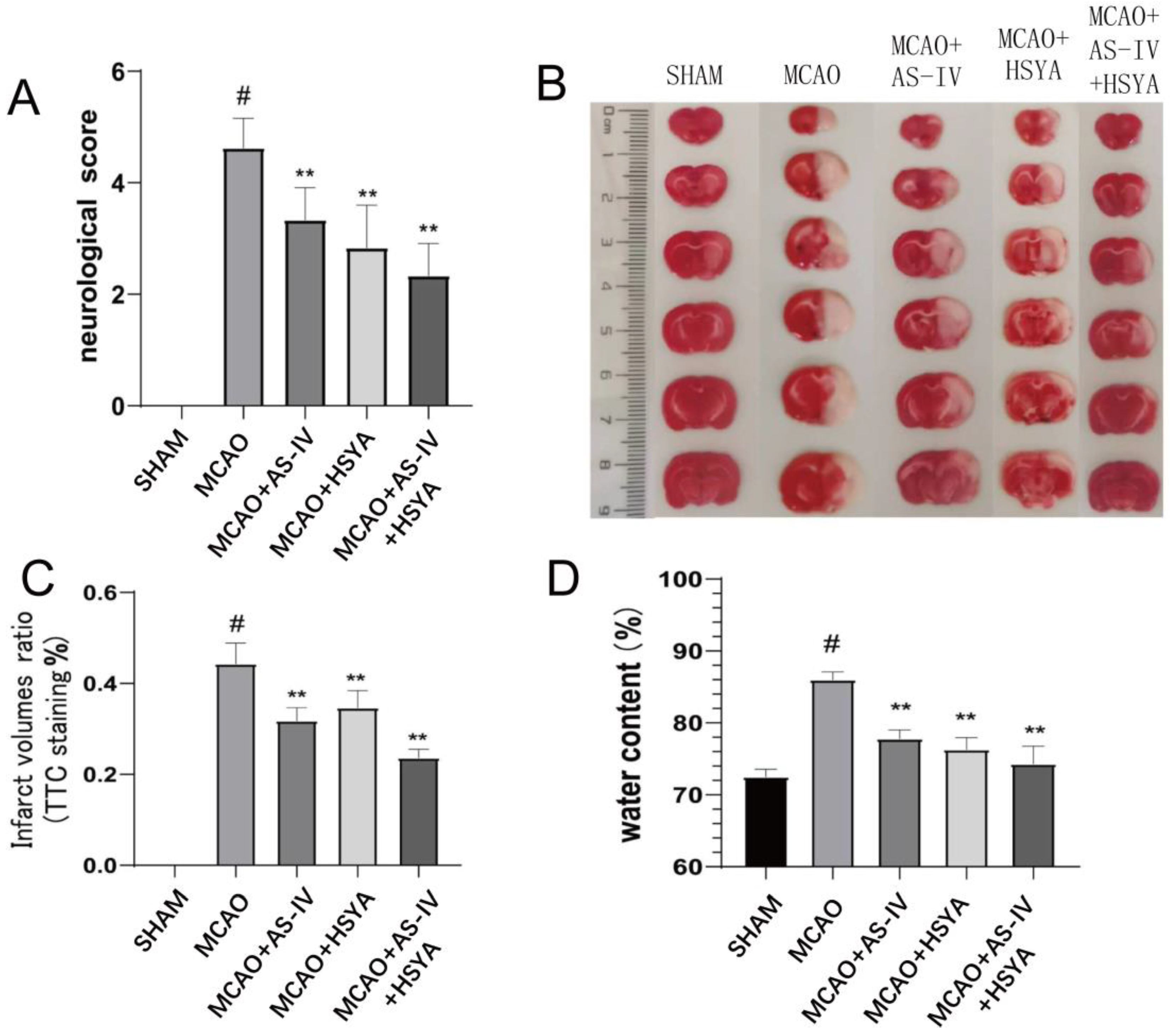



| Group | Neurobehavioral Score | TTC Infarct % | Water Content/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | |
| Sham | 0 | 0 | 0 | # | 0 | 0 | 0 | # | 42.17 | 1.47 | 0 | # |
| MCAO | 2.83 | 0.52 | - | 0.44 | 0.05 | - | 83.67 | 2.34 | - | |||
| MCAO+AS-IV | 1.75 | 0.61 | 0.002 | ** | 0.32 | 0.03 | 0 | ** | 70.17 | 5.12 | 0 | ** |
| MCAO+HSYA | 1.58 | 0.49 | 0 | ** | 0.35 | 0.04 | 0 | ** | 68.17 | 6.82 | 0 | ** |
| MCAO+AS-IV +HSYA | 1.42 | 0.49 | 0 | ** | 0.24 | 0.02 | 0 | ** | 57.33 | 2.58 | 0 | ** |
| F | 27.35 | 180.81 | 82.97 | |||||||||
| p | 0 | 0 | 0 | |||||||||
| Group | Neuron Content (%) | GSD Expressions | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | |
| Sham | 69.67 | 14.08 | 0 | # | 0.63 | 0.14 | 0 | # |
| MCAO | 29 | 7.16 | - | 2.60 | 0.52 | - | ||
| MCAO+AS-IV | 44.83 | 7.99 | 0.08 | 1.40 | 0.45 | 0 | ** | |
| MCAO+HSYA | 45.17 | 14.61 | 0.08 | 1.29 | 0.45 | 0 | ** | |
| MCAO+AS-IV+HSYA | 48 | 12.10 | 0.03 | * | 0.97 | 0.29 | 0 | ** |
| F | 9.43 | 21.59 | ||||||
| p | 0 | 0 | ||||||
| Group | MDA/(mol·mg−1) | SOD/(mol·mg−1) | IL-1β (pg·mg−1) | IL-18 (pg·mg−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | |
| Sham | 4.03 | 0.40 | 0 | # | 42.33 | 2.80 | 0 | # | 29.34 | 3.91 | 0 | # | 109.17 | 3.49 | 0 | # |
| MCAO | 8.65 | 0.64 | - | 24.17 | 3.19 | - | 24.51 | 3.20 | - | 155.23 | 1.60 | - | ||||
| MCAO+AS-IV | 7.23 | 0.23 | 0 | ** | 32.33 | 4.41 | 0.001 | ** | 32.60 | 2.48 | 0 | ** | 141.74 | 4.87 | 0 | ** |
| MCAO+HSYA | 6.70 | 0.34 | 0 | ** | 31.50 | 3.39 | 0.002 | ** | 33.99 | 1.94 | 0 | ** | 142.34 | 5.75 | 0 | ** |
| MCAO+AS-IV +HSYA | 5.93 | 0.16 | 0 | ** | 34.50 | 1.38 | 0 | ** | 43.54 | 1.59 | 0 | ** | 121.15 | 4.53 | 0 | ** |
| F | 110.60 | 25.06 | 43.48 | 110.97 | ||||||||||||
| p | 0 | 0 | 0 | 0 | ||||||||||||
| Group | MDA/(mol·mg−1) | SOD/(mol·mg−1) | IL-1β (pg·mg−1) | IL-18 (pg·mg−1) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | |
| Sham | 4.1 | 0.47 | 0 | # | 42.33 | 2.81 | 0 | # | 24.76 | 4.45 | 0 | # | 102.45 | 8.85 | 0 | # |
| MCAO | 5.15 | 0.14 | - | 28.83 | 3.37 | - | 32.90 | 2.19 | - | 140.33 | 7.92 | - | ||||
| MCAO+AS-IV | 5.1 | 0.38 | 1.00 | 32.33 | 3.14 | 0.18 | 29.87 | 0.98 | 0.25 | 123.62 | 2.53 | 0.001 | ** | |||
| MCAO+HSYA | 4.95 | 0.32 | 0.73 | 32.33 | 2.73 | 0.18 | 34.66 | 2.02 | 0.70 | 140.25 | 5.01 | 1 | ||||
| MCAO+AS-IV +HSYA | 4.7333 | 0.37 | 0.16 | 33.67 | 3.39 | 0.04 | * | 38.50 | 3.77 | 0.01 | * | 138.40 | 6.74 | 0.96 | ||
| F | 8.7 | 15.89 | 18.28 | 37.03 | ||||||||||||
| p | 0 | 0 | 0 | 0 | ||||||||||||
| Group | NF-KB | NLRP3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Sd | t Test | Sign | Mean | Sd | t Test | Sign | |
| Sham | 1.20 | 0.10 | 0 | # | 8.87 | 0.40 | 0 | # |
| MCAO | 5.23 | 0.25 | - | 27.30 | 1.61 | - | ||
| MCAO+AS-IV | 3.67 | 0.67 | 0.002 | ** | 21.33 | 2.31 | 0.004 | ** |
| MCAO+HSYA | 3.63 | 0.51 | 0.002 | ** | 24.00 | 1.00 | 0.009 | ** |
| MCAO+AS-IV+HSYA | 2.40 | 0.10 | 0 | ** | 16.67 | 2.08 | 0 | ** |
| F | 43.56 | 57.41 | ||||||
| p | 0 | 0 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Yan, Z.; Wan, H.; Yang, J.; Ding, Z.; He, Y. A Combination of Astragaloside IV and Hydroxysafflor Yellow A Attenuates Cerebral Ischemia-Reperfusion Injury via NF-κB/NLRP3/Caspase-1/GSDMD Pathway. Brain Sci. 2024, 14, 781. https://doi.org/10.3390/brainsci14080781
Hou Y, Yan Z, Wan H, Yang J, Ding Z, He Y. A Combination of Astragaloside IV and Hydroxysafflor Yellow A Attenuates Cerebral Ischemia-Reperfusion Injury via NF-κB/NLRP3/Caspase-1/GSDMD Pathway. Brain Sciences. 2024; 14(8):781. https://doi.org/10.3390/brainsci14080781
Chicago/Turabian StyleHou, Yongchun, Zi Yan, Haitong Wan, Jiehong Yang, Zhishan Ding, and Yu He. 2024. "A Combination of Astragaloside IV and Hydroxysafflor Yellow A Attenuates Cerebral Ischemia-Reperfusion Injury via NF-κB/NLRP3/Caspase-1/GSDMD Pathway" Brain Sciences 14, no. 8: 781. https://doi.org/10.3390/brainsci14080781




