From Infancy to Childhood: A Comprehensive Review of Event- and Task-Related Brain Oscillations
Abstract
:1. Introduction
2. Materials and Methods
- (a)
- Inclusion of event- or task-related oscillations in children, toddlers, or infants.
- (b)
- Recording of EEG activity while participants performed a task (i.e., not resting state EEG).
- (c)
- Examination of EEG power, phase, or other time-frequency analyses in response to task events, such as event-related increases or decreases.
- (d)
- No case studies or review articles.
- (e)
- Written in English.
3. Brain Oscillation from Infancy to Childhood
3.1. Delta Oscillations: The Foundation of Early Neural Development
3.2. Theta Oscillations: Bridging Perception and Cognition in Early Years
3.3. Alpha Activity: Markers of Attention and Rest in Developing Minds
3.4. Mu Rhythms: Mirroring Movement and Social Cognition
3.5. Beta Oscillations: The Rhythms of Active Thinking and Problem-Solving
3.6. Gamma Band: High-Frequency Insights into Advanced Cognitive Integration
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Higginbotham, H.; Yokota, Y.; Anton, E. Strategies for analyzing neuronal progenitor development and neuronal migration in the developing cerebral cortex. Cereb. Cortex 2011, 21, 1465–1474. [Google Scholar] [CrossRef]
- Ackerman, S. Discovering the Brain; National Academies Press (US): Washington, DC, USA, 1992. [Google Scholar]
- Alberts, B. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2017. [Google Scholar]
- Goodman, C.S.; Shatz, C.J. Developmental mechanisms that generate precise patterns of neuronal connectivity. Cell 1993, 72, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Penn, A.A.; Shatz, C.J. Brain waves and brain wiring: The role of endogenous and sensory-driven neural activity in development. Pediatr. Res. 1999, 45, 447–458. [Google Scholar] [CrossRef]
- Casey, B.J.; Tottenham, N.; Liston, C.; Durston, S. Imaging the developing brain: What have we learned about cognitive development? Trends Cogn. Sci. 2005, 9, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F., III; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef]
- Sowell, E.R.; Thompson, P.M.; Leonard, C.M.; Welcome, S.E.; Kan, E.; Toga, A.W. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 2004, 24, 8223–8231. [Google Scholar] [CrossRef] [PubMed]
- Sowell, E.R.; Peterson, B.S.; Thompson, P.M.; Welcome, S.E.; Henkenius, A.L.; Toga, A.W. Mapping cortical change across the human life span. Nat. Neurosci. 2003, 6, 309–315. [Google Scholar] [CrossRef]
- Dreyfus-Brisac, C. Ontogenesis of brain bioelectrical activity and sleep organization in neonates and infants. In Human Growth: Volume 3 Neurobiology and Nutrition; Springer: Berlin/Heidelberg, Germany, 1979; pp. 157–182. [Google Scholar]
- Vanhatalo, S.; Palva, J.M.; Andersson, S.; Rivera, C.; Voipio, J.; Kaila, K. Slow endogenous activity transients and developmental expression of K+–Cl− cotransporter 2 in the immature human cortex. Eur. J. Neurosci. 2005, 22, 2799–2804. [Google Scholar] [CrossRef]
- Ehlers, C.L.; Wills, D.N.; Phillips, E.; Havstad, J. Low voltage alpha EEG phenotype is associated with reduced amplitudes of alpha event-related oscillations, increased cortical phase synchrony, and a low level of response to alcohol. Int. J. Psychophysiol. 2015, 98, 65–75. [Google Scholar] [CrossRef]
- Eisermann, M.; Kaminska, A.; Moutard, M.-L.; Soufflet, C.; Plouin, P. Normal EEG in childhood: From neonates to adolescents. Neurophysiol. Clin./Clin. Neurophysiol. 2013, 43, 35–65. [Google Scholar] [CrossRef]
- Bhavnani, S.; Lockwood Estrin, G.; Haartsen, R.; Jensen, S.K.; Gliga, T.; Patel, V.; Johnson, M.H. EEG signatures of cognitive and social development of preschool children—A systematic review. PLoS ONE 2021, 16, e0247223. [Google Scholar] [CrossRef] [PubMed]
- Bașar, E. Brain-Body-Mind in the Nebulous Cartesian System: A Holistic Approach by Oscillations; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Whitford, T.J.; Rennie, C.J.; Grieve, S.M.; Clark, C.R.; Gordon, E.; Williams, L.M. Brain maturation in adolescence: Concurrent changes in neuroanatomy and neurophysiology. Hum. Brain Mapp. 2007, 28, 228–237. [Google Scholar] [CrossRef]
- Shaw, P.; Kabani, N.J.; Lerch, J.P.; Eckstrand, K.; Lenroot, R.; Gogtay, N.; Greenstein, D.; Clasen, L.; Evans, A.; Rapoport, J.L. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008, 28, 3586–3594. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, I.; Campbell, I.G. Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain Cogn. 2010, 72, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Segalowitz, S.J.; Santesso, D.L.; Jetha, M.K. Electrophysiological changes during adolescence: A review. Brain Cogn. 2010, 72, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Barriga-Paulino, C.I.; Flores, A.B.; Gómez, C.M. Developmental changes in the eeg rhythms of children and young adults. J. Psychophysiol. 2011, 25, 143–158. [Google Scholar] [CrossRef]
- Thorpe, S.G.; Cannon, E.N.; Fox, N.A. Spectral and source structural development of mu and alpha rhythms from infancy through adulthood. Clin. Neurophysiol. 2016, 127, 254–269. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, E.; Ruiz-Martínez, F.; Barriga Paulino, C.; Gómez, C.M. Frequency shift in topography of spontaneous brain rhythms from childhood to adulthood. Cogn. Neurodynamics 2017, 11, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Britto, P.R.; Lye, S.J.; Proulx, K.; Yousafzai, A.K.; Matthews, S.G.; Vaivada, T.; Perez-Escamilla, R.; Rao, N.; Ip, P.; Fernald, L.C. Nurturing care: Promoting early childhood development. Lancet 2017, 389, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Green, J.; Charman, T.; Pickles, A.; Wan, M.W.; Elsabbagh, M.; Slonims, V.; Taylor, C.; McNally, J.; Booth, R.; Gliga, T. Parent-mediated intervention versus no intervention for infants at high risk of autism: A parallel, single-blind, randomised trial. Lancet Psychiatry 2015, 2, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Basar, E. EEG-Brain Dynamics: Relation between EEG and Brain Evoked Potentials; Elsevier: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Başar, E. A review of alpha activity in integrative brain function: Fundamental physiology, sensory coding, cognition and pathology. Int. J. Psychophysiol. 2012, 86, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Güntekin, B. A short review of alpha activity in cognitive processes and in cognitive impairment. Int. J. Psychophysiol. 2012, 86, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Bullock, T.; McClune, M.; Achimowicz, J.; Iragui-Madoz, V.; Duckrow, R.; Spencer, S. Temporal fluctuations in coherence of brain waves. Proc. Natl. Acad. Sci. USA 1995, 92, 11568–11572. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Doppelmayr, M.; Schwaiger, J.; Auinger, P.; Winkler, T. Paradoxical’alpha synchronization in a memory task. Cogn. Brain Res. 1999, 7, 493–501. [Google Scholar] [CrossRef]
- Musacchia, G.; Ortiz-Mantilla, S.; Realpe-Bonilla, T.; Roesler, C.P.; Benasich, A.A. Infant auditory processing and event-related brain oscillations. JoVE (J. Vis. Exp.) 2015, 101, e52420. [Google Scholar]
- Pfurtscheller, G.; Da Silva, F.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Singer, W. Synchronization of cortical activity and its putative role in information processing and learning. Annu. Rev. Physiol. 1993, 55, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Başar-Eroglu, C.; Röschke, J.; Schütt, A. The EEG is a quasi-deterministic signal anticipating sensory-cognitive tasks. In Brain Dynamics: Progress and Perspectives; Springer: Berlin/Heidelberg, Germany, 1989; pp. 43–71. [Google Scholar]
- Başar-Eroglu, C.; Strüber, D.; Schürmann, M.; Stadler, M.; Başar, E. Gamma-band responses in the brain: A short review of psychophysiological correlates and functional significance. Int. J. Psychophysiol. 1996, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.M. Synchronous neural oscillations and cognitive processes. Trends Cogn. Sci. 2003, 7, 553–559. [Google Scholar] [CrossRef] [PubMed]
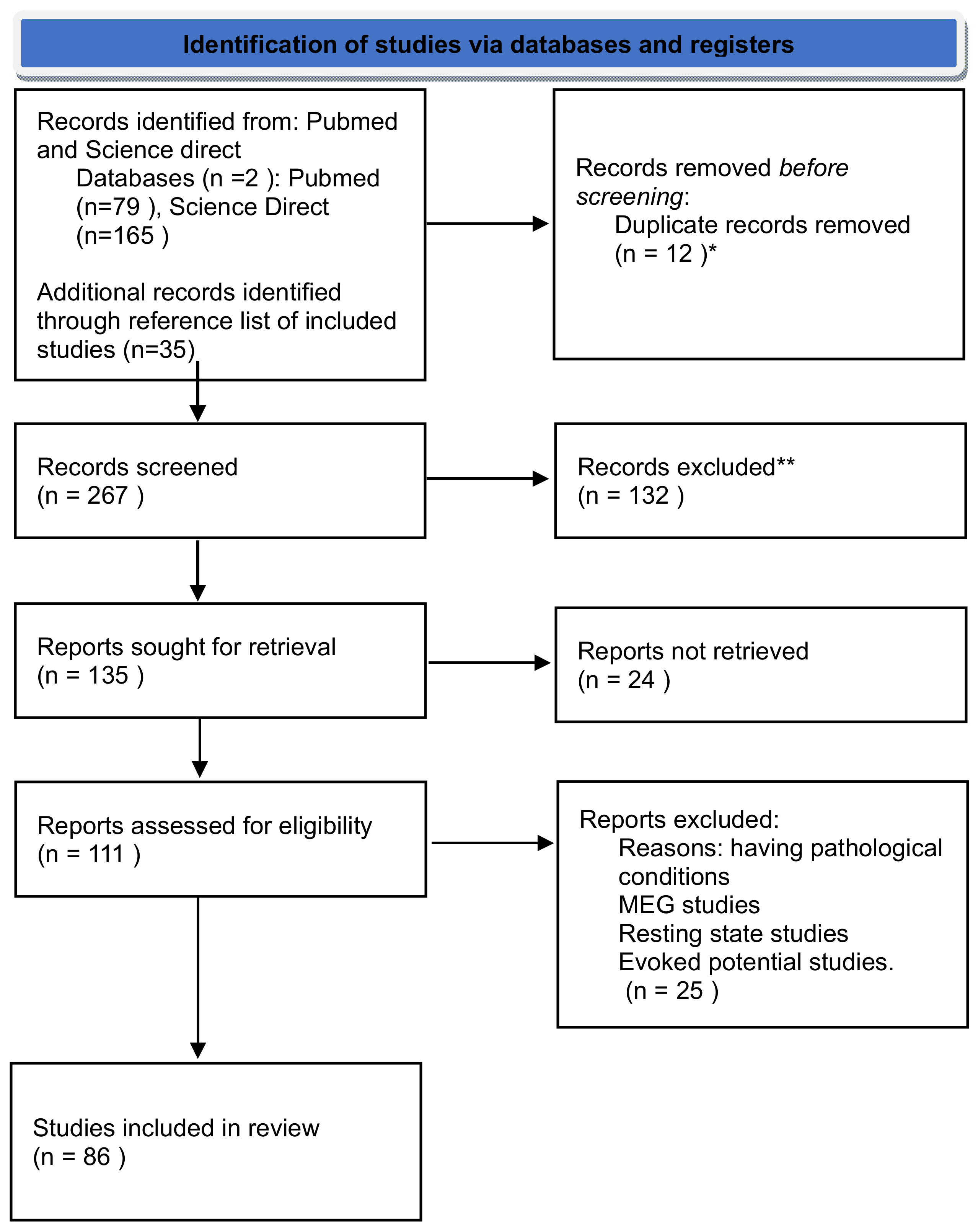
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Steriade, M. Brain Electrical Activity and Sensory Processing during Waking and Sleep States, Principles and Practice of Sleep Medicine; Kryger, M., Roth, T., Dement, W., Eds.; WB Saunders: Philadelphia, PA, USA, 2000. [Google Scholar]
- Babiloni, C.; Frisoni, G.; Steriade, M.; Bresciani, L.; Binetti, G.; Del Percio, C.; Geroldi, C.; Miniussi, C.; Nobili, F.; Rodriguez, G. Frontal white matter volume and delta EEG sources negatively correlate in awake subjects with mild cognitive impairment and Alzheimer’s disease. Clin. Neurophysiol. 2006, 117, 1113–1129. [Google Scholar] [CrossRef] [PubMed]
- Penolazzi, B.; Spironelli, C.; Angrilli, A. Delta EEG activity as a marker of dysfunctional linguistic processing in developmental dyslexia. Psychophysiology 2008, 45, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Spironelli, C.; Angrilli, A. Developmental aspects of language lateralization in delta, theta, alpha and beta EEG bands. Biol. Psychol. 2010, 85, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Başar, E. Review of evoked and event-related delta responses in the human brain. Int. J. Psychophysiol. 2016, 103, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, M.; Başar-Eroglu, C.; Kolev, V.; Başar, E. Delta responses and cognitive processing: Single-trial evaluations of human visual P300. Int. J. Psychophysiol. 2001, 39, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Parnefjord, R.; Başar, E. Evoked delta oscillations on the hearing threshold. In Brain Function and Oscillations: Integrative Brain Function. Neurophysiology and Cognitive Processes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; pp. 161–175. [Google Scholar]
- Knyazev, G.; Slobodskoj-Plusnin, J.Y.; Bocharov, A. Event-related delta and theta synchronization during explicit and implicit emotion processing. Neuroscience 2009, 164, 1588–1600. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.F.G.; Silvers, J.A. In due time: Neurodevelopmental considerations in the study of emotion regulation. In Emotion Regulation; Routledge: Abingdon, UK, 2018; pp. 93–116. [Google Scholar]
- Dehaene-Lambertz, G.; Hertz-Pannier, L.; Dubois, J. Nature and nurture in language acquisition: Anatomical and functional brain-imaging studies in infants. Trends Neurosci. 2006, 29, 367–373. [Google Scholar] [CrossRef]
- Obleser, J.; Kayser, C. Neural entrainment and attentional selection in the listening brain. Trends Cogn. Sci. 2019, 23, 913–926. [Google Scholar] [CrossRef] [PubMed]
- Kolev, V.; Başar-Eroglu, C.; Aksu, F.; Başar, E. EEG rhythmicities evoked by visual stimuli in three-year-old children. Int. J. Neurosci. 1994, 75, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, J.; Strüber, D.; Basar-Eroglu, C. Multistable perception in children: Prefrontal delta oscillations in the developing brain. Int. J. Psychophysiol. 2016, 103, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Attaheri, A.; Choisdealbha, Á.N.; Di Liberto, G.M.; Rocha, S.; Brusini, P.; Mead, N.; Olawole-Scott, H.; Boutris, P.; Gibbon, S.; Williams, I. Delta-and theta-band cortical tracking and phase-amplitude coupling to sung speech by infants. NeuroImage 2022, 247, 118698. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.F.; Poeppel, D. Discrimination of speech stimuli based on neuronal response phase patterns depends on acoustics but not comprehension. J. Neurophysiol. 2010, 104, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Von Stein, A.; Sarnthein, J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Saby, J.N.; Marshall, P.J. The utility of EEG band power analysis in the study of infancy and early childhood. Dev. Neuropsychol. 2012, 37, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-J. Neurophysiological and computational principles of cortical rhythms in cognition. Physiol. Rev. 2010, 90, 1195–1268. [Google Scholar] [CrossRef] [PubMed]
- Nanova, P.; Kolev, V.; Yordanova, J. Developmental gender differences in the synchronization of auditory event-related oscillations. Clin. Neurophysiol. 2011, 122, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Anaya, B.; Ostlund, B.; LoBue, V.; Buss, K.; Pérez-Edgar, K. Psychometric properties of infant EEG: Developmental stability, reliability, and construct validity of frontal alpha asymmetry and delta-beta coupling. Dev. Psychobiol. 2021, 63, e22178. [Google Scholar] [CrossRef]
- Anaya, B.; Vallorani, A.M.; Pérez-Edgar, K. Individual dynamics of delta–beta coupling: Using a multilevel framework to examine inter-and intraindividual differences in relation to social anxiety and behavioral inhibition. J. Child Psychol. Psychiatry 2021, 62, 771–779. [Google Scholar] [CrossRef]
- Basar, E.; Baar-Eroglu, C.; Rahn, E.; Schürmann, M. Sensory and cognitive components of brain resonance responses: An analysis of responsiveness in human and cat brain upon visual and auditory stimulation. Acta Oto-Laryngol. 1991, 111, 25–35. [Google Scholar] [CrossRef]
- Demiralp, T.; Başar-Eroglu, C.; Rahn, E.; Başar, E. Event-related theta rhythms in cat hippocampus and prefrontal cortex during an omitted stimulus paradigm. Int. J. Psychophysiol. 1994, 18, 35–48. [Google Scholar] [CrossRef]
- Harris, J.J.; Jolivet, R.; Attwell, D. Synaptic energy use and supply. Neuron 2012, 75, 762–777. [Google Scholar] [CrossRef] [PubMed]
- Köster, M.; Haese, A.; Czernochowski, D. Neuronal oscillations reveal the processes underlying intentional compared to incidental learning in children and young adults. PLoS ONE 2017, 12, e0182540. [Google Scholar] [CrossRef] [PubMed]
- Köster, M.; Langeloh, M.; Hoehl, S. Visually entrained theta oscillations increase for unexpected events in the infant brain. Psychol. Sci. 2019, 30, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Mallin, B.M.; Richards, J.E. Development of infant sustained attention and its relation to EEG oscillations: An EEG and cortical source analysis study. Dev. Sci. 2018, 21, e12562. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, E.V.; Stroganova, T.A.; Posikera, I.N. Theta synchronization during sustained anticipatory attention in infants over the second half of the first year of life. Int. J. Psychophysiol. 1999, 32, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Stroganova, T.A.; Orekhova, E.V.; Posikera, I.N. Externally and internally controlled attention in infants: An EEG study. Int. J. Psychophysiol. 1998, 30, 339–351. [Google Scholar] [CrossRef] [PubMed]
- van Noordt, S.; Heffer, T.; Willoughby, T. A developmental examination of medial frontal theta dynamics and inhibitory control. NeuroImage 2022, 246, 118765. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; van Schaik, J.; Poli, F.; Hunnius, S. How infant-directed actions enhance infants’ attention, learning, and exploration. Dev. Sci. 2022, 26, e13259. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M. Age and sex effects in the EEG: Development of the normal child. Clin. Neurophysiol. 2001, 112, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.A.; Wolfe, C.D. Changes in brain functioning from infancy to early childhood: Evidence from EEG power and coherence during working memory tasks. Dev. Neuropsychol. 2007, 31, 21–38. [Google Scholar] [CrossRef]
- Adam, N.; Blaye, A.; Gulbinaite, R.; Delorme, A.; Farrer, C. The role of midfrontal theta oscillations across the development of cognitive control in preschoolers and school-age children. Dev. Sci. 2020, 23, e12936. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-X.; Woltering, S.; Lewis, M.D. Developmental change in EEG theta activity in the medial prefrontal cortex during response control. NeuroImage 2014, 85, 873–887. [Google Scholar] [CrossRef]
- Orekhova, E.; Stroganova, T.; Posikera, I.; Elam, M. EEG theta rhythm in infants and preschool children. Clin. Neurophysiol. 2006, 117, 1047–1062. [Google Scholar] [CrossRef]
- Bosseler, A.N.; Taulu, S.; Pihko, E.; Mäkelä, J.P.; Imada, T.; Ahonen, A.; Kuhl, P.K. Theta brain rhythms index perceptual narrowing in infant speech perception. Front. Psychol. 2013, 4, 690. [Google Scholar] [CrossRef]
- Cavanagh, J.F.; Figueroa, C.M.; Cohen, M.X.; Frank, M.J. Frontal theta reflects uncertainty and unexpectedness during exploration and exploitation. Cereb. Cortex 2012, 22, 2575–2586. [Google Scholar] [CrossRef]
- Posner, M.I.; Tang, Y.-Y.; Lynch, G. Mechanisms of white matter change induced by meditation training. Front. Psychol. 2014, 5, 115924. [Google Scholar] [CrossRef] [PubMed]
- Conejero, Á.; Guerra, S.; Abundis-Gutiérrez, A.; Rueda, M.R. Frontal theta activation associated with error detection in toddlers: Influence of familial socioeconomic status. Dev. Sci. 2018, 21, e12494. [Google Scholar] [CrossRef] [PubMed]
- Köster, M.; Langeloh, M.; Michel, C.; Hoehl, S. Young infants process prediction errors at the theta rhythm. NeuroImage 2021, 236, 118074. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Endedijk, H.M.; Van Ede, F.; Hunnius, S. Theta oscillations in 4-year-olds are sensitive to task engagement and task demands. Sci. Rep. 2019, 9, 6049. [Google Scholar] [CrossRef]
- Ortiz-Mantilla, S.; Hämäläinen, J.A.; Musacchia, G.; Benasich, A.A. Enhancement of gamma oscillations indicates preferential processing of native over foreign phonemic contrasts in infants. J. Neurosci. 2013, 33, 18746–18754. [Google Scholar] [CrossRef]
- Fuentemilla, L.; Penny, W.D.; Cashdollar, N.; Bunzeck, N.; Düzel, E. Theta-coupled periodic replay in working memory. Curr. Biol. 2010, 20, 606–612. [Google Scholar] [CrossRef]
- Rutishauser, U.; Ross, I.B.; Mamelak, A.N.; Schuman, E.M. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 2010, 464, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Warden, M.R.; Miller, E.K. Phase-dependent neuronal coding of objects in short-term memory. Proc. Natl. Acad. Sci. USA 2009, 106, 21341–21346. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.A. Brain electrical activity associated with cognitive processing during a looking version of the A-not-B task. Infancy 2001, 2, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Morasch, K.C.; Bell, M.A. Patterns of brain-electrical activity during declarative memory performance in 10-month-old infants. Brain Cogn. 2009, 71, 215–222. [Google Scholar] [CrossRef]
- Ishii, R.; Shinosaki, K.; Ukai, S.; Inouye, T.; Ishihara, T.; Yoshimine, T.; Hirabuki, N.; Asada, H.; Kihara, T.; Robinson, S.E. Medial prefrontal cortex generates frontal midline theta rhythm. Neuroreport 1999, 10, 675–679. [Google Scholar] [CrossRef]
- Ellis, C.T.; Skalaban, L.J.; Yates, T.S.; Bejjanki, V.R.; Córdova, N.I.; Turk-Browne, N.B. Evidence of hippocampal learning in human infants. Curr. Biol. 2021, 31, 3358–3364.e4. [Google Scholar] [CrossRef]
- Matsuzawa, J.; Matsui, M.; Konishi, T.; Noguchi, K.; Gur, R.C.; Bilker, W.; Miyawaki, T. Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cereb. Cortex 2001, 11, 335–342. [Google Scholar] [CrossRef]
- Dehaene-Lambertz, G. The human infant brain: A neural architecture able to learn language. Psychon. Bull. Rev. 2017, 24, 48–55. [Google Scholar] [CrossRef]
- Raz, G.; Saxe, R. Learning in infancy is active, endogenously motivated, and depends on the prefrontal cortices. Annu. Rev. Dev. Psychol. 2020, 2, 247–268. [Google Scholar] [CrossRef]
- Schlichting, M.L.; Mumford, J.A.; Preston, A.R. Learning-related representational changes reveal dissociable integration and separation signatures in the hippocampus and prefrontal cortex. Nat. Commun. 2015, 6, 8151. [Google Scholar] [CrossRef]
- Nanova, P.; Kolev, V.; Yordanova, J. Effect of Proactive Mode of Processing on Event-related Oscillatory Brain Responses in Children. Int. J. Bioautomation 2018, 22, 253–262. [Google Scholar] [CrossRef]
- Gómez, C.M.; Munoz, V.; Rodríguez-Martínez, E.I.; Arjona, A.; Barriga-Paulino, C.I.; Pelegrina, S. Child and adolescent development of the brain oscillatory activity during a working memory task. Brain Cogn. 2023, 167, 105969. [Google Scholar] [CrossRef]
- Rajan, V.; Bell, M.A. Developmental changes in fact and source recall: Contributions from executive function and brain electrical activity. Dev. Cogn. Neurosci. 2015, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kuperberg, G.R.; Jaeger, T.F. What do we mean by prediction in language comprehension? Lang. Cogn. Neurosci. 2016, 31, 32–59. [Google Scholar] [CrossRef] [PubMed]
- Daube, C.; Ince, R.A.; Gross, J. Simple acoustic features can explain phoneme-based predictions of cortical responses to speech. Curr. Biol. 2019, 29, 1924–1937.e9. [Google Scholar] [CrossRef]
- Maulsby, R.L. An illustration of emotionally evoked theta rhythm in infancy: Hedonic hypersynchrony. Electroencephalogr. Clin. Neurophysiol. 1971, 31, 157–165. [Google Scholar] [CrossRef]
- Cuevas, K.; Raj, V.; Bell, M.A. A frequency band analysis of two-year-olds’ memory processes. Int. J. Psychophysiol. 2012, 83, 315–322. [Google Scholar] [CrossRef]
- Bazhenova, O.V.; Stroganova, T.A.; Doussard-Roosevelt, J.A.; Posikera, I.A.; Porges, S.W. Physiological responses of 5-month-old infants to smiling and blank faces. Int. J. Psychophysiol. 2007, 63, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The polyvagal theory: Phylogenetic contributions to social behavior. Physiol. Behav. 2003, 79, 503–513. [Google Scholar] [CrossRef]
- Bentley, P.; Vuilleumier, P.; Thiel, C.M.; Driver, J.; Dolan, R.J. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage 2003, 20, 58–70. [Google Scholar] [CrossRef]
- Wang, J.; Gao, D.; Li, D.; Desroches, A.S.; Liu, L.; Li, X. Theta–gamma coupling reflects the interaction of bottom-up and top-down processes in speech perception in children. Neuroimage 2014, 102, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.J.; Bar-Haim, Y.; Fox, N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002, 113, 1199–1208. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Babiloni, F.; Carducci, F.; Cappa, S.; Cincotti, F.; Del Percio, C.; Miniussi, C.; Moretti, D.V.; Pasqualetti, P.; Rossi, S. Human cortical EEG rhythms during long-term episodic memory task. A high-resolution EEG study of the HERA model. Neuroimage 2004, 21, 1576–1584. [Google Scholar] [CrossRef]
- Yordanova, J.; Kolev, V. Alpha response system in children: Changes with age. Int. J. Psychophysiol. 1997, 26, 411–430. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, J.; Kolev, V.; Bas¸ ar-Eroglu, C. Is the alpha rhythm a control parameter for brain responses? Biol. Cybern. 1997, 76, 471–480. [Google Scholar]
- Yordanova, J.Y.; Kolev, V.N. Developmental changes in the alpha response system. Electroencephalogr. Clin. Neurophysiol. 1996, 99, 527–538. [Google Scholar] [CrossRef]
- Chapeton, J.I.; Haque, R.; Wittig, J.H.; Inati, S.K.; Zaghloul, K.A. Large-scale communication in the human brain is rhythmically modulated through alpha coherence. Curr. Biol. 2019, 29, 2801–2811.e5. [Google Scholar] [CrossRef]
- Buzsáki, G.; Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef] [PubMed]
- Halgren, M.; Fabó, D.; Ulbert, I.; Madsen, J.R.; Erőss, L.; Doyle, W.K.; Devinsky, O.; Schomer, D.; Cash, S.S.; Halgren, E. Superficial slow rhythms integrate cortical processing in humans. Sci. Rep. 2018, 8, 2055. [Google Scholar] [CrossRef]
- Yakovlev, P.I.; Lecours, A.R. The myelogenetic cycles of regional maturation of the brain. In Regional Development of the Brain in Early Life; Minkowski, A., Ed.; Blackwell: Oxford, UK, 1967; pp. 3–70. [Google Scholar]
- Vollebregt, M.A.; Zumer, J.M.; Ter Huurne, N.; Castricum, J.; Buitelaar, J.K.; Jensen, O. Lateralized modulation of posterior alpha oscillations in children. NeuroImage 2015, 123, 245–252. [Google Scholar] [CrossRef]
- Vinck, M.; Oostenveld, R.; Van Wingerden, M.; Battaglia, F.; Pennartz, C.M. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011, 55, 1548–1565. [Google Scholar] [CrossRef]
- John, E.; Ahn, H.; Prichep, L.; Trepetin, M.; Brown, D.; Kaye, H. Developmental equations for the electroencephalogram. Science 1980, 210, 1255–1258. [Google Scholar] [CrossRef]
- Matoušek, M.; Petersén, I. Automatic evaluation of EEG background activity by means of age-dependent EEG quotients. Electroencephalogr. Clin. Neurophysiol. 1973, 35, 603–612. [Google Scholar] [CrossRef]
- Knyazev, G.G. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 2012, 36, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, E.V.; Stroganova, T.A.; Posikera, I.N. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clin. Neurophysiol. 2001, 112, 740–749. [Google Scholar] [CrossRef]
- Cuevas, K.; Cannon, E.N.; Yoo, K.; Fox, N.A. The infant EEG mu rhythm: Methodological considerations and best practices. Dev. Rev. 2014, 34, 26–43. [Google Scholar] [CrossRef]
- Debnath, R.; Salo, V.C.; Buzzell, G.A.; Yoo, K.H.; Fox, N.A. Mu rhythm desynchronization is specific to action execution and observation: Evidence from time-frequency and connectivity analysis. NeuroImage 2019, 184, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Güntekin, B.; Uzunlar, H.; Çalışoğlu, P.; Eroğlu-Ada, F.; Yıldırım, E.; Aktürk, T.; Atay, E.; Ceran, Ö. Theta and alpha oscillatory responses differentiate between six-to seven-year-old children and adults during successful visual and auditory memory encoding. Brain Res. 2020, 1747, 147042. [Google Scholar] [CrossRef]
- Hahn, G.; Ponce-Alvarez, A.; Deco, G.; Aertsen, A.; Kumar, A. Portraits of communication in neuronal networks. Nat. Rev. Neurosci. 2019, 20, 117–127. [Google Scholar] [CrossRef]
- Fries, P. Rhythms for cognition: Communication through coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Başar-Eroglu, C.; Kolev, V.; Ritter, B.; Aksu, F.; Başar, E. EEG, auditory evoked potentials and evoked rhythmicities in three-year-old children. Int. J. Neurosci. 1994, 75, 239–255. [Google Scholar] [CrossRef]
- Chatrian, G.E.; Petersen, M.C.; Lazarte, J.A. The blocking of the rolandic wicket rhythm and some central changes related to movement. Electroencephalogr. Clin. Neurophysiol. 1959, 11, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, W.N. Functional topography of the human mu rhythm. Electroencephalogr. Clin. Neurophysiol. 1978, 44, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C.; Krausz, G. Functional dissociation of lower and upper frequency mu rhythms in relation to voluntary limb movement. Clin. Neurophysiol. 2000, 111, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Marshall, P.J.; Young, T.; Meltzoff, A.N. Neural correlates of action observation and execution in 14-month-old infants: An event-related EEG desynchronization study. Dev. Sci. 2011, 14, 474–480. [Google Scholar] [CrossRef]
- Hao, J.; Feng, W.; Zhang, L.; Liao, Y. The post-movement beta rebound and motor-related Mu suppression in children. J. Mot. Behav. 2020, 52, 590–600. [Google Scholar] [CrossRef]
- Patzwald, C.; Matthes, D.; Elsner, B. Eighteen-month-olds integrate verbal cues into their action processing: Evidence from ERPs and mu power. Infant Behav. Dev. 2020, 58, 101414. [Google Scholar] [CrossRef]
- Culham, J.C.; Valyear, K.F. Human parietal cortex in action. Curr. Opin. Neurobiol. 2006, 16, 205–212. [Google Scholar] [CrossRef]
- Iacoboni, M. Visuo-motor integration and control in the human posterior parietal cortex: Evidence from TMS and fMRI. Neuropsychologia 2006, 44, 2691–2699. [Google Scholar] [CrossRef]
- Arnstein, D.; Cui, F.; Keysers, C.; Maurits, N.M.; Gazzola, V. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J. Neurosci. 2011, 31, 14243–14249. [Google Scholar] [CrossRef] [PubMed]
- Bryant, L.J.; Cuevas, K. Effects of active and observational experience on EEG activity during early childhood. Psychophysiology 2019, 56, e13360. [Google Scholar] [CrossRef]
- Fox, N.A.; Bakermans-Kranenburg, M.J.; Yoo, K.H.; Bowman, L.C.; Cannon, E.N.; Vanderwert, R.E.; Ferrari, P.F.; Van IJzendoorn, M.H. Assessing human mirror activity with EEG mu rhythm: A meta-analysis. Psychol. Bull. 2016, 142, 291. [Google Scholar] [CrossRef]
- Hari, R.; Salmelin, R. Human cortical oscillations: A neuromagnetic view through the skull. Trends Neurosci. 1997, 20, 44–49. [Google Scholar] [CrossRef]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition–timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef]
- van Wijk, B.C.; Beek, P.J.; Daffertshofer, A. Neural synchrony within the motor system: What have we learned so far? Front. Hum. Neurosci. 2012, 6, 252. [Google Scholar] [CrossRef]
- Mikhailova, A.; Orekhova, L.; Dyagileva, Y.O.; Mukhtarimova, T.; Pavlenko, V. Reactivity of the EEG μ rhythm on observing and performing actions in young children with different levels of receptive speech development. Neurosci. Behav. Physiol. 2021, 51, 85–92. [Google Scholar] [CrossRef]
- Ramachandran, V.S. The Tell-Tale Brain: A Neuroscientist’s Quest for What Makes Us Human; WW Norton & Company: New York, NY, USA, 2012. [Google Scholar]
- Meyer, M.; Hunnius, S.; Van Elk, M.; Van Ede, F.; Bekkering, H. Joint action modulates motor system involvement during action observation in 3-year-olds. Exp. Brain Res. 2011, 211, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Köster, M.; Langeloh, M.; Kliesch, C.; Kanngiesser, P.; Hoehl, S. Motor cortex activity during action observation predicts subsequent action imitation in human infants. NeuroImage 2020, 218, 116958. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Acar, Z.A.; Makeig, S.; Deak, G. EEG imaging of toddlers during dyadic turn-taking: Mu-rhythm modulation while producing or observing social actions. NeuroImage 2015, 112, 52–60. [Google Scholar] [CrossRef]
- Gerson, S.; Meyer, M.; Hunnius, S.; Bekkering, H. Unravelling the contributions of motor experience and conceptual knowledge in action perception: A training study. Sci. Rep. 2017, 7, 46761. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.A. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Res. Rev. 2005, 50, 57–68. [Google Scholar] [CrossRef]
- Belalov, V.; Dyagileva, Y.O.; Pavlenko, V.; Kochukhova, O.M. Neurophysiological analysis of speech perception in 2.5 to 3.5-year-old orphans and children raised in a family. Neurophysiology 2014, 46, 79–87. [Google Scholar] [CrossRef]
- Wróbel, A. Beta activity: A carrier for visual attention. Acta Neurobiol. Exp. 2000, 60, 247–260. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Hahne, A.; Eckstein, K.; Friederici, A.D. Brain signatures of syntactic and semantic processes during children’s language development. J. Cogn. Neurosci. 2004, 16, 1302–1318. [Google Scholar] [CrossRef]
- Davidson, D.J.; Indefrey, P. An inverse relation between event-related and time–frequency violation responses in sentence processing. Brain Res. 2007, 1158, 81–92. [Google Scholar] [CrossRef]
- Zeman, A. Consciousness. Brain 2001, 124 Pt 7, 1263–1289. [Google Scholar] [CrossRef]
- Csibra, G.; Davis, G.; Spratling, M.; Johnson, M. Gamma oscillations and object processing in the infant brain. Science 2000, 290, 1582–1585. [Google Scholar] [CrossRef] [PubMed]
- Jensen, O.; Kaiser, J.; Lachaux, J.-P. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007, 30, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Axmacher, N.; Henseler, M.M.; Jensen, O.; Weinreich, I.; Elger, C.E.; Fell, J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 3228–3233. [Google Scholar] [CrossRef] [PubMed]
- Farroni, T.; Csibra, G.; Simion, F.; Johnson, M.H. Eye contact detection in humans from birth. Proc. Natl. Acad. Sci. USA 2002, 99, 9602–9605. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, T.; Johnson, M.H.; Farroni, T.; Csibra, G. Social perception in the infant brain: Gamma oscillatory activity in response to eye gaze. Soc. Cogn. Affect. Neurosci. 2007, 2, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Leung, S.; Mareschal, D.; Rowsell, R.; Simpson, D.; Iaria, L.; Grbic, A.; Kaufman, J. Oscillatory activity in the infant brain and the representation of small numbers. Front. Syst. Neurosci. 2016, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Csibra, G.; Johnson, M.H. Representing occluded objects in the human infant brain. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, S140–S143. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Csibra, G.; Johnson, M.H. Oscillatory activity in the infant brain reflects object maintenance. Proc. Natl. Acad. Sci. USA 2005, 102, 15271–15274. [Google Scholar] [CrossRef] [PubMed]
- Southgate, V.; Csibra, G.; Kaufman, J.; Johnson, M.H. Distinct processing of objects and faces in the infant brain. J. Cogn. Neurosci. 2008, 20, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Reid, V.M.; Csibra, G.; Belsky, J.; Johnson, M.H. Neural correlates of the perception of goal-directed action in infants. Acta Psychol. 2007, 124, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Orekhova, E.V.; Butorina, A.V.; Sysoeva, O.V.; Prokofyev, A.O.; Nikolaeva, A.Y.; Stroganova, T.A. Frequency of gamma oscillations in humans is modulated by velocity of visual motion. J. Neurophysiol. 2015, 114, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, J.; Kolev, V.; Heinrich, H.; Woerner, W.; Banaschewski, T.; Rothenberger, A. Developmental event-related gamma oscillations: Effects of auditory attention. Eur. J. Neurosci. 2002, 16, 2214–2224. [Google Scholar] [CrossRef]
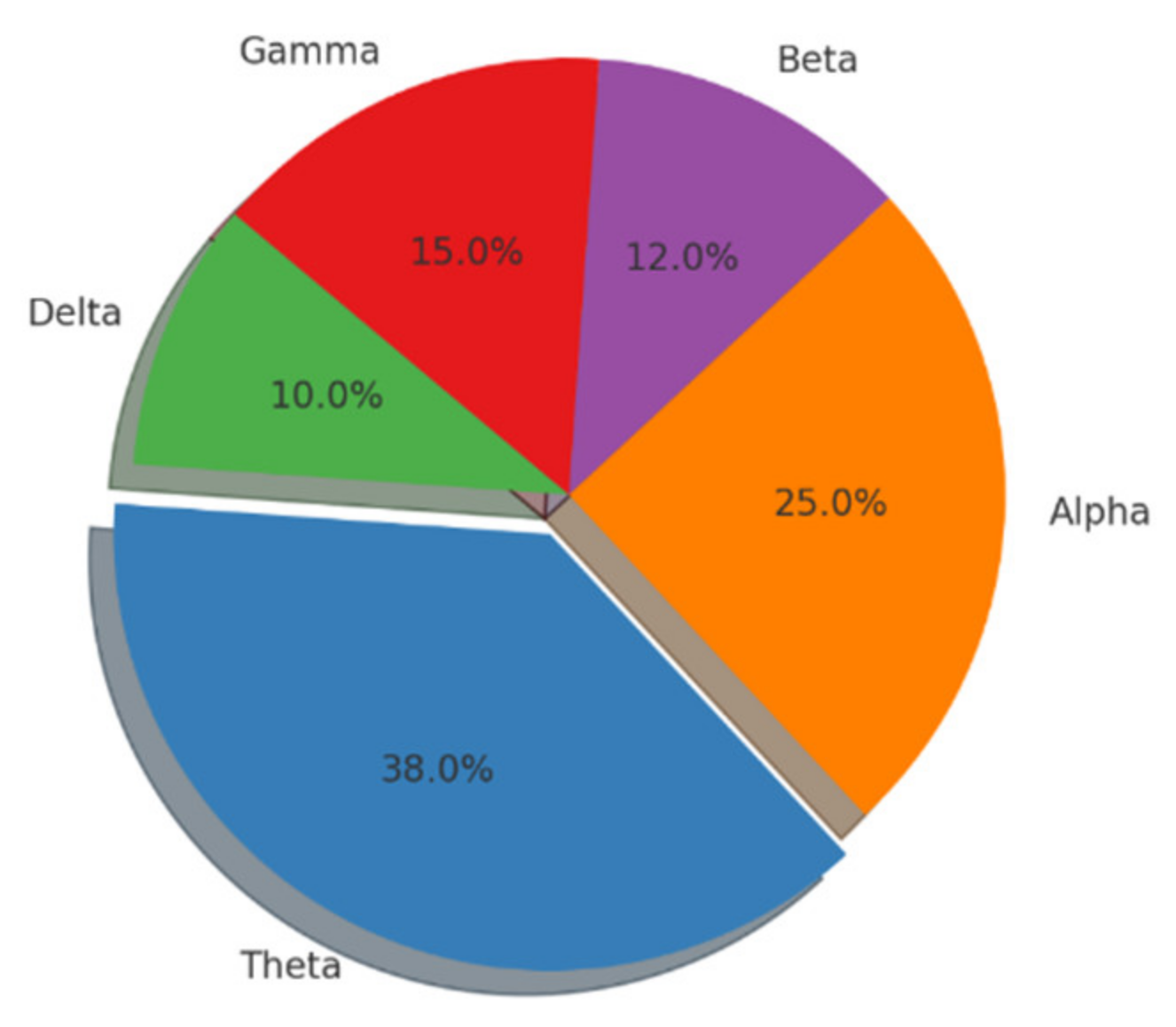
| Development Stages and Focused Oscillations | Modality and Paradigms | Inference | |
|---|---|---|---|
| Musacchia et al. (2015) | 211 infant recording sessions with 98.6% of data retention 57 participants Theta, beta, and gamma oscillations | The auditory stimuli in free-field. Stimuli are presented in two blocks that include a fundamental frequency of either 800 or 1200 Hz and 15 harmonics (6 dB roll-off per octave). | Time-frequency analyses of the EEG data that are collected from infants’ data show that stimulus evoked 1/f pattern neural synchrony like adults. The stimulus onset shows synchronous bursts of theta, beta, and gamma power in both the right and left auditory regions of the brain. Suggestion about the creation of a model to understand the integrity of early auditory processing mechanisms. |
| Güntekin et al. (2023) | 40 participants (children and young adults with rates of 1 in 2) Alpha oscillations | The day-night stroop task. Event-related spectral perturbation (ERSP) and event-related phase-locking analyses were performed. | Different alpha dynamics in children. Shifting of alpha power from posterior to anterior with age. The posterior sides of the brain are involved in the inhibitory processes. Increased alpha power in early time windows, decreased event-related desynchronization (ERD) in later time windows. |
| Gómez et al. (2023) | 239 participants (6–29 years) Delta, theta, and alpha oscillations | The Delayed-Match-to-Sample Test. ERSP in the range of 1–25 Hz. | Delta, theta (7–8 Hz) and alpha-2 (8.5–10 Hz) oscillations work for attentional engagement and focusing for working memory. Beta and low-frequency components work together for encoding, while alpha sharpens this process. Low-frequency component works to keep the WM trace active. Anticipatory attention is characterized by an increase in alpha and beta in late adolescents and adults. Behavioral responses which are represented with reaction time have the most consistent relationship with alpha-2. |
| Wang et al. (2014) | 17 children (mean age 10 years, 5 months) Theta and gamma oscillations | Speech perception paradigm consists of the match and mismatch conditions with auditory and visual stimulus. Theta–gamma coupling analysis. | The coupling of theta phase and gamma amplitude demonstrates the interaction of top-down and bottom-up processes in speech perception. Right hemisphere has higher theta–gamma coupling in the match conditions, and the left hemisphere has stronger theta–gamma coupling in the mismatch conditions. This hemispheric pattern is interpreted as a fast global processing strategy and a slow detailed strategy to adapt to congruent and incongruent conditions. |
| Ortiz-Mantilla et al. (2013) | 28 infants (6-month-olds) Theta and gamma band | English and Spanish syllable contrasts. Single-trial time frequency analysis. Temporal spectral evaluation (TSE). Intertrial phase locking (ITPL). | Increased delta/theta power and phase alignment could subserve auditory discrimination of syllabic information in 6-month-old infants. Preferential processing of specific native features are represented by frontal gamma power. Six-month-old infants can distinguish phoneme information across languages. |
| Maróti et al. (2019) | 22 children (mean age 6.5 years) Beta and gamma frequency range | Auditory stimulus includes the tones at the beta and gamma range in isochronous sequences. Steady-state evoked potentials. | Neural responses which are related with the entrainment associated with the different frequency oscillations do not develop at the same level. |
| Musacchia et al. (2017) | 49 infants A longitudinal study at the fourth and seventh months Theta and gamma oscillations | Active and passive auditory exposure. Go/no-go task. Passive oddball paradigm. Three different types of paired acoustic stimuli. TSE and ITPL. | A representation of maturation from 4 to 7 months, which is increased spead of event-related source response and decreased theta phase stability. Differentiation of theta and gamma oscillatory dynamics between active and passive auditory exposure protocols. Acoustic experience appears to promote more mature oscillatory and lateralized patterns for the auditory system. |
| Colomer et al. (2023) | 17 participants (9-month-olds) Theta and beta | Action observation task with familiar and novel conditions. Action execution task to engage the infants actively. Time–frequency analyses (4–20 Hz). Inter-Site Phase Clustering (ISPC) to perform phase-based connectivity analysis. | Infants’ brain has greater ISPC with age. It is suggested that the global connectivity at the whole brain might be related to maturation. Greater visual–motor coherence is shown in infants with better motor skills. That could have downstream consequences on how infants encode and learn others’ actions. |
| Hao et al. (2020) | 16 children (mean age 7.7) and 13 adults Mu and beta rhythms | Motor-related experiment. Event-related spectral pertubations (ERSPs). Inter-trial coherence (ITC). | The power of mu rhythm (8–14 Hz) decreases significantly during movement, and it returned to baseline after movement for both children and adults. Motor related mu supression develops early in children. Beta power (15–30 Hz) has different dynamics between children and adults. Movement induces beta ERD in both, but post-movement beta power rebound (PMBR) is observed only in adults. PMBR might be associated with a more prolonged motor development process. |
| Adam et al. (2020) | 87 children (52 preschool and 35 school children) Theta frequency range | Heart and flower task to evaluate cognitive control process. Behavioral results (response time and accuracy). Time–frequency analysis. Midfrontal theta peak latency. | Midfrontal theta power is interpreted as a generic indicator of cognitive control process that supports both inhibitory and flexibility functions. MFT might be accepted as a general neural mechanism to coordinate cognitive process across the development. |
| Cantiani et al. (2022) | A longitudinal study at seventh and ninth months 25 infants (mean age 8 months) | Three different rhythmic stimuli (African, Tabla, and speech). Steady-state evoked potentials (SS-EPs). | Low frequency cortical tracking of the stimulus envelope is already measurable at 4 months of age. There are no hemispheric differences in the magnitude of SS-EPs. There is left-lateralized SS-EPs at higher frequencies for speech stimulus. Infants can entrain like adults, but there is difference between frequency ranges for same stimulus. |
| Noordt et al. (2022) | 432 participants (224 females, 208 males) Theta | Go/no-go task. Raven’s progressive matrices to measure reasoning ability. Threshold free cluster enhancement and permutation testing to analyze spatial and temporal dynamics of theta power. Phase evaluation. Latent class analysis. | Greater non-phase locked theta power during response inhibition is associated with more efficient response control. Developmental changes in frontally mediated inhibitory control processes are represented by a greater non-phase locked theta power and a shift to less phase locked activity. |
| Antognini et al. (2019) | 20 toddlers (18 months), 27 toddlers (24 months) Mu | Three types of stimuli-related actions (auditory, visual and play material). | The lateralization of mu suppression towards the left hemisphere in response to action verbs in terms of similarities with language processing. Very strong occipital alpha suppression for action observation differs between age groups, whereas the lateralization of central activity is towards right hemisphere. Although the neural processing of action verbs and pseudoverbs is related to the left central cluster, the sensorimotor activity is shown just for action verbs. |
| Köster et al. (2021) | 36 infants (9-month-olds) Theta | The violation of expectation paradigm to evaluate neural proccesing of prediction error. Time–frequency analysis and event-related potentails | All scalp electrodes has increased activity in 4–5 Hz power in unexpected events. Theta substantially increased for the processing of prediction errors in the infant brain. Theta rhythms are located in the parietal regions in infants. Theta serves as a facilitation mehanism to integration of new information into existing networks. |
| Patzwald et al. (2020) | 38 infants (18-month-olds) Mu | Action observation with verbal cues. Mid-latency ERP 300–800 ms. Central mu power (6–9 Hz). | Reduced mu power is shown for congruent to incongruent stimuli. Central electrode sides (C3–C4) might evaluate as a primary channel of interest to study mu rhythm (6–9 Hz). Occipital channels are unavailable because of the widespread posterior alpha effect. |
| Bryant et al. (2019) | 21 children (mean age 4.89) Mu and beta | Two tool sets and plastic aquatic animal toys. Active and observational training sessions to handle the toys. Spectral analysis of signals. Time-locked ERD. | Central–parietal desynchronization during the action observation and execution represents neural mirroring, and 3- to 6-year-old children exhibit neural mirroring within mu ryhthm. Greater occipital alpha (8–10 Hz) ERD is found during perception of the active training task. This suggests that occipital alpha may be facilitating the processing of attended stimuli while suppressing attention to irrelevant information. |
| Angelini et al. (2023) | 17 infants (9-month-olds) Theta (4–5 Hz) and alpha (6–8 Hz) | Face-to-face live paradigm with a triadic social interaction. Time–frequency analysis. | Theta power increases in response to an unexpected event that is interpreted as a marker of information prediction about social behaviors. Higher alpha power is detected in infants in relation to the inhibition of interfering stimuli needed to sustain internally controlled attention. The live paradigm favors the expectation of a usual event that could have an enhanced effect on alpha power. |
| Ramos-Escobar et al. (2021) | 60 children (mean age: 9 years and 5 months) Syllable and word frequencies in ROIs | Audio-visual artificial language streams. Frequency tagging analysis of spectral power. Weighted Phase-Lag Index. | Frontal and parieto-occipital ROIs at target frequencies are detected with long-distance EEG phase synchronization. Word segmentation and meaning mapping might be modulated by attentional mechanisms to multimodal word learning. |
| Weiss et al. (2018) | 80 children (6 to 8 years old) Mu and alpha | Tactile stimulus. Flanker task. ERSP. | Anticipatory ERD of the mu rhythm is related to executive function skills, whereas it is not associated with the accuracy of children’s behavioral responses to tactile stimulation. |
| Mikhailova et al. (2021) | 36 children (17–41 months) Mu | Observation of actions (fake, real, and performing the action). Bayley test for speech comprehension. Time–frequency analysis. | Observing a real action situation led to greater power desynchronization in the μ range in the medial frontal and medial parietal loci compared to observing a fake action situation. Children with higher levels of speech comprehension showed greater EEG power desynchronization in the μ range when observing a real action in the frontal and parietal loci of the left hemisphere. |
| Cantiani et al. (2019) | 56 participants Typically developing (15 F/17 M) Age: 6 months and 20 months of age (longitudinal) High familial risk for Language and Learning Impairment (LLI) (11 F/13 M) Age: 6 months and 20 months of age (longitudinal) Delta/theta (2–12 Hz) Gamma (30–80 Hz) | Non-speech double oddball paradigm. Cluster permutation testing: Spectral power (TSE) Phase coherence (ITPL). Tests: BSID-III Language Development Survey (LDS) Communicative Development Inventories (CDIs). | Higher bilateral theta activation in auditory cortices in response to deviant stimuli in both groups Infants at risk for LLI: reduced gamma activity in left auditory cortex and higher gamma activity in right auditory cortex |
| Phelps et al. (2022) | 48 participants 24 bilinguals (8 F/16 M) Age: (M = 9.3 years, SD = 1.83) 24 monolinguals (11 F/13 M) Age: (M = 9.6 years, SD = 1.48) Low-frequency neural oscillations | Dichotic listening task. Multivariate temporal response function (mTRF). Speech envelopes. | Different EEG patterns in monolingual and bilingual children during encoding Weaker differentiation of linguistic distractors in bilingual children |
| Köster et al. (2020) | 42 participants (26 F/16 M) Age: 10-month-olds 36 participants (15 F/21 M) Age: 20-month-olds 2–14 Hz | Action demonstration videos and generalization test, followed by imitation test. Spectral perturbation analysis. | 20-month-old infants: increased 7–10 Hz activity at C3-C4 during action observation; desynchronization in 3–6 Hz peaked at posterior electrodes (P3, P4, Pz, P7, P8, O1, O2) |
| Ossmy et al. (2021) | 22 participants (11 F/11 M) Preschoolers Age: 3.09 to 5.49 years 22 participants (14 F/8 M) Adults Age: 19.37 to 26.40 years 6–9 Hz 8–13 Hz (Mu) | Videos of efficient and inefficient displays of tool use (multi-step actions). Eye-tracking, pupil dilation, EEG, and machine learning. Nonparametric cluster analysis. Event-related spectral perturbation (ERSP). | Increased event-related desynchronization in the mu frequency band at sensorimotor regions after action observation. Differential EEG responses to efficient versus inefficient initial grips observed only in adults. |
| Anderson et al. (2021) | 55 participants (31 F/24 M) Age: 6–12 months Alpha (6–9 Hz) | EEG data: baseline (1–2 min), 90 s of mother/infant play Behavioral data: parent/infant play, infant temperament. Fast Fourier Transform (FFT). Tests: The Infant Behavior Questionnaire (IBQ-R). | Significant positive correlation between baseline frontal alpha asymmetry (FAA) and object exploration Only the cuddliness subscale (in IBQ-R) was associated with a leftward shift in FAA. |
| Georgieva et al. (2020) | 14 participants (8 F/6 M) Age: (M = 338.85 days) Delta (1–3 Hz) Theta (3–6 Hz) Beta (∼15 Hz) | EEG data: resting state, spontaneous motion. Fast Fourier Transform (FFT). Cluster-Based Permutation Test. | Movement EEG scalp topology was similar to resting state EEG: high delta/theta power at posterior regions and high beta power at orbitofrontal regions. Upper limb movements characterized by increased beta power and generated more widespread artifacts. Decreased theta and alpha power at central in all motion types. |
| Ortiz-Mantilla et al. (2022) | 100 participants (47 F/53 M) Age: 4 months (longitudinal visits at 7, 9, 12, and 18 months) P1 Theta (frequency clusters in 2–6 Hz range) | Auditory go/no-go task (target stimulus is paired with a reward video). Intertrial Phase Synchrony. | Lower theta phase synchrony at 7 months is linked to better expressive language at 12 and 18 months and better receptive language at 9 months. |
| Buzzell et al. (2020) | 136 participants 68 receiving care as usual (CAUG) (35 F/33 M) 68 receiving foster care (FCG) (34 F/34 M) Age: (M age = 21.6 months) Mediofrontal theta (4–8 Hz) oscillations | Go/no-go task. Behavioral assessment of risk for psychopathology (HBQ). Theta power was calculated as the total power measure weighted by the average power time-frequency PCA loadings. | Higher error-related mediofrontal theta power in FCG compared to CAUG. |
| Rayson et al. (2019) | 23 participants (10 F/13 M, Age: 6.5 months (M = 200.91 days, SD = 5.86) 24 infants (13 F/11 M), Age: 9.5 months (M = 292.92 days, SD = 7.88) Alpha | Preferential looking stimuli. Gaze following stimuli. Event-related spectral perturbation analysis. | At both ages, alpha ERD was higher in the congruent condition than the incongruent condition. The higher the alpha ERD in the congruent condition, the more preference toward congruent stimuli (9.5-month-old infants). |
| Haartsen et al. (2020) | 73 participants (38 F/35 M, Age: (M = 302 days, SD = 13) EEG data for the second session consisted of 64 infants (9 drop-outs) Alpha oscillations Graph theory metrics (normalized weighted clustering coefficient, normalized weighted path length, and small-worldness index) | Presentation of naturalistic dynamic videos and moving toys (60 s each)—2 sessions with one-week interval. Phase lag index (PLI). Debiased weighted PLI (dbWPLI) measured using Fourier coefficients. | Intra-class correlations (ICCs) for whole brain connectivity were higher than ICCs for the other graph metrics. |
| Hoehl et al. (2014) | 24 participants (9 F/15 M) Age: 8 months and 29 days Alpha oscillations | EEG recording during eye-contact and no eye-contact conditions. Event-related spectral perturbation. | Infants responded with alpha desynchronization to eye-contact condition. In no eye-contact condition no alpha synchronization or desynchronization effect was observed. |
| Conejero et al. (2018) | 66 participants 52 toddlers (26 F/26 M) Age: (M = 16.75 months; SD = 0.67) 14 adults (13 F/1 M) Age: (M = 21.93; SD = 2.34) Error-related negativity (ERN) Theta (4–7 Hz) | 27 different animal head/body combinations (conceptual error condition). ERP. Time–frequency (power). | Higher fronto-central negativity for incorrect trials compared to correct trials in all participants. Significantly greater increase in theta power in the error condition compared to the correct condition in children of highly educated parents. SES significantly contributed to the amplitude of the ERN. |
| Jones et al. (2015) | 168 participants 88 infants (39 F/49 M) Age: 6 months 80 infants (40 F/40 M) Age: 12-month Theta (3 to 6 Hz) Alpha (6 to 9 Hz) | Two movies of 1-min duration: naturalistic social and non-social FFT. | Socially selective theta responses showed increased power and topographical extent in both Live Action and Movie formats between 6 and 12 months. Theta power has the potential to be a more sensitive measure of social brain development in the first year of life. |
| Thorpe et al. (2016) | 20 adults (11 F/9 M) Age: 18–21 years old, 47 participants (21 F/26 M, 9 drop-outs) Age: 45–68 months 50 participants (3 drop-outs) Age: 12 months Lower alpha band (8–10 Hz) Upper alpha band (10–12 Hz) Upper mu band (10–13 Hz) Lower mu band (8–10 Hz) | Grasp execution task. Power spectral density (PSD). Event-related desynchronization. | Both alpha bands’ topographies peak over occipital–parietal electrodes in all subjects. Strong peak over frontal channels in both alpha bands in adults; no evidence of such a frontal peak in younger subject groups. |
| Friedrich et al. (2017) | 107 participants (47 F/60 M) Age: 6–8 months 12–15 Hz | Learning phase and memory test (primed and unprimed word pairs were tested) ERP, FFT (late negativity, N400, spectral power of sleep spindle activity). | Evidence that infants as young as 6 months old can form semantic categories in long-term memory and associate them with word forms. The formation of perceptually based categories occur in the earlier stages of the sleep (NREM sleep stage 2). |
| Cuevas et al. (2011) | 20 healthy, full-term infants (longitudinally seen from 5 months to 10 months of age) 6–9 Hz | EEG and ECG recordings: Baseline A-not-B task EEG power Coherence. | Baseline: (a) Power increased with age in all electrode sites except for the frontal pole and lateral frontal; (b) coherence increased between frontal pole/medial frontal, medial frontal/lateral frontal, medial frontal/medial parietal, and medial frontal/occipital; (c) heart rate decreased with age. Task: (a) evidence for working memory processing related increase in EEG power in 5-month-old infants; (b) only 5–7-month-old infants showed more localized task-related changes in EEG power in medial parietal region; (c) HR showed no task related changes. |
| Meyer et al. (2011) | 7 participants (2 F/5 M) Age: 3-year-olds 17–21 Hz (beta) | Joint button-pressing game with three conditions: (1) joint action, (2) joint action observation, (3) individual action. Time–frequency representations. Discrete Fourier Transform (DFT) | Higher decrease in beta power in children during joint action observation when they were observing their own joint action partner. Enhanced motor system activity was observed at C3 electrode and was associated with fewer errors during task. |
| Swingler et al. (2017) | 388 participants (199 F/189 M) Age: at 5 months and 10 months of age (longitudinal) 6–9 Hz | EEG Data: Baseline Visual attention tasks at 5- and 10-month follow-ups. Behavioral data: Attention (glove puppet presentation). Maternal behavior (child-mother interaction). Medial frontal EEG power (F3/F4) DFT—natural logarithmic normalization. | Increased EEG power difference at 10 months at the right frontal medial region was linked with better task performance. Higher positivity during mother–infant interactions promote better attentional engagement. |
| Cuevas et al. (2012) | 122 participants (62 F/60 M) Age: 23 months–28 months (longitudinal) Theta (3–5 Hz) Alpha (6–9 Hz) Beta (10–12 Hz) | EEG Data: Baseline Verbal recall task Power—Discrete Fourier Transform (DFT). | Differences in theta and alpha responses during encoding and retrieval. Differences in theta, alpha, and beta responses in high and low performers. |
| Endedijk et al. (2017) | 29 participants (19 F/10 M) Age: (M = 52.48 months, SD = 1.94) Mu (7–12 Hz) Beta (16–20 Hz) | The color-naming task. Action observation task Cooperation task (double-tube task). The entrainment task. | 4-year-old children who exhibited greater motor system engagement during action observation, indicated by reduced beta-power, demonstrated higher success in early peer cooperation. |
| Liao et al. (2015) | 21 participants: 11 toddlers (7 F/4 M) Age: (M = 41 months, SD = 4) 10 mothers’ age: (M:35.5) Mu (7–9 Hz) Beta (15–18 Hz) | Component power spectral density (PSD). Component event-related spectral perturbations (ERSPs). IC clustering. | Age-matched head models indicated that mu sources were located in both the left and right sensorimotor cortex. When children observed their parents’ actions, there was a noticeable suppression of power in the mu (7–9 Hz) and beta (15–18 Hz) frequency ranges. Remarkably, the children’s mu suppression during action observation mirrored adult patterns in all observed properties. |
| Belalov et al. (2014) | 91 participants: 41 orphans (14 F/27 M) Age: (M = 36 months, SD = 2) 50 children raised in a family (19 F/31 M) Age: (M = 35 months, SD = 3) Theta (4–6 Hz) Alpha (7–10 Hz) Beta (11–29 Hz) Gamma (30–45 Hz) | EEG was recorded in three situations: Spontaneous EEG (eyes open): 60 s; while the child listened to a short poem: 20 s; while the child heard meaningless speech: 20 s. Spectral power density (SPD). Tests: The Language Scale of the Bayley Scales of Infant and Toddler Development III (BSID-III). | The Bayley assessment showed that children raised in orphanages had significant delays in language development compared to those raised in families. During the speech stimulus, alpha oscillations become desynchronized, while theta, beta, and gamma oscillations show increased synchronization. Children raised in families showed notable increases in gamma rhythm SPDs across 13 leads in both hemispheres. Conversely, orphans exhibited this increase in only 8 locations, predominantly localized in the left hemisphere. |
| Schneider et al. (2016) | 36 participants: 18 children (9 F/9 M) Age: (M = 10.94, SD = 0.94) 18 adults (9 F/9 M) Age: (M = 24.41, SD = 4.37) ERP (P600, N400) Theta (4–8 Hz) Beta (12–30 Hz) | Grammaticality judgment task. Event-related spectral perturbations (ERSPs). Fourier power spectra density (PSD). | Unlike adults, children exhibited a distinct pattern with a noticeable N400 effect and did not show a decrease in beta or theta activity in response to grammatical violations. |
| Orekhova et al. (2001) | 60 participants Age: (M = 37.3 rd week, SD = 1.96) Alpha (6.4–10 Hz) | EEG: Attention to an object in the visual field peek-a-boo game. Behavioral data: the total time of anticipatory attention was calculated across all 10 trials. | Infants who displayed longer periods of anticipatory attention had higher absolute spectral amplitude across a wide frequency range in both experimental tasks. Alpha synchronization in the posterior parietal cortex actively inhibits certain networks involved in maintaining attention to the peripheral visual field, rather than representing a passive or idle state. |
| Orekhova et al. (2006) | 47 participants: 28 infants (9 F/19 M) Age: (M = 9.2, SD = 1.3) 19 children (8 F/11 M) Age: (M = 5 years 5 months, SD = 359 days) Theta (3.6–5.6 Hz) Mu rhythms (6.0–8.8 Hz) | EEG was recorded during baseline (visual attention) and two test conditions: exploration of toys (manipulation), and attention to ‘social’ stimulation (speech). Event-related spectral power. | Theta responses increase in both infants and children during attention-requiring test conditions, with infants showing a broader topographical increase compared to children. (a) Theta frequency was observed at 3.6–5.6 Hz in the posterior temporal region and occipital–parietal–temporal regions of infants under attention conditions. (b) Theta frequency in the frontal region of children is observed to be in the range of 4–7.6 Hz. |
| Bell et al. (2007) | 50 participants (22 F/28 M) Age: (8 months, when the 25 children were 4.5 years of age, they joined the research again) 6–9 Hz Alpha (8–13 Hz) | Spontaneous EEG. Discrete Fourier transform (DFT) Coherence. Infant working memory/inhibitory control task. The Day-Night Stroop-like task. The Yes-No task. | (a) In infancy, working memory was associated with widespread changes in EEG power and coherence across the entire scalp, indicating broad cortical involvement. (b) By early childhood, these associations were more localized, focusing primarily on medial frontal areas for EEG power and involving specific pairs like medial frontal/posterior temporal, and medial frontal/occipital for EEG coherence. |
| Blankenship et al. (2018) | 242 participants (121 F/121 M) Age: (M = 6.64 years, SD = 0.44) Theta (4–7 Hz) Alpha (8–13 Hz) | Episodic memory (retrieval) tasks. Working memory tasks (A backward digit span (BDS) task). Tests: Woodcock–Johnson (WJ) III The Peabody Picture Vocabulary Test IV. | EEG alpha power in frontal regions and theta power in temporal regions during the working memory task were used to statistically predict both math and reading abilities. |
| Güntekin et al. (2020) | 34 participants: 16 children Age: (M = 6.69 years, SD = 0.48) 18 young adults Age (M = 21.32 years, SD = 3.28) Theta (4–7 Hz) Alpha (8–13 Hz) | The Boston Naming Test (BNT). Free and Cued Selective Reminding Test (FCSRT). Tests: Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV). | Frontocentral theta and alpha phase-locking play a crucial role in brain maturation and the achievement of successful memory performance. Young adults had higher theta and alpha phase-locking than children over the frontal and central locations. Children exhibited heightened theta phase-locking and increased left alpha power in response to remembered objects compared to forgotten objects. The children had higher parietal–occipital alpha phase-locking than the young adults. |
| Rajan et al. (2021) | 29 participants (19 F/10) Age: (M = 6.10 years, SD = 0.26) Theta (4–7 Hz) | Memory binding task. Discrete Fourier transform (DFT). Natural log (In) transform. | Theta rhythms play a significant role in memory-binding processes during middle childhood. |
| Stroganova et al. (2006) | 44 participants Age: (M = 19.6 weeks; SD= 2.59) Theta (3.6–5.2 Hz) | The attention tasks (ExA, EnA, and CA). Spectral analysis (Fast Fourier Transform). Spectral theta peak amplitude (SA). Tests: Bayley-2 scale | At five months of age, children who maintain attention on a hidden object demonstrate the emergence of a highly synchronized theta rhythm compared to their child stages. |
| Gomarus et al. (2006) | 18 participants (4 F/15 M) Age: (M = 10.4 years, SD = 1.5) ERPs Theta (4–8 Hz) Alpha (8–12 Hz). | Selective memory search task ERD, ERS. | Theta ERS was most evident during the most challenging task condition in the recognition set, while alpha ERD exhibited a load effect only during memorization. |
| Spironelli et al. (2010) | 70 participants: 28 children (14 F/14 M) Age: (M = 10.01 years, SD = 0.18) 22 young adults (11 F/11 M) Age: (M = 22.59 years, SD = 3.67) 20 middle-aged adults (7 F/13 M) Age: (M = 59.10 years, SD = 7.11) Delta (1–4 Hz) Theta (4–8 Hz) Alpha (8–13 Hz Beta (β1: 13–20 Hz, β2: 21–28 Hz) | Three linguistic tasks (orthographic, phonological, semantic). Spectral FFT analysis. | In adults, task-dependent right lateralization was observed in theta and alpha distributions, whereas children exhibited differences primarily between linguistic and non-linguistic tasks without clear lateralization patterns. |
| Jiang et al. (2017) | 37 participants (18 F/19 M; 10 excluded) Age: (4.5–5.5 years) Delta (1–3 Hz) Theta (3–8 Hz) Alpha (8–13 Hz) Beta (13–30 Hz) Gamma (30–70 Hz) | Event-related spectral perturbation (ERSP). | Negative stimuli, compared to neutral ones, induced greater theta ERS in children who showed improved cognitive efficiency with negative emotional content. |
| Morasch et al. (2009) | 48 participants (16 F/32 M) Age: (M = 10 months, 6 days) alpha (6–9 Hz) | Discrete Fourier transform (DFT) Tasks: The activity-matched baseline Encoding (modeling) Recall Test. | Infants who demonstrated successful ordered recall exhibited an increase in brain activity from the baseline to the task at the front temporal scalp areas. In contrast, infants who did not show ordered recall had no changes in brain activity from baseline to task. |
| Schneider et al. (2018) | 39 participants: 23 adults (14 F/9 M) (M = 24 years, SD = 4.3) 16 children (8 F/8 M) (M = 10.88 years, SD = 0.96 years) Theta, alpha, and beta = 3–30 Hz | Event-related spectral perturbation. | The increase in children’s theta power after 2400 ms over the frontal and right frontocentral areas during the processing of grammatically and semantically correct sentences significantly differed from that of adults. |
| Attaheri et al. (2022) | 60 participants Age: (longitudinal cohort: (M = 212.2 days SD = 7.2 to M = 333.1 days SD = 5.6)) Delta Theta | (mTRF) method in delta, theta, and alpha bands. Periodogram (PSD) analysis. Phase-amplitude coupling. (PAC) for delta–beta, delta–gamma, theta–beta, and theta–gamma coupling. | (a) PAC was significantly present at all ages, with both delta and theta bands driving the coupling. (b) Delta and theta were significant carrier phases, linking with higher-frequency amplitudes in the infant brain. |
| Meyer et al. (2023) | 23 infants (10 F/13 M) Age: 15.5–16.5 months (M = 15.9) Theta (4–5 Hz) | EEG recorded during demonstration video clips at one of the three conditions (normal, high, and variable) includes five goal-directed movement actions (acting on balls, cups, and rings). After EEG recording, 1 min. exploration phase started and infants had the opportunity to act on the objects themselves. Time–frequency analysis of Fz, FCz, Cz. | Infants with increased frontal theta activity were prone to investigating new objects within the present task framework. Variability has an important role in drawing infants’ attention to and thereby fostering their learning from infant-directed actions (IDAs). |
| Meyer et al. (2019) | 29 children (9 F/10 M) Age: 4 years (M = 52.48, SD = 1.94 months) Theta (3–6 Hz) | Tasks: No task, a color-naming task, an ımitation task. Theta band (3–6 Hz) power was calculated using a Fast Fourier Transform with multitaper method. | Frontocentral theta power was found higher when children engaged in tasks than when they were not involved in tasks. Higher theta power over left frontotemporal electrode sites when being engaged in the language-related task compared to the motor-related task Theta oscillations have a role in top-down control. |
| Grossmann et al. (2007) | 12 infants (5 girls), 4-month-olds Gamma oscillations (ERPs) | ||
| Köster et al. (2019) | 38 infants (14 F/24 M) Age: nine-month-olds (M = 9.4 months, SD = 7 days) Theta (3–5 Hz) Alpha (5–7 Hz) | Four classical violations of expectation paradigms. The picture sequences were visually flickered at a theta (4-Hz) or an alpha (6-Hz) frequency. Steady-state visually evoked potentials (SSVEPs) of posterior electrodes (O1, Oz, O2, P3, P4, Pz, P7, P8) were analyzed. | At unexpected events, the visually entrained theta oscillations increased. However, entrained alpha oscillations did not differ between outcomes. Expected and unexpected events are factors that cause a frequency-specific SSVEP response. |
| Tang et al. (2019) | 201 participants (124 F/77 M) 58 children (38 F/20 M) Age: 10–12 years (M = 10.83, SD = 0.82) 64 adolescents (39 F/25 M) Age: 14–17 years (M = 15.02, SD = 0.98) 79 young adults (47 F/32 M) Age: 18–28 (M = 19.42, SD = 1.92) Theta (4–7 Hz) | Social Exclusion Task (participants played the online ball-toss game Cyberball). ERSP between 200 to 600 ms at F3, FCz, P4. ITC from 100 to 400 ms. Event-related theta EEG power and phase coherence. | Adolescents and children showed the highest spectral power at 400–600 ms on the rejection part. However, adults’ spectral power was the highest theta spectral power at the not-my-turn event. Increased theta spectral power responses to both social exclusion and threats of exclusion shown in adolescent development (ITC); adolescents showed lower levels of left frontal theta synchrony in response to rejection compared to children and adults. |
| Orekhova et al. (1999) | 60 infants Age: 7–12 months born after 33.5–41 weeks gestation (M: 37.3, SD = 1.96) Theta 1 (3.6–4.8 Hz) Theta 2 (5.2–6.0 Hz) | EEG recorded in three attention conditions: looking without overt emotional reactions, the peek-a-boo game, and attention to the adult’s reappearance. | EEG theta rhythm (3.6–6 Hz) synchronized during internally controlled (anticipatory) attention. As infants become older, the attention-related slow theta synchronization tends to decrease. The fast theta rhythm appeared only during effortful concentration of attention. |
| Bazhenova et al. (2007) | 16 infants (9 F/7 M) Age: 20 weeks (M = 19.7; SD = 0.89) Theta (3.6–5.6 Hz) Alpha (6.0–9.0 Hz) | EEG recorded an adult’s smiling (SF) and a blank face (BF) in a face-to-face setting. | Looking at the adult’s BF causes increases in theta activity in the 4.8 Hz narrow band, observed over the majority of anterior and right posterior temporal scalp areas. The right hemisphere is more dominant to changes in a partner’s facial expressivity. |
| Yordanova et al. (2009) | 70 participants (37 F/33 M) 54 children (25 F/29 M) 16 young Adults (12 F/4 M) Age: M = 306.4, SD = 55.7 Theta (4–7 Hz) Alpha (8–14 Hz) | Three task conditions: passive listening condition (PLC), simple reaction task (SRT), serial learning reaction task (SLRT). EROs. | Results show that fast alpha oscillations are stronger in SLRT condition and more synchronized in children than adults. In the slower theta-to-alpha range, the phase synchronization increases with age. Developmental dynamics specific for theta, alpha-1, and alpha-2 frequencies differ in memory activation tasks. |
| Nanova et al. (2018) | 36 children (13 F/20 M) Age: 7–10 years Delta (0.5–4 Hz) Theta (4–7 Hz) Slow alpha (7–10 Hz) Fast alpha (10–14 Hz) | Sensorimotor task with fixed stimulus sequences. ERPs N1, P2, N2 and P3 amplitudes. Phase-synchronization of EROs. | Proactive processing mode was marked by increased pre-stimulus theta activity. Notable reduction in the temporal synchronization of event-related theta/alpha oscillations within 300 milliseconds after the stimulus. |
| Nanova et al. (2011) | 36 healthy children (18 F/18 M): Age: 7–8 years old (M = 7.7) (9 F/9 M) Age: 9–10 years old (M = 9.2) (9 F/9 M) Delta (0.5–4 Hz), Theta (4–7 Hz), Slow Alpha(7–10 Hz) Fast Alpha (10–14 Hz) | EEG recorded 3 different conditions: auditory stimulation, a passive listening condition (PLC), a simple reaction task (SRT), and a serial learning reaction task (SLRT). Phase-locking analysis. Auditory ERP. | Accelerated functional maturation of processing networks is frequency-specific and shown in increased girls’ phase-locking of the slower (delta, theta, and slow alpha) but not for faster (fast alpha) frequency bands. Between 7 and 10 years of age, the development of neurophysiological mechanisms crucial for early auditory information processing varies based on gender. |
| Ashkinazi et al. (2010) | 30 healthy children (M = 6.05, SD = 0.75): Age: 5–6 years old (n = 14) Age:6–7 years old (n = 16) Set: a plastic set (n = 14), a rigid set(n = 16) Theta (4–7 Hz) Alpha (8–13 Hz) | Resting-state EEG. Visual set paradigm. Spectral power analysis using FFT. | Higher alpha band spectral power (SP) has been shown at the plastic set rather than the rigid set. Both hemsipheres at occipital lobes showed increased alpha activity. |
| Chung et al. (2022) | 49 full-term infants (25 F/24 M): Age: 21 infants, 9-month-olds (M = 9.21, SD = 10.0 days) (8 F/13 M) Age: 28 infants, 12-month-olds (M = 12.21, SD = 17.8 days) (17 F/11 M) Mu (6- to 9-Hz) | EEG recorded during action observation conditions: grasp condition, cane-use condition. Action execution period: Allow the infant to attempt trials in grasping and using a cane. Interchannel phase coherence (ICPC). | At the 6–9 Hz frequency range phase, coherence of central–ocipital regions is higher than shorter distances such as central–parietal and central–frontal regions while observing grasping action. |
| Vollebregt et al. (2015) | 27 children (16 F/9 M): Age: 7–10 years (M = 9.11, SD = 1.29) Alpha (8–12 Hz) | Attention paradigm (Posner’s cueing paradigm). EEG recorded 2 min EO, 2 min EC, and during the task. | Healthy children exhibited adult-like alpha lateralization patterns during covert attention. Children who were less affected by spatial cueing showed stronger left alpha modulation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ünsal, E.; Duygun, R.; Yemeniciler, İ.; Bingöl, E.; Ceran, Ö.; Güntekin, B. From Infancy to Childhood: A Comprehensive Review of Event- and Task-Related Brain Oscillations. Brain Sci. 2024, 14, 837. https://doi.org/10.3390/brainsci14080837
Ünsal E, Duygun R, Yemeniciler İ, Bingöl E, Ceran Ö, Güntekin B. From Infancy to Childhood: A Comprehensive Review of Event- and Task-Related Brain Oscillations. Brain Sciences. 2024; 14(8):837. https://doi.org/10.3390/brainsci14080837
Chicago/Turabian StyleÜnsal, Esra, Rümeysa Duygun, İrem Yemeniciler, Elifnur Bingöl, Ömer Ceran, and Bahar Güntekin. 2024. "From Infancy to Childhood: A Comprehensive Review of Event- and Task-Related Brain Oscillations" Brain Sciences 14, no. 8: 837. https://doi.org/10.3390/brainsci14080837





