Individual Differences in Bodily Self-Consciousness and Its Neural Basis
Abstract
:1. Introduction
2. Individual Differences in BSC in Line with the Single-Level-of-Self-Processing Model
2.1. Interoceptive Processing
2.2. Exteroceptive Processing
2.3. Mental Self-Processing
2.4. Brief Summary
3. Individual Differences in BSC in Line with Interactions among Diverse Levels of Self-Processing
4. Neural Basis of Embodied Individual Differences in BSC
4.1. Interoceptive Processing
4.2. Exteroceptive Processing
4.3. Mental Self-Processing
4.4. Interaction of Multiple Levels of Self-Processing
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Park, H.D.; Blanke, O. Coupling Inner and Outer Body for Self-Consciousness. Trends Cogn. Sci. 2019, 23, 377–388. [Google Scholar] [CrossRef]
- Qin, P.; Wang, M.; Northoff, G. Linking bodily, environmental and mental states in the self-A three-level model based on a meta-analysis. Neurosci. Biobehav. Rev. 2020, 115, 77–95. [Google Scholar] [CrossRef]
- Adler, D.; Herbelin, B.; Similowski, T.; Blanke, O. Breathing and sense of self: Visuo-respiratory conflicts alter body self-consciousness. Respir. Physiol. Neurobiol. 2014, 203, 68–74. [Google Scholar] [CrossRef]
- Blanke, O.; Slater, M.; Serino, A. Behavioral, Neural, and Computational Principles of Bodily Self-Consciousness. Neuron 2015, 88, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M.; Hesse, M.D.; Boy, C.; Haggard, P.; Fink, G.R. Neural signatures of body ownership: A sensory network for bodily self-consciousness. Cereb. Cortex 2007, 17, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Schaller, K.; Iannotti, G.R.; Orepic, P.; Betka, S.; Haemmerli, J.; Boex, C.; Alcoba-Banqueri, S.; Garin, D.F.A.; Herbelin, B.; Park, H.D.; et al. The perspectives of mapping and monitoring of the sense of self in neurosurgical patients. Acta Neurochir. 2021, 163, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Aspell, J.E.; Heydrich, L.; Marillier, G.; Lavanchy, T.; Herbelin, B.; Blanke, O. Turning body and self inside out: Visualized heartbeats alter bodily self-consciousness and tactile perception. Psychol. Sci. 2013, 24, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Botvinick, M.; Cohen, J. Rubber hands ‘feel’ touch that eyes see. Nature 1998, 391, 145–166. [Google Scholar] [CrossRef]
- Lenggenhager, B.; Tadi, T.; Metzinger, T.; Blanke, O. Video ergo sum: Manipulating bodily self-consciousness. Science 2007, 317, 1096–1099. [Google Scholar] [CrossRef]
- Blanke, O. Multisensory brain mechanisms of bodily self-consciousness. Nat. Rev. Neurosci. 2012, 13, 556–571. [Google Scholar] [CrossRef]
- Tsakiris, M. The multisensory basis of the self: From body to identity to others. Q. J. Exp. Psychol. 2017, 70, 597–609. [Google Scholar] [CrossRef]
- Cowie, D.; Sterling, S.; Bremner, A.J. The development of multisensory body representation and awareness continues to 10 years of age: Evidence from the rubber hand illusion. J. Exp. Child. Psychol. 2016, 142, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Venskus, A.; Ferri, F.; Migliorati, D.; Spadone, S.; Costantini, M.; Hughes, G. Temporal binding window and sense of agency are related processes modifiable via occipital tACS. PLoS ONE 2021, 16, e0256987. [Google Scholar] [CrossRef]
- Reyno, S.M.; Simmons, M.; Kinley, J. A meta-analytic study examining the relationship between alexithymia and dissociation in psychiatric and nonclinical populations. Res. Psychother. 2020, 23, 439. [Google Scholar] [PubMed]
- Virtanen, N.; Tiippana, K.; Tervaniemi, M.; Poikonen, H.; Anttila, E.; Kaseva, K. Exploring body consciousness of dancers, athletes, and lightly physically active adults. Sci. Rep. 2022, 12, 8353. [Google Scholar] [CrossRef]
- Maister, L.; Tsakiris, M. My face, my heart: Cultural differences in integrated bodily self-awareness. Cogn. Neurosci. 2014, 5, 10–16. [Google Scholar] [CrossRef]
- Costantini, M.; Robinson, J.; Migliorati, D.; Donno, B.; Ferri, F.; Northoff, G. Temporal limits on rubber hand illusion reflect individuals’ temporal resolution in multisensory perception. Cognition 2016, 157, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. How do you feel--now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef]
- Farmer, H.; Maister, L.; Tsakiris, M. Change my body, change my mind: The effects of illusory ownership of an outgroup hand on implicit attitudes toward that outgroup. Front. Psychol. 2014, 4, 1016. [Google Scholar] [CrossRef]
- Pfeiffer, C.; Lopez, C.; Schmutz, V.; Duenas, J.A.; Martuzzi, R.; Blanke, O. Multisensory origin of the subjective first-person perspective: Visual, tactile, and vestibular mechanisms. PLoS ONE 2013, 8, e61751. [Google Scholar] [CrossRef]
- Dary, Z.; Lopez, C. Understanding the neural bases of bodily self-consciousness: Recent achievements and main challenges. Front. Integr. Neurosci. 2023, 17, 1145924. [Google Scholar] [CrossRef] [PubMed]
- David, N.; Fiori, F.; Aglioti, S.M. Susceptibility to the rubber hand illusion does not tell the whole body-awareness story. Cogn. Affect. Behav. Neurosci. 2014, 14, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zaidel, A.; Salomon, R. Multisensory decisions from self to world. Philos. Trans. R Soc. Lond B Biol. Sci. 2023, 378, 20220335. [Google Scholar] [CrossRef]
- Serino, A.; Alsmith, A.; Costantini, M.; Mandrigin, A.; Tajadura-Jimenez, A.; Lopez, C. Bodily ownership and self-location: Components of bodily self-consciousness. Conscious Cogn. 2013, 22, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Olivé, I.; Tempelmann, C.; Berthoz, A.; Heinze, H.J. Increased functional connectivity between superior colliculus and brain regions implicated in bodily self-consciousness during the rubber hand illusion. Hum. Brain Mapp. 2015, 36, 717–730. [Google Scholar] [CrossRef]
- King, J.A.; Geisler, D.; Ritschel, F.; Boehm, I.; Seidel, M.; Roschinski, B.; Soltwedel, L.; Zwipp, J.; Pfuhl, G.; Marxen, M.; et al. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biol. Psychiatry 2015, 77, 624–632. [Google Scholar] [CrossRef]
- Bernardoni, F.; King, J.A.; Geisler, D.; Stein, E.; Jaite, C.; Nätsch, D.; Tam, F.I.; Boehm, I.; Seidel, M.; Roessner, V.; et al. Weight restoration therapy rapidly reverses cortical thinning in anorexia nervosa: A longitudinal study. Neuroimage 2016, 130, 214–222. [Google Scholar] [CrossRef]
- Lavagnino, L.; Mwangi, B.; Cao, B.; Shott, M.E.; Soares, J.C.; Frank, G.K.W. Cortical thickness patterns as state biomarker of anorexia nervosa. Int. J. Eat Disord. 2018, 51, 241–249. [Google Scholar] [CrossRef]
- Candia-Rivera, D.; Annen, J.; Gosseries, O.; Martial, C.; Thibaut, A.; Laureys, S.; Tallon-Baudry, C. Neural Responses to Heartbeats Detect Residual Signs of Consciousness during Resting State in Postcomatose Patients. J. Neurosci. 2021, 41, 5251–5262. [Google Scholar] [CrossRef]
- Chong, J.S.X.; Ng, G.J.P.; Lee, S.C.; Zhou, J. Salience network connectivity in the insula is associated with individual differences in interoceptive accuracy. Brain Struct. Funct. 2017, 222, 1635–1644. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Nadeau, C.; Sorokopud-Jones, M.; Kornelsen, J. The Relationship Between Functional Connectivity and Interoceptive Sensibility. Brain Connect. 2022, 12, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Ma, Y. Cultural differences in human brain activity: A quantitative meta-analysis. Neuroimage 2014, 99, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.R.; Alain, C.; McIntosh, A.R. Individual Differences in Multisensory Processing Are Related to Broad Differences in the Balance of Local versus Distributed Information. J. Cogn. Neurosci. 2022, 34, 846–863. [Google Scholar] [CrossRef]
- Guterstam, A.; Björnsdotter, M.; Bergouignan, L.; Gentile, G.; Li, T.Q.; Ehrsson, H.H. Decoding illusory self-location from activity in the human hippocampus. Front. Hum. Neurosci. 2015, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- D’Argembeau, A.; Cassol, H.; Phillips, C.; Balteau, E.; Salmon, E.; Van der Linden, M. Brains creating stories of selves: The neural basis of autobiographical reasoning. Soc. Cogn. Affect. Neurosci. 2014, 9, 646–652. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Spence, C.; Passingham, R.E. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science 2004, 305, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Bowling, N.C.; Botan, V.; Santiesteban, I.; Ward, J.; Banissy, M.J. Atypical bodily self-awareness in vicarious pain responders. Philos. Trans. R Soc. Lond B Biol. Sci. 2019, 374, 20180361. [Google Scholar] [CrossRef]
- Engelen, T.; Solcà, M.; Tallon-Baudry, C. Interoceptive rhythms in the brain. Nat. Neurosci. 2023, 26, 1670–1684. [Google Scholar] [CrossRef]
- Faccini, J.; Joshi, V.; Del-Monte, J. Nightmares, interoceptive sensibility and nociception: An exploratory study in a general population. Sleep Med. 2023, 112, 209–215. [Google Scholar] [CrossRef]
- Ardizzi, M.; Ferri, F. Interoceptive influences on peripersonal space boundary. Cognition 2018, 177, 79–86. [Google Scholar] [CrossRef]
- Mai, S.; Wong, C.K.; Georgiou, E.; Pollatos, O. Interoception is associated with heartbeat-evoked brain potentials (HEPs) in adolescents. Biol. Psychol. 2018, 137, 24–33. [Google Scholar] [CrossRef]
- Ambrosini, E.; Finotti, G.; Azevedo, R.T.; Tsakiris, M.; Ferri, F. Seeing myself through my heart: Cortical processing of a single heartbeat speeds up self-face recognition. Biol. Psychol. 2019, 144, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M.; Tajadura-Jiménez, A.; Costantini, M. Just a heartbeat away from one’s body: Interoceptive sensitivity predicts malleability of body-representations. Proc. Biol. Sci. 2011, 278, 2470–2476. [Google Scholar] [CrossRef] [PubMed]
- Raimo, S.; Boccia, M.; Di Vita, A.; Cropano, M.; Guariglia, C.; Grossi, D.; Palermo, L. The Body Across Adulthood: On the Relation Between Interoception and Body Representations. Front. Neurosci. 2021, 15, 586684. [Google Scholar] [CrossRef] [PubMed]
- Longarzo, M.; Mele, G.; Alfano, V.; Salvatore, M.; Cavaliere, C. Gender Brain Structural Differences and Interoception. Front. Neurosci. 2021, 14, 586860. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.E.; Tsakiris, M. Going at the heart of social cognition: Is there a role for interoception in self-other distinction? Curr. Opin. Psychol. 2018, 24, 21–26. [Google Scholar] [CrossRef]
- Botan, V.; Salisbury, A.; Critchley, H.D.; Ward, J. Vicarious pain is an outcome of atypical body ownership: Evidence from the rubber hand illusion and enfacement illusion. Q. J. Exp. Psychol. 2021, 74, 1888–1899. [Google Scholar] [CrossRef]
- Ernst, M.O.; Banks, M.S. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 2002, 415, 429–433. [Google Scholar] [CrossRef]
- Chancel, M.; Hasenack, B.; Ehrsson, H.H. Integration of predictions and afferent signals in body ownership. Cognition 2021, 212, 104722. [Google Scholar] [CrossRef]
- Litwin, P. Extending Bayesian Models of the Rubber Hand Illusion. Multisens. Res. 2020, 33, 127–160. [Google Scholar] [CrossRef]
- Chancel, M.; Ehrsson, H.H.; Ma, W.J. Uncertainty-based inference of a common cause for body ownership. Elife 2022, 11, e77221. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tottenham, N. Exposure to the self-face facilitates identification of dynamic facial expressions: Influences on individual differences. Emotion 2013, 13, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism. Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef]
- Rosenberger, N.R. The process of discourse: Usages of a Japanese medical term. Soc. Sci. Med. 1992, 34, 237–247. [Google Scholar] [CrossRef]
- Ma-Kellams, C.; Blascovich, J.; McCall, C. Culture and the body: East-West differences in visceral perception. J. Pers. Soc. Psychol. 2012, 102, 718–728. [Google Scholar] [CrossRef]
- Lira, M.; Egito, J.H.; Dall’Agnol, P.A.; Amodio, D.M.; Gonçalves, Ó.F.; Boggio, P.S. The influence of skin colour on the experience of ownership in the rubber hand illusion. Sci. Rep. 2017, 7, 15745. [Google Scholar] [CrossRef]
- Kling, J.; Wängqvist, M.; Frisén, A. “This body is me”: Discovering the ways in which the body is salient in people’s identities. Body Image 2018, 24, 102–110. [Google Scholar] [CrossRef]
- Ainley, V.; Apps, M.A.; Fotopoulou, A.; Tsakiris, M. ‘Bodily precision’: A predictive coding account of individual differences in interoceptive accuracy. Philos. Trans. R Soc. Lond B Biol. Sci. 2016, 371, 20160003. [Google Scholar] [CrossRef]
- Fukushima, H.; Terasawa, Y.; Umeda, S. Association between interoception and empathy: Evidence from heartbeat-evoked brain potential. Int. J. Psychophysiol. 2011, 79, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Mul, C.L.; Stagg, S.D.; Herbelin, B.; Aspell, J.E. The Feeling of Me Feeling for You: Interoception, Alexithymia and Empathy in Autism. J. Autism. Dev. Disord. 2018, 48, 2953–2967. [Google Scholar] [CrossRef]
- Asai, T.; Mao, Z.; Sugimori, E.; Tanno, Y. Rubber hand illusion, empathy, and schizotypal experiences in terms of self-other representations. Conscious Cogn. 2021, 20, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Heydrich, L.; Walker, F.; Blättler, L.; Herbelin, B.; Blanke, O.; Aspell, J.E. Interoception and Empathy Impact Perspective Taking. Front. Psychol. 2021, 11, 599429. [Google Scholar] [CrossRef] [PubMed]
- Park, H.D.; Bernasconi, F.; Bello-Ruiz, J.; Pfeiffer, C.; Salomon, R.; Blanke, O. Transient Modulations of Neural Responses to Heartbeats Covary with Bodily Self-Consciousness. J. Neurosci. 2016, 36, 8453–8460. [Google Scholar] [CrossRef] [PubMed]
- Ceunen, E.; Vlaeyen, J.W.; Van Diest, I. On the Origin of Interoception. Front. Psychol. 2016, 7, 743. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Human feelings: Why are some more aware than others? Trends Cogn. Sci. 2004, 8, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Seth, A.K.; Barrett, A.B.; Suzuki, K.; Critchley, H.D. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 2015, 104, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Garfinkel, S.N.; Critchley, H.D. Threat and the Body: How the Heart Supports Fear Processing. Trends Cogn. Sci. 2016, 20, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Herbert, B.M.; Pollatos, O. The body in the mind: On the relationship between interoception and embodiment. Top. Cogn. Sci. 2012, 4, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.D.; Stefanovitch, I.; Evans, D.; Oliver, C.; Hawkins, A.; Dalgleish, T. Can you feel the beat? Interoceptive awareness is an interactive function of anxiety- and depression-specific symptom dimensions. Behav. Res. Ther. 2010, 48, 1133–1138. [Google Scholar] [CrossRef]
- Umeda, S.; Tochizawa, S.; Shibata, M.; Terasawa, Y. Prospective memory mediated by interoceptive accuracy: A psychophysiological approach. Philos. Trans. R Soc. Lond B Biol. Sci. 2016, 371, 20160005. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Eulenbruch, T.; Langer, C.; Banger, M. Interoceptive awareness, tension reduction expectancies and self-reported drinking behavior. Alcohol Alcohol. 2013, 48, 472–477. [Google Scholar] [CrossRef]
- Di Pellegrino, G.; Làdavas, E. Peripersonal space in the brain. Neuropsychologia 2015, 66, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sel, A.; Azevedo, R.T.; Tsakiris, M. Heartfelt Self: Cardio-Visual Integration Affects Self-Face Recognition and Interoceptive Cortical Processing. Cereb. Cortex 2017, 27, 5144–5155. [Google Scholar] [CrossRef]
- Grabauskaitė, A.; Baranauskas, M.; Griškova-Bulanova, I. Interoception and gender: What aspects should we pay attention to? Conscious Cogn. 2017, 48, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Barrett, L.F.; Simmons, W.K. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015, 16, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.A.; Taggart, P.; Sutton, P.M.; Groves, D.; Holdright, D.R.; Bradbury, D.; Brull, D.; Critchley, H.D. A cortical potential reflecting cardiac function. Proc. Natl. Acad. Sci. USA 2007, 104, 6818–6823. [Google Scholar] [CrossRef] [PubMed]
- Pollatos, O.; Schandry, R. Accuracy of heartbeat perception is reflected in the amplitude of the heartbeat-evoked brain potential. Psychophysiology 2004, 41, 476–482. [Google Scholar] [CrossRef]
- Luft, C.D.; Bhattacharya, J. Aroused with heart: Modulation of heartbeat evoked potential by arousal induction and its oscillatory correlates. Sci. Rep. 2015, 5, 15717. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, M.; Liu, Y.; Zhang, X.; Jiang, L.; Bao, H.; Qin, P.; Han, J. Brain State Relays Self-Processing and Heartbeat-Evoked Cortical Responses. Brain Sci. 2023, 13, 832. [Google Scholar] [CrossRef] [PubMed]
- Pollatos, O.; Kirsch, W.; Schandry, R. Brain structures involved in interoceptive awareness and cardioafferent signal processing: A dipole source localization study. Hum. Brain Mapp. 2005, 26, 54–64. [Google Scholar] [CrossRef]
- Katkin, E.S.; Cestaro, V.L.; Weitkunat, R. Individual differences in cortical evoked potentials as a function of heartbeat detection ability. Int. J. Neurosci. 1991, 61, 269–276. [Google Scholar] [CrossRef]
- Ueno, D.; Matsuoka, T.; Kato, Y.; Ayani, N.; Maeda, S.; Takeda, M.; Narumoto, J. Individual Differences in Interoceptive Accuracy Are Correlated With Salience Network Connectivity in Older Adults. Front. Aging Neurosci. 2020, 12, 592002. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Metzger, C.D.; Glover, G.H.; Duyn, J.H.; Heinze, H.J.; Walter, M. Association between heart rate variability and fluctuations in resting-state functional connectivity. Neuroimage 2013, 68, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Medford, N.; Critchley, H.D. Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Struct. Funct. 2010, 214, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Heydrich, L.; Blanke, O. Distinct illusory own-body perceptions caused by damage to posterior insula and extrastriate cortex. Brain 2013, 136, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Baier, B.; Karnath, H.O. Tight link between our sense of limb ownership and self-awareness of actions. Stroke 2008, 39, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhornitsky, S.; Li, C.S.; Le, T.M.; Joormann, J.; Li, C.R. Social anxiety, posterior insula activation, and autonomic response during self-initiated action in a Cyberball game. J. Affect. Disord. 2019, 255, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Re, A.; Perconti, P.; Malvica, S.; Castano, E. Did you hear your action? An ecological approach to the senses of ownership and agency. Perception 2023, 52, 129–145. [Google Scholar] [CrossRef]
- Aspell, J.E.; Lenggenhager, B.; Blanke, O. Multisensory Perception and Bodily Self-Consciousness: From Out-of-Body to Inside-Body Experience. In Neural Bases Multisensory Process; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Radziun, D.; Ehrsson, H.H. Auditory cues influence the rubber-hand illusion. J. Exp. Psychol. Hum. Percept. Perform. 2018, 44, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Blanke, O. Out of body experiences and their neural basis. BMJ 2004, 329, 1414–1415. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Schreyer, H.M.; Preuss, N.; Mast, F.W. Vestibular stimulation modifies the body schema. Neuropsychologia 2012, 50, 1830–1837. [Google Scholar] [CrossRef] [PubMed]
- Scandola, M.; Tidoni, E.; Avesani, R.; Brunelli, G.; Aglioti, S.M.; Moro, V. Rubber hand illusion induced by touching the face ipsilaterally to a deprived hand: Evidence for plastic “somatotopic” remapping in tetraplegics. Front. Hum. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Porciello, G.; Bufalari, I.; Minio-Paluello, I.; Di Pace, E.; Aglioti, S.M. The ‘Enfacement’ illusion: A window on the plasticity of the self. Cortex 2018, 104, 261–275. [Google Scholar] [PubMed]
- Finotti, G.; Menicagli, D.; Migliorati, D.; Costantini, M.; Ferri, F. Beyond peripersonal boundaries: Insights from crossmodal interactions. Cogn. Process 2024, 25, 121–132. [Google Scholar]
- Lanfranco, R.C.; Chancel, M.; Ehrsson, H.H. Quantifying body ownership information processing and perceptual bias in the rubber hand illusion. Cognition 2023, 238, 105491. [Google Scholar] [PubMed]
- Jola, C.; Davis, A.; Haggard, P. Proprioceptive integration and body representation: Insights into dancers’ expertise. Exp. Brain Res. 2011, 213, 257–265. [Google Scholar] [CrossRef]
- Hupfeld, K.E.; McGregor, H.R.; Hass, C.J.; Pasternak, O.; Seidler, R.D. Sensory system-specific associations between brain structure and balance. Neurobiol. Aging 2022, 119, 102–116. [Google Scholar]
- Beierholm, U.; Rohe, T.; Ferrari, A.; Stegle, O.; Noppeney, U. Using the past to estimate sensory uncertainty. Elife 2020, 9, e54172. [Google Scholar] [CrossRef]
- Chancel, M.; Ehrsson, H.H. Proprioceptive uncertainty promotes the rubber hand illusion. Cortex 2023, 165, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Noppeney, U. Multisensory Integration and Causal Inference in Typical and Atypical Populations. Adv. Exp. Med. Biol. 2024, 1437, 59–76. [Google Scholar] [PubMed]
- Rohde, M.; Van Dam, L.C.J.; Ernst, M. Statistically Optimal Multisensory Cue Integration: A Practical Tutorial. Multisens. Res. 2016, 29, 279–317. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.A.; Zemtsov, R.K.; Wallace, M.T. Individual differences in the multisensory temporal binding window predict susceptibility to audiovisual illusions. J. Exp. Psychol. Hum. Percept. Perform. 2012, 38, 1517–1529. [Google Scholar] [CrossRef] [PubMed]
- Samad, M.; Chung, A.J.; Shams, L. Perception of body ownership is driven by Bayesian sensory inference. PLoS ONE 2015, 10, e0117178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Acerbi, L.; Ma, W.J. The role of sensory uncertainty in simple contour integration. PLoS Comput. Biol. 2020, 16, e1006308. [Google Scholar] [CrossRef] [PubMed]
- Maister, L.; De Beukelaer, S.; Longo, M.R.; Tsakiris, M. The Self in the Mind’s Eye: Revealing How We Truly See Ourselves Through Reverse Correlation. Psychol. Sci. 2021, 32, 1965–1978. [Google Scholar] [CrossRef]
- Lou, H.C.; Luber, B.; Crupain, M.; Keenan, J.P.; Nowak, M.; Kjaer, T.W.; Sackeim, H.A.; Lisanby, S.H. Parietal cortex and representation of the mental Self. Proc. Natl. Acad. Sci. USA 2004, 101, 6827–6832. [Google Scholar] [CrossRef]
- Amodio, D.M.; Frith, C.D. Meeting of minds: The medial frontal cortex and social cognition. Nat. Rev. Neurosci. 2006, 7, 268–277. [Google Scholar] [CrossRef]
- Damasio, A. Feelings of emotion and the self. Ann. N. Y. Acad. Sci. 2003, 1001, 253–261. [Google Scholar] [CrossRef]
- Bréchet, L. Personal Memories and Bodily-Cues Influence Our Sense of Self. Front. Psychol. 2022, 13, 855450. [Google Scholar] [CrossRef] [PubMed]
- Habermas, T.; Köber, C. Autobiographical reasoning in life narratives buffers the effect of biographical disruptions on the sense of self-continuity. Memory 2015, 23, 664–674. [Google Scholar] [CrossRef]
- Schicktanz, S. Why the way we consider the body matters—Reflections on four bioethical perspectives on the human body. Philos. Ethics Humanit. Med. 2007, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Heine, S.J. Self as cultural product: An examination of East Asian and North American selves. Subcortical contributions to the sense of body ownership. J. Pers. 2001, 692, 881–906. [Google Scholar] [CrossRef]
- Terry, D.J.; Hogg, M.A.; White, K.M. The theory of planned behaviour: Self-identity, social identity and group norms. Br. J. Soc. Psychol. 1999, 38, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Grimm, S.; Duncan, N.W.; Fan, Y.; Huang, Z.; Lane, T.; Weng, X.; Bajbouj, M.; Northoff, G. Spontaneous activity in default-mode network predicts ascription of self-relatedness to stimuli. Soc. Cogn. Affect. Neurosci. 2016, 11, 693–702. [Google Scholar] [CrossRef]
- Maister, L.; Sebanz, N.; Knoblich, G.; Tsakiris, M. Experiencing ownership over a dark-skinned body reduces implicit racial bias. Cognition 2013, 128, 170–178. [Google Scholar] [CrossRef]
- Maister, L.; Slater, M.; Sanchez-Vives, M.V.; Tsakiris, M. Changing bodies changes minds: Owning another body affects social cognition. Trends Cogn. Sci. 2015, 19, 6–12. [Google Scholar] [CrossRef]
- Piran, N. Embodied possibilities and disruptions: The emergence of the Experience of Embodiment construct from qualitative studies with girls and women. Body Image 2016, 18, 43–60. [Google Scholar] [CrossRef]
- Moutoussis, M.; Trujillo-Barreto, N.J.; El-Deredy, W.; Dolan, R.J.; Friston, K.J. A formal model of interpersonal inference. Front. Hum. Neurosci. 2014, 8, 160. [Google Scholar] [CrossRef]
- Tajadura-Jiménez, A.; Grehl, S.; Tsakiris, M. The other in me: Interpersonal multisensory stimulation changes the mental representation of the self. PLoS ONE 2012, 7, e40682. [Google Scholar] [CrossRef] [PubMed]
- Desbordes, G. Self-related processing in mindfulness-based interventions. Neuron 2019, 28, 312–316. [Google Scholar] [CrossRef]
- Legrand, D. Subjective and physical dimensions of bodily self-consciousness, and their dis-integration in anorexia nervosa. Neuropsychologia 2010, 48, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Banellis, L.; Cruse, D. Skipping a Beat: Heartbeat-Evoked Potentials Reflect Predictions during Interoceptive-Exteroceptive Integration. Cereb. Cortex Commun. 2020, 1, tgaa060. [Google Scholar] [CrossRef]
- Seth, A.K. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 2013, 17, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Pezzulo, G. Why do you fear the bogeyman? An embodied predictive coding model of perceptual inference. Cogn. Affect. Behav. Neurosci. 2014, 14, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. The free-energy principle: A rough guide to the brain? Trends Cogn. Sci. 2009, 13, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; FitzGerald, T.H. Interoceptive inference: Homeostasis and decision-making. Trends Cogn. Sci. 2014, 18, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Tsakiris, M.; Carpenter, L.; James, D.; Fotopoulou, A. Hands only illusion: Multisensory integration elicits sense of ownership for body parts but not for non-corporeal objects. Exp. Brain Res. 2010, 204, 343–352. [Google Scholar] [CrossRef]
- Tajadura-Jiménez, A.; Tsakiris, M. Balancing the “inner” and the “outer” self: Interoceptive sensitivity modulates self-other boundaries. J. Exp. Psychol. Gen. 2014, 143, 736–744. [Google Scholar] [CrossRef]
- Shah, S.; Lambrecht, I.; O’Callaghan, A. Reigniting compassion in healthcare: Manaakitia Reflective Rounds. Intern. Med. J. 2017, 47, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Ruby, P.; Decety, J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. J. Cogn. Neurosci. 2004, 16, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, K.N.; Zaki, J.; Hanelin, J.; Ludlow, D.H.; Knierim, K.; Ramachandran, T.; Glover, G.H.; Mackey, S.C. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc. Cogn. Affect. Neurosci. 2008, 3, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Matuz-Budai, T.; Lábadi, B.; Kohn, E.; Matuz, A.; Zsidó, A.N.; Inhóf, O.; Kállai, J.; Szolcsányi, T.; Perlaki, G.; Orsi, G.; et al. Individual differences in the experience of body ownership are related to cortical thickness. Sci. Rep. 2022, 12, 808. [Google Scholar] [CrossRef] [PubMed]
- Seghezzi, S.; Giannini, G.; Zapparoli, L. Neurofunctional correlates of body-ownership and sense of agency: A meta-analytical account of self-consciousness. Cortex 2019, 121, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Farrer, C.; Franck, N.; Frith, C.D.; Decety, J.; Georgieff, N.; D’Amato, T.; Jeannerod, M. Neural correlates of action attribution in schizophrenia. Psychiatry Res. 2004, 131, 31–44. [Google Scholar] [CrossRef]
- Farrer, C.; Frith, C.D. Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency. Neuroimage 2002, 15, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Maurer, C.W.; LaFaver, K.; Ameli, R.; Epstein, S.A.; Hallett, M.; Horovitz, S.G. Impaired self-agency in functional movement disorders: A resting-state fMRI study. Neurology 2016, 87, 564–570. [Google Scholar] [CrossRef]
- Van Ombergen, A.; Wuyts, F.L.; Jeurissen, B.; Sijbers, J.; Vanhevel, F.; Jillings, S.; Parizel, P.M.; Sunaert, S.; Van de Heyning, P.H.; Dousset, V.; et al. Intrinsic functional connectivity reduces after first-time exposure to short-term gravitational alterations induced by parabolic flight. Sci. Rep. 2017, 7, 3061. [Google Scholar]
- Spadone, S.; Perrucci, M.G.; Di Cosmo, G.; Costantini, M.; Della Penna, S.; Ferri, F. Frontal and parietal background connectivity and their dynamic changes account for individual differences in the multisensory representation of peripersonal space. Sci. Rep. 2021, 11, 20533. [Google Scholar] [CrossRef]
- Betzel, R.F.; Byrge, L.; He, Y.; Goñi, J.; Zuo, X.N.; Sporns, O. Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage 2014, 102, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Bagarinao, E.; Watanabe, H.; Maesawa, S.; Mori, D.; Hara, K.; Kawabata, K.; Yoneyama, N.; Ohdake, R.; Imai, K.; Masuda, M.; et al. Reorganization of brain networks and its association with general cognitive performance over the adult lifespan. Sci. Rep. 2019, 9, 11352. [Google Scholar] [CrossRef]
- Pollatos, O.; Schandry, R.; Auer, D.P.; Kaufmann, C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 2007, 1141, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D.; Wiens, S.; Rotshtein, P.; Ohman, A.; Dolan, R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004, 7, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Caseras, X.; Murphy, K.; Mataix-Cols, D.; López-Solà, M.; Soriano-Mas, C.; Ortriz, H.; Pujol, J.; Torrubia, R. Anatomical and functional overlap within the insula and anterior cingulate cortex during interoception and phobic symptom provocation. Hum. Brain Mapp. 2013, 34, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Keenan, J.P.; Wheeler, M.A.; Gallup, G.G., Jr.; Pascual-Leone, A. Self-recognition and the right prefrontal cortex. Trends Cogn Sci. 2000, 4, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Saito, D.N.; Ban, M.; Shimada, K.; Okamoto, Y.; Kosaka, H.; Okazawa, H.; Asada, M.; Naito, E. Self-face recognition shares brain regions active during proprioceptive illusion in the right inferior fronto-parietal superior longitudinal fasciculus III network. Neuroscience 2017, 348, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Yantis, S. The Neural Basis of Selective Attention: Cortical Sources and Targets of Attentional Modulation. Curr. Dir. Psychol. Sci. 2008, 17, 86–90. [Google Scholar] [CrossRef]
- Driver, J.; Noesselt, T. Multisensory interplay reveals crossmodal influences on ‘sensory-specific’ brain regions, neural responses, and judgments. Neuron 2008, 57, 11–23. [Google Scholar] [CrossRef]
- Katayama, R.; Shiraki, R.; Ishii, S.; Yoshida, W. Belief inference for hierarchical hidden states in spatial navigation. Commun Biol. 2024, 7, 614. [Google Scholar] [CrossRef] [PubMed]
- Chiao, J.Y.; Harada, T.; Komeda, H.; Li, Z.; Mano, Y.; Saito, D.; Parrish, T.B.; Sadato, N.; Iidaka, T. Neural basis of individualistic and collectivistic views of self. Hum. Brain Mapp. 2009, 30, 2813–2820. [Google Scholar] [CrossRef]
- Binder, D.K.; Garcia, P.A.; Elangovan, G.K.; Barbaro, N.M. Characteristics of auras in patients undergoing temporal lobectomy. J. Neurosurg. 2009, 111, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J. The anatomy of language: A review of 100 fMRI studies published in 2009. Ann. N. Y. Acad. Sci. 2010, 1191, 62–88. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.; Cheung, B.; Davis, T.; Hughes, B. Mapping the self: A network approach for understanding psychological and neural representations of self-concept structure. J. Pers. Soc. Psychol. 2023, 124, 237–263. [Google Scholar] [CrossRef] [PubMed]
- Lanius, R.A.; Frewen, P.A.; Tursich, M.; Jetly, R.; McKinnon, M.C. Restoring large-scale brain networks in PTSD and related disorders: A proposal for neuroscientifically-informed treatment interventions. Eur. J. Psychotraumatol. 2015, 6, 27313. [Google Scholar] [CrossRef] [PubMed]
- Cabanis, M.; Pyka, M.; Mehl, S.; Müller, B.W.; Loos-Jankowiak, S.; Winterer, G.; Wölwer, W.; Musso, F.; Klingberg, S.; Rapp, A.M.; et al. The precuneus and the insula in self-attributional processes. Cogn. Affect. Behav. Neurosci. 2013, 13, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Immordino-Yang, M.H.; McColl, A.; Damasio, H.; Damasio, A. Neural correlates of admiration and compassion. Proc. Natl. Acad. Sci. USA 2009, 106, 8021–8026. [Google Scholar] [CrossRef] [PubMed]
- Singer, T.; Seymour, B.; O’Doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for pain involves the affective but not sensory components of pain. Science 2004, 303, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Seitz, R.J.; Nickel, J.; Azari, N.P. Functional modularity of the medial prefrontal cortex: Involvement in human empathy. Neuropsychology 2006, 20, 743–751. [Google Scholar] [CrossRef]
- LaVarco, A.; Ahmad, N.; Archer, Q.; Pardillo, M.; Nunez Castaneda, R.; Minervini, A.; Keenan, J.P. Self-Conscious Emotions and the Right Fronto-Temporal and Right Temporal Parietal Junction. Brain Sci. 2022, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Spitmaan, M.; Seo, H.; Lee, D.; Soltani, A. Multiple timescales of neural dynamics and integration of task-relevant signals across cortex. Proc. Natl. Acad. Sci. USA 2020, 117, 22522–22531. [Google Scholar] [CrossRef] [PubMed]
- Brang, D.; Taich, Z.J.; Hillyard, S.A.; Grabowecky, M.; Ramachandran, V.S. Parietal connectivity mediates multisensory facilitation. Neuroimage 2013, 78, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, M. Modulations of the experience of self and time. Conscious Cogn. 2015, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Millière, R.; Carhart-Harris, R.L.; Roseman, L.; Trautwein, F.M.; Berkovich-Ohana, A. Psychedelics, Meditation, and Self-Consciousness. Front. Psychol. 2018, 9, 1475. [Google Scholar] [CrossRef]
- Reutens, S.; Nielsen, O.; Sachdev, P. Depersonalization disorder. Curr. Opin. Psychiatry 2010, 23, 278–283. [Google Scholar] [CrossRef]
- Sarazine, J.; Heitschmidt, M.; Vondracek, H.; Sarris, S.; Marcinkowski, N.; Kleinpell, R. Mindfulness Workshops Effects on Nurses’ Burnout, Stress, and Mindfulness Skills. Holist. Nurs. Pract. 2021, 35, 10–18. [Google Scholar] [CrossRef]
- Dieguez, S.; Lopez, C. The bodily self: Insights from clinical and experimental research. Ann. Phys. Rehabil. Med. 2017, 60, 198–207. [Google Scholar] [CrossRef]
- Basagni, B.; Luzzatti, C.; De Tanti, A.; Bozzetti, F.; Crisi, G.; Pinardi, C.; Errante, A.; Fogassi, L. Some evidence on Gerstmann’s syndrome: A case study on a variant of the clinical disorder. Brain Cogn. 2021, 148, 105679. [Google Scholar] [CrossRef]
- Lopez, C.; Nakul, E.; Preuss, N.; Elzière, M.; Mast, F.W. Distorted own-body representations in patients with dizziness and during caloric vestibular stimulation. J. Neurol. 2018, 265, 86–94. [Google Scholar] [CrossRef]
- Nesti, A.; Rognini, G.; Herbelin, B.; Bülthoff, H.H.; Chuang, L.; Blanke, O. Modulation of vection latencies in the full-body illusion. PLoS ONE 2018, 13, e0209189. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Cai, X.L.; Weigl, M.; Bang, P.; Cheung, E.F.C.; Chan, R.C.K. Multisensory temporal binding window in autism spectrum disorders and schizophrenia spectrum disorders: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 86, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Langer, A.; Kaiser, J. Temporal integration of multisensory stimuli in autism spectrum disorder: A predictive coding perspective. J. Neural Transm. 2016, 123, 917–923. [Google Scholar] [CrossRef]
- Moseley, M.E.; Liu, C.; Rodriguez, S.; Brosnan, T. Advances in magnetic resonance neuroimaging. Neurol. Clin. 2009, 27, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Ehrsson, H.H.; Rosén, B.; Stockselius, A.; Ragnö, C.; Köhler, P.; Lundborg, G. Upper limb amputees can be induced to experience a rubber hand as their own. Brain 2008, 131, 3443–3452. [Google Scholar] [CrossRef] [PubMed]
| Manifestations of Individual Differences | Related Individual Differences in BSC | |
|---|---|---|
| Internal bodily signal perceptibility | Interoceptive accuracy | |
| Interoceptive sensibility |
| |
| Interoceptive awareness |
| |
| Multisensory processing principles | Bayesian CI principles (unisensory signal weighting) |
|
| Bayesian CI principles (multisensory processing) | ||
| Distinction mechanism of self-other | ||
| Personal traits shaped by environment | Personality affected by sociocultural constructs |
|
| Personal social identity | ||
| Interaction modes | Interaction between low-level self-processing |
|
| Interaction between low and high-level self-processing |
| |
| Level of Self-Processing | Corresponding Neural Basis | Brain Structure and Function |
|---|---|---|
| Interoceptive- processing |
| |
| Exteroceptive- processing |
|
|
| Mental-self-processing |
| |
| Multiple levels of self-processing |
|
|
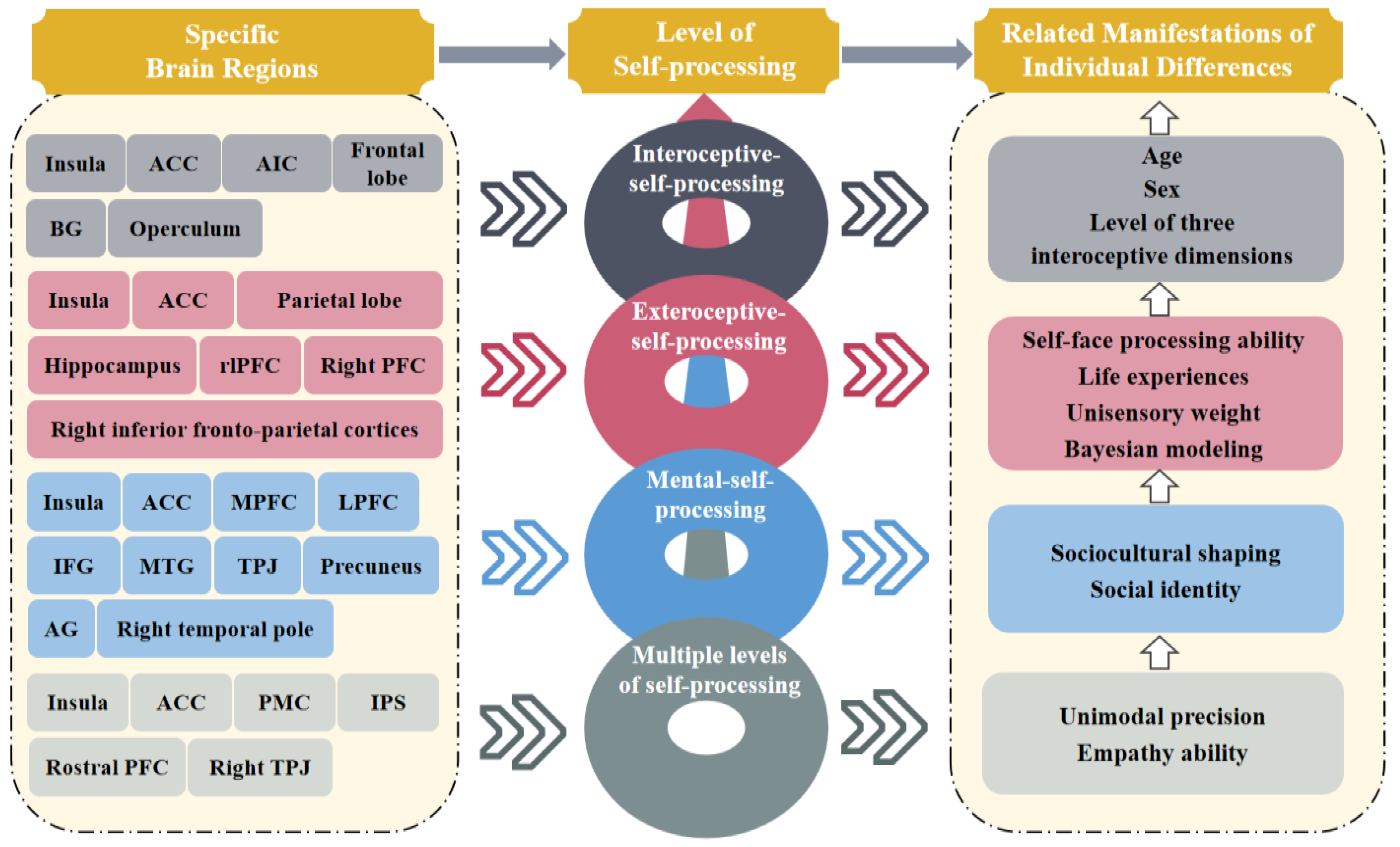
| Level of Self-Processing | Specific Areas of the Brain | Brain Network | Brain Activities | Personal Behavior and Mind | Bodily Experiences | Features of Consciousness |
|---|---|---|---|---|---|---|
| Interoceptive- processing | Insula (for the young)/ACC (for the elderly) | Salience network | Functional connectivity | Perception of heartbeat | Subjective perception of bodily awareness | Context- dependent (for the young)/Context- independent (for the elderly) |
| AIC | Salience network | Task-based activation | Performance accuracy on the interoceptive task | Interoceptive accuracy to bodily signals | Perceptibility and clarity of consciousness | |
| Frontal lobe/BG | Default mode network/(/) | Functional connectivity | Self-reported sensitivity to interoceptive information | Sensitivity to internal bodily signals | Sensibility of consciousness | |
| Visuospatial network | Visuospatial network | Functional connectivity | Maintaining normal cognitive function | Bodily representation ability | Degree of integration and association | |
| Operculum/right anterior insula | Sensorimotor/salience network | Gray matter volume | Attention to either heart-beat timing or external note quality | Awareness of bodily and stress responses | Accuracy of interoceptive awareness | |
| Exteroceptive- processing | Right PFC/right inferior frontoparietal cortices | Frontoparietal network | Task-based activation | Self-face recognition and facial expression processing | Sense of identification and ownership with one’s own body | Stable, self-bias, and continually updated |
| Insula/ACC | Salience network | Functional connectivity | Attention and cognitive control | Focus on sensory signals and bodily state | Integration of bottom-up and top-down sensory information | |
| ACC (bilateral caudal regions) | Salience network | Task-based activation | Integrating information over long timescales | Fine resolution of multisensory signals | Efficiency of multisensory processing | |
| Parietal lobe | Default mode network | Task-based activation | Receiving input from sensory-specific cortices | Perception of multisensory input | Representation of multisensory modalities | |
| Hippocampus | / | Task-based activation | Multisensory representation of the bodily self in space | Bodily self-location and out-of-body experience | Updatable and plastic | |
| rlPFC/hippocampus | Executive Control Network/(/) | Task-based activation | Performing inference tasks related to Bayesian modeling | / | Hierarchical and complex | |
| Mental-self- processing | dmPFC/LPFC/TPJ | Default mode/frontoparietal network/(/) | Task-based activation | Self-relevance encoding during social affective process | Sensitive to bodily information related to significant others | Context- dependent |
| ACC/vmPFC/bilateral insula/right temporal pole | Salience/default mode/(/)/(/) | Task-based activation | Self-relevance encoding during social affective process | Attention to self-focused bodily information | Context- independent | |
| dmPFC/MTG/IFG/AG | Default mode/auditory/language/visual recognition network | Task-based activation | Processing semantics and integrating personal identity | Sense of identity and distinction of bodily parts | Coherence and stability of self- consciousness | |
| vmPFC | Default mode network | Task-based activation | Caring about self-evaluation | Sensitive to bodily traits related to self-identity | Constructed by social identity | |
| Multiple levels of self-processing | Insula/ACC | Salience network | Task-based activation | Experiences of complex social emotions | Compassion for physical pain | High-level cognitive processing |
| PMC/IPS | Motor/frontoparietal network | Task-based activation | Preferentially processing self-related signals | Self-location and identification | Global unity and temporal continuity | |
| Rostral PFC | Default mode network | Task-based activation | Comprehending others’ intentions | / | Emotion- dependent | |
| Right TPJ | / | Task-based activation | Comprehension of self-conscious emotions | / | Impacted by emotions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Huang, Y.; Qin, P.; Wu, H. Individual Differences in Bodily Self-Consciousness and Its Neural Basis. Brain Sci. 2024, 14, 795. https://doi.org/10.3390/brainsci14080795
Wu H, Huang Y, Qin P, Wu H. Individual Differences in Bodily Self-Consciousness and Its Neural Basis. Brain Sciences. 2024; 14(8):795. https://doi.org/10.3390/brainsci14080795
Chicago/Turabian StyleWu, Haiyan, Ying Huang, Pengmin Qin, and Hang Wu. 2024. "Individual Differences in Bodily Self-Consciousness and Its Neural Basis" Brain Sciences 14, no. 8: 795. https://doi.org/10.3390/brainsci14080795






