Low-Grade Inflammation Associated with Major Depression Subtypes: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Medical and Psychiatric Assessment of Participant
2.3. Sleep Assessment and Examination
2.4. Statistical Analyses
3. Results
3.1. Polysomnography
3.2. MDD Groups (LGI, No-LGI)
3.2.1. Univariate Analyses
3.2.2. Multivariate Analyses
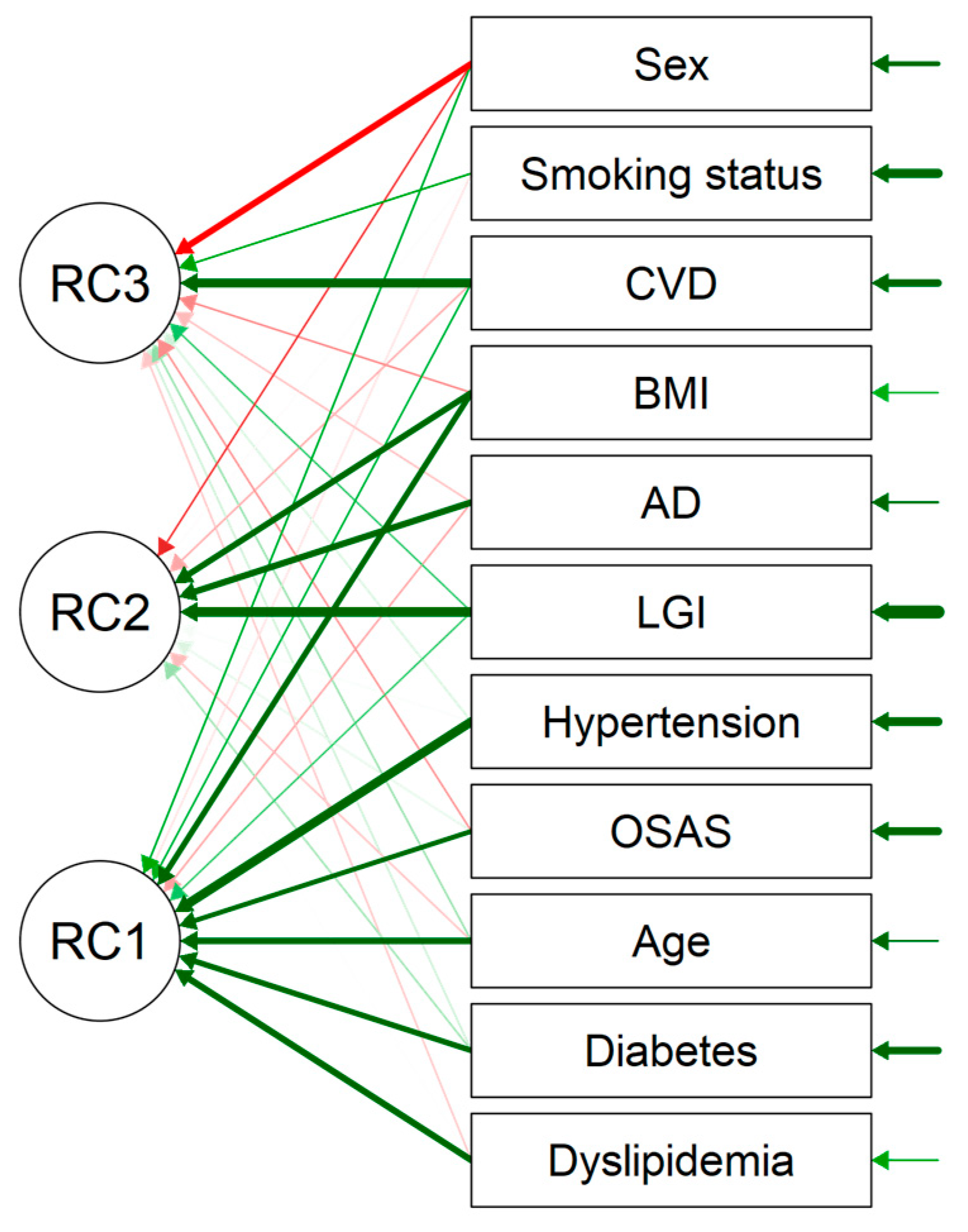
3.2.3. The PCA Analysis
3.2.4. Estimated Prevalence Calculation
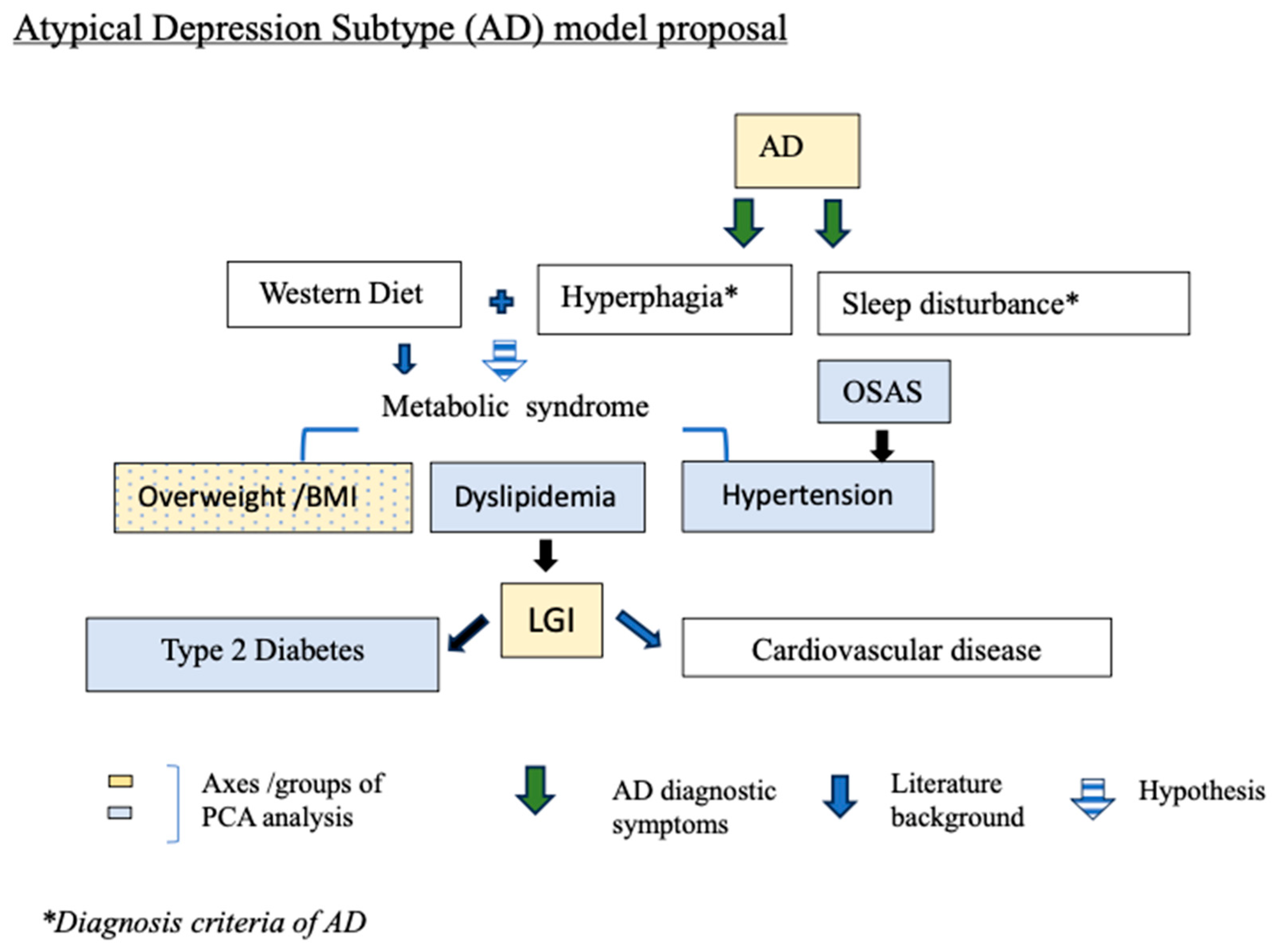
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Evans-Lacko, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Benjet, C.; Bruffaerts, R.; Chiu, W.T.; Florescu, S.; De Girolamo, G.; Gureje, O.; et al. Socio-Economic Variations in the Mental Health Treatment Gap for People with Anxiety, Mood, and Substance Use Disorders: Results from the WHO World Mental Health (WMH) Surveys. Psychol. Med. 2018, 48, 1560–1571. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; WHO: Geneva, Switzerland, 2017.
- Diagnostic and Statistical Manual of Mental Disorders. Available online: https://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 (accessed on 30 April 2024).
- Osimo, E.F.; Baxter, L.J.; Lewis, G.; Jones, P.B.; Khandaker, G.M. Prevalence of Low-Grade Inflammation in Depression: A Systematic Review and Meta-Analysis of CRP Levels. Psychol. Med. 2019, 49, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Osimo, E.F.; Cardinal, R.N.; Jones, P.B.; Khandaker, G.M. Prevalence and Correlates of Low-Grade Systemic Inflammation in Adult Psychiatric Inpatients: An Electronic Health Record-Based Study. Psychoneuroendocrinology 2018, 91, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Valkanova, V.; Ebmeier, K.P.; Allan, C.L. CRP, IL-6 and Depression: A Systematic Review and Meta-Analysis of Longitudinal Studies. J. Affect. Disord. 2013, 150, 736–744. [Google Scholar] [CrossRef]
- Hiles, S.A.; Baker, A.L.; De Malmanche, T.; Attia, J. Interleukin-6, C-Reactive Protein and Interleukin-10 after Antidepressant Treatment in People with Depression: A Meta-Analysis. Psychol. Med. 2012, 42, 2015–2026. [Google Scholar] [CrossRef]
- Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Dębowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of Antidepressant Treatment on Peripheral Inflammation Markers—A Meta-Analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Haroon, E.; Daguanno, A.W.; Woolwine, B.J.; Goldsmith, D.R.; Baer, W.M.; Wommack, E.C.; Felger, J.C.; Miller, A.H. Antidepressant Treatment Resistance Is Associated with Increased Inflammatory Markers in Patients with Major Depressive Disorder. Psychoneuroendocrinology 2018, 95, 43–49. [Google Scholar] [CrossRef]
- Miola, A.; Dal Porto, V.; Tadmor, T.; Croatto, G.; Scocco, P.; Manchia, M.; Carvalho, A.F.; Maes, M.; Vieta, E.; Sambataro, F.; et al. Increased C-reactive Protein Concentration and Suicidal Behavior in People with Psychiatric Disorders: A Systematic Review and Meta-analysis. Acta Psychiatr. Scand. 2021, 144, 537–552. [Google Scholar] [CrossRef]
- Zalli, A.; Jovanova, O.; Hoogendijk, W.J.G.; Tiemeier, H.; Carvalho, L.A. Low-Grade Inflammation Predicts Persistence of Depressive Symptoms. Psychopharmacology 2016, 233, 1669–1678. [Google Scholar] [CrossRef]
- Freeman, M.P.; Lee, H.; Savella, G.M.; Sosinsky, A.Z.; Marfurt, S.P.; Murphy, S.K.; Cohen, L.S. Predictors of Depressive Relapse in Women Undergoing Infertility Treatment. J. Women’s Health 2018, 27, 1408–1414. [Google Scholar] [CrossRef]
- Huffman, J.C.; Celano, C.M.; Beach, S.R.; Motiwala, S.R.; Januzzi, J.L. Depression and Cardiac Disease: Epidemiology, Mechanisms, and Diagnosis. Cardiovasc. Psychiatry Neurol. 2013, 2013, 695925. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Capuron, L.; Miller, A.H. Cytokines Sing the Blues: Inflammation and the Pathogenesis of Depression. Trends Immunol. 2006, 27, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, Sickness Behavior, and Depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef]
- Capuron, L. Neurobehavioral Effects of Interferon-α in Cancer Patients Phenomenology and Paroxetine Responsiveness of Symptom Dimensions. Neuropsychopharmacology 2002, 26, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. Do Cytokines Really Sing the Blues? Cerebrum 2013, 2013, 10. [Google Scholar] [PubMed]
- Uher, R.; Tansey, K.E.; Dew, T.; Maier, W.; Mors, O.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A.; et al. An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment with Escitalopram and Nortriptyline. Am. J. Psychiatry 2014, 171, 1278–1286. [Google Scholar] [CrossRef]
- Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Greer, T.; Grannemann, B.; Soyombo, A.; Mayes, T.L.; Rush, A.J.; Trivedi, M.H. Can C-Reactive Protein Inform Antidepressant Medication Selection in Depressed Outpatients? Findings from the CO-MED Trial. Psychoneuroendocrinology 2017, 78, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Han, K.-M.; Ham, B.-J. How Inflammation Affects the Brain in Depression: A Review of Functional and Structural MRI Studies. J. Clin. Neurol. 2021, 17, 503. [Google Scholar] [CrossRef]
- Thase, M.E. Recognition and Diagnosis of Atypical Depression. J. Clin. Psychiatry 2007, 68 (Suppl. S8), 11–16. [Google Scholar]
- Łojko, D.; Rybakowski, J.K. Atypical Depression: Current Perspectives. Neuropsychiatr. Dis. Treat. 2017, 13, 2447–2456. [Google Scholar] [CrossRef] [PubMed]
- Lamers, F.; Vogelzangs, N.; Merikangas, K.R.; De Jonge, P.; Beekman, A.T.F.; Penninx, B.W.J.H. Evidence for a Differential Role of HPA-Axis Function, Inflammation and Metabolic Syndrome in Melancholic versus Atypical Depression. Mol. Psychiatry 2013, 18, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Antonijevic, I. Depressive Disorders—Is It Time to Endorse Different Pathophysiologies? Psychoneuroendocrinology 2006, 31, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Glaus, J.; Von Känel, R.; Lasserre, A.M.; Strippoli, M.-P.F.; Vandeleur, C.L.; Castelao, E.; Gholam-Rezaee, M.; Marangoni, C.; Wagner, E.-Y.N.; Marques-Vidal, P.; et al. Mood Disorders and Circulating Levels of Inflammatory Markers in a Longitudinal Population-Based Study. Psychol. Med. 2018, 48, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, A.M.; Strippoli, M.-P.F.; Glaus, J.; Gholam-Rezaee, M.; Vandeleur, C.L.; Castelao, E.; Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; Preisig, M. Prospective Associations of Depression Subtypes with Cardio-Metabolic Risk Factors in the General Population. Mol. Psychiatry 2017, 22, 1026–1034. [Google Scholar] [CrossRef]
- Lamers, F.; Milaneschi, Y.; De Jonge, P.; Giltay, E.J.; Penninx, B.W.J.H. Metabolic and Inflammatory Markers: Associations with Individual Depressive Symptoms. Psychol. Med. 2018, 48, 1102–1110. [Google Scholar] [CrossRef]
- Baune, B.T.; Stuart, M.; Gilmour, A.; Wersching, H.; Heindel, W.; Arolt, V.; Berger, K. The Relationship between Subtypes of Depression and Cardiovascular Disease: A Systematic Review of Biological Models. Transl. Psychiatry 2012, 2, e92. [Google Scholar] [CrossRef] [PubMed]
- Hickman, R.J.; Khambaty, T.; Stewart, J.C. C-Reactive Protein Is Elevated in Atypical but Not Nonatypical Depression: Data from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. J. Behav. Med. 2014, 37, 621–629. [Google Scholar] [CrossRef]
- Karlović, D.; Serretti, A.; Vrkić, N.; Martinac, M.; Marčinko, D. Serum Concentrations of CRP, IL-6, TNF-α and Cortisol in Major Depressive Disorder with Melancholic or Atypical Features. Psychiatry Res. 2012, 198, 74–80. [Google Scholar] [CrossRef]
- Mohamed, A.E.; El-Latif, R.R.A.; Youssef, A.M.; Ibrahim, A.S. C-Reactive Protein and Clinical Subtypes of Major Depressive Disorder at Zagazig University Hospitals. Middle East. Curr. Psychiatry 2020, 27, 35. [Google Scholar] [CrossRef]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in Sleep Disturbance: A Review on a Bidirectional Relationship, Mechanisms and Treatment. J. Cell. Mol. Medi 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Ballesio, A. Inflammatory Hypotheses of Sleep Disturbance—Depression Link: Update and Research Agenda. Brain Behav. Immun.—Health 2023, 31, 100647. [Google Scholar] [CrossRef]
- Quinn, B. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Primary Care Version. Prim. Care Companion J. Clin. Psychiatry 1999, 1, 54–55. [Google Scholar] [CrossRef]
- Werneck, A.O.; Christofaro, D.G.D.; Ritti-Dias, R.M.; Cucato, G.G.; Stubbs, B.; Oyeyemi, A.L.; Conceição, R.D.O.; Santos, R.D.; Bittencourt, M.S. Does Physical Activity Influence the Association between Depressive Symptoms and Low-Grade Inflammation in Adults? A Study of 8048 Adults. Physiol. Behav. 2020, 223, 112967. [Google Scholar] [CrossRef] [PubMed]
- Kushida, C.A.; Littner, M.R.; Morgenthaler, T.; Alessi, C.A.; Bailey, D.; Coleman, J.; Friedman, L.; Hirshkowitz, M.; Kapen, S.; Kramer, M.; et al. Practice Parameters for the Indications for Polysomnography and Related Procedures: An Update for 2005. Sleep 2005, 28, 499–523. [Google Scholar] [CrossRef]
- StataCorp, Version 14. Stata 18 Base Reference Manual. Stata Press Software: College Station, TX, USA, 2023.
- IBM Corp, Version 29.0.2.0. IBM SPSS Statistics for Windows. IBM Corp: Armonk, NY, USA, 2023.
- Aronson, D.; Avizohar, O.; Levy, Y.; Bartha, P.; Jacob, G.; Markiewicz, W. Factor Analysis of Risk Variables Associated with Low-Grade Inflammation. Atherosclerosis 2008, 200, 206–212. [Google Scholar] [CrossRef]
- Paiva, C.; Beserra, B.; Reis, C.; Dorea, J.; Da Costa, T.; Amato, A. Consumption of Coffee or Caffeine and Serum Concentration of Inflammatory Markers: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Javaheri, S.; Redline, S. Insomnia and Risk of Cardiovascular Disease. Chest 2017, 152, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative Meta-Analysis of Interleukins 6 and 1β, Tumour Necrosis Factor α and C-Reactive Protein in Patients with Major Depressive Disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Kaptoge, S.; Di Angelantonio, E.; Lowe, G.; Pepys, M.B.; Thompson, S.G.; Collins, R.; Danesh, J. C-Reactive Protein Concentration and Risk of Coronary Heart Disease, Stroke, and Mortality: An Individual Participant Meta-Analysis. Lancet 2010, 375, 132–140. [Google Scholar] [CrossRef]
- Andaku, D.K.; D’Almeida, V.; Carneiro, G.; Hix, S.; Tufik, S.; Togeiro, S.M. Sleepiness, Inflammation and Oxidative Stress Markers in Middle-Aged Males with Obstructive Sleep Apnea without Metabolic Syndrome: A Cross-Sectional Study. Respir. Res. 2015, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Cortez, A.F.; Muxfeldt, E.S.; Cardoso, C.R.L.; Salles, G.F. Prognostic Value of C-Reactive Protein in Resistant Hypertension. Am. J. Hypertens. 2016, 29, 992–1000. [Google Scholar] [CrossRef]
- Jahn, C.; Gouveris, H.; Matthias, C. Systemic Inflammation in Patients with Compromised Upper Airway Anatomy and Primary Snoring or Mild Obstructive Sleep Apnea. Eur. Arch. Otorhinolaryngol. 2016, 273, 3429–3433. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Somers, V.K.; Punjabi, N.M.; Winkelman, J.W. Restless Legs Syndrome and Cardiovascular Disease: A Research Roadmap. Sleep Med. 2017, 31, 10–17. [Google Scholar] [CrossRef]
- Kronish, I.M.; Rieckmann, N.; Shimbo, D.; Burg, M.; Davidson, K.W. Aspirin Adherence, Aspirin Dosage, and C-Reactive Protein in the First 3 Months after Acute Coronary Syndrome. Am. J. Cardiol. 2010, 106, 1090–1094. [Google Scholar] [CrossRef]
- Wichniak, A.; Wierzbicka, A.; Walęcka, M.; Jernajczyk, W. Effects of Antidepressants on Sleep. Curr. Psychiatry Rep. 2017, 19, 63. [Google Scholar] [CrossRef]
- Wilson, S.; Argyropoulos, S. Antidepressants and Sleep: A Qualitative Review of the Literature. Drugs 2005, 65, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, S.; Sredni, B.; Carasso, R.L.; Kenigsbuch-Sredni, D. REM Sleep Deprivation in Rats Results in Inflammation and Interleukin-17 Elevation. J. Interferon Cytokine Res. 2009, 29, 393–398. [Google Scholar] [CrossRef]
- Chen, C.-N. Sleep, Depression and Antidepressants. Br. J. Psychiatry 1979, 135, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Sánchez-de-la-Torre, M.; White, D.P.; Azarbarzin, A. Hypoxic Burden in Obstructive Sleep Apnea: Present and Future. Arch. Bronconeumol. 2023, 59, 36–43. [Google Scholar] [CrossRef]
- Hein, M.; Wacquier, B.; Conenna, M.; Lanquart, J.-P.; Point, C. Impact of Intermittent Hypoxia Related to Obstructive Sleep Apnoea Syndrome on Low-Grade Inflammation in Hypertensive Patients: Potential Implications for Cardiovascular Risk. Life 2024, 14, 592. [Google Scholar] [CrossRef] [PubMed]
- Lyons, M.M.; Bhatt, N.Y.; Pack, A.I.; Magalang, U.J. Global Burden of Sleep-disordered Breathing and Its Implications. Respirology 2020, 25, 690–702. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. [Google Scholar] [CrossRef]
- Ljunggren, M.; Zhou, X.; Theorell-Haglöw, J.; Janson, C.; Franklin, K.A.; Emilsson, Ö.; Lindberg, E. Sleep Apnea Indices Associated with Markers of Inflammation and Cardiovascular Disease: A Proteomic Study in the MUSTACHE Cohort. Ann. ATS 2024, 21, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Hein, M.; Lanquart, J.-P.; Mungo, A.; Loas, G. Cardiovascular Risk Associated with Co-Morbid Insomnia and Sleep Apnoea (COMISA) in Type 2 Diabetics. Sleep Sci. 2022, 15, 184–194. [Google Scholar] [CrossRef] [PubMed]
- WHO Depressive Disorder. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 1 March 2024).
- Lasserre, A.M.; Strippoli, M.-P.F.; Marques-Vidal, P.; Williams, L.J.; Jacka, F.N.; Vandeleur, C.L.; Vollenweider, P.; Preisig, M. Dietary Patterns Are Differentially Associated with Atypical and Melancholic Subtypes of Depression. Nutrients 2021, 13, 768. [Google Scholar] [CrossRef]
- Giugliano, D.; Ceriello, A.; Esposito, K. The Effects of Diet on Inflammation. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Martín-Rodríguez, A.; Ramos-Campo, D.J.; Redondo-Flórez, L.; Tornero-Aguilera, J.F. The Burden of Carbohydrates in Health and Disease. Nutrients 2022, 14, 3809. [Google Scholar] [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 226. [Google Scholar] [CrossRef]
- Myles, I.A. Fast Food Fever: Reviewing the Impacts of the Western Diet on Immunity. Nutr. J. 2014, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Minihane, A.M.; Vinoy, S.; Russell, W.R.; Baka, A.; Roche, H.M.; Tuohy, K.M.; Teeling, J.L.; Blaak, E.E.; Fenech, M.; Vauzour, D.; et al. Low-Grade Inflammation, Diet Composition and Health: Current Research Evidence and Its Translation. Br. J. Nutr. 2015, 114, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Stromsnes, K.; Correas, A.G.; Lehmann, J.; Gambini, J.; Olaso-Gonzalez, G. Anti-Inflammatory Properties of Diet: Role in Healthy Aging. Biomedicines 2021, 9, 922. [Google Scholar] [CrossRef]
- Christ, A.; Lauterbach, M.; Latz, E. Western Diet and the Immune System: An Inflammatory Connection. Immunity 2019, 51, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Ostadkarampour, M.; Putnins, E.E. Monoamine Oxidase Inhibitors: A Review of Their Anti-Inflammatory Therapeutic Potential and Mechanisms of Action. Front. Pharmacol. 2021, 12, 676239. [Google Scholar] [CrossRef]
- Thase, M.E. The Role of Monoamine Oxidase Inhibitors in Depression Treatment Guidelines. J. Clin. Psychiatry 2012, 73, 10–16. [Google Scholar] [CrossRef]
- Singh, T.; Williams, K. Atypical Depression. Psychiatry 2006, 3, 33–39. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W.F. Comparison of Beck Depression Inventories-IA and-II in Psychiatric Outpatients. J. Personal. Assess. 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Morin, C.M. Insomnia: Psychological Assessment and Management; Treatment manuals for practitioners; Guilford Press: New York, NY, USA, 1993; ISBN 9780898622102. [Google Scholar]
- Iber, C.; Ancoli-Israel, S.; Chesson, A. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st ed.; American Academy of Sleep Medicine: Westchester, IL, USA, 2007. [Google Scholar]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Fleetham, J.; Ayas, N.; Bradley, D.; Ferguson, K.; Fitzpatrick, M.; George, C.; Hanly, P.; Hill, F.; Kimoff, J.; Kryger, M.; et al. Canadian Thoracic Society Guidelines: Diagnosis and Treatment of Sleep Disordered Breathing in Adults. Can. Respir. J. 2006, 13, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Ferri, R.; Koo, B.B.; Picchietti, D.L.; Fulda, S. Periodic Leg Movements during Sleep: Phenotype, Neurophysiology, and Clinical Significance. Sleep Med. 2017, 31, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Haba-Rubio, J.; Marti-Soler, H.; Tobback, N.; Andries, D.; Marques-Vidal, P.; Vollenweider, P.; Preisig, M.; Heinzer, R. Clinical Significance of Periodic Limb Movements during Sleep: The HypnoLaus Study. Sleep Med. 2018, 41, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Picchietti, D.L.; Garcia-Borreguero, D.; Ondo, W.G.; Walters, A.S.; Winkelman, J.W.; Zucconi, M.; Ferri, R.; Trenkwalder, C.; Lee, H.B. Restless Legs Syndrome/Willis–Ekbom Disease Diagnostic Criteria: Updated International Restless Legs Syndrome Study Group (IRLSSG) Consensus Criteria—History, Rationale, Description, and Significance. Sleep Med. 2014, 15, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Edinger, J.D.; Bonnet, M.H.; Bootzin, R.R.; Doghramji, K.; Dorsey, C.M.; Espie, C.A.; Jamieson, A.O.; McCall, W.V.; Morin, C.M.; Stepanski, E.J. Derivation of Research Diagnostic Criteria for Insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep 2004, 27, 1567–1596. [Google Scholar] [CrossRef]
- Hein, M.; Lanquart, J.; Loas, G.; Hubain, P.; Linkowski, P. Insomnia with Short Sleep Duration as Risk Factor for Type 2 Diabetes: A Systematic Review of the Literature. Rev. Med. Brux. 2020, 41, 98–104. [Google Scholar] [CrossRef]
| Median (P25–P75) Whole Sample (n = 765) | Median (P25–P75) Subjects without LGI (n = 538) | Median (P25–P75) Subjects with LGI (n = 227) | Wilcoxon Test p-Value | ba1 (ES) | p-Value Adjusted | |
|---|---|---|---|---|---|---|
| Sleep latency (min) | 33.0 (18.0–63.0) | 32.5 (18.0–65.5) | 34.0 (19.0–58.5) | 0.879 | 3 (3.2) | 0.341 |
| Sleep efficiency (%) | 79.2 (70.5–85.9) | 79.3 (70.5–86.3) | 79.1 (70.6–85.1) | 0.470 | 0.2 (1.1) | 0.837 |
| Sleep period time (min) | 445.0 (409.7–482.5) | 445.0 (407.0–485.0) | 445.0 (416.5–480.5) | 0.687 | −3.5 (5.3) | 0.512 |
| Total sleep time (min) | 392.7 (344.5–431.0) | 394.5 (345.0–435.3) | 386.5 (344.0–425.0) | 0.355 | −7.6 (5.9) | 0.196 |
| % Stage 1 | 7.0 (4.7–9.7) | 7.1 (4.7–9.6) | 6.8 (4.7–10.0) | 0.783 | −0.2 (0.3) | 0.492 |
| % Stage 2 | 54.1 (47.1–61.1) | 54.4 (47.1–61.1) | 53.9 (46.5–61.1) | 0.926 | −1.1 (1.0) | 0.295 |
| % Stage 3 | 6.7 (1.0–14.0) | 6.5 (1.1–13.6) | 7.8 (0.8–15.4) | 0.652 | 0.4 (1.0) | 0.714 |
| % REM | 16.7 (12.3–20.9) | 17.1 (12.8–21.4) | 15.5 (10.9–19.7) | 0.001 | −1.6 (0.6) | 0.006 |
| REM latency (min) | 88.5 (63.0–150.5) | 83.0 (61.3–136.0) | 105.0 (72.5–183.0) | <0.001 | 12.0 (5.3) | 0.024 |
| % wake after sleep onset | 10.4 (5.8–17.2) | 9.9 (5.8–16.8) | 11.3 (5.7–18.1) | 0.113 | 1.6 (0.8) | 0.055 |
| Number of awakenings | 27 (19–39) | 27 (19–39) | 27 (19–41) | 0.276 | 1.0 (1.3) | 0.448 |
| Micro-arousal index | 9 (6–14) | 9 (6–13) | 9 (6–16) | 0.159 | 1.0 (0.6) | 0.105 |
| Apnoea–hypopnoea index | 3 (1–8) | 3 (1–7) | 3 (1–11) | 0.013 | 0.2 (0.4) | 0.568 |
| Oxygen desaturation index | 1 (0–4) | 1 (0–4) | 2 (0–6) | 0.001 | 1 (0.3) | <0.001 |
| Total time under 90% of SaO2 (min) | 0.3 (0.0–11.0) | 0.0 (0.0–5.7) | 1.5 (0.0–21.0) | <0.001 | 1.5 (0.5) | 0.001 |
| PLMS index | 1 (0–7) | 2 (0–8) | 1 (0–7) | 0.019 | −0.7 (0.4) | 0.113 |
| Variables | Categories | % | Subjects without LGI | Subjects with LGI | p-Value Chi2 | OR (CI 95%) | p-Value |
|---|---|---|---|---|---|---|---|
| Gender | Female (n = 416) male (n = 349) | 54.4% 45.6% | 49.8% 50.2% | 65.2% 34.8% | <0.001 | 1 0.53 (0.38 to 0.73) | <0.001 |
| Age (years) | <40 (n = 299) ≥40 (n = 466) | 39.1% 60.9% | 41.6% 58.4% | 33.0% 67.0% | 0.026 | 1 1.45 (1.04 to 2.00) | 0.026 |
| BMI (kg/m2) | <25 (n = 302) ≥25 (n = 463) | 39.5% 60.5% | 46.8% 53.2% | 22.0% 78.0% | <0.001 | 1 3.12 (2.18 to 4.46) | <0.001 |
| Antidepressant therapy | No (n = 455) Yes (n = 310) | 59.5% 40.5% | 61.5% 38.5% | 54.6% 45.4% | 0.076 | 1 1.33 (0.97 to 1.82) | 0.076 |
| Benzodiazepine receptor agonists | No (n = 599) Yes (n = 166) | 78.3% 21.7% | 79.0% 21.0% | 76.7% 23.3% | 0.472 | 1 1.15 (0.79 to 1.66) | 0.473 |
| Smoking | No (n = 576) Yes (n = 189) | 75.3% 24.7% | 77.3% 22.7% | 70.5% 29.5% | 0.045 | 1 1.43 (1.01 to 2.02) | 0.046 |
| Alcohol | No (n = 526) Yes (n = 239) | 68.8% 31.2% | 68.2% 31.8% | 70.0% 30.0% | 0.618 | 1 0.92 (0.66 to 1.29) | 0.618 |
| Caffeine | No (n = 181) Yes (n = 584) | 23.7% 76.3% | 23.1% 76.9% | 25.1% 74.9% | 0.540 | 1 0.89 (0.62 to 1.28) | 0.540 |
| Type 2 diabetes | No (n = 681) Yes (n = 84) | 89.0% 11.0% | 91.8% 8.2% | 82.4% 17.6% | <0.001 | 1 2.40 (1.52 to 3.80) | <0.001 |
| Dyslipidemia | No (n = 434) Yes (n = 331) | 56.7% 43.3% | 60.0% 40.0% | 48.9% 51.1% | 0.005 | 1 1.57 (1.15 to 2.15) | 0.005 |
| Hypertension | No (n = 488) Yes (n = 277) | 63.8% 36.2% | 68.0% 32.0% | 53.7% 46.3% | <0.001 | 1 1.83 (1.33 to 2.52) | <0.001 |
| Cardiovascular comorbidities | No (n = 697) Yes (n = 68) | 91.1% 8.9% | 92.6% 7.4% | 87.7% 12.3% | 0.030 | 1 1.75 (1.05 to 2.92) | 0.031 |
| Aspirin therapy | No (n = 710) Yes (n = 55) | 92.8% 7.2% | 93.1% 6.9% | 92.1% 7.9% | 0.607 | 1 1.17 (0.65 to 2.10) | 0.607 |
| OSAS | No (n = 487) With TO2 90% < 10 min (n = 149) With TO2 ≥ 10 min (n = 129) | 63.7% 19.5% 16.8% | 65.4% 19.9% 14.7% | 59.5% 18.5% 22.0% | 0.046 | 1 1.02 (0.68 to 1.54) 1.65 (1.10 to 2.48) | 0.048 |
| Insomnia disorder | No (n = 186) Without short sleep duration (n = 397) With short sleep duration (n = 182) | 24.3% 51.9% 23.8% | 25.3% 51.1% 23.6% | 22.0% 53.7% 24.3% | 0.627 | 1 1.21 (0.82 to 1.78) 1.18 (0.75 to 1.85) | 0.628 |
| Sleep movement disorders | No (n = 631) Moderate to severe PLMs alone (n = 46) RLS alone or combined with PLMs (n = 88) | 82.5% 6.0% 11.5% | 82.2% 6.5% 11.3% | 83.3% 4.8% 11.9% | 0.671 | 1 0.73 (0.37 to 1.48) 1.04 (0.64 to 1.68) | 0.673 |
| EDS | No (n = 366) Yes (n = 399) | 47.8% 52.2% | 49.6% 50.4% | 43.6% 56.4% | 0.128 | 1 1.27 (0.93 to 1.74) | 0.128 |
| Depression severity | Mild to moderate (n = 549) severe (n = 216) | 71.8% 28.2% | 72.7% 27.3% | 69.6% 30.4% | 0.388 | 1 1.16 (0.83 to 1.63) | 0.389 |
| Depression subtype | OD (n = 596) AD (n = 169) | 77.9% 22.1% | 80.3% 19.7% | 72.3% 27.7% | 0.014 | 1 1.57 (1.09 to 2.24) | 0.015 |
| LGI | No (n = 538) Yes (n = 227) | 70.3% 29.7% | |||||
| Median (P25–P75) | Wilcoxon test | ||||||
| Age (years) | 43 (33–52) | 42 (33–51) | 45 (36–53) | 0.071 | |||
| BMI (kg/m2) | 26.6 (22.9–31.1) | 25.5 (22.2–29.1) | 30.5 (25.6–36.2) | <0.001 | |||
| CRP (mg/L) | 1.6 (0.8–3.5) | 1.1 (0.7–1.8) | 5.2 (3.8–7.2) | <0.001 | |||
| ESS | 11 (7–14) | 11 (7–14) | 12 (7–15) | 0.431 | |||
| ISI | 18 (15–21) | 18 (14–21) | 18 (15–21) | 0.430 | |||
| BDI | 12 (9–16) | 12 (10–16) | 13 (9–17) | 0.445 |
| Variables | Model 1 OR Adjusted (CI 95%) | p-Value | Model 2 OR Adjusted (CI 95%) | p-Value | Model 3 OR Adjusted (CI 95%) | p-Value | Model 4 OR Adjusted (CI 95%) | p-Value |
|---|---|---|---|---|---|---|---|---|
| MDD | 0.007 | 0.007 | 0.045 | 0.047 | ||||
| OD | 1 | 1 | 1 | 1 | ||||
| AD | 1.67 (1.15 to 2.41) | 1.66 (1.15 to 2.41) | 1.48 (1.01 to 2 2.18) | 1.48 (1.01 to 2.18) |
| Component Characteristics | |||
|---|---|---|---|
| Eigenvalue | Proportion Var. | Cumulative | |
| PC1 | 2.422 | 0.220 | 0.220 |
| PC2 | 1.304 | 0.119 | 0.339 |
| PC3 | 1.098 | 0.100 | 0.439 |
| Component Loadings | ||||
|---|---|---|---|---|
| PC1 | PC2 | PC3 | Uniqueness | |
| LGI | 0.235 | 0.715 | 0.236 | 0.379 |
| AD | 0.584 | 0.624 | ||
| CVD | 0.326 | 0.686 | 0.403 | |
| Dyslipidemia | 0.586 | 0.648 | ||
| Diabetes | 0.551 | 0.676 | ||
| Smoking status | 0.386 | 0.848 | ||
| Sex | −0.587 | 0.420 | ||
| BMI | 0.554 | 0.569 | 0.332 | |
| Age | 0.585 | 0.629 | ||
| OSAS | 0.518 | 0.698 | ||
| Hypertension | 0.691 | 0.519 | ||
| Non-LGI (0) LGI(1) | ||||
|---|---|---|---|---|
| AD = 1, OD = 0 | 0 | 1 | Total | |
| 0 | Observed | 436 | 160 | 596 |
| %per line | 73.2% | 26.8% | 100.0% | |
| 1 | Observed | 107 | 57 | 164 |
| %per line | 65.2% | 34.8% | 100.0% | |
| Total | Observed | 543 | 217 | |
| %per line | 71.4% | 28.6% | 100.0% | |
| Tests χ2 | ||
|---|---|---|
| Valeur | ddl | |
| χ2 | 3.42 | 1 |
| N | 765 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernier, V.; Alsaleh, G.; Point, C.; Wacquier, B.; Lanquart, J.-P.; Loas, G.; Hein, M. Low-Grade Inflammation Associated with Major Depression Subtypes: A Cross-Sectional Study. Brain Sci. 2024, 14, 850. https://doi.org/10.3390/brainsci14090850
Bernier V, Alsaleh G, Point C, Wacquier B, Lanquart J-P, Loas G, Hein M. Low-Grade Inflammation Associated with Major Depression Subtypes: A Cross-Sectional Study. Brain Sciences. 2024; 14(9):850. https://doi.org/10.3390/brainsci14090850
Chicago/Turabian StyleBernier, Veronique, Ghada Alsaleh, Camille Point, Benjamin Wacquier, Jean-Pol Lanquart, Gwenolé Loas, and Matthieu Hein. 2024. "Low-Grade Inflammation Associated with Major Depression Subtypes: A Cross-Sectional Study" Brain Sciences 14, no. 9: 850. https://doi.org/10.3390/brainsci14090850
APA StyleBernier, V., Alsaleh, G., Point, C., Wacquier, B., Lanquart, J.-P., Loas, G., & Hein, M. (2024). Low-Grade Inflammation Associated with Major Depression Subtypes: A Cross-Sectional Study. Brain Sciences, 14(9), 850. https://doi.org/10.3390/brainsci14090850






