The Relationships of Specific Cognitive Control Abilities with Objective and Subjective Sleep Parameters in Mild Cognitive Impairment: Revealing the Association between Cognitive Planning and Sleep Duration
Abstract
:1. Introduction
1.1. Aims and Hypotheses of the Present Study
1.2. Based on the Theoretical Framework, the Following Hypotheses Were Formulated
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Ethics
2.6. Procedure
2.7. Instruments
2.7.1. Cognitive Measures
2.7.2. D-KEFS Verbal Fluency Test, Standard Form–D-KEFS VF, SF
2.7.3. D-KEFS Color–Word Interference Test, Standard Form–D-KEFS C-WIT, SF
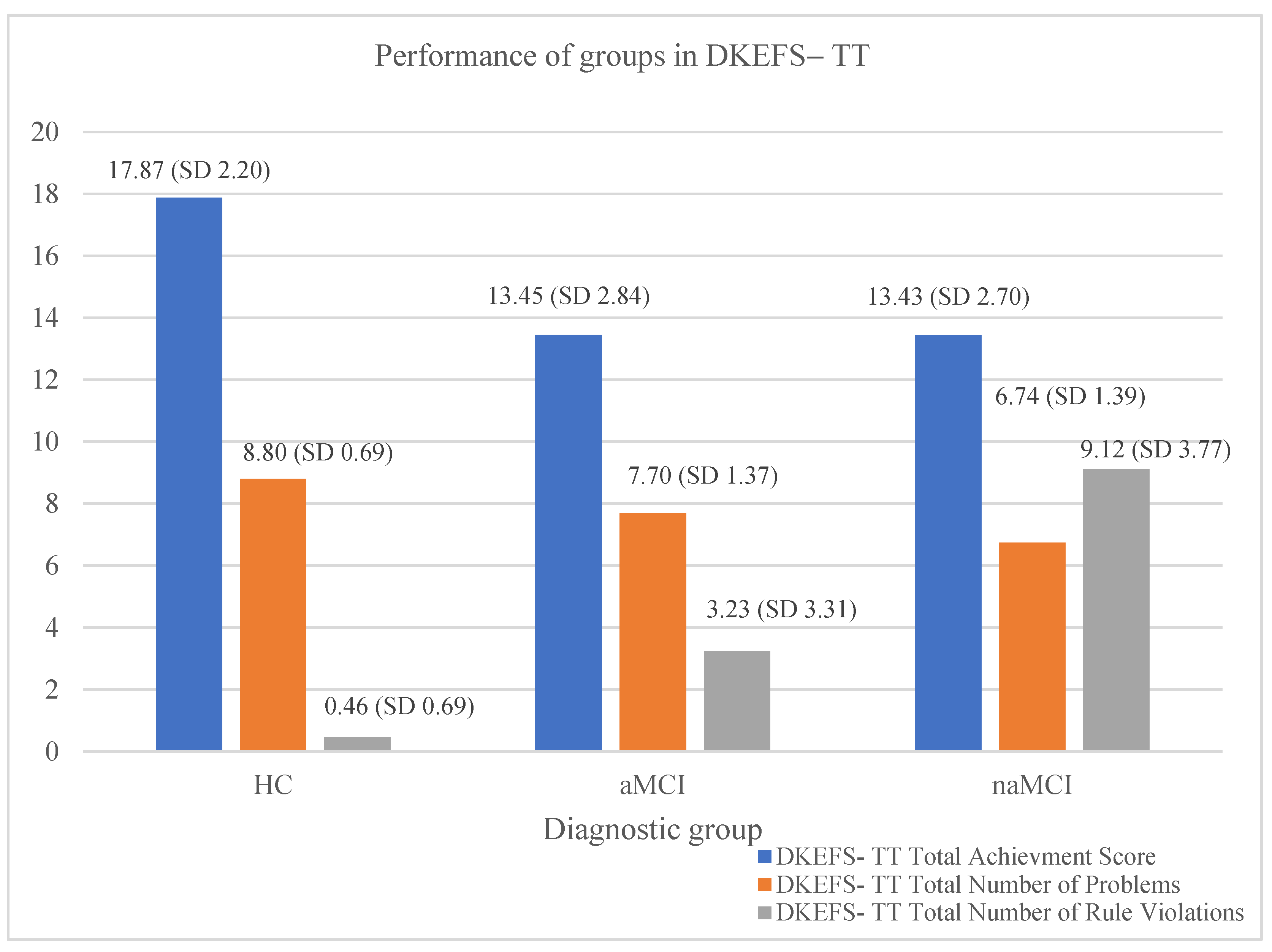
2.7.4. D-KEFS Tower Test, Standard Form–D-KEFS–TT, SF
2.8. Sleep Measures
2.8.1. Athens Insomnia Scale
2.8.2. Stop-Bang Questionnaire
2.8.3. The Pittsburgh Sleep Quality Index (PSQI)
2.8.4. Sleep Diary
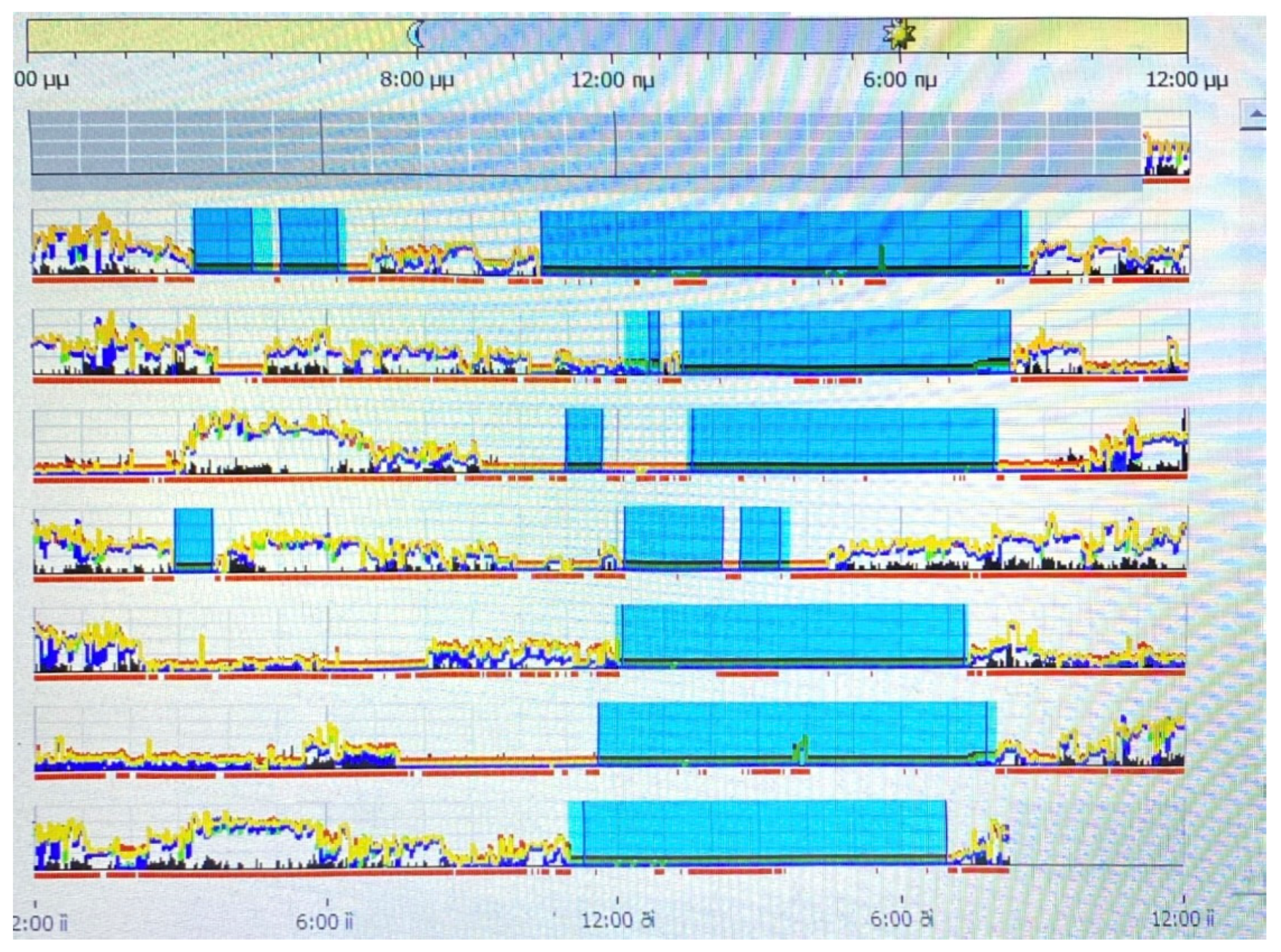
2.8.5. Objective Sleep Measures
Actigraphy
2.9. Statistical Analysis
3. Results
3.1. Cognitive Control Performance
3.2. Subjective Sleep Measures: Group Differences in AIS, Stop-Bang, and PSQI Diary
3.3. Objective Sleep Measures: Group Differences in Actigraphy
3.4. Correlations between All Variables of Interest
3.5. Mediation Analyses
4. Discussion
4.1. Cognitive Control Differences among Diagnostic Groups
4.2. Sleep Disturbances and MCI
4.3. Connection between Sleep Duration and Cognitive Planning
5. Limitations
6. Conclusions
7. Future Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, R.C. Mild Cognitive Impairment as a Diagnostic Entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild Cognitive Impairment: A Concept in Evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef]
- Costa, A.R.; Moreira, P.S.; Machado, A.; Sousa, N. Sleep Disturbances and Cognitive Impairment: A Comprehensive Review Exploring the Link Between MCI and Sleep Quality. Nat. Sci. Sleep 2023, 15, 103–120. [Google Scholar]
- Lima, N.C.; Kirov, R.; de Almondes, K.M. Impairment of Executive Functions Due to Sleep Alterations: An Integrative Review on the Use of P300. Front. Neurosci. 2022, 16, 906492. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.B.; Rosselli, M. The Elusive Nature of Executive Functions: A Review of Our Current Understanding. Neuropsychol. Rev. 2007, 17, 213–233. [Google Scholar] [CrossRef]
- Luo, Y.; Qiao, M.; Liang, Y.; Chen, C.; Zeng, L.; Wang, L.; Wu, W. Functional Brain Connectivity in Mild Cognitive Impairment with Sleep Disorders: A Study Based on Resting-State Functional Magnetic Resonance Imaging. Front. Aging Neurosci. 2022, 14, 812664. [Google Scholar] [CrossRef] [PubMed]
- Randhi, B.; Gutlapalli, S.D.; Pu, J.; Zaidi, M.F.; Patel, M.; Atluri, L.M.; Gonzalez, N.A.; Sakhamuri, N.; Athiyaman, S.; Hamid, P. Sleep Disorders in Mild Cognitive Impairment. Cureus 2023, 15, e36202. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Van Hoecke, L.; Vandenbroucke, R.E. The Impact of Systemic Inflammation on Alzheimer’s Disease Pathology. Front. Immunol. 2022, 12, 796867. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- Yang, T.; Shen, B.; Wu, A.; Tang, X.; Chen, W.; Zhang, Z.; Chen, B.; Guo, Z.; Liu, X. Abnormal Functional Connectivity of the Amygdala in Mild Cognitive Impairment Patients with Depression Symptoms Revealed by Resting-State fMRI. Front. Psychiatry 2021, 12, 533428. [Google Scholar] [CrossRef]
- Song, J. Amygdala activity and amygdala-hippocampus connectivity: Metabolic diseases, dementia, and neuropsychiatric issues. Biomed. Pharmacother. 2023, 162, 114647. [Google Scholar] [CrossRef]
- Toader, C.; Dobrin, N.; Brehar, F.M.; Popa, C.; Covache-Busuioc, R.A.; Glavan, L.A.; Costin, H.P.; Bratu, B.G.; Corlatescu, A.D.; Popa, A.A.; et al. From Recognition to Remedy: The Significance of Biomarkers in Neurodegenerative Disease Pathology. Int. J. Mol. Sci. 2023, 24, 16119. [Google Scholar] [CrossRef]
- Sen, A.; Tai, X.Y. Sleep Duration and Executive Function in Adults. Curr. Neurol. Neurosci. Rep. 2023, 23, 801–813. [Google Scholar] [CrossRef]
- Okuda, M.; Noda, A.; Iwamoto, K.; Nakashima, H.; Takeda, K.; Miyata, S.; Yasuma, F.; Ozaki, N.; Shimouchi, A. Effects of long sleep time and irregular sleep–wake rhythm on cognitive function in older people. Sci. Rep. 2021, 11, 7039. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175191. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2022. [Google Scholar]
- Fountoulakis, K.N.; Tsolaki, M.; Iacovides, A.; Yesavage, J.; O’Hara, R.; Kazis, A.; Ierodiakonou, C. The Validation of the Short Form of the Geriatric Depression Scale (GDS) in Greece. Aging Clin. Exp. Res. 1999, 11, 367–372. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulos, I.A.; Sinoff, G.; Alegakis, A.; Kounalakis, D.; Antonopoulou, M.; Lionis, C. The Short Anxiety Screening Test in Greek: Translation and validation. Ann. Gen. Psychiatry 2010, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Sinoff, G.; Ore, L.; Zlotogorsky, D.; Tamir, A. Short Anxiety Screening Test--a brief instrument for detecting anxiety in the elderly. Int. J. Geriatr. Psychiatry 1999, 14, 1062–1071. [Google Scholar] [CrossRef]
- Politis, A.M.; Mayer, L.S.; Passa, M.; Maillis, A.; Lyketsos, C.G. Validity and reliability of the newly translated Hellenic Neuropsychiatric Inventory (H-NPI) applied to Greek outpatients with Alzheimer’s disease: A study of disturbing behaviors among referrals to a memory clinic. Int. J. Geriatr. Psychiatry 2004, 19, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L.; Mega, M.; Gray, K.; Rosenberg-Thompson, S.; Carusi, D.A.; Gornbein, J. The Neuropsychiatric Inventory: Comprehensive Assessment of Psychopathology in Dementia. Neurology 1994, 44, 2308. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, K.N.; Tsolaki, M.; Chantzi, H.; Kazis, A.D. Mini Mental State Examination (MMSE): A validation study in Greece. Am. J. Alzheimer’s Dis. Other Demen. 2000, 15, 342–345. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Poptsi, E.; Moraitou, D.; Eleftheriou, M.; Kounti-Zafeiropoulou, F.; Papasozomenou, C.; Agogiatou, C.; Bakoglidou, E.; Batsila, G.; Liapi, D.; Markou, N.; et al. Normative Data for the Montreal Cognitive Assessment in Greek Older Adults with Subjective Cognitive Decline, Mild Cognitive Impairment and Dementia. J. Geriatr. Psychiatry Neurol. 2019, 32, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Kounti, F.; Tsolaki, M.; Kiosseoglou, G. Functional cognitive assessment scale (FUCAS): A new scale to assess executive cognitive function in daily life activities in patients with dementia and mild cognitive impairment. Hum. Psychopharmacol. 2006, 21, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef]
- Kosta-Tsolaki, M.; Poptsi, E.; Aggogiatou, C.; Kounti, F.; Zafeiropoulos, S.; Markou, N. Computer-Based Cognitive Training Versus Paper and Pencil Training: Which Is More Effective? A Randomized Controlled Trial in People with Mild Cognitive Impairment. JSM Alzheimer’s Dis. Relat. Dement. 2017, 4, 1032. [Google Scholar]
- Delis, D.C.; Kaplan, E.; Kramer, J.H. Delis-Kaplan Executive Function System (D–KEFS) [Database record]. APA PsycTests 2001, 10, t15082. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Paul, R.H.; Ozonoff, A.; Cohen, R.A. Evaluating elements of executive functioning as predictors of instrumental activities of daily living (IADLs). Arch. Clin. Neuropsychol. 2006, 21, 311320. [Google Scholar] [CrossRef] [PubMed]
- Fine, E.M.; Delis, D.C. Delis–kaplan executive functioning system. In Encyclopedia of Clinical Neuropsychology.; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Soldatos, C.R.; Dikeos, D.G.; Paparrigopoulos, T.J. Athens Insomnia Scale: Validation of an instrument based on ICD-10 criteria. J. Psychosom. Res. 2000, 48, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang questionnaire: A practical approach to screen for obstructive sleep apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Miskedaki, A.; Bacopoulou, F.; Vlachakis, D.; Artemiadis, A.; Chrousos, G.P.; Darviri, C. Validation of the STOP-Bang Questionnaire in Greek Patients Suffering from Obstructive Sleep Apnea. Adv. Exp. Med. Biol. 2021, 1337, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Perantoni, E.; Steiropoulos, P.; Siopi, D.; Amfilochiou, A.; Michailidis, V.; Christoforatou, K.; Tsara, V. Validation of the Greek version of Pittsburgh Sleep Quality Questionnaire in a sleep lab population. Eur. Respir. J. 2012, 40 (Suppl. 56), P903. [Google Scholar]
- Kotronoulas, G.C.; Papadopoulou, C.N.; Papapetrou, A.; Patiraki, E. Psychometric evaluation and feasibility of the Greek Pittsburgh Sleep Quality Index (GR-PSQI) in patients with cancer receiving chemotherapy. Support. Care Cancer 2011, 19, 1831–1840. [Google Scholar] [CrossRef]
- Petropoulakos, K.; Papakonstantinou, V.; Pentsi, S.; Souzou, E.; Dimitriadis, Z.; Billis, E.; Koumantakis, G.; Poulis, I.; Spanos, S. Validity and Reliability of the Greek Version of Pittsburgh Sleep Quality Index in Chronic Non-Specific Low Back Pain Patients. Healthcare 2024, 12, 557. [Google Scholar] [CrossRef]
- Edinger, J.D.; Means, M.K.; Carney, C.E.; Manber, R. Psychological and Behavioral Treatments for Insomnia II. In Principles and Practice of Sleep Medicine; Elsevier: Amsterdam, The Netherlands, 2011; pp. 884–904. [Google Scholar] [CrossRef]
- Pigot, H.; Lefebvre, B.; Meunier, J.; Kerhervé, B.; Mayers, A.; Giroux, S. The role of intelligent habitats in upholding elders in residence. WIT Trans. Biomed. Health 2003, 6, 497–506. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 24.0; IBM Corp: Armonk, NY, USA, 2016. [Google Scholar]
- Jeffreys, H. Theory of Probability, 3rd ed.; MR0187257; Oxford University Press: New York, NY, USA, 1961; p. 432. [Google Scholar]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.; Ganguli, M.; Gloss, D.; Gronseth, G.; Sager, M.; Stevens, J.; Rae-Grant, A. Practice Guideline Update Summary: Mild Cognitive Impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef]
- Roberts, R.O.; Aakre, J.A.; Cha, R.H.; Mielke, M.M.; Geda, Y.E.; Boeve, B.F.; Machulda, M.M.; Ivnik, R.J.; Knopman, D.S.; Petersen, R.C. Prevalence and Outcomes of Mild Cognitive Impairment in a Population-Based Study. Neurology 2014, 82, 959–967. [Google Scholar]
- Kirova, A.M.; Bays, R.B.; Lagalwar, S. Working Memory and Executive Function Decline across Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. BioMed Res. Int. 2015, 748212. [Google Scholar] [CrossRef]
- Wang, F.; Liu, C.-J. Cognitive Processes in Rule Violation. Adv. Cogn. Psychol. 2024, 20, 35–43. [Google Scholar] [CrossRef]
- Possin, K.L.; Brambati, S.M.; Rosen, H.J.; Johnson, J.K.; Pa, J.; Weiner, M.W.; Miller, B.L.; Kramer, J.H. Rule Violation Errors are Associated with Right Lateral Prefrontal Cortex Atrophy in Neurodegenerative Disease. J. Int. Neuropsychol. Soc. 2009, 15, 354–364. [Google Scholar] [CrossRef]
- Geda, Y.E. Mild Cognitive Impairment in Older Adults. Curr. Psychiatry Rep. 2012, 14, 320–327. [Google Scholar] [CrossRef]
- Ozzoude, M.; Varriano, B.; Beaton, D.; Ramirez, J.; Adamo, S.; Holmes, M.F.; Scott, C.J.M.; Gao, F.; Sunderland, K.M.; McLaughlin, P.; et al. White Matter Hyperintensities and Smaller Cortical Thickness are Associated with Neuropsychiatric Symptoms in Neurodegenerative and Cerebrovascular Diseases. Alzheimer’s Res. Ther. 2023, 15, 114. [Google Scholar] [CrossRef]
- Misquitta, K.; Dadar, M.; Collins, D.L.; Tartaglia, M.C.; Alzheimer’s Disease Neuroimaging Initiative. White Matter Hyperintensities and Neuropsychiatric Symptoms in Mild Cognitive Impairment and Alzheimer’s Disease. NeuroImage Clin. 2020, 28, 102367. [Google Scholar] [CrossRef]
- Li, X.; Shen, M.; Jin, Y.; Jia, S.; Zhou, Z.; Han, Z.; Zhang, X.; Tong, X.; Jiao, J. The Effect of Cerebral Small Vessel Disease on the Subtypes of Mild Cognitive Impairment. Front. Psychiatry 2021, 12, 685965. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kong, I.J.; Choi, J.; Baek, J.Y.; Kim, E.J.; Shin, Y.I.; Ko, M.H.; Shin, Y.B.; Shin, M.J. Neural Compensatory Response During Complex Cognitive Function Tasks in Mild Cognitive Impairment: A Near-Infrared Spectroscopy Study. Neural Plast. 2019, 2019, 7845104. [Google Scholar] [CrossRef]
- Baruth, J.M.; Salgado, M.F.; Joseph, B.; Singh, B.; Nunez, N.A. Association between sleep disturbances and mild cognitive impairment: Clinical and research considerations: Commentary on “Late-life sleep duration associated with amnestic mild cognitive impairment” by Yuan et al. Int. Psychogeriatr. 2023, 35, 403–406. [Google Scholar] [CrossRef]
- Mayer, G.; Frohnhofen, H.; Jokisch, M.; Hermann, D.M.; Gronewold, J. Associations of sleep disorders with all-cause MCI/dementia and different types of dementia—Clinical evidence, potential pathomechanisms and treatment options: A narrative review. Front. Neurosci. 2024, 18, 1372326. [Google Scholar] [CrossRef] [PubMed]
- Basta, M.; Simos, P.; Vgontzas, A.; Koutentaki, E.; Tziraki, S.; Zaganas, I.; Panagiotakis, S.; Kapetanaki, S.; Fountoulakis, N.; Lionis, C. Associations between sleep duration and cognitive impairment in mild cognitive impairment. J. Sleep Res. 2019, 28, e12864. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, M.; Guo, Y.; Liu, Y.; Dong, X. Sleep structure assessed by objective measurement in patients with mild cognitive impairment: A meta-analysis. Sleep Med. 2024, 113, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Li, T.; Zhang, L.; Shi, L.; Liao, J.; Li, W.; Cheng, G.; Tan, W.; Rong, S. Characteristics of sleep structure assessed by objective measurements in patients with amnestic mild cognitive impairment: A meta-analysis. Front. Neurol. 2020, 11, 577126. [Google Scholar] [CrossRef] [PubMed]
- Knopper, R.W.; Hansen, B. Locus Coeruleus and the Defensive Activation Theory of Rapid Eye Movement Sleep: A Mechanistic Perspective. Front. Neurosci. 2023, 17, 1094812. [Google Scholar] [CrossRef]
- Friston, K.J.; FitzGerald, T.; Rigoli, F.; Schwartenbeck, P.; Pezzulo, G. Active Inference: A Process Theory. Neural Comput. 2017, 29, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Eagleman, D.M.; Vaughn, D.A. The Defensive Activation Theory: REM Sleep as a Mechanism to Prevent Takeover of the Visual Cortex. Front. Neurosci. 2021, 21, 632853. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Raho, E.M.; Sensi, M.; Di Lorenzo, F.; Fadiga, L.; Koch, G. A New Perspective on Positive Symptoms: Expression of Damage or Self-Defence Mechanism of the Brain? Neurol. Sci. 2024, 45, 2347–2351. [Google Scholar] [CrossRef]
- Meerlo, P.; Havekes, R.; Steiger, A.; Peters, K. Short-term sleep deprivation primes the brain for neurodegeneration by increasing cortical amyloid-β levels. Proc. Natl. Acad. Sci. USA 2009, 106, 14564–14569. [Google Scholar] [CrossRef]
- Basta, M.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Antypa, D.; Li, Y.; Zaganas, I.; Panagiotakis, S.; Karagkouni, E.; Simos, P. Basal cortisol levels are increased in patients with mild cognitive impairment: Role of insomnia and short sleep duration. J. Alzheimer’s Dis. 2022, 87, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Espinosa, M.P.; Atienza, M.; Cantero, J.L. Sleep deficits in mild cognitive impairment are related to increased levels of plasma amyloid-β and cortical thinning. Neuroimage 2014, 98, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.Y.; Chen, C.; Manohar, S.; Husain, M. Impact of sleep duration on executive function and brain structure. Commun. Biol. 2022, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.P.; Stickgold, R. Sleep, memory, and plasticity. Annu. Rev. Psychol. 2022, 73, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2023, 24, 12–23. [Google Scholar] [CrossRef]
- Rasch, B.; Born, J. About sleep’s role in memory. Trends Cogn. Sci. 2024, 28, 187–195. [Google Scholar] [CrossRef]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.C.; Ong, J.L.; Leong, R.L.; Gooley, J.J.; Chee, M.W. Cognitive performance and sleep quality in relation to mild cognitive impairment. Neurobiol. Aging 2022, 115, 81–89. [Google Scholar]
- Lee, H.J.; Lee, D.A.; Shin, K.J.; Park, K.M. Glymphatic system dysfunction in obstructive sleep apnea evidenced by DTI-ALPS. Sleep Med. 2022, 89, 176–181. [Google Scholar] [CrossRef]
- Ramirez, J.; Holmes, M.F.; Berezuk, C.; Kwan, D.; Tan, B.; Beaton, D.; Scott, C.J.M.; Ozzoude, M.; Gao, F.; Yu, D.; et al. MRI-visible perivascular space volumes, sleep duration and daytime dysfunction in adults with cerebrovascular disease. Sleep Med. 2021, 83, 83–88. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Lucey, B.P.; Holtzman, D.M.; Morris, J.C.; Bateman, R.J. Sleep, amyloid, and Alzheimer’s disease: Interaction by genotype. JAMA 2023, 329, 1042–1050. [Google Scholar]

| HC | aMCI | naMCI | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 70.28 (5.02) | 71.09 (4.98) | 69.07 (4.18) |
| Education | 11.98 (3.09) | 12.41 (3.18) | 12.57 (3.39) |
| Gender (f/m) | 36/10 | 51/24 | 39/19 |
| HC | aMCI | naMCI | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| AIS Score | 5.26 (3.58) | 6.44 (4.53) | 4.76 (4.47) |
| Stop-Bang Score | 3.11 (1.10) | 2.79 (1.11) | 2.69 (1.30) |
| PSQI Score | 5.65 (2.08) | 5.60 (2.66) | 4.74 (2.05) |
| HC | aMCI | naMCI | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Sleep efficiency (SE) | 77.61 (8.58) | 77.76 (10.58) | 76.74 (8.91) |
| Wake after sleep onset (WASO) | 1.79 (0.35) | 1.76 (0.31) | 1.81 (0.29) |
| Total Achievement Score | Total Number of Problems | Total Number of Rule Violations | |
|---|---|---|---|
| Total sleep time | 0.158 * | 0.169 * | −0.229 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batzikosta, A.; Moraitou, D.; Steiropoulos, P.; Papantoniou, G.; Kougioumtzis, G.A.; Katsouri, I.-G.; Sofologi, M.; Tsolaki, M. The Relationships of Specific Cognitive Control Abilities with Objective and Subjective Sleep Parameters in Mild Cognitive Impairment: Revealing the Association between Cognitive Planning and Sleep Duration. Brain Sci. 2024, 14, 813. https://doi.org/10.3390/brainsci14080813
Batzikosta A, Moraitou D, Steiropoulos P, Papantoniou G, Kougioumtzis GA, Katsouri I-G, Sofologi M, Tsolaki M. The Relationships of Specific Cognitive Control Abilities with Objective and Subjective Sleep Parameters in Mild Cognitive Impairment: Revealing the Association between Cognitive Planning and Sleep Duration. Brain Sciences. 2024; 14(8):813. https://doi.org/10.3390/brainsci14080813
Chicago/Turabian StyleBatzikosta, Areti, Despina Moraitou, Paschalis Steiropoulos, Georgia Papantoniou, Georgios A. Kougioumtzis, Ioanna-Giannoula Katsouri, Maria Sofologi, and Magda Tsolaki. 2024. "The Relationships of Specific Cognitive Control Abilities with Objective and Subjective Sleep Parameters in Mild Cognitive Impairment: Revealing the Association between Cognitive Planning and Sleep Duration" Brain Sciences 14, no. 8: 813. https://doi.org/10.3390/brainsci14080813
APA StyleBatzikosta, A., Moraitou, D., Steiropoulos, P., Papantoniou, G., Kougioumtzis, G. A., Katsouri, I.-G., Sofologi, M., & Tsolaki, M. (2024). The Relationships of Specific Cognitive Control Abilities with Objective and Subjective Sleep Parameters in Mild Cognitive Impairment: Revealing the Association between Cognitive Planning and Sleep Duration. Brain Sciences, 14(8), 813. https://doi.org/10.3390/brainsci14080813












