Comprehensive Analysis of Age- and Sex-Related Expression of the Chaperone Protein Sigma-1R in the Mouse Brain
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Antibodies and Reagents
2.3. Plasmid Constructs
2.4. Cell Culture and siRNA Assay
2.5. Tissue Homogenization
2.6. Western Blotting (WB)
2.7. Densitometry Analysis
2.8. Perfusion and Cryo-Sectioning
2.9. Immunohistochemistry (IHC) & Antibody Validation
2.10. Confocal Imaging and Image Analyses
2.11. Statistical Analysis
3. Results
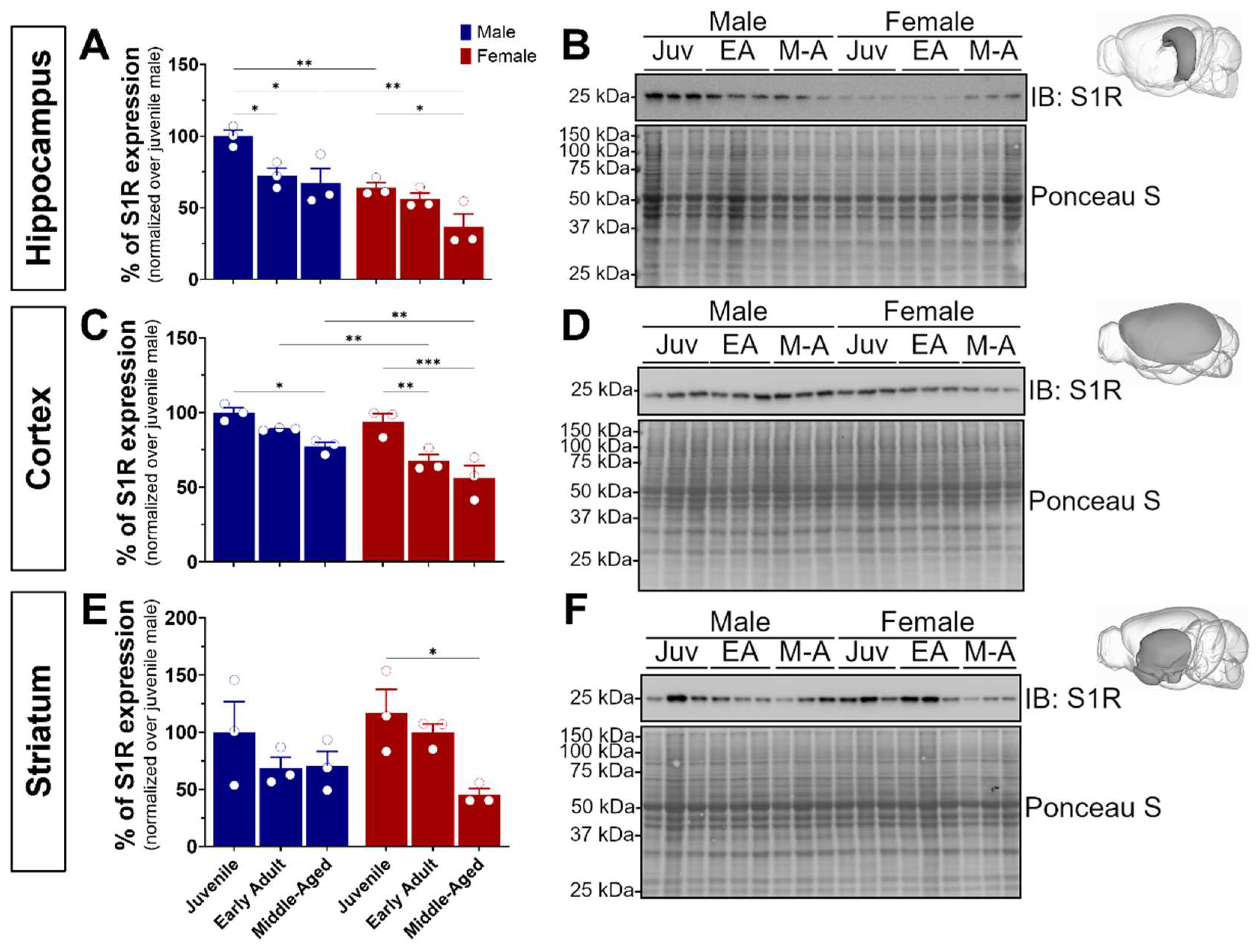
3.1. S1R Is Differentially Expressed in Males and Females and Reduces during Aging in the Hippocampus, Cortex, and Striatum
3.2. Histological Analysis of S1R in Brain Tissue and Antibody Validation
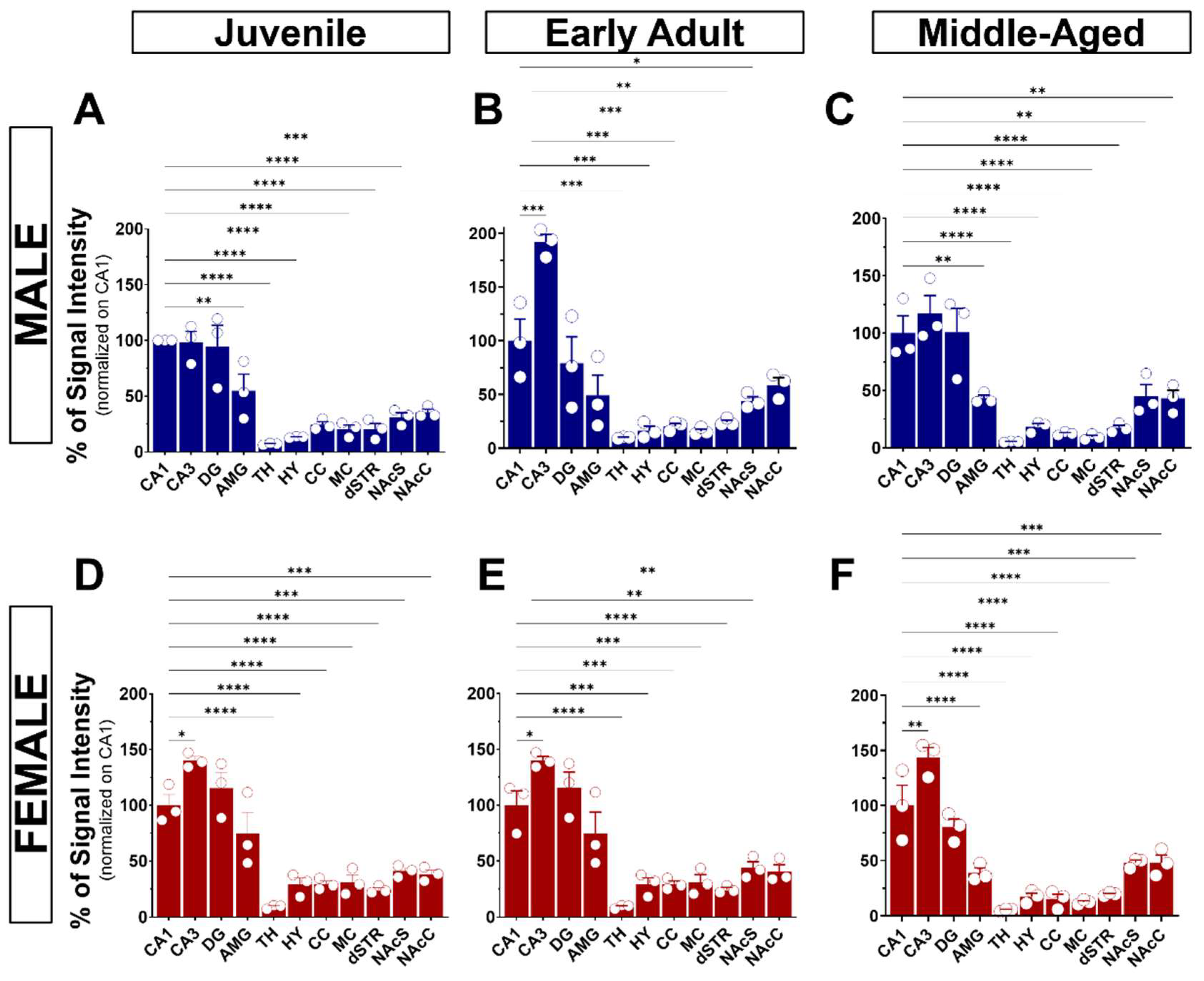
3.3. General Observation of S1R Distribution in the Mouse Brain
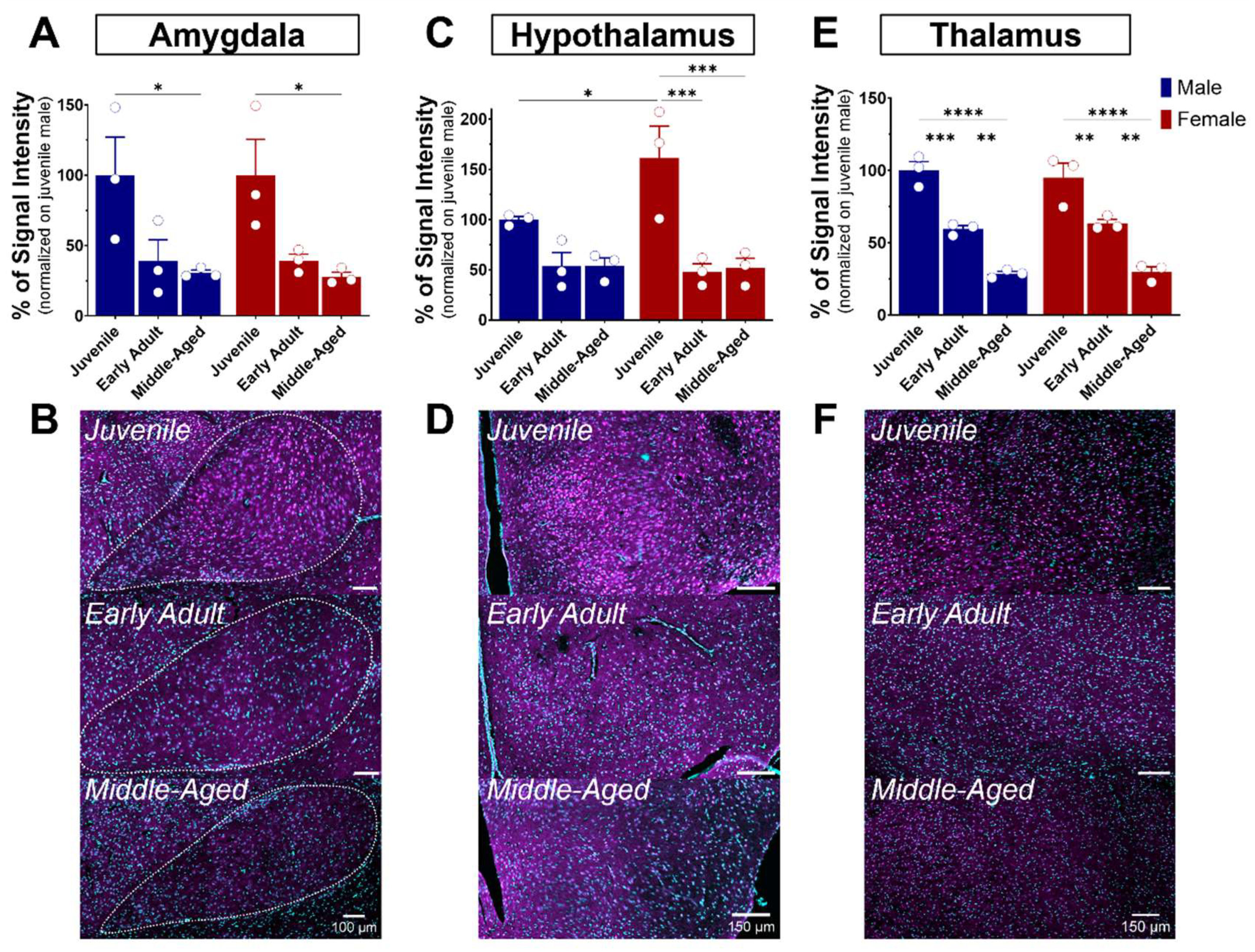
3.4. S1R Expression and Distribution in the Hippocampus, Amygdala, Hypothalamus, and Thalamus
3.4.1. The Hippocampus
3.4.2. The Amygdala
3.4.3. The Hypothalamus
3.4.4. The Thalamus
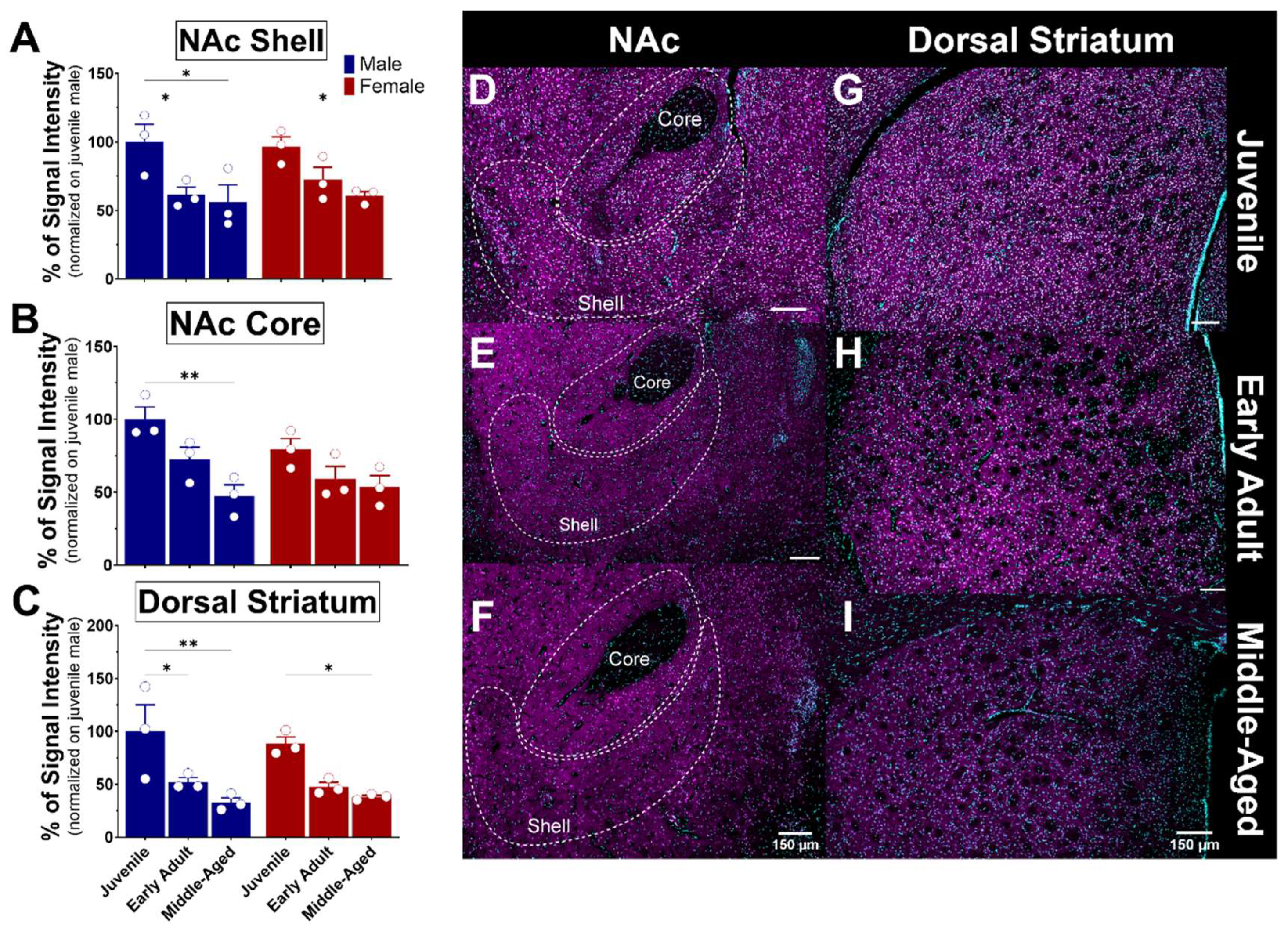
3.5. S1R Expression and Distribution in the Cortex and Striatum
3.5.1. The Motor and Cingulate Cortex
3.5.2. The Striatum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramakrishnan, N.K.; Visser, A.K.D.; Rybczynska, A.A.; Nyakas, C.J.; Luiten, P.G.M.; Kwizera, C.; Sijbesma, J.W.A.; Elsinga, P.H.; Ishiwata, K.; Dierckx, R.A.J.O.; et al. Sigma-1 Agonist Binding in the Aging Rat Brain: A MicroPET Study with [11C]SA4503. Mol. Imaging Biol. 2015, 18, 588–597. [Google Scholar] [CrossRef]
- Kourrich, S. Sigma-1 Receptor and Neuronal Excitability. Handb. Exp. Pharmacol. 2017, 244, 109–130. [Google Scholar] [CrossRef] [PubMed]
- Lachance, V.; Bélanger, S.-M.; Hay, C.; Le Corvec, V.; Banouvong, V.; Lapalme, M.; Tarmoun, K.; Beaucaire, G.; Lussier, M.P.; Kourrich, S. Overview of Sigma-1R Subcellular Specific Biological Functions and Role in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 1971. [Google Scholar] [CrossRef]
- Morales-Lázaro, S.L.; González-Ramírez, R.; Rosenbaum, T. Molecular Interplay Between the Sigma-1 Receptor, Steroids, and Ion Channels. Front. Pharmacol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, N.; Keller, E.; Maurice, T. Behavioural phenotyping of knockout mice for the sigma-1 (σ1) chaperone protein revealed gender-related anxiety, depressive-like and memory alterations. J. Psychopharmacol. 2011, 25, 960–975. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.L.; Fang, M.; Zhao, Y.X.; Liu, X.Y. Roles of sigma-1 receptors in Alzheimer’s disease. Int. J. Clin. Exp. Med. 2015, 8, 4808–4820. [Google Scholar]
- Mavlyutov, T.A.; Guo, L.-W.; Epstein, M.L.; Ruoho, A.E. Role of the Sigma-1 receptor in Amyotrophic Lateral Sclerosis (ALS). J. Pharmacol. Sci. 2015, 127, 10–16. [Google Scholar] [CrossRef]
- Maurice, T.; Martin-Fardon, R.; Romieu, P.; Matsumoto, R.R. Sigma1 (σ1) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci. Biobehav. Rev. 2002, 26, 499–527. [Google Scholar] [CrossRef]
- Ren, P.; Wang, J.; Li, N.; Li, G.; Ma, H.; Zhao, Y.; Li, Y. Sigma-1 Receptors in Depression: Mechanism and Therapeutic Development. Front. Pharmacol. 2022, 13, 925879. [Google Scholar] [CrossRef]
- Maurice, T.; Su, T.-P.; Privat, A. Sigma1 (σ1) receptor agonists and neurosteroids attenuate β25–35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience 1998, 83, 413–428. [Google Scholar] [CrossRef]
- Su, T.-P.; London, E.D.; Jaffe, J.H. Steroid Binding at σ Receptors Suggests a Link Between Endocrine, Nervous, and Immune Systems. Science 1988, 240, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hajipour, A.R.; Sievert, M.K.; Arbabian, M.; Ruoho, A.E. Characterization of the Cocaine Binding Site on the Sigma-1 Receptor. Biochemistry 2007, 46, 3532–3542. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Qin, W.; Tian, S.; Xu, Q.; Wold, E.A.; Zhou, J.; Zhen, X.-C. Small Molecules Selectively Targeting Sigma-1 Receptor for the Treatment of Neurological Diseases. J. Med. Chem. 2020, 63, 15187–15217. [Google Scholar] [CrossRef]
- Itzhak, Y. Repeated methamphetamine-treatment alters brain δ receptors. Eur. J. Pharmacol. 1993, 230, 243–244. [Google Scholar] [CrossRef]
- Kourrich, S.; Su, T.-P.; Fujimoto, M.; Bonci, A. The sigma-1 receptor: Roles in neuronal plasticity and disease. Trends Neurosci. 2012, 35, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Delint-Ramirez, I.; Garcia-Oscos, F.; Segev, A.; Kourrich, S. Cocaine engages a non-canonical, dopamine-independent, mechanism that controls neuronal excitability in the nucleus accumbens. Mol. Psychiatry 2020, 25, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.-P. Sigma-1 Receptor Chaperones at the ER- Mitochondrion Interface Regulate Ca2+ Signaling and Cell Survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.-P. Intracellular Dynamics of σ-1 Receptors (σ1Binding Sites) in NG108-15 Cells. J. Pharmacol. Exp. Ther. 2003, 306, 726–733. [Google Scholar] [CrossRef]
- Esiri, M. Ageing and the brain. J. Pathol. 2007, 211, 181–187. [Google Scholar] [CrossRef]
- Azam, S.; Haque, E.; Balakrishnan, R.; Kim, I.-S.; Choi, D.-K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef]
- Young, J.E.; Wu, M.; Hunsberger, H.C. Editorial: Sex and gender differences in neurodegenerative diseases. Front. Neurosci. 2023, 17, 1175674. [Google Scholar] [CrossRef]
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Park. Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Danis, M. Sex-Specific Role of Sigma-1 Receptor in Alzheimer’s Disease. Master’s Thesis, University of Ottawa, Ottawa, ON, Canada, 11 January 2021. [Google Scholar]
- Kawamura, K.; Kimura, Y.; Tsukada, H.; Kobayashi, T.; Nishiyama, S.; Kakiuchi, T.; Ohba, H.; Harada, N.; Matsuno, K.; Ishii, K.; et al. An increase of sigma1 receptors in the aged monkey brain. Neurobiol. Aging 2002, 24, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Phan, V. Preserved sigma1 (σ1) receptor expression and behavioral efficacy in the aged C57BL/6 mouse. Neurobiol. Aging 2003, 24, 865–881. [Google Scholar] [CrossRef]
- Gassmann, M.; Grenacher, B.; Rohde, B.; Vogel, J. Quantifying Western blots: Pitfalls of densitometry. Electrophoresis 2009, 30, 1845–1855. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Ismail, N.; Garas, P.; Blaustein, J.D. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-α expression in CD-1 female mice. Horm. Behav. 2011, 59, 565–571. [Google Scholar] [CrossRef]
- Hendrickx, J.O.; De Moudt, S.; Calus, E.; De Deyn, P.P.; Van Dam, D.; De Meyer, G.R. Age-related cognitive decline in spatial learning and memory of C57BL/6J mice. Behav. Brain Res. 2021, 418, 113649. [Google Scholar] [CrossRef]
- Bakker, R.; Tiesinga, P.; Kötter, R. The Scalable Brain Atlas: Instant Web-Based Access to Public Brain Atlases and Related Content. Neuroinformatics 2015, 13, 353–366. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef]
- Jurkowski, M.P.; Bettio, L.; Woo, E.K.; Patten, A.; Yau, S.-Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Front. Cell. Neurosci. 2020, 14, 576444. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, S.-J.; Kwon, O.-B.; Lee, J.H.; Kim, J.-H. Inhibitory networks of the amygdala for emotional memory. Front. Neural Circuits 2013, 7, 129. [Google Scholar] [CrossRef]
- Babaev, O.; Chatain, C.P.; Krueger-Burg, D. Inhibition in the amygdala anxiety circuitry. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hrybouski, S.; Aghamohammadi-Sereshki, A.; Madan, C.R.; Shafer, A.T.; Baron, C.A.; Seres, P.; Beaulieu, C.; Olsen, F.; Malykhin, N.V. Amygdala subnuclei response and connectivity during emotional processing. NeuroImage 2016, 133, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Bear, M.H.; Reddy, V.; Bollu, P.C. Neuroanatomy, Hypothalamus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Swaab, D.F.; Hofman, M.A.; Lucassen, P.J.; Purba, J.S.; Raadsheer, F.C.; Nes, J. Functional neuroanatomy and neuropathology of the human hypothalamus. Anat. Embryol. 1993, 187, 317–330. [Google Scholar] [CrossRef]
- Stein, T.; Moritz, C.; Quigley, M.; Cordes, D.; Haughton, V.; Meyerand, E. Functional Connectivity in the Thalamus and Hippocampus Studied with Functional MR Imaging. Am. J. Neuroradiol. 2000, 21, 1397–1401. [Google Scholar] [PubMed]
- Javed, K.; Reddy, V.; Lui, F. Neuroanatomy, Cerebral Cortex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jawabri, K.H.; Sharma, S. Physiology, Cerebral Cortex Functions. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Alheid, G.; Heimer, L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 1988, 27, 1–39. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, M.; Wang, Y.; Wang, Y. Nucleus accumbens in the pathogenesis of major depressive disorder: A brief review. Brain Res. Bull. 2023, 196, 68–75. [Google Scholar] [CrossRef]
- Smith, A.D.; Bolam, J.P. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990, 13, 259–265. [Google Scholar] [CrossRef]
- Park, Y.-S.; Sammartino, F.; Young, N.A.; Corrigan, J.; Krishna, V.; Rezai, A.R. Anatomic Review of the Ventral Capsule/Ventral Striatum and the Nucleus Accumbens to Guide Target Selection for Deep Brain Stimulation for Obsessive-Compulsive Disorder. World Neurosurg. 2019, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Melief, E.J.; McKinley, J.W.; Lam, J.Y.; Whiteley, N.M.; Gibson, A.W.; Neumaier, J.F.; Henschen, C.W.; Palmiter, R.D.; Bamford, N.S.; Darvas, M. Loss of glutamate signaling from the thalamus to dorsal striatum impairs motor function and slows the execution of learned behaviors. npj Park. Dis. 2018, 4, 23. [Google Scholar] [CrossRef]
- Balleine, B.W.; Delgado, M.R.; Hikosaka, O. The Role of the Dorsal Striatum in Reward and Decision-Making. J. Neurosci. 2007, 27, 8161–8165. [Google Scholar] [CrossRef] [PubMed]
- Kerkenberg, N.; Wachsmuth, L.; Zhang, M.; Schettler, C.; Ponimaskin, E.; Faber, C.; Baune, B.T.; Zhang, W.; Hohoff, C. Brain microstructural changes in mice persist in adulthood and are modulated by the palmitoyl acyltransferase ZDHHC7. Eur. J. Neurosci. 2021, 54, 5951–5967. [Google Scholar] [CrossRef] [PubMed]
- Pascarella, G.; Antonelli, L.; Narzi, D.; Battista, T.; Fiorillo, A.; Colotti, G.; Guidoni, L.; Morea, V.; Ilari, A. Investigation of the Entry Pathway and Molecular Nature of σ1 Receptor Ligands. Int. J. Mol. Sci. 2023, 24, 6367. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Chu, U.B.; Ramachandran, S.; Grawoig, D.; Guo, L.-W.; Hajipour, A.R.; Ruoho, A.E. Juxtaposition of the Steroid Binding Domain-like I and II Regions Constitutes a Ligand Binding Site in the σ-1 Receptor. J. Biol. Chem. 2008, 283, 19646–19656. [Google Scholar] [CrossRef]
- Pal, A.; Hajipour, A.R.; Fontanilla, D.; Ramachandran, S.; Chu, U.B.; Mavlyutov, T.; Ruoho, A.E. Identification of Regions of the σ-1 Receptor Ligand Binding Site Using a Novel Photoprobe. Mol. Pharmacol. 2007, 72, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Zhemkov, V.; A Ditlev, J.; Lee, W.-R.; Wilson, M.; Liou, J.; Rosen, M.K.; Bezprozvanny, I. The role of sigma 1 receptor in organization of endoplasmic reticulum signaling microdomains. eLife 2021, 10, e65192. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.-J. Normal Aging Induces Changes in the Brain and Neurodegeneration Progress: Review of the Structural, Biochemical, Metabolic, Cellular, and Molecular Changes. Front. Aging Neurosci. 2022, 14, 931536. [Google Scholar] [CrossRef]
- Charalampopoulos, I.; Alexaki, V.; Tsatsanis, C.; Minas, V.; Dermitzaki, E.; Lasaridis, I.; Vardouli, L.; Stournaras, C.; Margioris, A.N.; Castanas, E.; et al. Neurosteroids as Endogenous Inhibitors of Neuronal Cell Apoptosis in Aging. Ann. N. Y. Acad. Sci. 2006, 1088, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Weindruch, R.; Prolla, T.A. Gene-expression profile of the ageing brain in mice. Nat. Genet. 2000, 25, 294–297. [Google Scholar] [CrossRef]
- Crouzier, L.; Couly, S.; Roques, C.; Peter, C.; Belkhiter, R.; Jacquemin, M.A.; Bonetto, A.; Delprat, B.; Maurice, T. Sigma-1 (σ1) receptor activity is necessary for physiological brain plasticity in mice. Eur. Neuropsychopharmacol. 2020, 39, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Denoth-Lippuner, A.; Jessberger, S. Formation and integration of new neurons in the adult hippocampus. Nat. Rev. Neurosci. 2021, 22, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Ngwenya, L.B.; Heyworth, N.C.; Shwe, Y.; Moore, T.L.; Rosene, D.L. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front. Syst. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, M.; Fulmore, C.A.; Tartt, A.N.; Simeon, L.R.; Pavlova, I.; Poposka, V.; Rosoklija, G.B.; Stankov, A.; Arango, V.; Dwork, A.J.; et al. Human Hippocampal Neurogenesis Persists throughout Aging. Cell Stem Cell 2018, 22, 589–599.e5. [Google Scholar] [CrossRef]
- Magnusson, K.R. The aging of the NMDA receptor complex. Front. Biosci. 1998, 3, e70–e80. [Google Scholar] [CrossRef]
- Sheng, M.; Cummings, J.; Roldan, L.A.; Jan, Y.N.; Jan, L.Y. Changing subunit composition of heteromeric NMDA receptors during development of rat cortex. Nature 1994, 368, 144–147. [Google Scholar] [CrossRef]
- Hussain, S.G.; Ramaiah, K.V. Reduced eIF2α phosphorylation and increased proapoptotic proteins in aging. Biochem. Biophys. Res. Commun. 2007, 355, 365–370. [Google Scholar] [CrossRef]
- Mori, T.; Hayashi, T.; Hayashi, E.; Su, T.-P. Sigma-1 Receptor Chaperone at the ER-Mitochondrion Interface Mediates the Mitochondrion-ER-Nucleus Signaling for Cellular Survival. PLoS ONE 2013, 8, e76941. [Google Scholar] [CrossRef]





| Brain Regions | Female | Male | ||||
|---|---|---|---|---|---|---|
| Juvenile | Early Adult | Middle-Aged | Juvenile | Early Adult | Middle-Aged | |
| Hippocampus | ||||||
| CA1 | 73.56 ± 7.13 | 51.59 ± 6.61 | 39.61 ± 7.25 | 100 | 43.72 ± 8.75 | 38.94 ± 5.87 |
| CA3 | 103.07 ± 2.74 | 70.91 ± 9.5 | 56.87 ± 3.58 | 98.19 ± 10.02 | 83.83 ± 3.26 | 45.64 ± 6.03 |
| DG | 84.91 ± 10.74 | 32.09 ± 2.10 | 31.83 ± 2.93 | 94.54 ± 18.97 | 34.55 ± 10.75 | 39.27 ± 8.02 |
| Thalamus | 6.86 ±0.73 | 4.58 ± 0.19 | 2.15 ± 0.25 | 7.22 ± 0.44 | 4.30 ± 0.16 | 2.06 ± 0.11 |
| Amygdala | 55.02 ± 13.97 | 21.60 ± 2.56 | 15.34 ± 1.76 | 54.95 ± 14.87 | 21.46 ± 8.29 | 16.83 ± 1.06 |
| Hypothalamus | 21.52 ± 4.20 | 6.42 ± 1.05 | 6.92 ± 1.28 | 13.33 ± 0.41 | 7.15 ± 1.81 | 7.17 ± 1.06 |
| Cortex | ||||||
| Cingulate | 21.61 ± 2.05 | 10.94 ± 2.09 | 5.94 ± 1.84 | 24.42 ± 2.74 | 8.96 ± 1.04 | 4.87 ± 0.35 |
| Motor | 22.95 ± 4.84 | 8.40 ± 0.6 | 4.98 ± 0.49 | 20.42 ± 3.83 | 6.95 ± 0.81 | 3.66 ± 0.57 |
| Striatum | ||||||
| Dorsal | 18.01 ± 1.33 | 9.74 ± 0.86 | 7.78 ± 0.31 | 20.38 ± 5.12 | 10.65 ± 0.84 | 6.69 ± 0.90 |
| NAc Shell | 30.22 ± 2.20 | 22.66 ± 2.83 | 18.98 ± 1.00 | 31.27 ± 4.05 | 19.22 ± 1.76 | 17.59 ± 3.89 |
| NAc Core | 28.21 ± 2.65 | 20.94 ± 3.11 | 19.05 ± 2.75 | 35.55 ± 2.98 | 25.75 ± 2.97 | 16.80 ± 2.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarmoun, K.; Lachance, V.; Le Corvec, V.; Bélanger, S.-M.; Beaucaire, G.; Kourrich, S. Comprehensive Analysis of Age- and Sex-Related Expression of the Chaperone Protein Sigma-1R in the Mouse Brain. Brain Sci. 2024, 14, 881. https://doi.org/10.3390/brainsci14090881
Tarmoun K, Lachance V, Le Corvec V, Bélanger S-M, Beaucaire G, Kourrich S. Comprehensive Analysis of Age- and Sex-Related Expression of the Chaperone Protein Sigma-1R in the Mouse Brain. Brain Sciences. 2024; 14(9):881. https://doi.org/10.3390/brainsci14090881
Chicago/Turabian StyleTarmoun, Khadija, Véronik Lachance, Victoria Le Corvec, Sara-Maude Bélanger, Guillaume Beaucaire, and Saïd Kourrich. 2024. "Comprehensive Analysis of Age- and Sex-Related Expression of the Chaperone Protein Sigma-1R in the Mouse Brain" Brain Sciences 14, no. 9: 881. https://doi.org/10.3390/brainsci14090881
APA StyleTarmoun, K., Lachance, V., Le Corvec, V., Bélanger, S.-M., Beaucaire, G., & Kourrich, S. (2024). Comprehensive Analysis of Age- and Sex-Related Expression of the Chaperone Protein Sigma-1R in the Mouse Brain. Brain Sciences, 14(9), 881. https://doi.org/10.3390/brainsci14090881








