Neurophysiological Oscillatory Mechanisms Underlying the Effect of Mirror Visual Feedback-Induced Illusion of Hand Movements on Nociception and Cortical Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
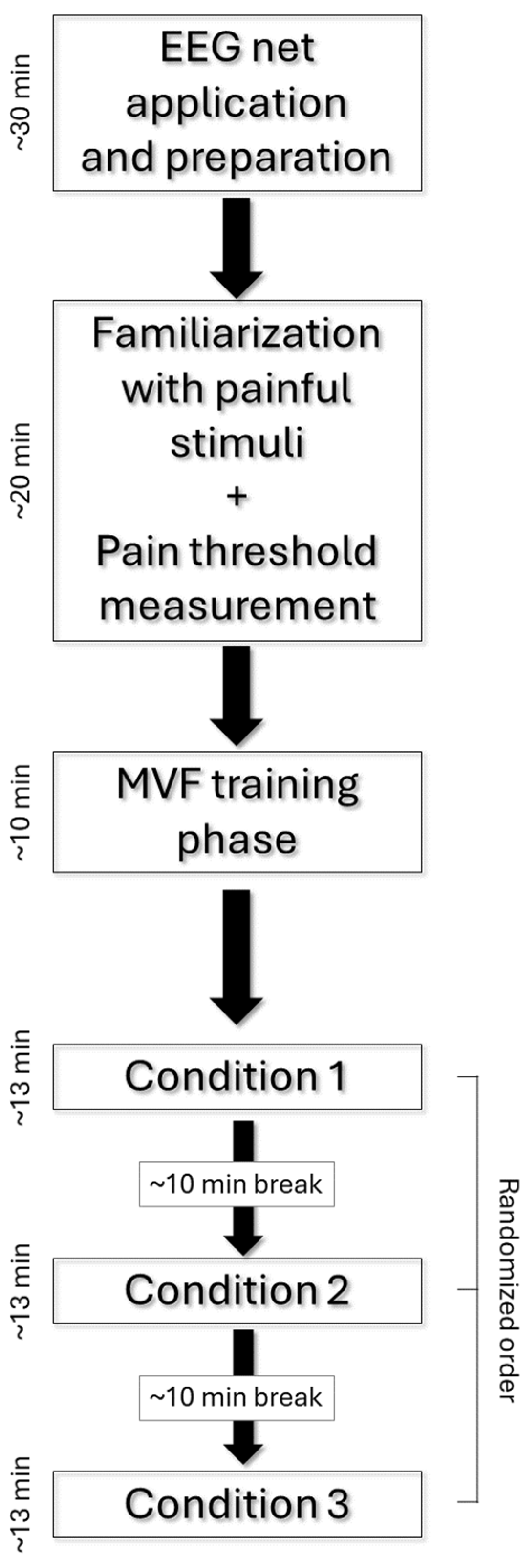
2.2. Experimental Procedure
2.3. Electrical Stimulations and Pain Threshold Detection
2.4. EEG Recording and Preprocessing
2.5. Cortical Sources Estimation
2.6. Alpha ERD/ERS Calculation
2.7. Statistical Analysis for the ERD/ERS Scalp Distribution
2.8. Statistical Analysis for the Alpha ERD/ERS Cortical Sources
2.9. Control Analysis
3. Results
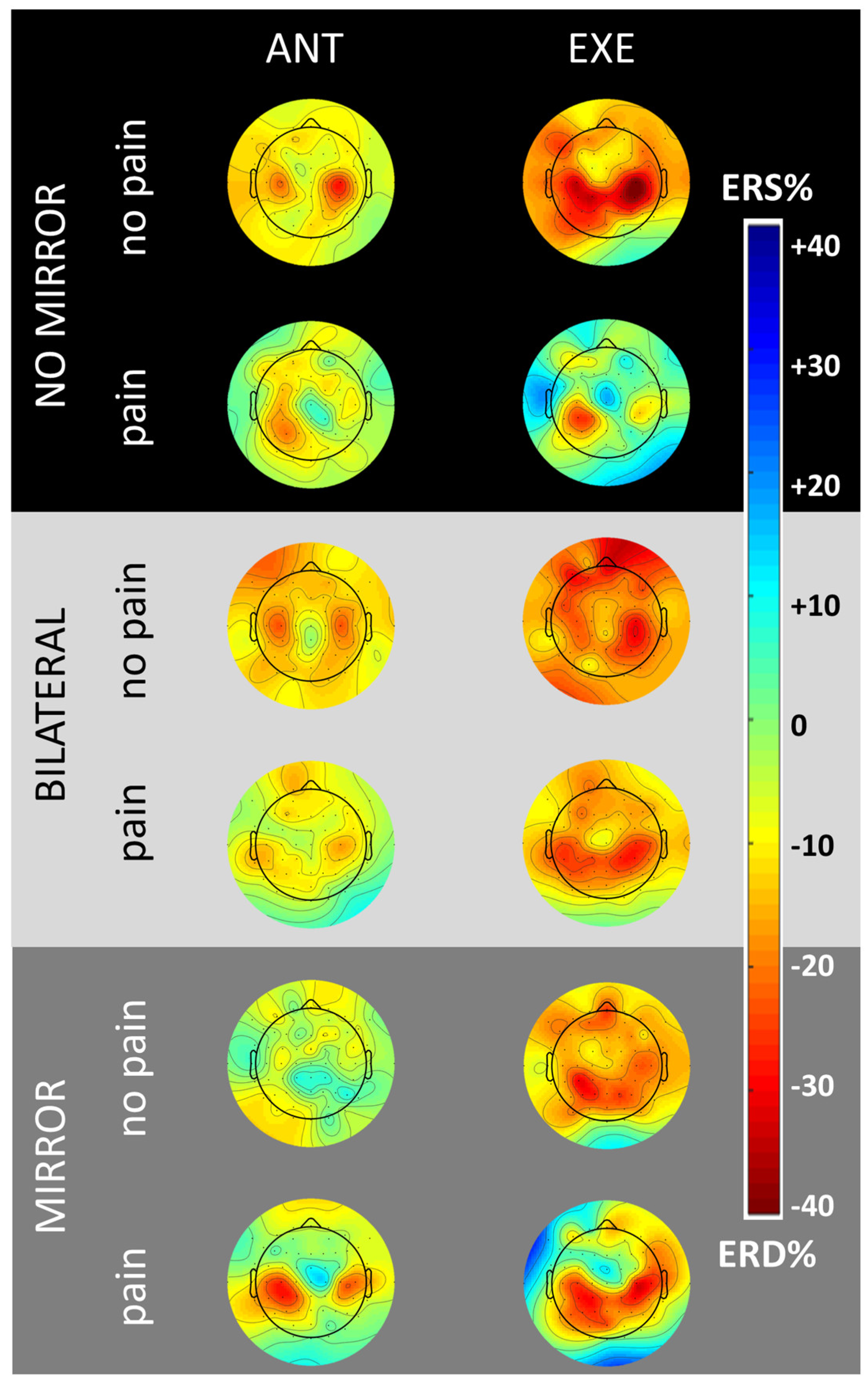
3.1. Scalp Topography
3.2. Control Conditions without MVF
3.3. Experimental Condition with MVF
4. Discussion
4.1. Effects of MVF-Induced Movement Illusion on Cortical Activity and Pain
4.2. Clinical Implications and Future Perspectives
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortiz-Catalan, M.; Guðmundsdóttir, R.A.; Kristoffersen, M.B.; Zepeda-Echavarria, A.; Caine-Winterberger, K.; Kulbacka-Ortiz, K.; Widehammar, C.; Eriksson, K.; Stockselius, A.; Ragnö, C.; et al. Phantom Motor Execution Facilitated by Machine Learning and Augmented Reality as Treatment for Phantom Limb Pain: A Single Group, Clinical Trial in Patients with Chronic Intractable Phantom Limb Pain. Lancet 2016, 388, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, X.; Guo, X.; Chen, S.; Wang, H.; Cui, X.; Rong, J.; Jia, J. Effects of Camera-Based Mirror Visual Feedback Therapy for Patients Who Had a Stroke and the Neural Mechanisms Involved: Protocol of a Multicentre Randomised Control Study. BMJ Open 2019, 9, e022828. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.S.; Rogers-Ramachandran, D. Synaesthesia in Phantom Limbs Induced with Mirrors. Proc. R. Soc. Lond. B Biol. Sci. 1997, 263, 377–386. [Google Scholar] [CrossRef]
- Herrador Colmenero, L.; Perez Marmol, J.M.; Martí-García, C.; Querol Zaldivar, M.D.; Tapia Haro, R.M.; Castro Sánchez, A.M.; Aguilar-Ferrándiz, M.E. Effectiveness of Mirror Therapy, Motor Imagery, and Virtual Feedback on Phantom Limb Pain Following Amputation: A Systematic Review. Prosthet. Orthot. Int. 2018, 42, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Arya, K.N. Underlying Neural Mechanisms of Mirror Therapy: Implications for Motor Rehabilitation in Stroke. Neurol. India 2016, 64, 38. [Google Scholar] [CrossRef] [PubMed]
- Deconinck, F.J.A.; Smorenburg, A.R.P.; Benham, A.; Ledebt, A.; Feltham, M.G.; Savelsbergh, G.J.P. Reflections on Mirror Therapy: A Systematic Review of the Effect of Mirror Visual Feedback on the Brain. Neurorehabil. Neural Repair 2015, 29, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Finn, S.B.; Perry, B.N.; Clasing, J.E.; Walters, L.S.; Jarzombek, S.L.; Curran, S.; Rouhanian, M.; Keszler, M.S.; Hussey-Andersen, L.K.; Weeks, S.R.; et al. A Randomized, Controlled Trial of Mirror Therapy for Upper Extremity Phantom Limb Pain in Male Amputees. Front. Neurol. 2017, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Thøgersen, M.; Andoh, J.; Milde, C.; Graven-Nielsen, T.; Flor, H.; Petrini, L. Individualized Augmented Reality Training Reduces Phantom Pain and Cortical Reorganization in Amputees: A Proof of Concept Study. J. Pain 2020, 21, 1257–1269. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Jeong, W.S.; Kim, K.Y. Effects of Mirror Therapy on Subacute Stroke Patients’ Brain Waves and Upper Extremity Functions. J. Phys. Ther. Sci. 2012, 24, 1119–1122. [Google Scholar] [CrossRef]
- Bartur, G.; Pratt, H.; Frenkel-Toledo, S.; Soroker, N. Neurophysiological Effects of Mirror Visual Feedback in Stroke Patients with Unilateral Hemispheric Damage. Brain Res. 2018, 1700, 170–180. [Google Scholar] [CrossRef]
- Dohle, C.; Püllen, J.; Nakaten, A.; Küst, J.; Rietz, C.; Karbe, H. Mirror Therapy Promotes Recovery from Severe Hemiparesis: A Randomized Controlled Trial. Neurorehabil. Neural Repair 2009, 23, 209–217. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.S.; Haigh, R.C.; Ring, E.F.J.; Halligan, P.W.; Wall, P.D.; Blake, D.R. A Controlled Pilot Study of the Utility of Mirror Visual Feedback in the Treatment of Complex Regional Pain Syndrome (Type 1). Rheumatology 2003, 42, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Vladimir Tichelaar, Y.I.G.; Geertzen, J.H.B.; Keizer, D.; Paul van Wilgen, C. Mirror Box Therapy Added to Cognitive Behavioural Therapy in Three Chronic Complex Regional Pain Syndrome Type I Patients: A Pilot Study. Int. J. Rehabil. Res. 2007, 30, 181. [Google Scholar] [CrossRef] [PubMed]
- Bullock, K.; Won, A.S.; Bailenson, J.; Friedman, R. Virtual Reality-Delivered Mirror Visual Feedback and Exposure Therapy for FND: A Midpoint Report of a Randomized Controlled Feasibility Study. J. Neuropsychiatry Clin. Neurosci. 2020, 32, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Fong, K.N.K. Enhancing Mirror Visual Feedback with Intermittent Theta Burst Stimulation in Healthy Adults. Restor. Neurol. Neurosci. 2019, 37, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Merians, A.S.; Tunik, E.; Fluet, G.G.; Qiu, Q.; Adamovich, S.V. Innovative Approaches to the Rehabilitation of Upper Extremity Hemiparesis Using Virtual Environments. Eur. J. Phys. Rehabil. Med. 2009, 45, 123–133. [Google Scholar] [PubMed]
- Shinoura, N.; Suzuki, Y.; Watanabe, Y.; Yamada, R.; Tabei, Y.; Saito, K.; Yagi, K. Mirror Therapy Activates Outside of Cerebellum and Ipsilateral M1. NeuroRehabilitation 2008, 23, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Murayama, T.; Takasugi, J.; Monma, M.; Oga, M. Mirror Observation of Finger Action Enhances Activity in Anterior Intraparietal Sulcus: A Functional Magnetic Resonance Imaging Study. J. Jpn. Phys. Ther. Assoc. 2013, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Imai, I.; Takeda, K.; Shiomi, T.; Taniguchi, T.; Kato, H. Sensorimotor Cortex Activation during Mirror Therapy in Healthy Right-Handed Subjects: A Study with Near-Infrared Spectroscopy. J. Phys. Ther. Sci. 2008, 20, 141–145. [Google Scholar] [CrossRef][Green Version]
- Inagaki, Y.; Seki, K.; Makino, H.; Matsuo, Y.; Miyamoto, T.; Ikoma, K. Exploring Hemodynamic Responses Using Mirror Visual Feedback with Electromyogram-Triggered Stimulation and Functional Near-Infrared Spectroscopy. Front. Hum. Neurosci. 2019, 13, 60. [Google Scholar] [CrossRef]
- Fukumura, K.; Sugawara, K.; Tanabe, S.; Ushiba, J.; Tomita, Y. Influence of Mirror Therapy on Human Motor Cortex. Int. J. Neurosci. 2007, 117, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Aziz-Zadeh, L.; Maeda, F.; Zaidel, E.; Mazziotta, J.; Iacoboni, M. Lateralization in Motor Facilitation during Action Observation: A TMS Study. Exp. Brain Res. 2002, 144, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Garry, M.I.; Loftus, A.; Summers, J.J. Mirror, Mirror on the Wall: Viewing a Mirror Reflection of Unilateral Hand Movements Facilitates Ipsilateral M1 Excitability. Exp. Brain Res. 2005, 163, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, C.; Wang, J.; dos Santos, L.F.; Mauritz, K.-H.; Brunetti, M.; Dohle, C. Different Effects of the Mirror Illusion on Motor and Somatosensory Processing. Restor. Neurol. Neurosci. 2014, 32, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Hamzei, F.; Läppchen, C.H.; Glauche, V.; Mader, I.; Rijntjes, M.; Weiller, C. Functional Plasticity Induced by Mirror Training: The Mirror as the Element Connecting Both Hands to One Hemisphere. Neurorehabil. Neural Repair 2012, 26, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Matthys, K.; Smits, M.; Van der Geest, J.N.; Van der Lugt, A.; Seurinck, R.; Stam, H.J.; Selles, R.W. Mirror-Induced Visual Illusion of Hand Movements: A Functional Magnetic Resonance Imaging Study. Arch. Phys. Med. Rehabil. 2009, 90, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Seidel, S.; Kasprian, G.; Furtner, J.; Schöpf, V.; Essmeister, M.; Sycha, T.; Auff, E.; Prayer, D. Mirror Therapy in Lower Limb Amputees—A Look Beyond Primary Motor Cortex Reorganization. RöFo Fortschritte Auf Dem Geb. Röntgenstrahlen Bildgeb. Verfahr. 2011, 183, 1051–1057. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, B.-H. Mirror Therapy Combined With Biofeedback Functional Electrical Stimulation for Motor Recovery of Upper Extremities after Stroke: A Pilot Randomized Controlled Trial. Occup. Ther. Int. 2015, 22, 51–60. [Google Scholar] [CrossRef]
- Light, G.A.; Williams, L.E.; Minow, F.; Sprock, J.; Rissling, A.; Sharp, R.; Swerdlow, N.R.; Braff, D.L. Electroencephalography (EEG) and Event-Related Potentials (ERPs) with Human Participants. Curr. Protoc. Neurosci. 2010, 52, 6.25.1–6.25.24. [Google Scholar] [CrossRef]
- Debnath, R.; Franz, E.A. Perception of Hand Movement by Mirror Reflection Evokes Brain Activation in the Motor Cortex Contralateral to a Non-Moving Hand. Cortex J. Devoted Study Nerv. Syst. Behav. 2016, 81, 118–125. [Google Scholar] [CrossRef]
- Touzalin-Chretien, P.; Dufour, A. Motor Cortex Activation Induced by a Mirror: Evidence from Lateralized Readiness Potentials. J. Neurophysiol. 2008, 100, 19–23. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-Related EEG/MEG Synchronization and Desynchronization: Basic Principles. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Babiloni, C.; Del Percio, C.; Arendt-Nielsen, L.; Soricelli, A.; Romani, G.L.; Rossini, P.M.; Capotosto, P. Cortical EEG Alpha Rhythms Reflect Task-Specific Somatosensory and Motor Interactions in Humans. Clin. Neurophysiol. 2014, 125, 1936–1945. [Google Scholar] [CrossRef]
- Neuper, C.; Wörtz, M.; Pfurtscheller, G. ERD/ERS Patterns Reflecting Sensorimotor Activation and Deactivation. Prog. Brain Res. 2006, 159, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Babiloni, C.; Capotosto, P.; Brancucci, A.; Del Percio, C.; Petrini, L.; Buttiglione, M.; Cibelli, G.; Romani, G.L.; Rossini, P.M.; Arendt-Nielsen, L. Cortical Alpha Rhythms Are Related to the Anticipation of Sensorimotor Interaction between Painful Stimuli and Movements: A High-Resolution EEG Study. J. Pain 2008, 9, 902–911. [Google Scholar] [CrossRef]
- Babiloni, C.; Capotosto, P.; Del Percio, C.; Babiloni, F.; Petrini, L.; Buttiglione, M.; Cibelli, G.; Marusiak, J.; Romani, G.L.; Arendt-Nielsen, L.; et al. Sensorimotor Interaction between Somatosensory Painful Stimuli and Motor Sequences Affects Both Anticipatory Alpha Rhythms and Behavior as a Function of the Event Side. Brain Res. Bull. 2010, 81, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Bartur, G.; Pratt, H.; Dickstein, R.; Frenkel-Toledo, S.; Geva, A.; Soroker, N. Electrophysiological Manifestations of Mirror Visual Feedback during Manual Movement. Brain Res. 2015, 1606, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-M.; Li, P.-C.; Fan, S.-C. Delayed Mirror Visual Feedback Presented Using a Novel Mirror Therapy System Enhances Cortical Activation in Healthy Adults. J. NeuroEngineering Rehabil. 2015, 12, 56. [Google Scholar] [CrossRef][Green Version]
- Rizzo, M.; Petrini, L.; Del Percio, C.; Lopez, S.; Arendt-Nielsen, L.; Babiloni, C. Mirror Visual Feedback during Unilateral Finger Movements Is Related to the Desynchronization of Cortical Electroencephalographic Somatomotor Alpha Rhythms. Psychophysiology 2022, 59, e14116. [Google Scholar] [CrossRef]
- Foell, J.; Bekrater-Bodmann, R.; McCabe, C.S.; Flor, H. Sensorimotor Incongruence and Body Perception: An Experimental Investigation. Front. Hum. Neurosci. 2013, 7, 310. [Google Scholar] [CrossRef]
- McCabe, C.S.; Haigh, R.C.; Halligan, P.W.; Blake, D.R. Simulating Sensory–Motor Incongruence in Healthy Volunteers: Implications for a Cortical Model of Pain. Rheumatology 2005, 44, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Katayama, O.; Osumi, M.; Kodama, T.; Morioka, S. Dysesthesia Symptoms Produced by Sensorimotor Incongruence in Healthy Volunteers: An Electroencephalogram Study. J. Pain Res. 2016, 9, 1197–1204. [Google Scholar] [CrossRef][Green Version]
- McCabe, C.S.; Cohen, H.; Blake, D.R. Somaesthetic Disturbances in Fibromyalgia Are Exaggerated by Sensory–Motor Conflict: Implications for Chronicity of the Disease? Rheumatology 2007, 46, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Katayama, O.; Nishi, Y.; Osumi, M.; Takamura, Y.; Kodama, T.; Morioka, S. Neural Activities behind the Influence of Sensorimotor Incongruence on Dysesthesia and Motor Control. Neurosci. Lett. 2019, 698, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nishigami, T.; Nakano, H.; Osumi, M.; Tsujishita, M.; Mibu, A.; Ushida, T. Central Neural Mechanisms of Interindividual Difference in Discomfort during Sensorimotor Incongruence in Healthy Volunteers: An Experimental Study. Rheumatology 2014, 53, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Daenen, L.; Nijs, J.; Roussel, N.; Wouters, K.; Van Loo, M.; Cras, P. Sensorimotor Incongruence Exacerbates Symptoms in Patients with Chronic Whiplash Associated Disorders: An Experimental Study. Rheumatology 2012, 51, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Araujo, H.F.; Kaplan, J.; Damasio, A. Cortical Midline Structures and Autobiographical-Self Processes: An Activation-Likelihood Estimation Meta-Analysis. Front. Hum. Neurosci. 2013, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Cavanna, A.E.; Trimble, M.R. The Precuneus: A Review of Its Functional Anatomy and Behavioural Correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef] [PubMed]
- Goffaux, P.; Girard-Tremblay, L.; Marchand, S.; Daigle, K.; Whittingstall, K. Individual Differences in Pain Sensitivity Vary as a Function of Precuneus Reactivity. Brain Topogr. 2014, 27, 366–374. [Google Scholar] [CrossRef]
- Northoff, G.; Bermpohl, F. Cortical Midline Structures and the Self. Trends Cogn. Sci. 2004, 8, 102–107. [Google Scholar] [CrossRef]
- Northoff, G.; Heinzel, A.; de Greck, M.; Bermpohl, F.; Dobrowolny, H.; Panksepp, J. Self-Referential Processing in Our Brain—A Meta-Analysis of Imaging Studies on the Self. NeuroImage 2006, 31, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Kida, T.; Wasaka, T.; Inui, K.; Akatsuka, K.; Nakata, H.; Kakigi, R. Centrifugal Regulation of Human Cortical Responses to a Task-Relevant Somatosensory Signal Triggering Voluntary Movement. NeuroImage 2006, 32, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Simms, E.; Clark, C.R.; Paul, R.H.; Rowe, D.; Gordon, E. The Test-Retest Reliability of a Standardized Neurocognitive and Neurophysiological Test Battery: “Neuromarker”. Int. J. Neurosci. 2005, 115, 1605–1630. [Google Scholar] [CrossRef] [PubMed]
- Justen, C.; Herbert, C. Snap Your Fingers! An ERP/sLORETA Study Investigating Implicit Processing of Self- vs. Other-Related Movement Sounds Using the Passive Oddball Paradigm. Front. Hum. Neurosci. 2016, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cai, M.M.; Xiao, P.; Luo, F.; Iannetti, G.D. Human Brain Responses to Concomitant Stimulation of Aδ and C Nociceptors. J. Neurosci. 2014, 34, 11439–11451. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, P.; Zhang, R.; Chen, M.; Shi, L.; Gao, J.; Hu, Y. The Influence of Different EEG References on Scalp EEG Functional Network Analysis During Hand Movement Tasks. Front. Hum. Neurosci. 2020, 14, 367. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.; Sejnowski, T. An Informatio-Maximization Approach to Blind Separation and Blind Deconvolution. Neural Comput. 1995, 7, 1129–1159. [Google Scholar] [CrossRef]
- Klimesch, W. EEG Alpha and Theta Oscillations Reflect Cognitive and Memory Performance: A rKlimesch, W. EEG Alpha and Theta Oscillations Reflect Cognitive and Memory Performance: A Review and Analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Corcoran, A.W.; Alday, P.M.; Schlesewsky, M.; Bornkessel-Schlesewsky, I. Toward a Reliable, Automated Method of Individual Alpha Frequency (IAF) Quantification. Psychophysiology 2018, 55, e13064. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D. Discrete, 3D Distributed Linear Imaging Methods of Electric Neuronal Activity. Part 1: Exact, Zero Error Localization. arXiv 2007, arXiv:0710.3341. [Google Scholar]
- Pascual-Marqui, R.D.; Esslen, M.; Kochi, K.; Lehmann, D. Functional Imaging with Low-Resolution Brain Electromagnetic Tomography (LORETA): A Review. Methods Find. Exp. Clin. Pharmacol. 2002, 24, 91–95. [Google Scholar] [PubMed]
- Pascual-Marqui, R.D.; Lehmann, D.; Koenig, T.; Kochi, K.; Merlo, M.C.G.; Hell, D.; Koukkou, M. Low Resolution Brain Electromagnetic Tomography (LORETA) Functional Imaging in Acute, Neuroleptic-Naive, First-Episode, Productive Schizophrenia. Psychiatry Res. Neuroimaging 1999, 90, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Hu, Y.; Mao, Y.; Babiloni, C. Widespread Cortical α-ERD Accompanying Visual Oddball Target Stimuli Is Frequency but Non-Modality Specific. Behav. Brain Res. 2015, 295, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Neuper, C.; Flotzinger, D.; Pregenzer, M. EEG-Based Discrimination between Imagination of Right and Left Hand Movement. Electroencephalogr. Clin. Neurophysiol. 1997, 103, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Aranibar, A. Evaluation of Event-Related Desynchronization (ERD) Preceding and Following Voluntary Self-Paced Movement. Electroencephalogr. Clin. Neurophysiol. 1979, 46, 138–146. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Neuper, C. Event-Related Synchronization of Mu Rhythm in the EEG over the Cortical Hand Area in Man. Neurosci. Lett. 1994, 174, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, G. Summary of the N1-P2 Cortical Auditory Evoked Potential to Estimate the Auditory Threshold in Adults. Semin. Hear. 2016, 37, 1–8. [Google Scholar] [CrossRef]
- Mouraux, A.; Iannetti, G.D.; Plaghki, L. Low Intensity Intra-Epidermal Electrical Stimulation Can Activate Aδ-Nociceptors Selectively. Pain 2010, 150, 199–207. [Google Scholar] [CrossRef]
- Fabrizi, L.; Verriotis, M.; Williams, G.; Lee, A.; Meek, J.; Olhede, S.; Fitzgerald, M. Encoding of Mechanical Nociception Differs in the Adult and Infant Brain. Sci. Rep. 2016, 6, 28642. [Google Scholar] [CrossRef]
- Nichols, T.E.; Holmes, A.P. Nonparametric Permutation Tests for Functional Neuroimaging: A Primer with Examples. Hum. Brain Mapp. 2001, 25, 1–25. [Google Scholar] [CrossRef]
- Bian, Y.; Qi, H.; Zhao, L.; Ming, D.; Guo, T.; Fu, X. Dynamic Visual Guidance with Complex Task Improves Intracortical Source Activities during Motor Imagery. NeuroReport 2019, 30, 645–652. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. In Breakthroughs in Statistics: Methodology and Distribution; Kotz, S., Johnson, N.L., Eds.; Springer: New York, NY, USA, 1992; pp. 196–202. ISBN 978-1-4612-4380-9. [Google Scholar]
- Tomczak, M.; Tomczak, E. The Need to Report Effect Size Estimates Revisited. An Overview of Some Recommended Measures of Effect Size. 2014, 1, 19–25. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- Peng, K.; Steele, S.C.; Becerra, L.; Borsook, D. Brodmann Area 10: Collating, Integrating and High Level Processing of Nociception and Pain. Prog. Neurobiol. 2018, 161, 1–22. [Google Scholar] [CrossRef]
- Tolomeo, S.; Christmas, D.; Jentzsch, I.; Johnston, B.; Sprengelmeyer, R.; Matthews, K.; Douglas Steele, J. A Causal Role for the Anterior Mid-Cingulate Cortex in Negative Affect and Cognitive Control. Brain 2016, 139, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Vanneste, S. Burst and Tonic Spinal Cord Stimulation: Different and Common Brain Mechanisms. Neuromodulation J. Int. Neuromodulation Soc. 2016, 19, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, B.; Bentley, D.E.; Elliott, R.; Youell, P.; Watson, A.; Derbyshire, S.W.G.; Frackowiak, R.S.J.; Friston, K.J.; Jones, A.K.P. Attention to Pain Localization and Unpleasantness Discriminates the Functions of the Medial and Lateral Pain Systems. Eur. J. Neurosci. 2005, 21, 3133–3142. [Google Scholar] [CrossRef] [PubMed]
- Price, D.D. Psychological and Neural Mechanisms of the Affective Dimension of Pain. Science 2000, 288, 1769–1772. [Google Scholar] [CrossRef]
- Fauchon, C.; Faillenot, I.; Quesada, C.; Meunier, D.; Chouchou, F.; Garcia-Larrea, L.; Peyron, R. Brain Activity Sustaining the Modulation of Pain by Empathetic Comments. Sci. Rep. 2019, 9, 8398. [Google Scholar] [CrossRef]
- Broyd, S.J.; Demanuele, C.; Debener, S.; Helps, S.K.; James, C.J.; Sonuga-Barke, E.J.S. Default-Mode Brain Dysfunction in Mental Disorders: A Systematic Review. Neurosci. Biobehav. Rev. 2009, 33, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Gusnard, D.A.; Raichle, M.E. Searching for a Baseline: Functional Imaging and the Resting Human Brain. Nat. Rev. Neurosci. 2001, 2, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, E.; Pinel, P.; Dehaene, S.; Kleinschmidt, A. The Enigma of Gerstmann’s Syndrome Revisited: A Telling Tale of the Vicissitudes of Neuropsychology. Brain 2010, 133, 320–332. [Google Scholar] [CrossRef]
- Rusconi, E.; Walsh, V.; Butterworth, B. Dexterity with Numbers: rTMS over Left Angular Gyrus Disrupts Finger Gnosis and Number Processing. Neuropsychologia 2005, 43, 1609–1624. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Del Percio, C.; Petrini, L.; Lopez, S.; Arendt-Nielsen, L.; Babiloni, C. Cortical Sources of Electroencephalographic Alpha Rhythms Related to the Anticipation and Experience of Mirror Visual Feedback-Induced Illusion of Finger Movements. Psychophysiology 2023, 60, e14281. [Google Scholar] [CrossRef]
- Carino-Escobar, R.; Carrillo-Mora, P.; Valdes, R.; Rodríguez Barragán, M.; Hernandez-Arenas, C.; Quinzaños-Fresnedo, J.; Galicia Alvarado, M.; Cantillo-Negrete, J. Longitudinal Analysis of Stroke Patients’ Brain Rhythms during an Intervention with a Brain-Computer Interface. Neural Plast. 2019, 2019, 7084618. [Google Scholar] [CrossRef]
- Gandolfi, M.; Formaggio, E.; Geroin, C.; Storti, S.F.; Boscolo Galazzo, I.; Waldner, A.; Manganotti, P.; Smania, N. Electroencephalographic Changes of Brain Oscillatory Activity after Upper Limb Somatic Sensation Training in a Patient with Somatosensory Deficit after Stroke. Clin. EEG Neurosci. 2015, 46, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Jochumsen, M.; Rovsing, C.; Rovsing, H.; Cremoux, S.; Signal, N.; Allen, K.; Taylor, D.; Niazi, I.K. Quantification of Movement-Related EEG Correlates Associated with Motor Training: A Study on Movement-Related Cortical Potentials and Sensorimotor Rhythms. Front. Hum. Neurosci. 2017, 11, 604. [Google Scholar] [CrossRef]
- Carson, R.G. Neural Pathways Mediating Bilateral Interactions between the Upper Limbs. Brain Res. Brain Res. Rev. 2005, 49, 641–662. [Google Scholar] [CrossRef]
- Yavuzer, G.; Selles, R.; Sezer, N.; Sütbeyaz, S.; Bussmann, J.B.; Köseoğlu, F.; Atay, M.B.; Stam, H.J. Mirror Therapy Improves Hand Function in Subacute Stroke: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2008, 89, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Martini, M. Real, Rubber or Virtual: The Vision of “One’s Own” Body as a Means for Pain Modulation. A Narrative Review. Conscious. Cogn. 2016, 43, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Matamala-Gomez, M.; Donegan, T.; Bottiroli, S.; Sandrini, G.; Sanchez-Vives, M.V.; Tassorelli, C. Immersive Virtual Reality and Virtual Embodiment for Pain Relief. Front. Hum. Neurosci. 2019, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, E.; Güntekin, B.; Hanoğlu, L.; Algun, C. EEG Alpha Activity Increased in Response to Transcutaneous Electrical Nervous Stimulation in Young Healthy Subjects but Not in the Healthy Elderly. PeerJ 2020, 8, e8330. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs-Graham, E.; Kurz, M.J.; Gehringer, J.E.; Wilson, T.W. The Functional Role of Post-Movement Beta Oscillations in Motor Termination. Brain Struct. Funct. 2017, 222, 3075–3086. [Google Scholar] [CrossRef] [PubMed]
- Körmendi, J.; Ferentzi, E.; Weiss, B.; Nagy, Z. Topography of Movement-Related Delta and Theta Brain Oscillations. Brain Topogr. 2021, 34, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Tomasevic, L.; Herz, D.M.; Larsen, K.M.; Siebner, H.R. Theta Activity in the Left Dorsal Premotor Cortex during Action Re-Evaluation and Motor Reprogramming. Front. Hum. Neurosci. 2018, 12, 364. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Reimer, J.; Penn, R.; Ojakangas, C.L.; Hatsopoulos, N.G. Fast and Slow Oscillations in Human Primary Motor Cortex Predict Oncoming Behaviorally Relevant Cues. Neuron 2010, 65, 461–471. [Google Scholar] [CrossRef]
- Schramm, S.; Albers, L.; Ille, S.; Schröder, A.; Meyer, B.; Sollmann, N.; Krieg, S.M. Navigated Transcranial Magnetic Stimulation of the Supplementary Motor Cortex Disrupts Fine Motor Skills in Healthy Adults. Sci. Rep. 2019, 9, 17744. [Google Scholar] [CrossRef]


| ANTICIPATION | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Conditions Comparisons | BAs | Cluster Size | Region | Hemisphere | MNI Coordinates | T Value | Wilcoxon’s r | ||
| x | y | z | |||||||
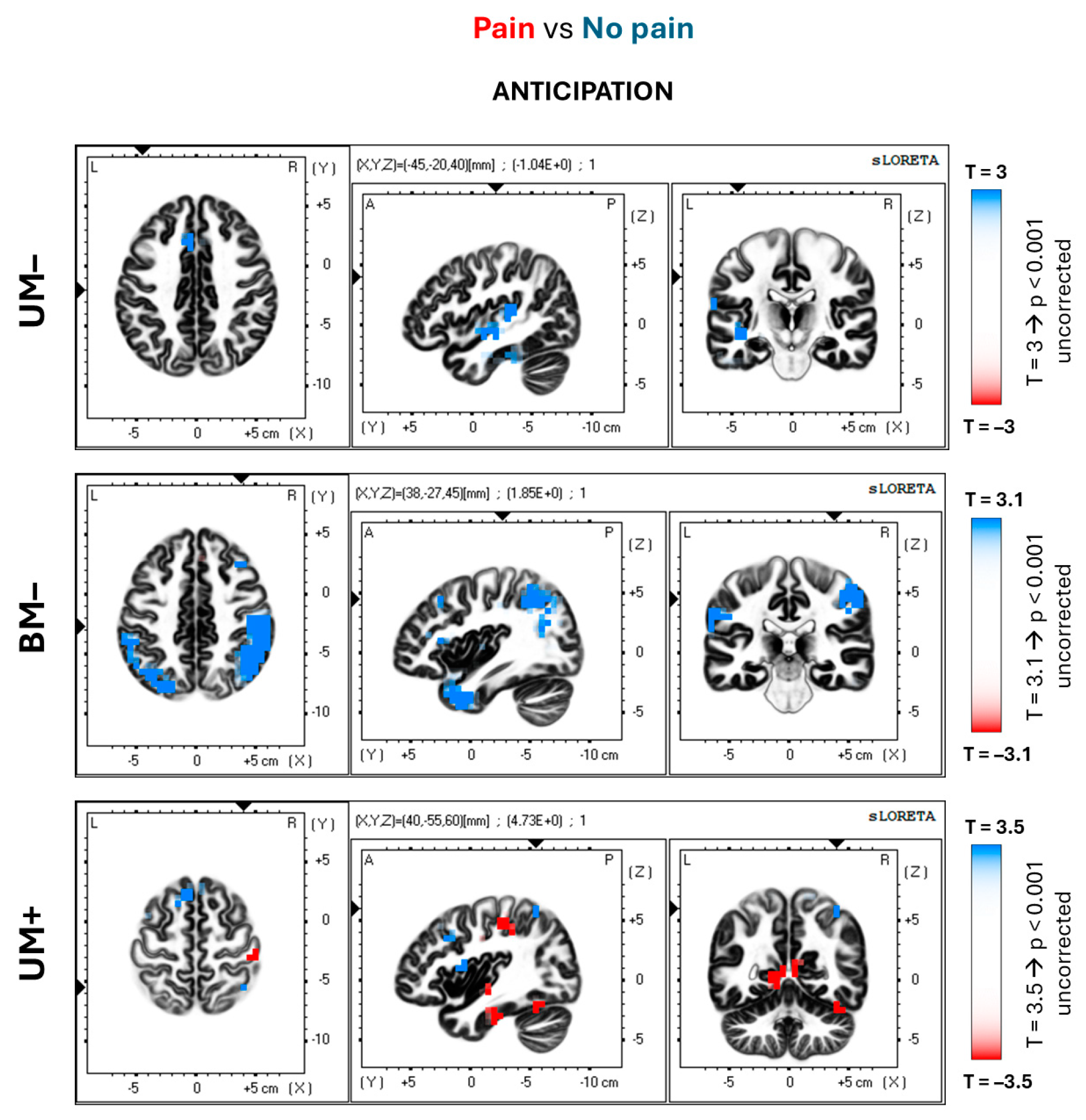
| UM– | 32 | 5 (9) | Anterior cingulate | L | −5 | 20 | 40 | 4.36 ** | −0.87 |
| (Pain vs. No pain) | 40 | 3 (8) | Inferior parietal lobule | L | −65 | −25 | 20 | 3.95 ** | −0.81 |
| BM– (Pain vs. No pain) | 1–2–3, 4 | 13 (36) | Central gyrus | L/R | 60 | −30 | 45 | 4.61 ** | −0.84 |
| 40 | 113 | Inferior parietal lobule | L/R | −65 | −40 | 35 | 4.46 ** | −0.89 | |
| 44–45 | 15 | Inferior frontal gyrus | L/R | −60 | 5 | 15 | 3.36 * | −0.81 | |
| UM+ | 30 | 2 (8) | Posterior cingulate | R | 5 | −55 | 5 | −4.16 ** | 0.94 |
| (Pain vs. No pain) | 40 | 1 (5) | Inferior parietal lobule | R | 40 | −55 | 60 | −4.73 ** | 0.93 |
| EXECUTION | |||||||||
| Conditions Comparisons | BAs | Cluster Size | Region | Hemisphere | MNI Coordinates | T Value | Wilcoxon’s r | ||
| x | y | z | |||||||
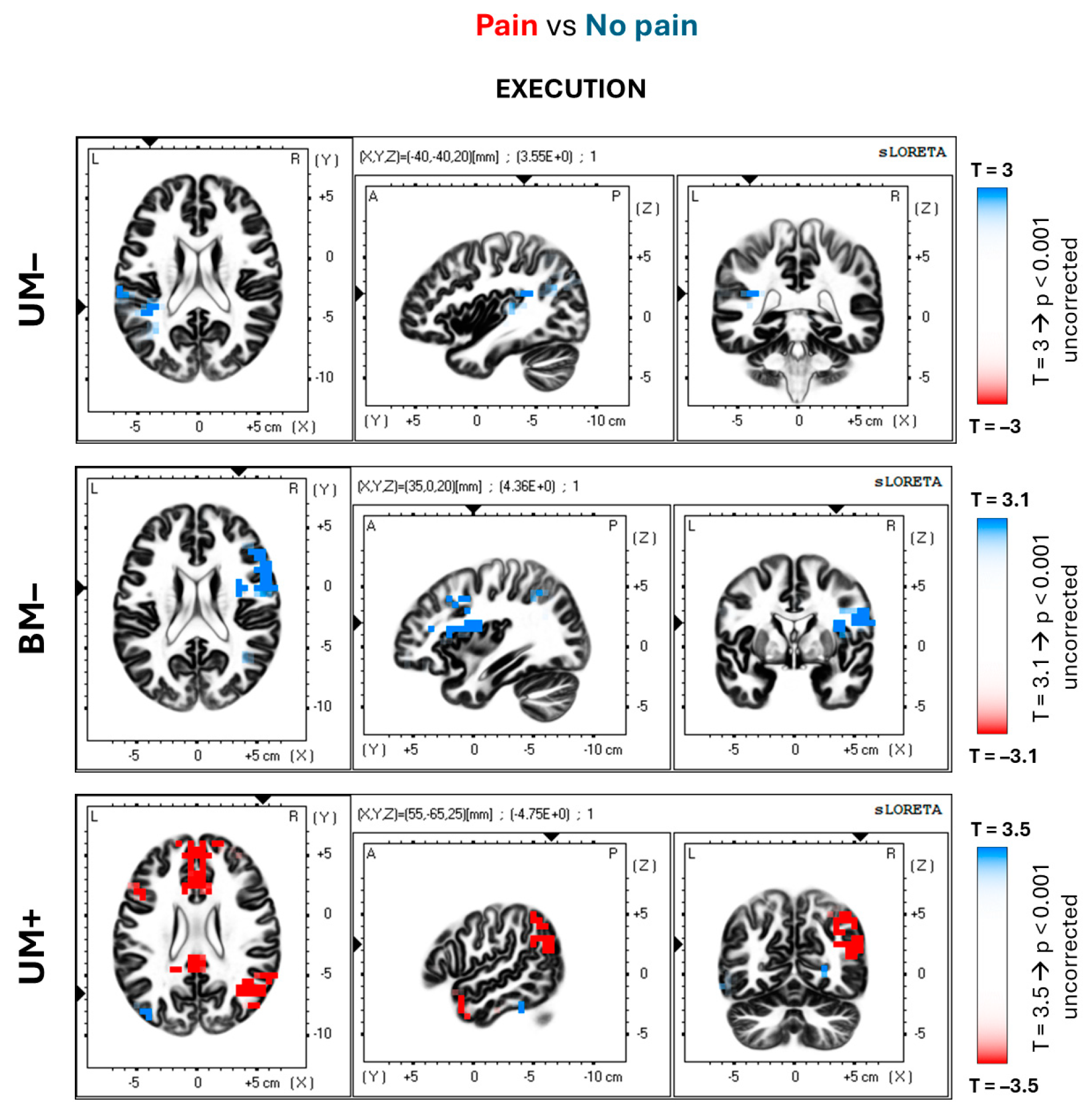
| UM– (Pain vs. No pain) | 40 | 5 | Inferior parietal lobule | L | −55 | −25 | 15 | 3.34 n.s. | −0.75 |
| BM– (Pain vs. No pain) | 9–46 | 7 (21) | Superior frontal gyrus | R | 55 | 15 | 30 | 4.11 ** | −0.85 |
| 32 | 7 | Anterior cingulate | R | 15 | 45 | −5 | 3.67 * | −0.81 | |
| 44–45 | 35 | Inferior frontal gyrus | R | 60 | 15 | 15 | 3.79 * | −0.9 | |
| UM+ (Pain vs. No pain) | 10 | 2 (19) | Medial frontal gyrus | R | 10 | 50 | 15 | −4.31 ** | 0.94 |
| 19 | 5 | Precuneus | R | 30 | −75 | 35 | −3.87 * | 0.84 | |
| 24–32 | 2 (11) | Anterior cingulate | L/R | 5 | 25 | 15 | −4.66 ** | 0.85 | |
| 39 | 2 (17) | Angular gyrus | R | 55 | −65 | 25 | −4.75 ** | 0.93 | |
| 40 | 3 (10) | Inferior parietal lobule | R | 45 | −55 | 45 | −4.67 ** | 0.86 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, M.; Petrini, L.; Del Percio, C.; Arendt-Nielsen, L.; Babiloni, C. Neurophysiological Oscillatory Mechanisms Underlying the Effect of Mirror Visual Feedback-Induced Illusion of Hand Movements on Nociception and Cortical Activation. Brain Sci. 2024, 14, 696. https://doi.org/10.3390/brainsci14070696
Rizzo M, Petrini L, Del Percio C, Arendt-Nielsen L, Babiloni C. Neurophysiological Oscillatory Mechanisms Underlying the Effect of Mirror Visual Feedback-Induced Illusion of Hand Movements on Nociception and Cortical Activation. Brain Sciences. 2024; 14(7):696. https://doi.org/10.3390/brainsci14070696
Chicago/Turabian StyleRizzo, Marco, Laura Petrini, Claudio Del Percio, Lars Arendt-Nielsen, and Claudio Babiloni. 2024. "Neurophysiological Oscillatory Mechanisms Underlying the Effect of Mirror Visual Feedback-Induced Illusion of Hand Movements on Nociception and Cortical Activation" Brain Sciences 14, no. 7: 696. https://doi.org/10.3390/brainsci14070696
APA StyleRizzo, M., Petrini, L., Del Percio, C., Arendt-Nielsen, L., & Babiloni, C. (2024). Neurophysiological Oscillatory Mechanisms Underlying the Effect of Mirror Visual Feedback-Induced Illusion of Hand Movements on Nociception and Cortical Activation. Brain Sciences, 14(7), 696. https://doi.org/10.3390/brainsci14070696







