Gender Differences in Prefrontal Cortex Response to Negative Emotional Stimuli in Drivers
Abstract
:1. Introduction
2. Method
2.1. Participants and Procedure
2.2. Material
2.2.1. Multidimensional Driving Style Inventory (MDSI)
2.2.2. Pictures
2.2.3. fNIR Recording and Data Analysis
2.2.4. Statistical Analysis
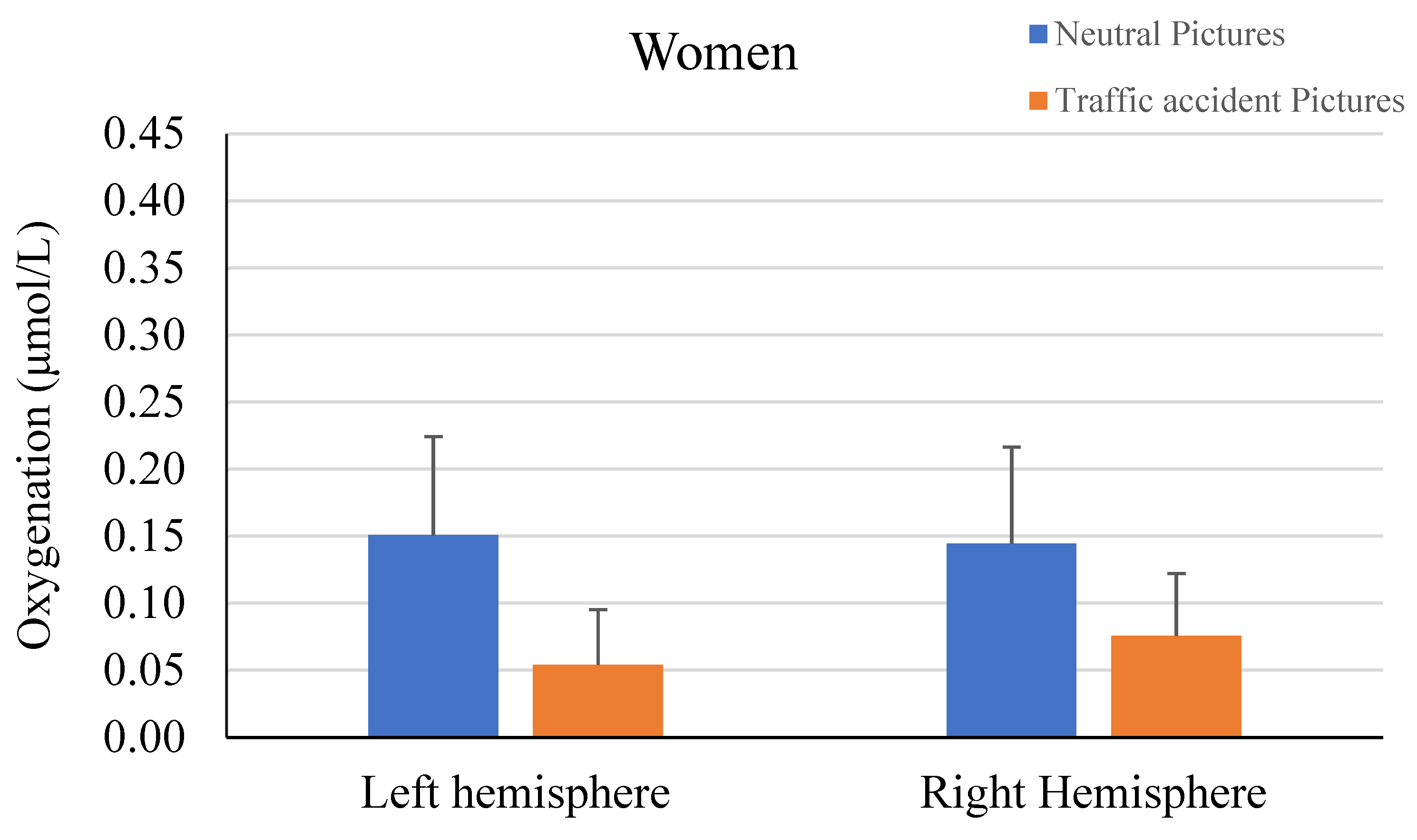
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations General Assembly. Improving Global Road Safety. GA Res. 74/299, 12, U.N. GAOR, 74th Sess., Supp. No. 49, (Vol. I), U.N. Doc. A/74/49 (Vol. III), at 26-33. 2020. Available online: https://documents.un.org/doc/undoc/gen/n20/226/30/pdf/n2022630.pdf (accessed on 22 April 2024).
- Singh, S. Critical Reasons for Crashes Investigated in the National Motor Vehicle Crash Causation Survey; Traffic Safety Facts Crash Stats; Report No. DOT HS 812 506; National Highway Traffic Safety Administration: Washington, DC, USA, 2018. [Google Scholar]
- Elander, J.; West, R.; French, D. Behavioral correlates of individual differences in road-traffic crash risk: An examination of methods and findings. Psychol. Bull. 1993, 113, 279–294. [Google Scholar] [CrossRef] [PubMed]
- French, D.J.; West, R.J.; Elander, J.; Wilding, J.M. Decision-making style, driving style, and self-reported involvement in road traffic accidents. Ergonomics 1993, 36, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Gulian, E.; Matthews, G.; Glendon, A.I.; Davies, D.R.; Debney, L.M. Dimensions of driver stress. Ergonomics 1989, 32, 585–602. [Google Scholar] [CrossRef]
- Reason, J.; Manstead, A.; Stradling, S.; Baxter, J.; Campbell, K. Errors and violations on the roads: A real distinction? Ergonomics 1990, 33, 1315–1332. [Google Scholar] [CrossRef]
- Taubman-Ben-Ari, O.; Mikulincer, M.; Gillath, O. The multidimensional driving style inventory—Scale construct and validation. Accid. Anal. Prev. 2004, 36, 323–332. [Google Scholar] [CrossRef]
- Taubman-Ben-Ari, O.; Skvirsky, V. The multidimensional driving style inventory a decade later: Review of the literature and re-evaluation of the scale. Accid. Anal. Prev. 2016, 93, 179–188. [Google Scholar] [CrossRef]
- Roid, E.; Siebert, F.W.; Oehl, M.; Höger, R. Introducing a multivariate model for predicting driving performance: The role of driving anger and personal characteristics. J. Saf. Res. 2013, 47, 47–56. [Google Scholar] [CrossRef]
- Trógolo, M.A.; Melchior, F.; Medrano, L.A. The role of difficulties in emotion regulation on driving behavior. J. Behav. Health Soc. Issues 2014, 6, 107–117. [Google Scholar] [CrossRef]
- Navon–Eyal, M.; Taubman–Ben-Ari, O. Can emotion regulation explain the association between age and driving styles? Transp. Res. Part F Traffic Psychol. Behav. 2020, 74, 439–445. [Google Scholar] [CrossRef]
- Trick, L.M.; Brandigampola, S.; Enns, J.T. How fleeting emotions affect hazard perception and steering while driving: The impact of image arousal and valence. Accid. Anal. Prev. 2012, 45, 222–229. [Google Scholar] [CrossRef]
- Jeon, M.; Zhang, W. Sadder but wiser? Effects of negative emotions on risk perception, driving performance, and perceived workload. Proc. Hum. Factors Ergon. Soc. Annu. Meet. 2013, 57, 1849–1853. [Google Scholar] [CrossRef]
- Braun, M.; Weber, F.; Alt, F. Affective Automotive User Interfaces–Reviewing the State of Driver Affect Research and Emotion Regulation in the Car. ACM Comput. Surv. 2021, 54, 1–26. [Google Scholar] [CrossRef]
- Šeibokaitė, L.; Endriulaitienė, A.; Sullman, M.J.; Markšaitytė, R.; Žardeckaitė-Matulaitienė, K. Difficulties in emotion regulation and risky driving among Lithuanian drivers. Traffic Inj. Prev. 2017, 18, 688–693. [Google Scholar] [CrossRef]
- Eherenfreund-Hager, A.; Taubman–Ben-Ari, O.; Toledo, T.; Farah, H. The effect of positive and negative emotions on young drivers: A simulator study. Transp. Res. Part F Traffic Psychol. Behav. 2017, 49, 236–243. [Google Scholar] [CrossRef]
- Hancock, G.M.; Hancock, P.A.; Janelle, C.M. The impact of emotions and predominant emotion regulation technique on driving performance. Work 2012, 41 (Suppl. S1), 3608–3611. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.P.; Chapman, P.; Bailey, K. The influence of image valence on visual attention and perception of risk in drivers. Accid. Anal. Prev. 2014, 73, 296–304. [Google Scholar] [CrossRef]
- Holman, A.C.; Popușoi, S.A. How you deal with your emotions is how you drive. Emotion regulation strategies, traffic offenses, and the mediating role of driving styles. Sustainability 2020, 12, 4929. [Google Scholar] [CrossRef]
- Harris, H.; Nass, C. Emotion regulation for frustrating driving contexts. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Vancouver, BC, Canada, 7–12 May 2011; pp. 749–752. [Google Scholar] [CrossRef]
- Pizzo, A.; Lausi, G.; Burrai, J.; Quaglieri, A.; Mari, E.; D’Alessio, I.; Barchielli, B.; Cordellieri, P.; Giannini, A.M.; Cricenti, C. Emotion behind the Wheel: Unravelling the Impact of Emotional (dys). Regulation on Young Driving Behaviour—A Systematic Review. Sustainability 2024, 16, 3384. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Gross, J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005, 9, 242–249. [Google Scholar] [CrossRef]
- Mitchell, D.G. The nexus between decision making and emotion regulation: A review of convergent neurocognitive substrates. Behav. Brain Res. 2011, 217, 215–231. [Google Scholar] [CrossRef]
- Ochsner, K.N.; Silvers, J.A.; Buhle, J.T. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012, 1251, E1–E24. [Google Scholar] [CrossRef] [PubMed]
- Kohn, N.; Eickhoff, S.B.; Scheller, M.; Laird, A.R.; Fox, P.T.; Habel, U. Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. Neuroimage 2014, 87, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Balada, F.; Blanch, A.; Aluja, A. Arousal and Habituation Effects (Excitability) on Startle Responses to the International Affective Picture Systems (IAPS). J. Psychophysiol. 2014, 28, 233–241. [Google Scholar] [CrossRef]
- Aluja, A.; Blanch, A.; Blanco, E.; Balada, F. Affective Modulation of the Startle Reflex and the Reinforcement Sensitivity Theory of Personality: The Role of Sensitivity to Reward. Physiol. Behav. 2015, 138, 332–339. [Google Scholar] [CrossRef]
- Aluja, A.; Balada, F.; Blanco, E.; Lucas, I.; Blanch, A. Startle Reflex Modulation by Affective Face “Emoji” Pictographs. Psychol. Res. 2020, 84, 15–22. [Google Scholar] [CrossRef]
- Ray, R.D.; McRae, K.; Ochsner, K.N.; Gross, J.J. Cognitive reappraisal of negative affect: Converging evidence from EMG and self-report. Emotion 2010, 10, 587–592. [Google Scholar] [CrossRef]
- Jackson, D.C.; Mueller, C.J.; Dolski, I.; Dalton, K.M.; Nitschke, J.B.; Urry, H.L.; Rosenkranz, M.A.; Ryff, C.D.; Singer, B.H.; Davidson, R.J. Now you feel it, now you don’t: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol. Sci. 2003, 14, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Hamann, S.; Canli, T. Individual differences in emotion processing. Curr. Opin. Neurobiol. 2004, 14, 233–238. [Google Scholar] [CrossRef]
- Quaresima, V.; Bisconti, S.; Ferrari, M. A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain Lang. 2012, 121, 79–89. [Google Scholar] [CrossRef]
- Westgarth, M.M.; Hogan, C.A.; Neumann, D.L.; Shum, D.H. A systematic review of studies that used NIRS to measure neural activation during emotion processing in healthy individuals. Soc. Cogn. Affect. Neurosci. 2021, 16, 345–369. [Google Scholar] [CrossRef]
- Doi, H.; Nishitani, S.; Shinohara, K. NIRS as a tool for assaying emotional function in the prefrontal cortex. Front. Hum. Neurosci. 2013, 7, 770. [Google Scholar] [CrossRef]
- Bendall, R.C.; Eachus, P.; Thompson, C. A Brief Review of Research Using Near-Infrared Spectroscopy to Measure Activation of the Prefrontal Cortex during Emotional Processing: The Importance of Experimental Design. Front. Hum. Neurosci. 2016, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.K.; Chan, A.S. A systematic review of the application of functional near-infrared spectroscopy to the study of cerebral hemodynamics in healthy aging. Neuropsychol. Rev. 2021, 31, 139–166. [Google Scholar] [CrossRef]
- Harada, H.; Nashihara, H.; Morozumi, K.; Ota, H.; Hatakeyama, E. A comparison of cerebral activity in the prefrontal region between young adults and the elderly while driving. J. Physiol. Anthropol. 2007, 26, 409–414. [Google Scholar] [CrossRef]
- Opitz, P.C.; Rauch, L.C.; Terry, D.P.; Urry, H.L. Prefrontal mediation of age differences in cognitive reappraisal. Neurobiol. Aging 2012, 33, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Padilla García, J.L.; Castro Ramírez, C.; Doncel, P.; Ben-Ari, O.T. Adaptation of the multidimensional driving styles inventory for Spanish drivers: Convergent and predictive validity evidence for detecting safe and unsafe driving styles. Accid. Anal. Prev. 2020, 136, 105413. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual; Technical Report A-8; University of Florida: Gainesville, FL, USA, 2008. [Google Scholar]
- Marchewka, A.; Żurawski, Ł.; Jednoróg, K.; Grabowska, A. The Nencki Affective Picture System (NAPS): Introduction to a novel, standardized, wide-range, high-quality, realistic picture database. Behav. Res. Methods 2014, 46, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, H.; Shewokis, P.A.; Curtin, A.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B. Using MazeSuite and Functional Near Infrared Spectroscopy to Study Learning in Spatial Navigation. J. Vis. Exp. 2011, 56, e3443. [Google Scholar] [CrossRef]
- Ayaz, H.; Shewokis, P.A.; Bunce, S.C.; Onaral, B. Functional Near Infrared Spectroscopy-Based Brain Computer Interface. U.S. Patent US9946344B2, 17 April 2018. Available online: https://patents.google.com/patent/US9946344B2/en/ (accessed on 22 April 2024).
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Publishing Company Reading, Mass: Menlo Park, CA, USA; London, UK; Amsterdam, The Netherlands; Don Mills, ON, Canada; Sydney, Australia, 1977. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Reber, J.; Tranel, D. Sex differences in the functional lateralization of emotion and decision making in the human brain. J. Neurosci. Res. 2017, 95, 270–278. [Google Scholar] [CrossRef]
- Drzewiecki, C.M.; Juraska, J.M. The structural reorganization of the prefrontal cortex during adolescence as a framework for vulnerability to the environment. Pharmacol. Biochem. Behav. 2020, 199, 173044. [Google Scholar] [CrossRef]
- Dreher, J.-C.; Schmidt, P.J.; Kohn, P.; Furman, D.; Rubinow, D.; Berman, K.F. Menstrual cycle phase modulates reward-related neural function in women. Proc. Natl. Acad. Sci. USA 2007, 104, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Dan, R.; Canetti, L.; Keadan, T.; Segman, R.; Weinstock, M.; Bonne, O.; Reuveni, I.; Goelman, G. Sex Differences during Emotion Processing Are Dependent on the Menstrual Cycle Phase. Psychoneuroendocrinology 2019, 100, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Jin, X.; Niu, D.; Zhang, L.; Jiang, S.Y.; Ruan, H.D.; Ho, G.W. Sex differences in hemispheric lateralization of attentional networks. Psychol. Res. 2021, 85, 2697–2709. [Google Scholar] [CrossRef]
- Fan, J.; McCandliss, B.D.; Fossella, J.; Flombaum, J.I.; Posner, M.I. The activation of attentional networks. Neuroimage 2005, 26, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Posner, M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012, 35, 73–89. [Google Scholar] [CrossRef]
- De Joux, N.; Russell, P.N.; Helton, W.S. A functional near-infrared spectroscopy study of sustained attention to local and global target features. Brain Cogn. 2013, 81, 370–375. [Google Scholar] [CrossRef]
- Li, S.; Cai, Y.; Liu, J.; Li, D.; Feng, Z.; Chen, C.; Xue, G. Dissociated roles of the parietal and frontal cortices in the scope and control of attention during visual working memory. NeuroImage 2017, 149, 210–219. [Google Scholar] [CrossRef]
- Manelis, A.; Huppert, T.J.; Rodgers, E.; Swartz, H.A.; Phillips, M.L. The role of the right prefrontal cortex in recognition of facial emotional expressions in depressed individuals: fNIRS study. J. Affect. Disord. 2019, 258, 151–158. [Google Scholar] [CrossRef]
- Poó, F.M.; Ledesma, R.D. A study on the relationship between personality and driving styles. Traffic Inj. Prev. 2013, 14, 346–352. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, W.; Ge, Y.; Sun, X.; Zhang, K. Effect of personality traits on driving style: Psychometric adaption of the multidimensional driving style inventory in a Chinese sample. PLoS ONE 2018, 13, e0202126. [Google Scholar] [CrossRef]
- Eysenck, H.J. Personality. In The Neuropsychology of Individual Differences; Elsevier: Amsterdam, The Netherlands, 1994; pp. 151–207. [Google Scholar] [CrossRef]
- Eysenck, H.J. The Biological Basis of Personality; Transaction Publ.: Piscataway, NJ, USA, 1967. [Google Scholar]
- Madsen, M.K.; McMahon, B.; Andersen, S.B.; Siebner, H.R.; Knudsen, G.M.; Fisher, P.M. Threat-related amygdala functional connectivity is associated with 5-HTTLPR genotype and neuroticism. Soc. Cogn. Affect. Neurosci. 2016, 11, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Hu, K. Investigations into ventral prefrontal cortex using mediation models. J. Neurosci. Res. 2020, 98, 632–642. [Google Scholar] [CrossRef]
- Yamasaki, H.; LaBar, K.S.; McCarthy, G. Dissociable prefrontal brain systems for attention and emotion. Proc. Natl. Acad. Sci. USA 2002, 99, 11447–11451. [Google Scholar] [CrossRef] [PubMed]
- Viinikainen, M.; Jääskeläinen, I.P.; Alexandrov, Y.; Balk, M.H.; Autti, T.; Sams, M. Nonlinear relationship between emotional valence and brain activity: Evidence of separate negative and positive valence dimensions. Hum. Brain Mapp. 2010, 31, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Qiu, X.; Li, S.; Mo, L.; Xu, F.; Zhang, D. Different Roles of the Left and Right Ventrolateral Prefrontal Cortex in Cognitive Reappraisal: An Online Transcranial Magnetic Stimulation Study. Front. Hum. Neurosci. 2022, 16, 928077. [Google Scholar] [CrossRef]
- Foy, H.J.; Runham, P.; Chapman, P. Prefrontal cortex activation and young driver behaviour: A fNIRS study. PLoS ONE 2016, 11, e0156512. [Google Scholar] [CrossRef]




| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p< | α | ||
| Age | 44.38 | 12.98 | 38.89 | 10.60 | 3.30 | 0.001 | ||
| MDSI | Reckless | 14.47 | 6.81 | 12.48 | 5.56 | 2.28 | 0.024 | 0.88 |
| Anxious | 9.08 | 4.19 | 8.98 | 4.14 | 0.17 | 0.867 | 0.80 | |
| Careful | 32.81 | 4.63 | 32.79 | 6.48 | 0.04 | 0.972 | 0.80 | |
| Angry | 8.57 | 3.47 | 8.73 | 3.47 | −0.32 | 0.749 | 0.77 | |
| Dissociative | 14.47 | 4.46 | 14.55 | 4.63 | −0.11 | 0.910 | 0.71 | |
| Distress Reduction | 17.05 | 3.15 | 17.04 | 3.84 | 0.03 | 0.976 | 0.67 | |
| MDSI Factor | 0.05 | 0.99 | −0.07 | 1.01 | 0.87 | 0.384 | ||
| Oxygenation Change | Lateral Left | 0.15 | 0.47 | 0.06 | 0.53 | 1.23 | 0.221 | -- |
| Rostral Left | 0.21 | 0.52 | 0.14 | 0.46 | 0.92 | 0.359 | -- | |
| Rostral Right | 0.18 | 0.48 | 0.16 | 0.44 | 0.30 | 0.764 | -- | |
| Lateral Right | 0.12 | 0.52 | 0.06 | 0.48 | 0.78 | 0.438 | -- | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balada, F.; Aluja, A.; García, Ó.; Aymamí, N.; García, L.F. Gender Differences in Prefrontal Cortex Response to Negative Emotional Stimuli in Drivers. Brain Sci. 2024, 14, 884. https://doi.org/10.3390/brainsci14090884
Balada F, Aluja A, García Ó, Aymamí N, García LF. Gender Differences in Prefrontal Cortex Response to Negative Emotional Stimuli in Drivers. Brain Sciences. 2024; 14(9):884. https://doi.org/10.3390/brainsci14090884
Chicago/Turabian StyleBalada, Ferran, Anton Aluja, Óscar García, Neus Aymamí, and Luis F. García. 2024. "Gender Differences in Prefrontal Cortex Response to Negative Emotional Stimuli in Drivers" Brain Sciences 14, no. 9: 884. https://doi.org/10.3390/brainsci14090884






