The Neuroprotective and Anxiolytic Effects of Magnesium Sulfate on Retinal Dopaminergic Neurons in 6-OHDA-Induced Parkinsonian Rats: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Grouping
2.3. Drug Treatment In Vivo
2.3.1. 6-Hydroxydopamine (6-OHDA)
2.3.2. Magnesium Sulfate
2.4. Apomorphine (APO)-Induced Rotation Test
2.5. Open-Field Test
2.6. Immunofluorescence Staining
2.7. Estimation of Magnesium Ion Content in Rat Retinal Tissues
2.8. Western Blot Analysis of Magnesium Ion Transporter Expression in Retinal Tissue
2.9. Statistical Analysis
3. Results
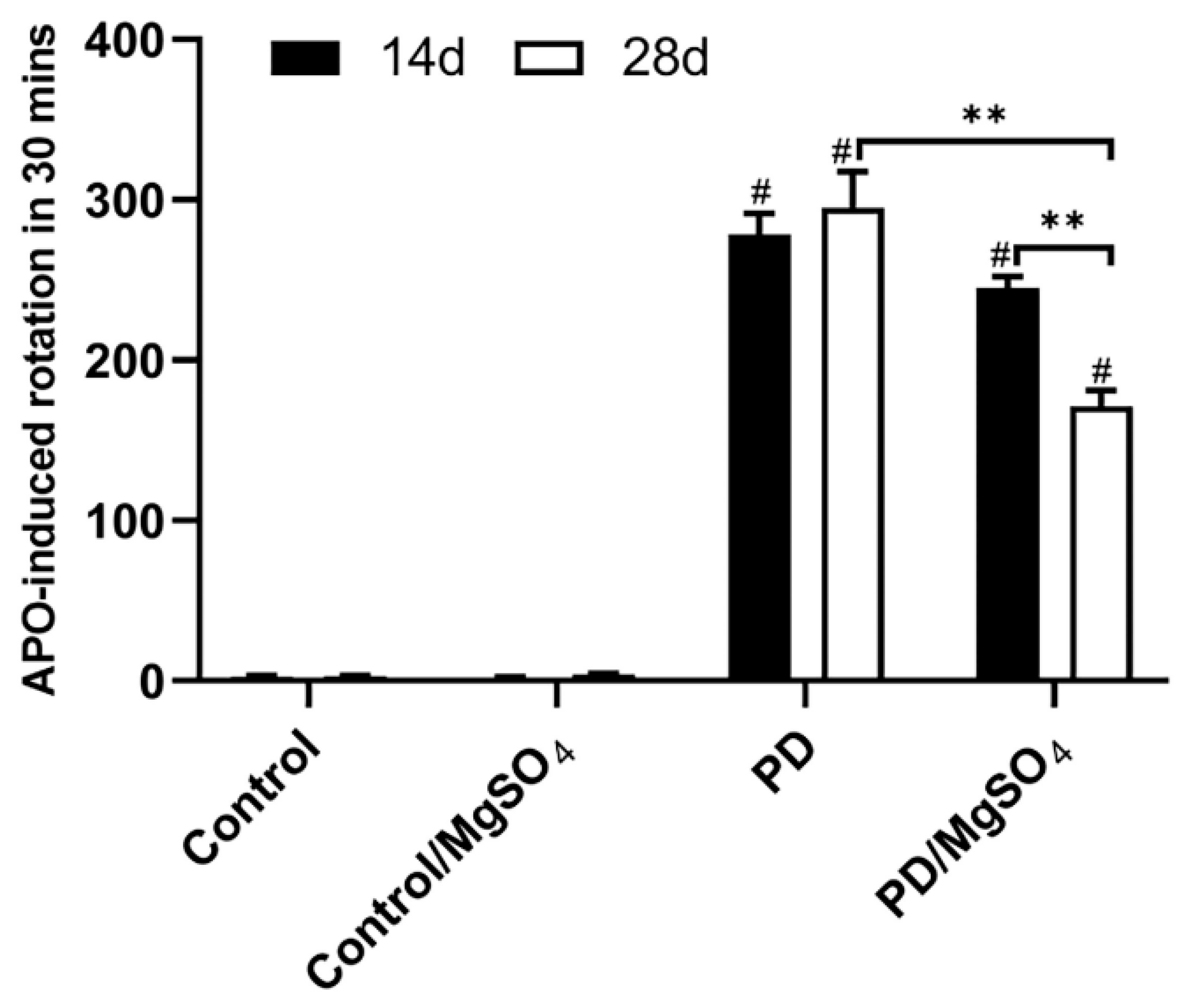
3.1. Successful Establishment of the 6-OHDA-Induced Rat Model of PD
3.2. Magnesium Sulfate Improved Anxiety State and Behavior in a 6-OHDA-Induced Rat Model of PD
3.3. Magnesium Sulfate Effectively Protected Dopaminergic Neurons in Rats’ Retina
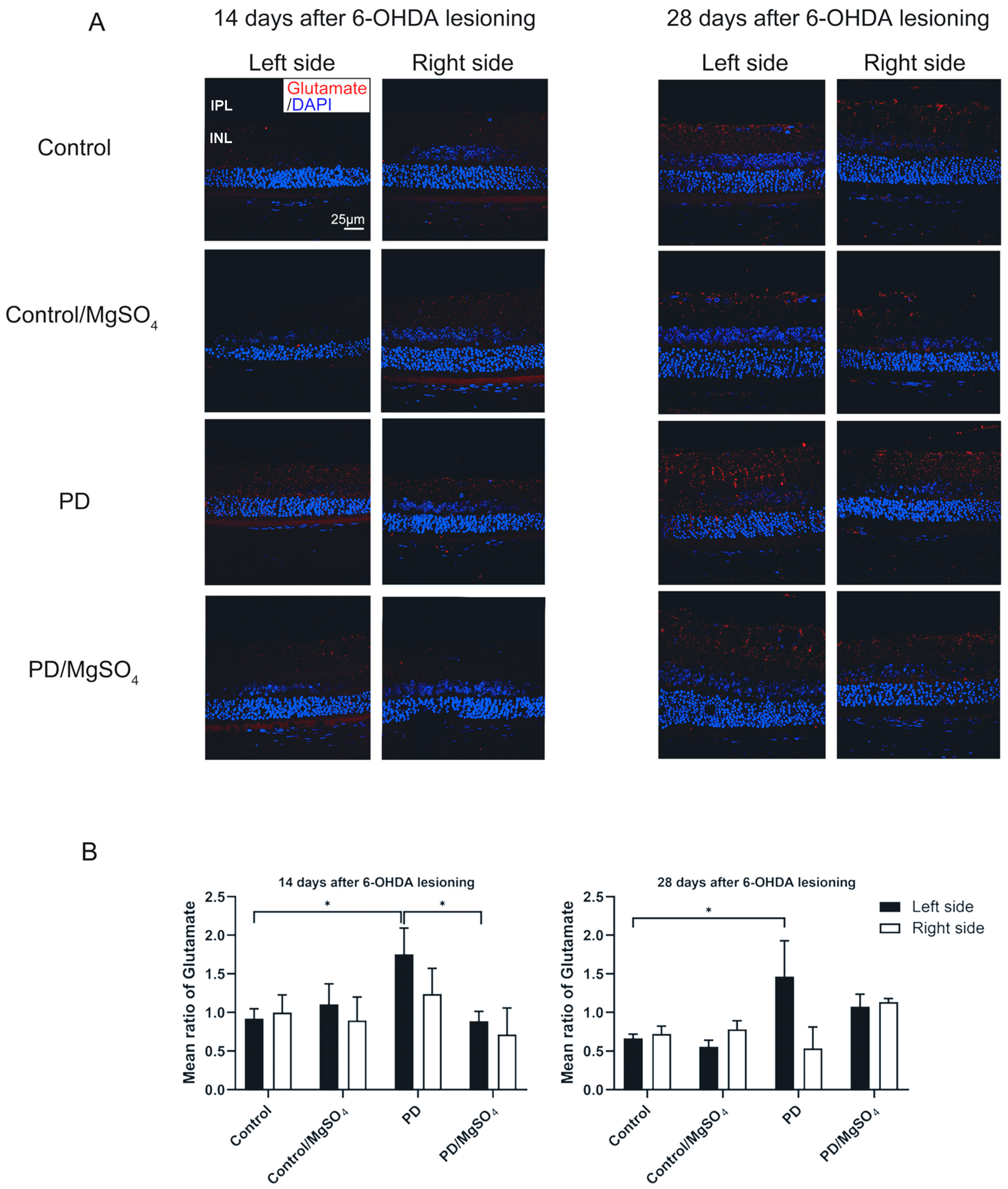
3.4. Magnesium Sulfate Reduced Retinal Glutamate Content in Rats’ Retina
3.5. Correlation between TH Fluorescence Intensity and Glutamate Fluorescence Intensity in Rat Retinal Tissue
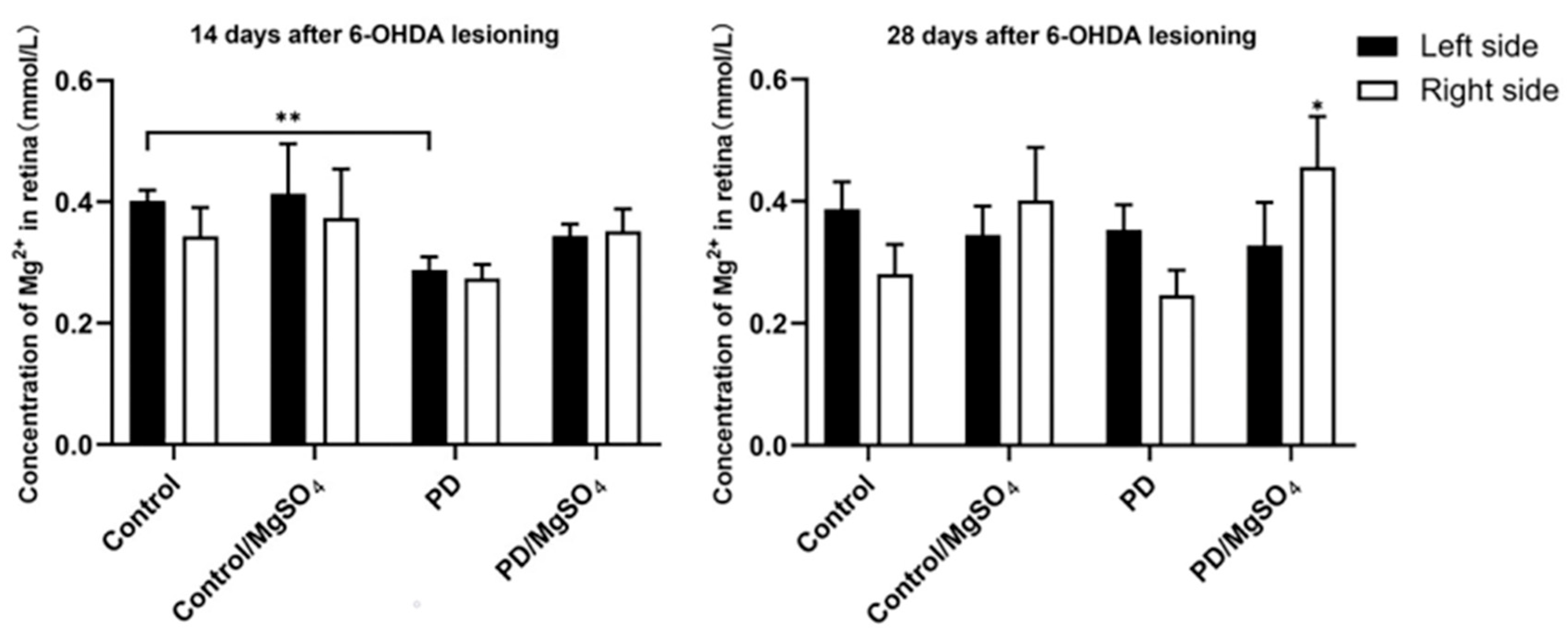
3.6. Magnesium Ion Content in the Retina
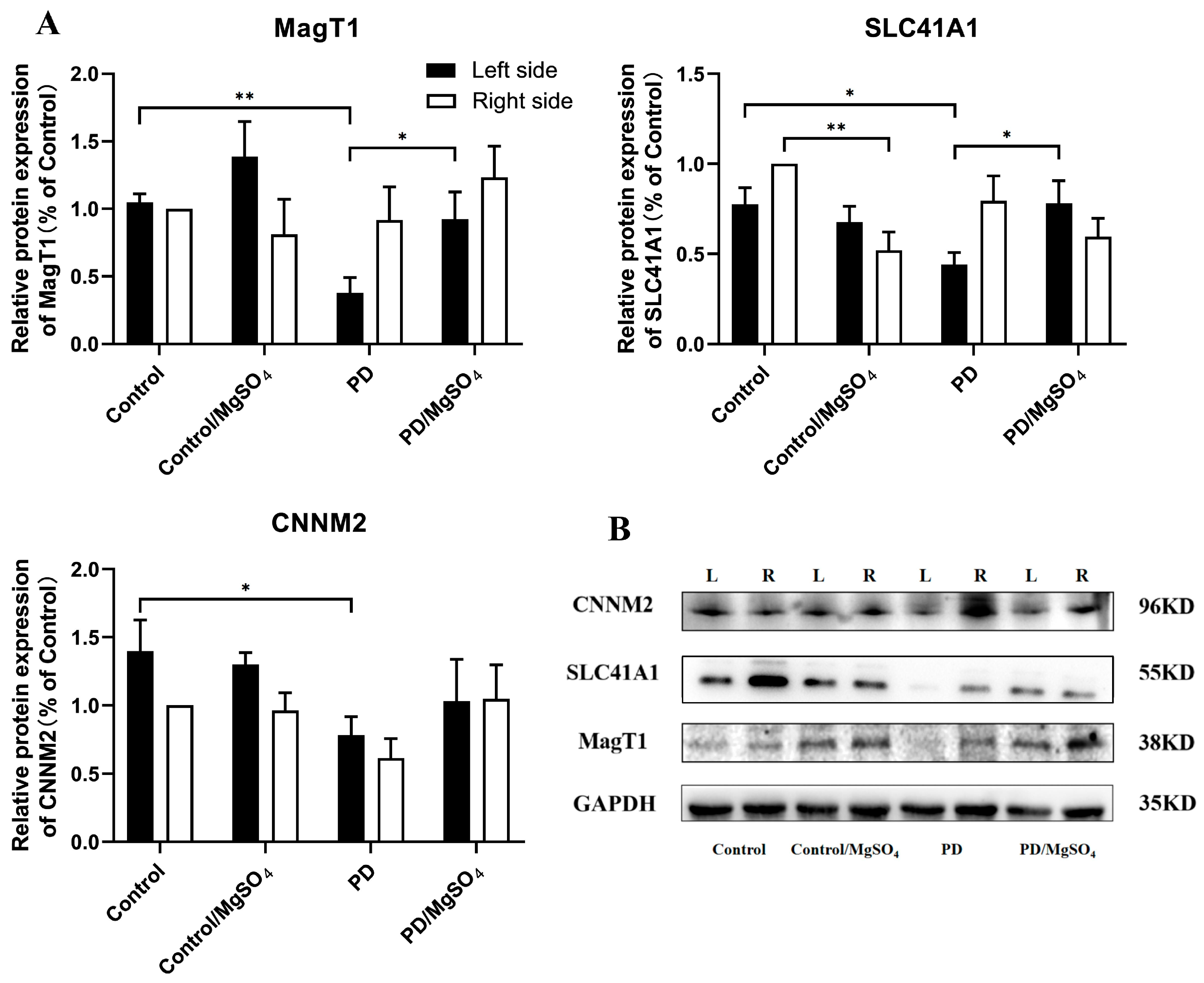
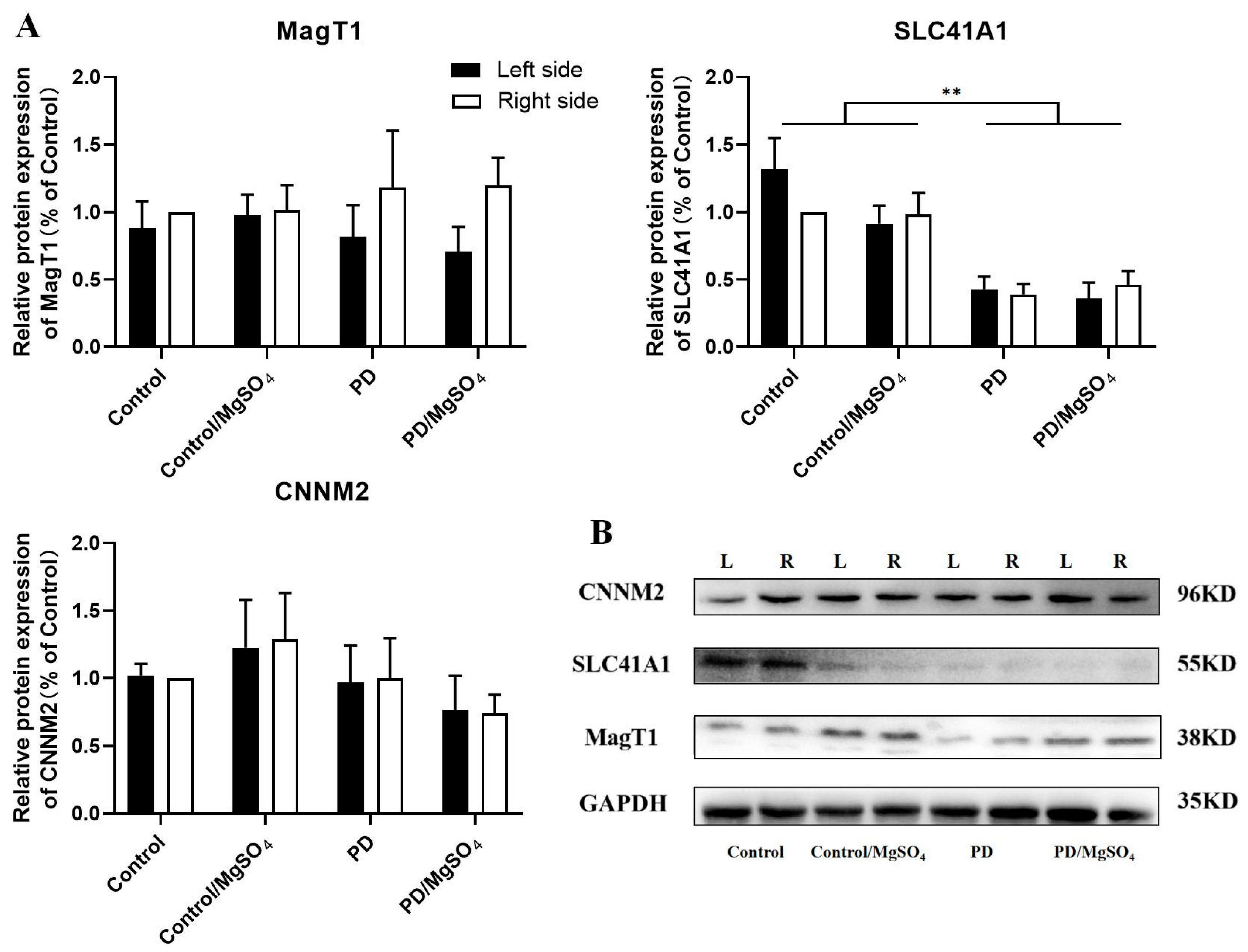
3.7. Expression Levels of Magnesium Ion Transporters Detected by Western Blots
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D.L. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. S1), 318–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tian, Y.; Si, H. Evaluation of olfactory function and related factors in patients with Parkinson’s disease. Chin. J. Neuroimmunol. Neurol. 2019, 26, 5. [Google Scholar]
- Huang, L.; Lin, L. The basic and clinical studies of retinal changes in Parkinson’s disease. Int. Rev. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Yamanaka, R.; Shindo, Y.; Oka, K. Magnesium Is a Key Player in Neuronal Maturation and Neuropathology. Int. J. Mol. Sci. 2019, 20, 3439. [Google Scholar] [CrossRef]
- Tomita, A.; Zhang, M.; Jin, F.; Zhuang, W.; Takeda, H.; Maruyama, T.; Osawa, M.; Hashimoto, K.-I.; Kawasaki, H.; Ito, K.; et al. ATP-dependent modulation of MgtE in Mg2+ homeostasis. Nat. Commun. 2017, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Alimonti, A.; Senofonte, O.; Pino, A.; Violante, N.; Petrucci, F.; Sancesario, G.; Forte, G. Metal changes in CSF and peripheral compartments of parkinsonian patients. J. Neurol. Sci. 2006, 248, 23–30. [Google Scholar] [CrossRef]
- Yasui, M.; Kihira, T.; Ota, K. Calcium, magnesium and aluminum concentrations in Parkinson’s disease. Neurotoxicology 1992, 13, 593–600. [Google Scholar]
- Shen, Y.; Dai, L.; Tian, H.; Xu, R.; Li, F.; Li, Z.; Zhou, J.; Wang, L.; Dong, J.; Sun, L. Treatment of Magnesium-L-Threonate Elevates the Magnesium Level in the Cerebrospinal Fluid and Attenuates Motor Deficits and Dopamine Neuron Loss in a Mouse Model of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2019, 15, 3143–3153. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Yamanaka, R.; Hotta, K.; Oka, K. Inhibition of Mg2+ Extrusion Attenuates Glutamate Excitotoxicity in Cultured Rat Hippocampal Neurons. Nutrients 2020, 12, 2768. [Google Scholar] [CrossRef]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, R.J.M. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Hosgorler, F.; Kizildag, S.; Ates, M.; Argon, A.; Koc, B.; Kandis, S.; Guvendi, G.; Ilgin, R.; Uysal, N. The Chronic Use of Magnesium Decreases VEGF Levels in the Uterine Tissue in Rats. Biol. Trace Elem. Res. 2020, 196, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, F.; Liu, X.; Lv, W.; Shang, H.; Wang, H.; Zhu, X.; Wu, J.; Chang, Y.; Chen, L. The bluebook for Parkinsonian Disorders in China. Chin. J. Clin. Neurosci. 2024, 32, 1–41. [Google Scholar]
- Li, Z.; Tian, T. Light Therapy Promoting Dopamine Release by Stimulating Retina in Parkinson Disease. JAMA Neurol. 2017, 74, 1267–1268. [Google Scholar] [CrossRef] [PubMed]
- Lambuk, L.; Jafri, A.J.A.; Arfuzir, N.N.N.; Iezhitsa, I.; Agarwal, R.; Rozali, K.N.B.; Agarwal, P.; Bakar, N.S.; Kutty, M.K.; Yusof, A.P.M.; et al. Neuroprotective Effect of Magnesium Acetyltaurate Against NMDA-Induced Excitotoxicity in Rat Retina. Neurotox. Res. 2017, 31, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 3rd ed.; Elsevier: San Diego, CA, USA, 2007. [Google Scholar]
- Nishijima, H.; Kimura, T.; Mori, F.; Wakabayashi, K.; Kinoshita, I.; Nakamura, T.; Kon, T.; Suzuki, C.; Tomiyama, M. Effects of Aging on Levo-Dihydroxyphenylalanine- Induced Dyskinesia in a Rat Model of Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 650350. [Google Scholar] [CrossRef]
- Lin, L.; Yan, M.; Wu, B.; Lin, R.; Zheng, Z. Expression of magnesium transporter SLC41A1 in the striatum of 6-hydroxydopamine-induced parkinsonian rats. Brain Res. Bull. 2018, 142, 338–343. [Google Scholar] [CrossRef]
- Lin, L.; Ke, Z.; Lv, M.; Lin, R.; Wu, B.; Zheng, Z. Effects of MgSO4 and magnesium transporters on 6-hydroxydopamine-induced SH-SY5Y cells. Life Sci. 2017, 172, 48–54. [Google Scholar] [CrossRef]
- Shindo, Y.; Yamanaka, R.; Suzuki, K.; Hotta, K.; Oka, K. Intracellular magnesium level determines cell viability in the MPP+ model of Parkinson’s disease. Biochim. Biophys. Acta 2015, 1853, 3182–3191. [Google Scholar] [CrossRef]
- Antipova, V.; Holzmann, C.; Hawlitschka, A.; Witt, M.; Wree, A. Antidepressant-Like Properties of Intrastriatal Botulinum Neurotoxin-A Injection in a Unilateral 6-OHDA Rat Model of Parkinson’s Disease. Toxins 2021, 13, 505. [Google Scholar] [CrossRef]
- Hajizade Ghonsulakandi, S.; Sheikh, M.; Dehghan Shasaltaneh, M.; Chopani, S.; Naghdi, N. The Association Between Effective Dose of Magnesium and Mild Compulsive Exercise on Spatial Learning, Memory, and Motor Activity of Adult Male Rats. Biol. Trace Elem. Res. 2017, 178, 235–245. [Google Scholar] [CrossRef]
- Lin, L.; Tong, Y. The role of retinal dopamine system in visual information transmission. Int. Rev. Ophthalmol. 2011, 35, 5. [Google Scholar]
- Jackson, C.R.; Capozzi, M.; Dai, H.; McMahon, D.G. Circadian perinatal photoperiod has enduring effects on retinal dopamine and visual function. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 4627–4633. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ma, J.; Yuan, L. β-Methylphenylalanine exerts neuroprotective effects in a Parkinson’s disease model by protecting against tyrosine hydroxylase depletion. J. Cell Mol. Med. 2020, 24, 9871–9880. [Google Scholar] [CrossRef]
- Sarre, S.; Yuan, H.; Jonkers, N.; Van Hemelrijck, A.; Ebinger, G.; Michotte, Y. In vivo characterization of somatodendritic dopamine release in the substantia nigra of 6-hydroxydopamine-lesioned rats. J. Neurochem. 2004, 90, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Iovino, L.; Tremblay, M.E.; Civiero, L. Glutamate-induced excitotoxicity in Parkinson’s disease: The role of glial cells. J. Pharmacol. Sci. 2020, 144, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Meng, X.; Wu, X.; Tong, H.; Zhang, X.; Qu, S. Regulation of glutamate transporter trafficking by Nedd4-2 in a Parkinson’s disease model. Cell Death Dis. 2017, 8, e2574. [Google Scholar] [CrossRef]
- Chotibut, T.; Davis, R.W.; Arnold, J.C.; Frenchek, Z.; Gurwara, S.; Bondada, V.; Geddes, J.W.; Salvatore, M.F. Ceftriaxone increases glutamate uptake and reduces striatal tyrosine hydroxylase loss in 6-OHDA Parkinson’s model. Mol. Neurobiol. 2014, 49, 1282–1292. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Davis, R.W.; Arnold, J.C.; Chotibut, T. Transient striatal GLT-1 blockade increases EAAC1 expression, glutamate reuptake, and decreases tyrosine hydroxylase phosphorylation at ser19. Exp. Neurol. 2012, 234, 428–436. [Google Scholar] [CrossRef]
- Koç, E.R.; Ilhan, A.; Zübeyde, A.; Acar, B.; Gürler, M.; Altuntaş, A.; Karapirli, M.; Bodur, A.S. A comparison of hair and serum trace elements in patients with Alzheimer disease and healthy participants. Turk. J. Med. Sci. 2015, 45, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Rajput, K.; Afridi, H.I.; Kazi, T.G.; Talpur, F.N.; Baig, J.A. Sodium, Potassium, Calcium, and Magnesium in the Scalp Hair and Blood Samples Related to the Clinical Stages of the Parkinson’s Disease. Biol. Trace Elem. Res. 2021, 199, 2582–2589. [Google Scholar] [CrossRef] [PubMed]
- Golts, N.; Snyder, H.; Frasier, M.; Theisler, C.; Choi, P.; Wolozin, B. Magnesium inhibits spontaneous and iron-induced aggregation of alpha-synuclein. J. Biol. Chem. 2002, 277, 16116–16123. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, M.; Wu, P.; Cornell, R. SLC41A1 and TRPM7 in magnesium homeostasis and genetic risk for Parkinson’s disease. J. Neurol. Neuromed. 2016, 1, 23–28. [Google Scholar]
- Miyake, Y.; Tanaka, K.; Fukushima, W.; Sasaki, S.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T.; Kawamura, N.; et al. Dietary intake of metals and risk of Parkinson’s disease: A case-control study in Japan. J. Neurol. Sci. 2011, 306, 98–102. [Google Scholar] [CrossRef]
- Meng, T. Relationships between the Retina-Midbrain-Striatum-Pineal Gland Axis and Parkinson’s Disease. Ph.D. Thesis, Fujian Medical University, Fuzhou, China, 2012. [Google Scholar]
- Zhu, Q.; Sun, S.G.; Zhang, Y.J.; Cao, X.B. Determination and significance of calcium and magnesium ions in erythrocytes of monkey model of Parkinson’s disease. J. Brain Nerv. Dis. 2005. [Google Scholar] [CrossRef]
- Forrester, J.; Peters, A. Nerve fibres in optic nerve of rat. Nature 1967, 214, 245–247. [Google Scholar] [CrossRef]
- Shindo, Y.; Yamanaka, R.; Suzuki, K.; Hotta, K.; Oka, K. Altered expression of Mg2+ transport proteins during Parkinson’s disease-like dopaminergic cell degeneration in PC12 cells. Biochim. Biophys. Acta 2016, 1863, 1979–1984. [Google Scholar] [CrossRef]
- Kolisek, M.; Sponder, G.; Mastrototaro, L.; Smorodchenko, A.; Launay, P.; Vormann, J.; Schweigel-Röntgen, M. Substitution p.A350V in Na+/Mg2+ exchanger SLC41A1, potentially associated with Parkinson’s disease, is a gain-of-function mutation. PLoS ONE 2013, 8, e71096. [Google Scholar] [CrossRef]



| Antibody | The Catalog Number |
|---|---|
| SCL41A1 | NBP1-59693 |
| MagT1 | PA5-44929 |
| CNNM2 | PA5-43015 |
| GAPDH | AC002 |
| TH | MA1-24654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Lin, R.; Zhang, C.; Zheng, S.; Wang, Y.; Wu, Z.; Chen, S.; Shen, Y.; Zhang, G.; Qi, Y.; et al. The Neuroprotective and Anxiolytic Effects of Magnesium Sulfate on Retinal Dopaminergic Neurons in 6-OHDA-Induced Parkinsonian Rats: A Pilot Study. Brain Sci. 2024, 14, 861. https://doi.org/10.3390/brainsci14090861
Huang L, Lin R, Zhang C, Zheng S, Wang Y, Wu Z, Chen S, Shen Y, Zhang G, Qi Y, et al. The Neuroprotective and Anxiolytic Effects of Magnesium Sulfate on Retinal Dopaminergic Neurons in 6-OHDA-Induced Parkinsonian Rats: A Pilot Study. Brain Sciences. 2024; 14(9):861. https://doi.org/10.3390/brainsci14090861
Chicago/Turabian StyleHuang, Leyi, Renxi Lin, Chunying Zhang, Shaoqing Zheng, Yiyang Wang, Zeyu Wu, Sihao Chen, Yihan Shen, Guoheng Zhang, Yuanlin Qi, and et al. 2024. "The Neuroprotective and Anxiolytic Effects of Magnesium Sulfate on Retinal Dopaminergic Neurons in 6-OHDA-Induced Parkinsonian Rats: A Pilot Study" Brain Sciences 14, no. 9: 861. https://doi.org/10.3390/brainsci14090861






