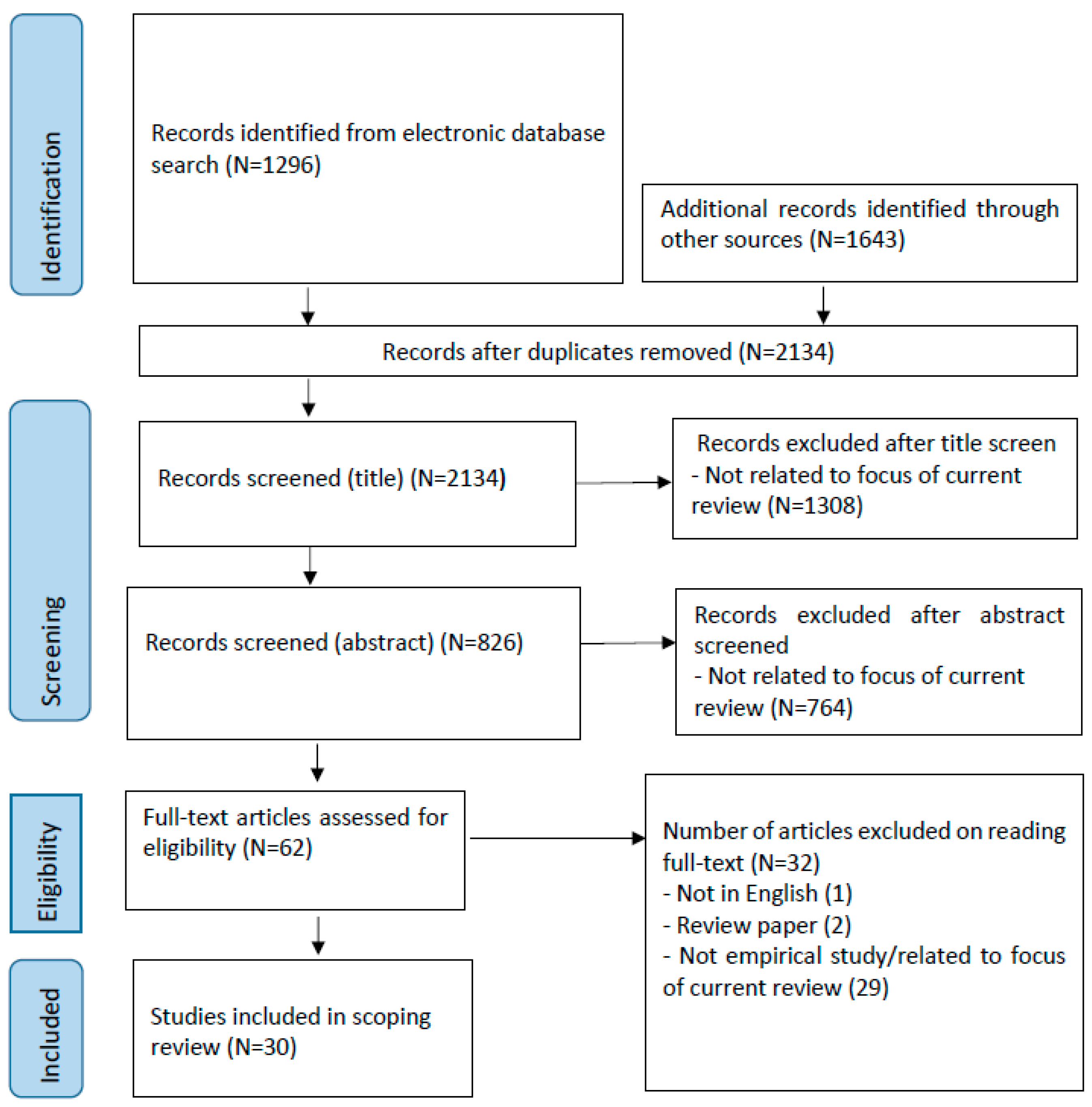
Prediction of Clinical Outcomes in Psychotic Disorders Using Artificial Intelligence Methods: A Scoping Review
Abstract
1. Introduction
2. Methodology
2.1. Search Strategies
2.2. Inclusion and Exclusion Criteria
2.3. Data Collection
2.4. Quality Assessment
2.5. Data Analysis
3. Results
3.1. General Features of Studies
3.2. Predictive Accuracy and AI Models Used for Prediction of Clinical Outcomes
3.3. Predictors of Prognosis
3.3.1. Predictors of Negative Outcomes
Demographic Data
Social Factors
Illness Course and Symptoms
Treatment
3.3.2. Predictors of Positive Outcomes
Demographics
Social Factors
Illness Course and Symptoms
Treatment
3.3.3. Biological Predictors of Clinical Outcomes Based on MRI and Genotyping Data
4. Discussion
4.1. Clinical Outcomes and Predictive Variables Examined
4.2. Predictive Accuracy of AI Methods for Clinical Outcomes
4.3. Specific Predictors of the Clinical Outcomes
4.4. Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perälä, J.; Suvisaari, J.; Saarni, S.I.; Kuoppasalmi, K.; Isometsä, E.; Pirkola, S.; Partonen, T.; Tuulio-Henriksson, A.; Hintikka, J.; Kieseppä, T. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch. Gen. Psychiatry 2007, 64, 19–28. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Casey, P.; Casey, P.R.; Kelly, B. Fish’s Clinical Psychopathology: Signs and Symptoms in Psychiatry; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- McCutcheon, R.A.; Krystal, J.H.; Howes, O.D. Dopamine and glutamate in schizophrenia: Biology, symptoms and treatment. World Psychiatry 2020, 19, 15–33. [Google Scholar] [CrossRef]
- Lewis, D.A.; Gonzalez-Burgos, G. Pathophysiologically based treatment interventions in schizophrenia. Nat. Med. 2006, 12, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- GBD Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P. Premature mortality of people with severe mental illness: A renewed focus for a new era. Ir. J. Psychol. Med. 2023, 40, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Bjarke, J.; Sinkeviciute, I.; Kroken, R.A.; Løberg, E.-M.; Jørgensen, H.A.; Johnsen, E.; Gjestad, R. Different response patterns in hallucinations and delusions to antipsychotic treatment. Nord. J. Psychiatry 2020, 74, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Kinon, B.J. The group of treatment resistant schizophrenias. Heterogeneity in treatment resistant schizophrenia (TRS). Front. Psychiatry 2019, 9, 757. [Google Scholar] [CrossRef]
- Mizuno, Y.; McCutcheon, R.A.; Brugger, S.P.; Howes, O.D. Heterogeneity and efficacy of antipsychotic treatment for schizophrenia with or without treatment resistance: A meta-analysis. Neuropsychopharmacology 2020, 45, 622–631. [Google Scholar] [CrossRef]
- Coutts, F.; Koutsouleris, N.; McGuire, P. Psychotic disorders as a framework for precision psychiatry. Nat. Rev. Neurol. 2023, 19, 221–234. [Google Scholar] [CrossRef]
- Ponce-Correa, F.; Caqueo-Urízar, A.; Berrios, R.; Escobar-Soler, C. Defining recovery in schizophrenia: A review of outcome studies. Psychiatry Res. 2023, 322, 115134. [Google Scholar] [CrossRef]
- Desalegn, D.; Girma, S.; Abdeta, T. Quality of life and its association with psychiatric symptoms and socio-demographic characteristics among people with schizophrenia: A hospital-based cross-sectional study. PLoS ONE 2020, 15, e0229514. [Google Scholar] [CrossRef]
- Hadzi Boskovic, D.; Smith-Palmer, J.; Pöhlmann, J.; Pollock, R.F.; Hwang, S.; Bruhn, D. Systematic Literature Review of Studies Reporting Measures of Functional Outcome or Quality of Life in People with Negative Symptoms of Schizophrenia. Patient Relat. Outcome Meas. 2024, 15, 199–217. [Google Scholar] [CrossRef]
- Brims, F.J.; Meniawy, T.M.; Duffus, I.; de Fonseka, D.; Segal, A.; Creaney, J.; Maskell, N.; Lake, R.A.; de Klerk, N.; Nowak, A.K. A novel clinical prediction model for prognosis in malignant pleural mesothelioma using decision tree analysis. J. Thorac. Oncol. 2016, 11, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Schoene, A.M.; Ji, S.; Ananiadou, S. Natural language processing applied to mental illness detection: A narrative review. NPJ Digit. Med. 2022, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Abd-Alrazaq, A.; Alhuwail, D.; Schneider, J.; Toro, C.T.; Ahmed, A.; Alzubaidi, M.; Alajlani, M.; Househ, M. The performance of artificial intelligence-driven technologies in diagnosing mental disorders: An umbrella review. NPJ Digit. Med. 2022, 5, 87. [Google Scholar] [CrossRef]
- Hauser, T.U.; Skvortsova, V.; De Choudhury, M.; Koutsouleris, N. The promise of a model-based psychiatry: Building computational models of mental ill health. Lancet Digit. Health 2022, 4, e816–e828. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J. From here to human-level AI. Artif. Intell. 2007, 171, 1174–1182. [Google Scholar] [CrossRef]
- Samoili, S.; Cobo, M.L.; Gómez, E.; De Prato, G.; Martínez-Plumed, F.; Delipetrev, B. AI Watch. Defining Artificial Intelligence. In Towards an Operational Definition and Taxonomy of Artificial Intelligence; Joint Research Centre: Brussels, Belgium, 2020. [Google Scholar]
- McCarthy, J. What is Artificial Intelligence; Stanford University: Stanford, CA, USA, 2007; Available online: https://www-formal.stanford.edu/jmc/whatisai/ (accessed on 25 June 2024).
- Morales, E.F.; Escalante, H.J. A brief introduction to supervised, unsupervised, and reinforcement learning. In Biosignal Processing and Classification Using Computational Learning and Intelligence; Elsevier: Amsterdam, The Netherlands, 2022; pp. 111–129. [Google Scholar]
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine learning and deep learning. Electron. Mark. 2021, 31, 685–695. [Google Scholar] [CrossRef]
- Tate, A.E.; McCabe, R.C.; Larsson, H.; Lundström, S.; Lichtenstein, P.; Kuja-Halkola, R. Predicting mental health problems in adolescence using machine learning techniques. PLoS ONE 2020, 15, e0230389. [Google Scholar] [CrossRef]
- Garriga, R.; Mas, J.; Abraha, S.; Nolan, J.; Harrison, O.; Tadros, G.; Matic, A. Machine learning model to predict mental health crises from electronic health records. Nat. Med. 2022, 28, 1240–1248. [Google Scholar] [CrossRef]
- Blessing, E.M.; Murty, V.P.; Zeng, B.; Wang, J.; Davachi, L.; Goff, D.C. Anterior hippocampal–cortical functional connectivity distinguishes antipsychotic naïve first-episode psychosis patients from controls and may predict response to second-generation antipsychotic treatment. Schizophr. Bull. 2020, 46, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Cho, R.Y.; Chen, D.; Xiu, M.; Wang, L.; Soares, J.C.; Zhang, X.Y. Treatment response prediction and individualized identification of first-episode drug-naive schizophrenia using brain functional connectivity. Mol. Psychiatry 2020, 25, 906–913. [Google Scholar] [CrossRef]
- Goodwin, M.S.; Mazefsky, C.A.; Ioannidis, S.; Erdogmus, D.; Siegel, M. Predicting aggression to others in youth with autism using a wearable biosensor. Autism Res. 2019, 12, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Ambrosen, K.S.; Skjerbæk, M.W.; Foldager, J.; Axelsen, M.C.; Bak, N.; Arvastson, L.; Christensen, S.R.; Johansen, L.B.; Raghava, J.M.; Oranje, B. A machine-learning framework for robust and reliable prediction of short-and long-term treatment response in initially antipsychotic-naïve schizophrenia patients based on multimodal neuropsychiatric data. Transl. Psychiatry 2020, 10, 276. [Google Scholar] [CrossRef]
- Cui, L.B.; Zhang, Y.J.; Lu, H.L.; Liu, L.; Zhang, H.J.; Fu, Y.F.; Wu, X.S.; Xu, Y.Q.; Li, X.S.; Qiao, Y.T. Thalamus radiomics-based disease identification and prediction of early treatment response for schizophrenia. Front. Neurosci. 2021, 15, 682777. [Google Scholar] [CrossRef] [PubMed]
- Koutsouleris, N.; Kahn, R.S.; Chekroud, A.M.; Leucht, S.; Falkai, P.; Wobrock, T.; Derks, E.M.; Fleischhacker, W.W.; Hasan, A. Multisite prediction of 4-week and 52-week treatment outcomes in patients with first-episode psychosis: A machine learning approach. Lancet Psychiatry 2016, 3, 935–946. [Google Scholar] [CrossRef]
- Leighton, S.P.; Upthegrove, R.; Krishnadas, R.; Benros, M.E.; Broome, M.R.; Gkoutos, G.V.; Liddle, P.F.; Singh, S.P.; Everard, L.; Jones, P.B. Development and validation of multivariable prediction models of remission, recovery, and quality of life outcomes in people with first episode psychosis: A machine learning approach. Lancet Digit. Health 2019, 1, e261–e270. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Lin, C.-H.; Lane, H.-Y. Prediction of functional outcomes of schizophrenia with genetic biomarkers using a bagging ensemble machine learning method with feature selection. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hui, C.L.; Honer, W.G.; Lee, E.H.; Chang, W.; Chan, S.K.; Chen, E.S.; Pang, E.P.; Lui, S.S.; Chung, D.W.; Yeung, W. Predicting first-episode psychosis patients who will never relapse over 10 years. Psychol. Med. 2019, 49, 2206–2214. [Google Scholar] [CrossRef]
- Li, L.; Rami, F.Z.; Lee, B.M.; Kim, W.-S.; Kang, C.Y.; Kim, S.-W.; Lee, B.J.; Kim, J.J.; Yu, J.-C.; Lee, K.Y. Predictors of full recovery in patients with early stage schizophrenia spectrum disorders. Psychiatry Res. 2023, 320, 115035. [Google Scholar] [CrossRef]
- Bozzatello, P.; Bellino, S.; Rocca, P. Predictive factors of treatment resistance in first episode of psychosis: A systematic review. Front. Psychiatry 2019, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Carbon, M.; Correll, C.U. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin. Neurosci. 2014, 16, 205–524. [Google Scholar] [CrossRef] [PubMed]
- Arrasate, M.; González-Ortega, I.; García-Alocén, A.; Alberich, S.; Zorrilla, I.; González-Pinto, A. Prognostic value of affective symptoms in first-admission psychotic patients. Int. J. Mol. Sci. 2016, 17, 1039. [Google Scholar] [CrossRef]
- Magrangeas, T.T.; Kolliakou, A.; Sanyal, J.; Patel, R.; Stewart, R. Investigating the relationship between thought interference, somatic passivity and outcomes in patients with psychosis: A natural language processing approach using a clinical records search platform in south London. BMJ Open 2022, 12, e057433. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. Critical Appraisal Tools. 2020. Available online: https://jbi.global/critical-appraisal-tools (accessed on 1 February 2024).
- Kottaram, A.; Johnston, L.A.; Tian, Y.; Ganella, E.P.; Laskaris, L.; Cocchi, L.; McGorry, P.; Pantelis, C.; Kotagiri, R.; Cropley, V. Predicting individual improvement in schizophrenia symptom severity at 1-year follow-up: Comparison of connectomic, structural, and clinical predictors. Hum. Brain Mapp. 2020, 41, 3342–3357. [Google Scholar] [CrossRef]
- Soldatos, R.F.; Cearns, M.; Nielsen, M.Ø.; Kollias, C.; Xenaki, L.-A.; Stefanatou, P.; Ralli, I.; Dimitrakopoulos, S.; Hatzimanolis, A.; Kosteletos, I. Prediction of early symptom remission in two independent samples of first-episode psychosis patients using machine learning. Schizophr. Bull. 2022, 48, 122–133. [Google Scholar] [CrossRef]
- Ebdrup, B.H.; Axelsen, M.C.; Bak, N.; Fagerlund, B.; Oranje, B.; Raghava, J.M.; Nielsen, M.Ø.; Rostrup, E.; Hansen, L.K.; Glenthøj, B.Y. Accuracy of diagnostic classification algorithms using cognitive-, electrophysiological-, and neuroanatomical data in antipsychotic-naïve schizophrenia patients. Psychol. Med. 2019, 49, 2754–2763. [Google Scholar] [CrossRef]
- Fond, G.; Bulzacka, E.; Boucekine, M.; Schürhoff, F.; Berna, F.; Godin, O.; Aouizerate, B.; Capdevielle, D.; Chereau, I.; d’Amato, T. Machine learning for predicting psychotic relapse at 2 years in schizophrenia in the national FACE-SZ cohort. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 8–18. [Google Scholar] [CrossRef]
- van Hooijdonk, C.F.; van der Pluijm, M.; de Vries, B.M.; Cysouw, M.; Alizadeh, B.Z.; Simons, C.J.; van Amelsvoort, T.A.; Booij, J.; Selten, J.-P.; de Haan, L. The association between clinical, sociodemographic, familial, and environmental factors and treatment resistance in schizophrenia: A machine-learning-based approach. Schizophr. Res. 2023, 262, 132–141. [Google Scholar] [CrossRef]
- Modai, I.; Saban, N.I.; Stoler, M.; Valevski, A.; Saban, N. Sensitivity profile of 41 psychiatric parameters determined by neural network in relation to 8-week outcome. Comput. Hum. Behav. 1995, 11, 181–190. [Google Scholar] [CrossRef]
- Homan, P.; Argyelan, M.; DeRosse, P.; Szeszko, P.R.; Gallego, J.A.; Hanna, L.; Robinson, D.G.; Kane, J.M.; Lencz, T.; Malhotra, A.K. Structural similarity networks predict clinical outcome in early-phase psychosis. Neuropsychopharmacology 2019, 44, 915–922. [Google Scholar] [CrossRef]
- Sarpal, D.K.; Argyelan, M.; Robinson, D.G.; Szeszko, P.R.; Karlsgodt, K.H.; John, M.; Weissman, N.; Gallego, J.A.; Kane, J.M.; Lencz, T. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am. J. Psychiatry 2016, 173, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Smucny, J.; Davidson, I.; Carter, C.S. Comparing machine and deep learning-based algorithms for prediction of clinical improvement in psychosis with functional magnetic resonance imaging. Hum. Brain Mapp. 2021, 42, 1197–1205. [Google Scholar] [CrossRef]
- Lamichhane, B.; Zhou, J.; Sano, A. Psychotic Relapse Prediction in Schizophrenia Patients Using a Personalized Mobile Sensing-Based Supervised Deep Learning Model. IEEE J. Biomed. Health Inform. 2023, 27, 3246–3257. [Google Scholar] [CrossRef]
- Leighton, S.P.; Krishnadas, R.; Chung, K.; Blair, A.; Brown, S.; Clark, S.; Sowerbutts, K.; Schwannauer, M.; Cavanagh, J.; Gumley, A.I. Predicting one-year outcome in first episode psychosis using machine learning. PLoS ONE 2019, 14, e0212846. [Google Scholar] [CrossRef]
- Mourao-Miranda, J.; Reinders, A.; Rocha-Rego, V.; Lappin, J.; Rondina, J.; Morgan, C.; Morgan, K.D.; Fearon, P.; Jones, P.B.; Doody, G.A. Individualized prediction of illness course at the first psychotic episode: A support vector machine MRI study. Psychol. Med. 2012, 42, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.B.; Fu, Y.F.; Liu, L.; Wu, X.S.; Xi, Y.B.; Wang, H.N.; Qin, W.; Yin, H. Baseline structural and functional magnetic resonance imaging predicts early treatment response in schizophrenia with radiomics strategy. Eur. J. Neurosci. 2021, 53, 1961–1975. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Zhang, Y.; Wen, H.; Huang, J.; Shen, Y.; Li, H. A random forest model for predicting social functional improvement in Chinese patients with schizophrenia after 3 months of atypical antipsychotic monopharmacy: A cohort study. Neuropsychiatr. Dis. Treat. 2021, 17, 847–857. [Google Scholar] [CrossRef]
- Liu, W.; Fang, P.; Guo, F.; Qiao, Y.; Zhu, Y.; Wang, H. Graph-Theory-Based Degree Centrality Combined with Machine Learning Algorithms Can Predict Response to Treatment with Antipsychotic Medications in Patients with First-Episode Schizophrenia. Dis. Markers 2022, 2022, 10853002. [Google Scholar] [CrossRef]
- Wang, M.; Hu, K.; Fan, L.; Yan, H.; Li, P.; Jiang, T.; Liu, B. Predicting Treatment Response in Schizophrenia With Magnetic Resonance Imaging and Polygenic Risk Score. Front. Genet. 2022, 13, 848205. [Google Scholar] [CrossRef]
- Lin, E.; Lin, C.-H.; Lane, H.-Y. Applying a bagging ensemble machine learning approach to predict functional outcome of schizophrenia with clinical symptoms and cognitive functions. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Wu, C.-S.; Luedtke, A.R.; Sadikova, E.; Tsai, H.-J.; Liao, S.-C.; Liu, C.-C.; Gau, S.S.-F.; VanderWeele, T.J.; Kessler, R.C. Development and validation of a machine learning individualized treatment rule in first-episode schizophrenia. JAMA Netw. Open 2020, 3, e1921660. [Google Scholar] [CrossRef] [PubMed]
- de Nijs, J.; Burger, T.J.; Janssen, R.J.; Kia, S.M.; van Opstal, D.P.; de Koning, M.B.; de Haan, L.; Cahn, W.; Schnack, H.G. Individualized prediction of three-and six-year outcomes of psychosis in a longitudinal multicenter study: A machine learning approach. Npj Schizophr. 2021, 7, 34. [Google Scholar] [CrossRef]
- Schie, B.v. Assessing the Role of Cytokines in Psychosis Prognosis Prediction: A Machine Learning Approach. Master’s Thesis, Utrecht University, Utrecht, The Netherlands, 2022. [Google Scholar]
- Podichetty, J.T.; Silvola, R.M.; Rodriguez-Romero, V.; Bergstrom, R.F.; Vakilynejad, M.; Bies, R.R.; Stratford, R.E., Jr. Application of machine learning to predict reduction in total PANSS score and enrich enrollment in schizophrenia clinical trials. Clin. Transl. Sci. 2021, 14, 1864–1874. [Google Scholar] [CrossRef]
- Talpalaru, A.; Bhagwat, N.; Devenyi, G.A.; Lepage, M.; Chakravarty, M.M. Identifying schizophrenia subgroups using clustering and supervised learning. Schizophr. Res. 2019, 214, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Indrayan, A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011, 48, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Allwright, S. What Is a Good Balanced Accuracy Score? Simply Explainedat Is a Good Balanced Accuracy Score? Simply Explained. 2023. Available online: https://stephenallwright.com/balanced-accuracy/#:~:text=Much%20like%20accuracy%2C%20balanced%20accuracy,Between%200.7%20and%200.9%20%2D%20Good (accessed on 17 February 2023).
- Glen, S. RMSE: Root Mean Square Error. Statistics How To 2023. Available online: https://www.statisticshowto.com/probability-and-statistics/regression-analysis/rmse-root-mean-square-error/ (accessed on 17 February 2023).
- Ellison-Wright, I.; Glahn, D.C.; Laird, A.R.; Thelen, S.M.; Bullmore, E. The anatomy of first-episode and chronic schizophrenia: An anatomical likelihood estimation meta-analysis. Am. J. Psychiatry 2008, 165, 1015–1023. [Google Scholar] [CrossRef]
- Díaz-Caneja, C.M.; Pina-Camacho, L.; Rodríguez-Quiroga, A.; Fraguas, D.; Parellada, M.; Arango, C. Predictors of outcome in early-onset psychosis: A systematic review. Npj Schizophr. 2015, 1, 1–10. [Google Scholar] [CrossRef]
- Bernardini, F.; Attademo, L.; Cleary, S.D.; Luther, C.; Shim, R.S.; Quartesan, R.; Compton, M.T. Risk prediction models in psychiatry: Toward a new frontier for the prevention of mental illnesses. J. Clin. Psychiatry 2017, 78, 18451. [Google Scholar] [CrossRef]
- Sajjadian, M.; Lam, R.W.; Milev, R.; Rotzinger, S.; Frey, B.N.; Soares, C.N.; Parikh, S.V.; Foster, J.A.; Turecki, G.; Müller, D.J. Machine learning in the prediction of depression treatment outcomes: A systematic review and meta-analysis. Psychol. Med. 2021, 51, 2742–2751. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.E.; Zantvoord, J.B.; Wezenberg, B.N.; Bockting, C.L.; van Wingen, G.A. Magnetic resonance imaging for individual prediction of treatment response in major depressive disorder: A systematic review and meta-analysis. Transl. Psychiatry 2021, 11, 168. [Google Scholar] [CrossRef]
- de Siqueira Rotenberg, L.; Borges-Júnior, R.G.; Lafer, B.; Salvini, R.; da Silva Dias, R. Exploring machine learning to predict depressive relapses of bipolar disorder patients. J. Affect. Disord. 2021, 295, 681–687. [Google Scholar] [CrossRef]
- Shao, Y.; Cheng, Y.; Gottipati, S.; Zeng-Treitler, Q. Outcome Prediction for Patients with Bipolar Disorder Using Prodromal and Onset Data. Appl. Sci. 2023, 13, 1552. [Google Scholar] [CrossRef]
- Soria, C.; Arroyo, Y.; Torres, A.M.; Redondo, M.; Basar, C.; Mateo, J. Method for Classifying Schizophrenia Patients Based on Machine Learning. J. Clin. Med. 2023, 12, 4375. [Google Scholar] [CrossRef]
- Ferrara, M.; Franchini, G.; Funaro, M.; Cutroni, M.; Valier, B.; Toffanin, T.; Palagini, L.; Zerbinati, L.; Folesani, F.; Murri, M.B.; et al. Machine Learning and Non-Affective Psychosis: Identification, Differential Diagnosis, and Treatment. Curr. Psychiatry Rep. 2022, 24, 925–936. [Google Scholar] [CrossRef]
- Del Fabro, L.; Bondi, E.; Serio, F.; Maggioni, E.; D’Agostino, A.; Brambilla, P. Machine learning methods to predict outcomes of pharmacological treatment in psychosis. Transl. Psychiatry 2023, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Usall, J.; Haro, J.; Ochoa, S.; Márquez, M.; Araya, S.; Group, N. Influence of gender on social outcome in schizophrenia. Acta Psychiatr. Scand. 2002, 106, 337–342. [Google Scholar] [CrossRef]
- Clemmensen, L.; Vernal, D.L.; Steinhausen, H.-C. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry 2012, 12, 1–16. [Google Scholar] [CrossRef]
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef]
- Li, R.; Ma, X.; Wang, G.; Yang, J.; Wang, C. Why sex differences in schizophrenia? J. Transl. Neurosci. 2016, 1, 37. [Google Scholar]
- Hatzimanolis, A.; Stefanatou, P.; Kattoulas, E.; Ralli, I.; Dimitrakopoulos, S.; Foteli, S.; Kosteletos, I.; Mantonakis, L.; Selakovic, M.; Soldatos, R.-F. Familial and socioeconomic contributions to premorbid functioning in psychosis: Impact on age at onset and treatment response. Eur. Psychiatry 2020, 63, e44. [Google Scholar] [CrossRef]
- Strauss, J.S.; Carpenter, W.T. The prediction of outcome in schizophrenia: II. Relationships between predictor and outcome variables: A report from the WHO International Pilot Study of Schizophrenia. Arch. Gen. Psychiatry 1974, 31, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.S.; Rekhi, G.; Lee, J. Associations of living arrangements with symptoms and functioning in schizophrenia. BMC Psychiatry 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Czepielewski, L.S.; Alliende, L.M.; Castañeda, C.P.; Castro, M.; Guinjoan, S.M.; Massuda, R.; Berberian, A.A.; Fonseca, A.O.; Gadelha, A.; Bressan, R. Effects of socioeconomic status in cognition of people with schizophrenia: Results from a Latin American collaboration network with 1175 subjects. Psychol. Med. 2022, 52, 2177–2188. [Google Scholar] [CrossRef]
- Sweeney, S.; Air, T.; Zannettino, L.; Galletly, C. Psychosis, socioeconomic disadvantage, and health service use in South Australia: Findings from the second Australian national survey of psychosis. Front. Public Health 2015, 3, 259. [Google Scholar] [CrossRef]
- Xu, L.; Guo, Y.; Cao, Q.; Li, X.; Mei, T.; Ma, Z.; Tang, X.; Ji, Z.; Yang, L.; Liu, J. Predictors of outcome in early onset schizophrenia: A 10-year follow-up study. BMC Psychiatry 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Ortiz, B.B.; Higuchi, C.H.; Noto, C.; Joyce, D.W.; Correll, C.U.; Bressan, R.A.; Gadelha, A. A symptom combination predicting treatment-resistant schizophrenia–A strategy for real-world clinical practice. Schizophr. Res. 2020, 218, 195–200. [Google Scholar] [CrossRef]
- Rannikko, I.; Murray, G.K.; Juola, P.; Salo, H.; Haapea, M.; Miettunen, J.; Veijola, J.; Barnett, J.H.; Husa, A.P.; Jones, P.B. Poor premorbid school performance, but not severity of illness, predicts cognitive decline in schizophrenia in midlife. Schizophr. Res. Cogn. 2015, 2, 120–126. [Google Scholar] [CrossRef]
- Hendryx, M.; Green, C.A.; Perrin, N.A. Social support, activities, and recovery from serious mental illness: STARS study findings. J. Behav. Health Serv. Res. 2009, 36, 320–329. [Google Scholar] [CrossRef]
- Alvarez-Jimenez, M.; Gleeson, J.; Henry, L.; Harrigan, S.; Harris, M.; Amminger, G.; Killackey, E.; Yung, A.; Herrman, H.; Jackson, H. Prediction of a single psychotic episode: A 7.5-year, prospective study in first-episode psychosis. Schizophr. Res. 2011, 125, 236–246. [Google Scholar] [CrossRef]
- Nyer, M.; Kasckow, J.; Fellows, I.; Lawrence, E.C.; Golshan, S.; Solorzano, E.; Zisook, S. The relationship of marital status and clinical characteristics in middle-aged and older patients with schizophrenia and depressive symptoms. Ann Clin Psychiatry 2010, 22, 172–179. [Google Scholar] [PubMed]
- Ran, M.-S.; Wong, Y.-L.I.; Yang, S.-Y.; Ho, P.S.; Mao, W.-J.; Li, J.; Chan, C.L.-W. Marriage and outcomes of people with schizophrenia in rural China: 14-year follow-up study. Schizophr. Res. 2017, 182, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.; Rosenheck, R. Socioeconomic status and the effectiveness of treatment for first-episode psychosis. Health Serv. Res. 2021, 56, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, M.; Roos, J.L. Physical exercise and the patient with schizophrenia. Aust. J. Gen. Pract. 2020, 49, 803–808. [Google Scholar] [CrossRef]
- Moges, S.; Belete, T.; Mekonen, T.; Menberu, M. Lifetime relapse and its associated factors among people with schizophrenia spectrum disorders who are on follow up at Comprehensive Specialized Hospitals in Amhara region, Ethiopia: A cross-sectional study. Int. J. Ment. Health Syst. 2021, 15, 42. [Google Scholar] [CrossRef]
- Lin, D.; Joshi, K.; Keenan, A.; Shepherd, J.; Bailey, H.; Berry, M.; Wright, J.; Meakin, S.; Benson, C.; Kim, E. Associations between relapses and psychosocial outcomes in patients with schizophrenia in real-world settings in the United States. Front. Psychiatry 2021, 12, 695672. [Google Scholar] [CrossRef]
- Emsley, R.; Chiliza, B.; Asmal, L. The evidence for illness progression after relapse in schizophrenia. Schizophr. Res. 2013, 148, 117–121. [Google Scholar] [CrossRef]
- Oomen, P.P.; Begemann, M.J.; Brand, B.A.; de Haan, L.; Veling, W.; Koops, S.; van Os, J.; Smit, F.; Bakker, P.R.; van Beveren, N. Longitudinal clinical and functional outcome in distinct cognitive subgroups of first-episode psychosis: A cluster analysis. Psychol. Med. 2023, 53, 2317–2327. [Google Scholar] [CrossRef]
- Liddle, P.F. The core deficit of classical schizophrenia: Implications for predicting the functional outcome of psychotic illness and developing effective treatments. Can. J. Psychiatry 2019, 64, 680–685. [Google Scholar] [CrossRef]
- Mäkinen, J.; Miettunen, J.; Isohanni, M.; Koponen, H. Negative symptoms in schizophrenia—A review. Nord. J. Psychiatry 2008, 62, 334–341. [Google Scholar] [CrossRef]
- Szulc, A.; Dudek, D.; Samochowiec, J.; Wojnar, M.; Heitzman, J.; Gałecki, P. Recommendations for the treatment of schizophrenia with negative symptoms. Standards of pharmacotherapy by the Polish Psychiatric Association (Polskie Towarzystwo Psychiatryczne), part 2. Psychiatr. Pol. 2019, 53, 525–540. [Google Scholar] [CrossRef]
- Lang, F.; Koesters, M.; Lang, S.; Becker, T.; Jaeger, M. Psychopathological long-term outcome of schizophrenia—A review. Acta Psychiatr. Scand. 2013, 127, 173–182. [Google Scholar] [CrossRef]
- Kotov, R.; Foti, D.; Li, K.; Bromet, E.J.; Hajcak, G.; Ruggero, C.J. Validating dimensions of psychosis symptomatology: Neural correlates and 20-year outcomes. J. Abnorm. Psychol. 2016, 125, 1103–1119. [Google Scholar] [CrossRef]
- McGinty, J.; Upthegrove, R. Depressive symptoms during first episode psychosis and functional outcome: A systematic review and meta-analysis. Schizophr. Res. 2020, 218, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Shah, N.; Johnston, M.; Stitt, L.; Thakar, M. Predictors of long-term outcome of first-episode schizophrenia: A ten-year follow-up study. Indian J. Psychiatry 2010, 52, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Godin, O.; Leboyer, M.; Schürhoff, F.; Llorca, P.-M.; Boyer, L.; Andre, M.; Andrianarisoa, M.; Aouizerate, B.; Berna, F.; Capdevielle, D. Metabolic syndrome and illness severity predict relapse at 1-year follow-up in schizophrenia: The FACE-SZ cohort. J. Clin. Psychiatry 2018, 79, 11814. [Google Scholar] [CrossRef] [PubMed]
- Foiselle, M.; Barbosa, S.; Godin, O.; Wu, C.-L.; Boukouaci, W.; Andre, M.; Aouizerate, B.; Berna, F.; Barau, C.; Capdevielle, D. Immuno-metabolic profile of patients with psychotic disorders and metabolic syndrome. Results from the FACE-SZ cohort. Brain Behav. Immun-Health 2022, 22, 100436. [Google Scholar] [CrossRef]
- Elie, D.; Poirier, M.; Chianetta, J.; Durand, M.; Grégoire, C.; Grignon, S. Cognitive effects of antipsychotic dosage and polypharmacy: A study with the BACS in patients with schizophrenia and schizoaffective disorder. J. Psychopharmacol. 2010, 24, 1037–1044. [Google Scholar] [CrossRef]
- Leucht, S.; Wahlbeck, K.; Hamann, J.; Kissling, W. New generation antipsychotics versus low-potency conventional antipsychotics: A systematic review and meta-analysis. Lancet 2003, 361, 1581–1589. [Google Scholar] [CrossRef]
- Leucht, S.; Corves, C.; Arbter, D.; Engel, R.R.; Li, C.; Davis, J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: A meta-analysis. Lancet 2009, 373, 31–41. [Google Scholar] [CrossRef]
- Kolakowska, T.; Williams, A.; Ardern, M.; Reveley, M.; Jambor, K.; Gelder, M.; Mandelbrote, B. Schizophrenia with good and poor outcome. I: Early clinical features, response to neuroleptics and signs of organic dysfunction. Br. J. Psychiatry 1985, 146, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Noguchi, H.; Hashimoto, R.; Nakabayashi, T.; Omori, M.; Takahashi, S.; Tsukue, R.; Anami, K.; Hirabayashi, N.; Harada, S. Antipsychotic medication and cognitive function in schizophrenia. Schizophr. Res. 2006, 86, 138–146. [Google Scholar] [CrossRef]
- Siskind, D.; McCartney, L.; Goldschlager, R.; Kisely, S. Clozapine v. first-and second-generation antipsychotics in treatment-refractory schizophrenia: Systematic review and meta-analysis. Br. J. Psychiatry 2016, 209, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Grover, S.; Chakrabarti, S. Effectiveness of clozapine on quality of life and functioning in patients with treatment-resistant schizophrenia. Nord. J. Psychiatry 2021, 75, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Tor, P.C.; Tan, X.W.; Martin, D.; Loo, C. Comparative outcomes in electroconvulsive therapy (ECT): A naturalistic comparison between outcomes in psychosis, mania, depression, psychotic depression and catatonia. Eur. Neuropsychopharmacol. 2021, 51, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Zipursky, R.B.; Zhang-Wong, J.; Lambe, E.K.; Bean, G.; Beiser, M. MRI correlates of treatment response in first episode psychosis. Schizophr. Res. 1998, 30, 81–90. [Google Scholar] [CrossRef]
- Mitelman, S.A.; Newmark, R.E.; Torosjan, Y.; Chu, K.-W.; Brickman, A.M.; Haznedar, M.M.; Hazlett, E.A.; Tang, C.Y.; Shihabuddin, L.; Buchsbaum, M.S. White matter fractional anisotropy and outcome in schizophrenia. Schizophr. Res. 2006, 87, 138–159. [Google Scholar] [CrossRef]
- Kasparek, T.; Prikryl, R.; Schwarz, D.; Kucerova, H.; Marecek, R.; Mikl, M.; Vanicek, J.; Ceskova, E. Gray matter morphology and the level of functioning in one-year follow-up of first-episode schizophrenia patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 1438–1446. [Google Scholar] [CrossRef]
- Mané, A.; Falcon, C.; Mateos, J.J.; Fernandez-Egea, E.; Horga, G.; Lomeña, F.; Bargalló, N.; Prats-Galino, A.; Bernardo, M.; Parellada, E. Progressive gray matter changes in first episode schizophrenia: A 4-year longitudinal magnetic resonance study using VBM. Schizophr. Res. 2009, 114, 136–143. [Google Scholar] [CrossRef]
- Wassink, T.H.; Andreasen, N.C.; Nopoulos, P.; Flaum, M. Cerebellar morphology as a predictor of symptom and psychosocial outcome in schizophrenia. Biol. Psychiatry 1999, 45, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Jääskeläinen, E.; Juola, P.; Kurtti, J.; Haapea, M.; Kyllönen, M.; Miettunen, J.; Tanskanen, P.; Murray, G.; Huhtaniska, S.; Barnes, A. Associations between brain morphology and outcome in schizophrenia in a general population sample. Eur. Psychiatry 2014, 29, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Lappin, J.; Morgan, C.; Chalavi, S.; Morgan, K.; Reinders, A.; Fearon, P.; Heslin, M.; Zanelli, J.; Jones, P.; Murray, R. Bilateral hippocampal increase following first-episode psychosis is associated with good clinical, functional and cognitive outcomes. Psychol. Med. 2014, 44, 1279–1291. [Google Scholar] [CrossRef]
- Mehta, U.M.; Ibrahim, F.A.; Sharma, M.S.; Venkatasubramanian, G.; Thirthalli, J.; Bharath, R.D.; Bolo, N.R.; Gangadhar, B.N.; Keshavan, M.S. Resting-state functional connectivity predictors of treatment response in schizophrenia–A systematic review and meta-analysis. Schizophr. Res. 2021, 237, 153–165. [Google Scholar] [CrossRef]
- Doucet, G.E.; Moser, D.A.; Luber, M.J.; Leibu, E.; Frangou, S. Baseline brain structural and functional predictors of clinical outcome in the early course of schizophrenia. Mol. Psychiatry 2020, 25, 863–872. [Google Scholar] [CrossRef]
- Sarpal, D.K.; Robinson, D.G.; Lencz, T.; Argyelan, M.; Ikuta, T.; Karlsgodt, K.; Gallego, J.A.; Kane, J.M.; Szeszko, P.R.; Malhotra, A.K. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 2015, 72, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, W.; Xu, J.; Zhang, F.; Yu, H.; Luo, C.; Wang, L.; Chen, X.; Shan, B.; Xu, L. Altered resting-state regional homogeneity after 13 weeks of paliperidone injection treatment in schizophrenia patients. Psychiatry Res. Neuroimaging 2016, 258, 37–43. [Google Scholar] [CrossRef]
- Li, H.; Guo, W.; Liu, F.; Chen, J.; Su, Q.; Zhang, Z.; Fan, X.; Zhao, J. Enhanced baseline activity in the left ventromedial putamen predicts individual treatment response in drug-naive, first-episode schizophrenia: Results from two independent study samples. EBioMedicine 2019, 46, 248–255. [Google Scholar] [CrossRef]
- Sambataro, F.; Blasi, G.; Fazio, L.; Caforio, G.; Taurisano, P.; Romano, R.; Di Giorgio, A.; Gelao, B.; Lo Bianco, L.; Papazacharias, A. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology 2010, 35, 904–912. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, F.; Guo, W.; Chen, J.; Su, Q.; Zhang, Z.; Li, H.; Fan, X.; Zhao, J. Disrupted asymmetry of inter-and intra-hemispheric functional connectivity in patients with drug-naive, first-episode schizophrenia and their unaffected siblings. EBioMedicine 2018, 36, 429–435. [Google Scholar] [CrossRef]
- Kraguljac, N.V.; White, D.M.; Hadley, N.; Hadley, J.A.; Ver Hoef, L.; Davis, E.; Lahti, A.C. Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: A longitudinal resting state functional MRI study. Schizophr. Bull. 2016, 42, 1046–1055. [Google Scholar] [CrossRef] [PubMed]

| Authors/ Year | Clinical Outcomes | AUC If Available | Predictors of Clinical Outcomes |
|---|---|---|---|
| Ambrosen et al., 2020 [29] | Symptomatic improvement | Best algorithms for treatment response: logistic regression (long term), SVM with L1 regularization (short term). | |
| Blessing et al., 2019 [26] | Symptomatic improvement | 0.95 (random forest) | Anteromedial hippocampal functional connectivity with right superior frontal gyrus, right posterior insular–opercular cortex and left pre- and postcentral gyrus predicted treatment response. |
| Cao et al., 2018 [27] | Symptomatic improvement | Functional connectivity between superior temporal cortex and other cortical areas. | |
| Cui et al., 2021 [54] | Symptomatic improvement | Twelve features (three cortical features and nine functional connections) remained in the prediction model. | |
| Cui et al., 2021 [30] | Symptomatic improvement | 0.72 (SVM) | Four features from 4019 radiomics features were identified. |
| Ebdrup et al., 2019 [44] | Symptomatic improvement | No variable predicted symptom remission after six weeks. | |
| Fond et al., 2019 [45] | Psychotic relapse | High hospitalization rate, use of first-generation and higher-dose antipsychotics, metabolic syndrome, CDSS, GAF, Buss and Perry anger score, PANSS for positive and depressed subscales. | |
| Homan et al., 2019 [48] | Symptom status | Implicated notes were in the (1) prefrontal cortices; (2) posterior cingulate cortex; and (3) the precentral, superior temporal, and middle cingulate cortex. | |
| Kottaram et al., 2019 [42] | Psychotic symptoms at 1 year | 0.78–0.85 (linear R) | Predictive factors for worsening positive symptoms included hyper-dynamism and hypo-connectivity, while predictive factors for worsening negative symptoms included hypo-dynamism and hyper-connectivity. |
| Koutsouleris et al., 2016 [31] | Good versus poor outcome based on GAF | Poor outcome predictors included male gender; unemployment; poor educational status; recurrent relapses; suicidality; unmet needs in CAN including relationships, activities, psychological distress, money, information, accommodation, and sexual expression; Haloperidol treatment; lower baseline scores for PANSS item positive symptoms; conceptual disorganization; and hyperactivity. Good outcome predictors include greater GAF scores, positive MANSA scores for job, leisure, friendship, and health. | |
| Lamichhane et al., 2023 [51] | Relapse | Changes in conversation, volume, and distance travelled were predictors of relapse. | |
| Leighton et al., 2019 [52] | Employment/education status, point, period symptom remission | 0.88 (logistic R) 0.63–0.65 (logistic R) | Positive predictors were baseline functioning, white ethnicity, living with family, employment, having relationships, PANSS scores for excitement, depression, and poor rapport. Negative predictors included rented accommodation, PANSS for suspiciousness, hostility, delusions, social withdrawal, somatic concern, abstract thinking difficulty, and unusual thought content. |
| Leighton et al., 2019 [32] | Symptom status, vocational recovery, QOL | 0.70–0.74 (logistic R) | Predictors were higher education, staying in own or parents’ home, employment, no self-harm, good social support, and insight. Negative predictors were hallucinations, unusual thought content, adolescent social withdrawal, and substance use. |
| Li et al., 2021 [55] | Social functioning | 0.81 (random forest) | Positive predictors at 3 months included female gender; younger age; being unmarried; being employed; first episode; outpatient treatment; shorter relapse duration; lesser relapse; lower baseline social functioning score; fewer comorbidities; and more severe PANSS, CDSS, and CGI scores. |
| Lin et al., 2021 [58] | Social functioning, QOL | Quality of life was best predicted with the Scale for the Assessments of Negative Symptoms and 17-item Hamilton Depression Rating Scale. GAF was best predicted with a PANSS-positive item and the Scale for the Assessments of Negative Symptoms. | |
| Lin et al., 2021 [33] | Social functioning, QOL | M5 prime algorithm identified G72 rs2391191 and MET rs2237717 as quality-of-life predictors, while AKT1 rs1130233 predicted GAF. | |
| Liu et al., 2022 [56] | Symptomatic improvement | 0.93 (SVM) | Reduced degree centrality was found in subcortical gray matter structures. Post treatment, changes in degree centrality correlated with PANSS changes, with negative correlations in the right and left putamens. |
| Magrangeas et al., 2022 [39] | Negative outcomes | Predictors of negative outcomes within 2 years included younger age, black ethnicity, staying in poorer neighborhoods, and psychotic symptoms. | |
| Modai et al., 1995 [47] | Social functioning | Predictors of positive outcomes at 8 weeks included higher socioeconomic class; positive symptoms; and receiving psychotherapy, electroconvulsive therapy, Clozapine, or noradrenergic antidepressants. Other factors were older age onset, high premorbid level, axis II diagnosis, and frequent hospitalization. Predictors of negative outcomes at 8 weeks included negative symptoms, duration of last hospitalization stay, low potency antipsychotics, requiring community aid, resistant depression, and OCD. | |
| Mourao-Miranda et al., 2012 [53] | Course of illness | Anatomical regions that discriminated continuous course vs. episodic course and the control included the parahippocampal gyri, basal ganglia, cingulate, and thalami. | |
| Nijs et al., 2021 [60] services | Symptomatic improvement, social functioning | Predictors of outcomes included older age, self-harm, lack of activity, emotional withdrawal, delusions, unusual thought content, PANSS for depression, flat affect, motor retardation, lack of spontaneity, hallucinatory behaviors, suspiciousness, abstract thinking difficulty, and poor judgement and insight. | |
| Podichetty et al., 2021 [62] | Symptomatic improvement | 0.65 (random forest) | Predictors were poor attention, depression, preoccupation, volition impairment, abstract thinking difficulty, stereotyped thinking, anxiety, abnormal thought content, excitement, and observed depression. |
| Sarpal et al., 2016 [49] | Clinical Global Impression, symptomatic improvement | 0.78 (COX R) | A total of 91 connections were associated with treatment response. Greater connectivity with striatal subdivision at posterior regions and lower striatal connectivity at frontal regions were associated with better response. |
| Schie, 2022 [61] | Symptomatic improvement | 0.58–0.67 (neural network) | Non-remitted patients were more likely to be older, males, living alone, or unemployed, and to have higher weight and greater substance use. Amongst cytokines, cytokine IL-18 was a predictor. |
| Soldatos et al., 2022 [43] | Symptomatic improvement | 0.68 (SVM) | Predictive factors of non-remission at 4–6 weeks included PSP and GAF scores; PANSS scores for delusions; social avoidance; passive/apathetic social withdrawal; blunted affect; emotional withdrawal; poor rapport; delusions; lack of spontaneity; and poor flow of conversation, judgement, and insight. |
| Smucny et al., 2020 [50] | Symptomatic improvement | Activation of the dorsolateral prefrontal cortex was the most predictive factor. | |
| Talpalaru et al., 2019 [63] | Symptom status: (1) high; (2) positive; (3) mild | 0.61–0.81 (random forest) | Paracingulate gyri and the left anterior cingulate differentiated between groups: right insula, middle temporal gyri, and left temporal poles of the superior temporal affected in groups 1 and 2; left insula affected in groups 2 and 3. |
| Van Hooijdonk et al., 2023 [46] | Treatment resistance | 0.69 (random forest) | Predictors of poor treatment response in schizophrenia included poor premorbid functioning, not being married, younger age of illness onset, childhood sexual trauma, lower education level, greater use of substances, and staying in non-urban environments. |
| Wang et al., 2022 [57] | Responders versus non-responders | 0.86 (gradient boosting) | Predictors associated were grey matter volume, cortical thickness, aberrant amplitude low-frequency fluctuation, cortical thickness and volume, surface area, curvature, and sulcal depth. |
| Wu et al., 2020 [59] | Symptomatic improvements | Predictors included age; number of hospitalizations and emergency room/clinics visits; and the use of benzodiazepines, mood stabilizers, and antiepileptics. |
| Authors | Machine Learning Models | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Logistic r | Support Vector Machine | Linear r | Random Forest | KNN | Bagging Ensemble | Decision Tree | NN | Naïve Bayes | AB | Gradient Boosting | COX r | |
| Ambrosen et al., 2020 [29] | ||||||||||||
| Diagnostic classification (Balanced accuracy) | 63.8% | 50.4% | 64.2% | |||||||||
| Long-term response (Balanced accuracy) | 50.3% | 50% | 49.7% | |||||||||
| Blessing et al., 2019 [26] FC (AUC) | 0.95 | |||||||||||
| FC (Accuracy) | 89% | |||||||||||
| Cao et al., 2018 [27] CFC (Balanced accuracy) | 82.5% | |||||||||||
| MRI (Balanced accuracy) | 57.4% | |||||||||||
| Cui et al., 2021 [54] Functional MRI | 80.38% | |||||||||||
| Structural MRI | 69.68% | |||||||||||
| Functional and Structural MRI) (accuracy) | 85.03% | |||||||||||
| Cui et al., 2021 [30] | ||||||||||||
| Dataset 1 (accuracy) | 68.36% | |||||||||||
| Dataset 2 (accuracy) | 65.21% | |||||||||||
| AUC | 0.72 | |||||||||||
| Ebdrup et al., 2019 [44] | ||||||||||||
| Cognition (accuracy) | 62% | 64% | 56% | 48% | 59% | |||||||
| EEG (accuracy) | 66% | 64% | 49% | 50% | 48% | |||||||
| MRI (accuracy) | 67% | 64% | 67% | 63% | 61% | |||||||
| Diffusion tensor imaging (accuracy) | 66% | 63% | 65% | 52% | 55% | |||||||
| Clinical modality (accuracy) | 62% | 60% | 64% | 56% | 67% | |||||||
| Fond et al., 2019 [45] | ||||||||||||
| Relapse (Accuracy) | 63.8% | |||||||||||
| F/up withdrawal (Accuracy) | 52.4% | |||||||||||
| Kottaram et al., 2019 [42] | ||||||||||||
| Positive symptoms (AUC) | 0.85 | |||||||||||
| Negative symptoms (AUC) | 0.83 | |||||||||||
| BPRS (AUC) | 0.78 | |||||||||||
| Koutsouleris et al., 2016 [31] | ||||||||||||
| GAF at 4 weeks (Balanced accuracy) | 69.6–72.1% | |||||||||||
| GAF at 52 weeks (Balanced accuracy) | 67.7–71.5% | |||||||||||
| Leighton et al., 2019 [52] | ||||||||||||
| Functional status (AUC) | 0.88 | |||||||||||
| Point remission (AUC) | 0.65 | |||||||||||
| Period remission (AUC) | 0.63 | |||||||||||
| Leighton et al., 2019 [32] | ||||||||||||
| Symptom recovery (AUC) | 0.70 | |||||||||||
| Social recovery (AUC) | 0.73 | |||||||||||
| Vocational recovery (AUC) | 0.74 | |||||||||||
| Quality of life (AUC) | 0.70 | |||||||||||
| Li et al., 2021 [55] (AUC) | 0.81 | |||||||||||
| Lin et al., 2021 [58] | ||||||||||||
| QOLS(RMSE) | 6.44 | 6.56 | 7.16 | 6.43–6.44 | 6.49 | |||||||
| GAF (RMSE) | 7.91 | 7.96 | 8.45 | 7.78–7.81 | 7.84 | |||||||
| Lin et al., 2021 [33] | ||||||||||||
| QOLS(RMSE) | 8.88 | 8.78 | 9.43 | 8.68–8.71 | 8.87 | |||||||
| GAF (RMSE) | 10.08 | 9.70 | 10.50 | 9.70–9.78 | 10.06 | |||||||
| Liu et al., 2022 [56] (AUC) | 0.93 | |||||||||||
| Mourao-Miranda et al., 2012 [53] (Accuracy) | 67–70% | |||||||||||
| Nijs et al., 2021 [60] | ||||||||||||
| GAF (3 year) (Balanced accuracy) | 53–69.7% | |||||||||||
| GAF (6 year) (Balanced accuracy) | 54.4–69.3% | |||||||||||
| Podichetty et al., 2021 [62](AUC) | 0.65 | |||||||||||
| Sarpal et al., 2016 [49] (AUC) | 0.78 | |||||||||||
| Schie, 2022 [61] | ||||||||||||
| Symptoms (4 weeks) (AUC) | 0.58 | |||||||||||
| Symptoms (10 weeks) (AUC) | 0.67 | |||||||||||
| Soldatos et al., 2022 [43](AUC) | 0.68 | |||||||||||
| Smucny et al., 2020 [50] (Accuracy) | 63.7% | 63.6% | 63.4% | 60.8% | 66.7% | 70% | 67.4% | 62.9% | ||||
| Talpalaru et al., 2019 [63] | ||||||||||||
| Schizophrenia vs. c (AUC) | 0.69 | 0.71 | 0.75 | |||||||||
| High symptoms vs. c (AUC) | 0.74 | 0.80 | 0.81 | |||||||||
| Positive symptoms vs. c (AUC) | 0.61 | 0.61 | 0.61 | |||||||||
| Mild symptoms vs. c (AUC) | 0.65 | 0.78 | 0.63 | |||||||||
| Van Hooijdonk et al., 2023 [46] (AUC) | 0.69 | |||||||||||
| Wang et al., 2022 [57] Structural MRI (AUC) | 0.86 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tay, J.L.; Htun, K.K.; Sim, K. Prediction of Clinical Outcomes in Psychotic Disorders Using Artificial Intelligence Methods: A Scoping Review. Brain Sci. 2024, 14, 878. https://doi.org/10.3390/brainsci14090878
Tay JL, Htun KK, Sim K. Prediction of Clinical Outcomes in Psychotic Disorders Using Artificial Intelligence Methods: A Scoping Review. Brain Sciences. 2024; 14(9):878. https://doi.org/10.3390/brainsci14090878
Chicago/Turabian StyleTay, Jing Ling, Kyawt Kyawt Htun, and Kang Sim. 2024. "Prediction of Clinical Outcomes in Psychotic Disorders Using Artificial Intelligence Methods: A Scoping Review" Brain Sciences 14, no. 9: 878. https://doi.org/10.3390/brainsci14090878
APA StyleTay, J. L., Htun, K. K., & Sim, K. (2024). Prediction of Clinical Outcomes in Psychotic Disorders Using Artificial Intelligence Methods: A Scoping Review. Brain Sciences, 14(9), 878. https://doi.org/10.3390/brainsci14090878







