A Two-Hit Approach Inducing Flurothyl Seizures in Fmr1 Knockout Mice Impacts Anxiety and Repetitive Behaviors
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Seizure Induction
2.3. Open Field
2.4. Elevated Plus Maze
2.5. Nose Poke
2.6. Social Partition
2.7. Delay Fear Conditioning
2.8. Pre-Pulse Inhibition
2.9. Western Blot Analysis
2.10. Statistical Analysis
3. Results
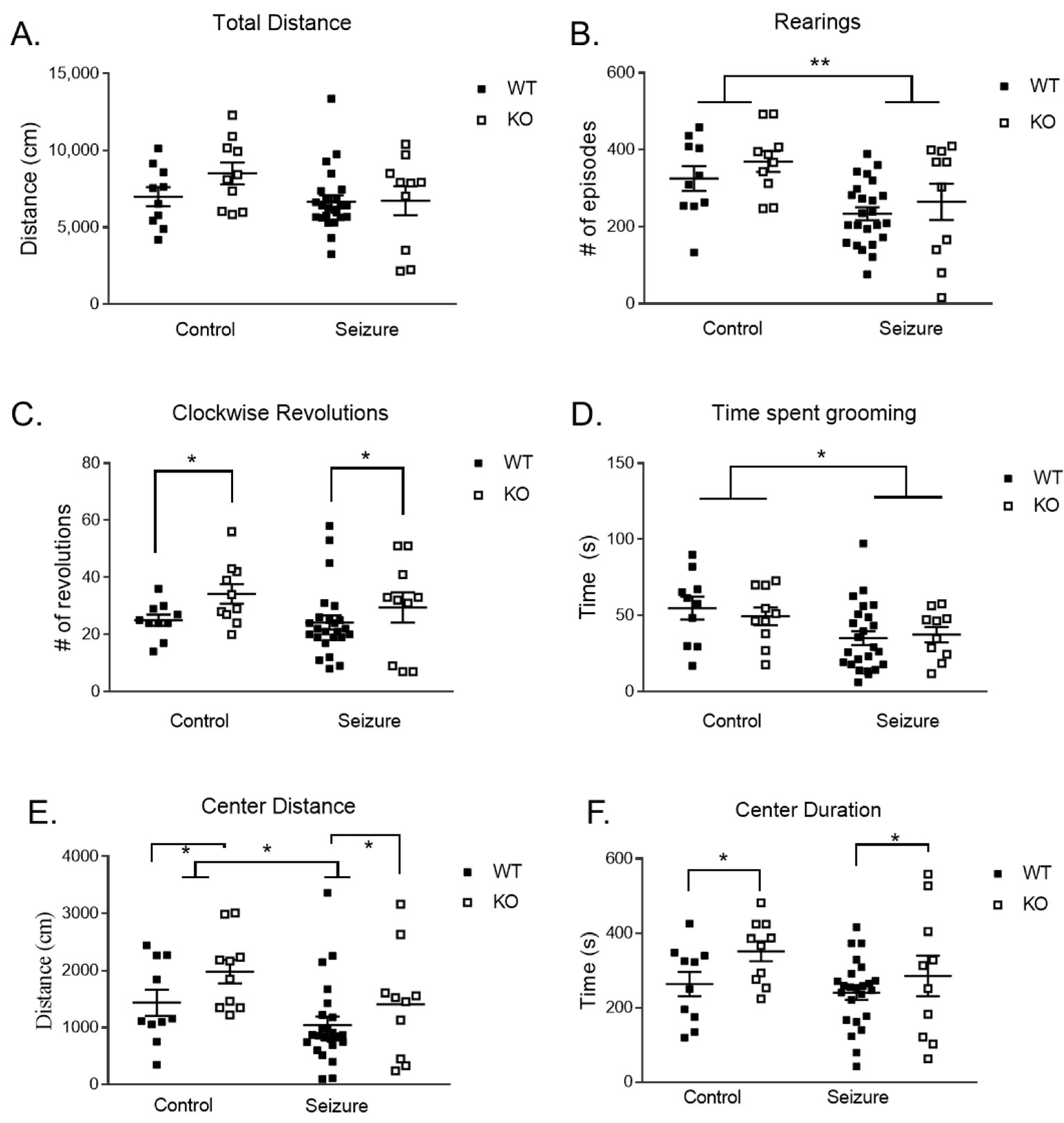
3.1. Early-Life Seizures Decreased Locomotor and Stereotypic Behavior but Did Not Impact Anxiety-like Behavior in the Open Field
3.2. Early-Life Seizures Decreased Locomotor Activity but Did Not Affect Anxiety-like Behavior in the Elevated Plus Maze
3.3. Early-Life Seizures Decrease Exploratory Behavior in Fmr1 KO Mice in the Nose Poke Task
3.4. Early-Life Seizures Reduce Social Behavior in the Social Partition Task
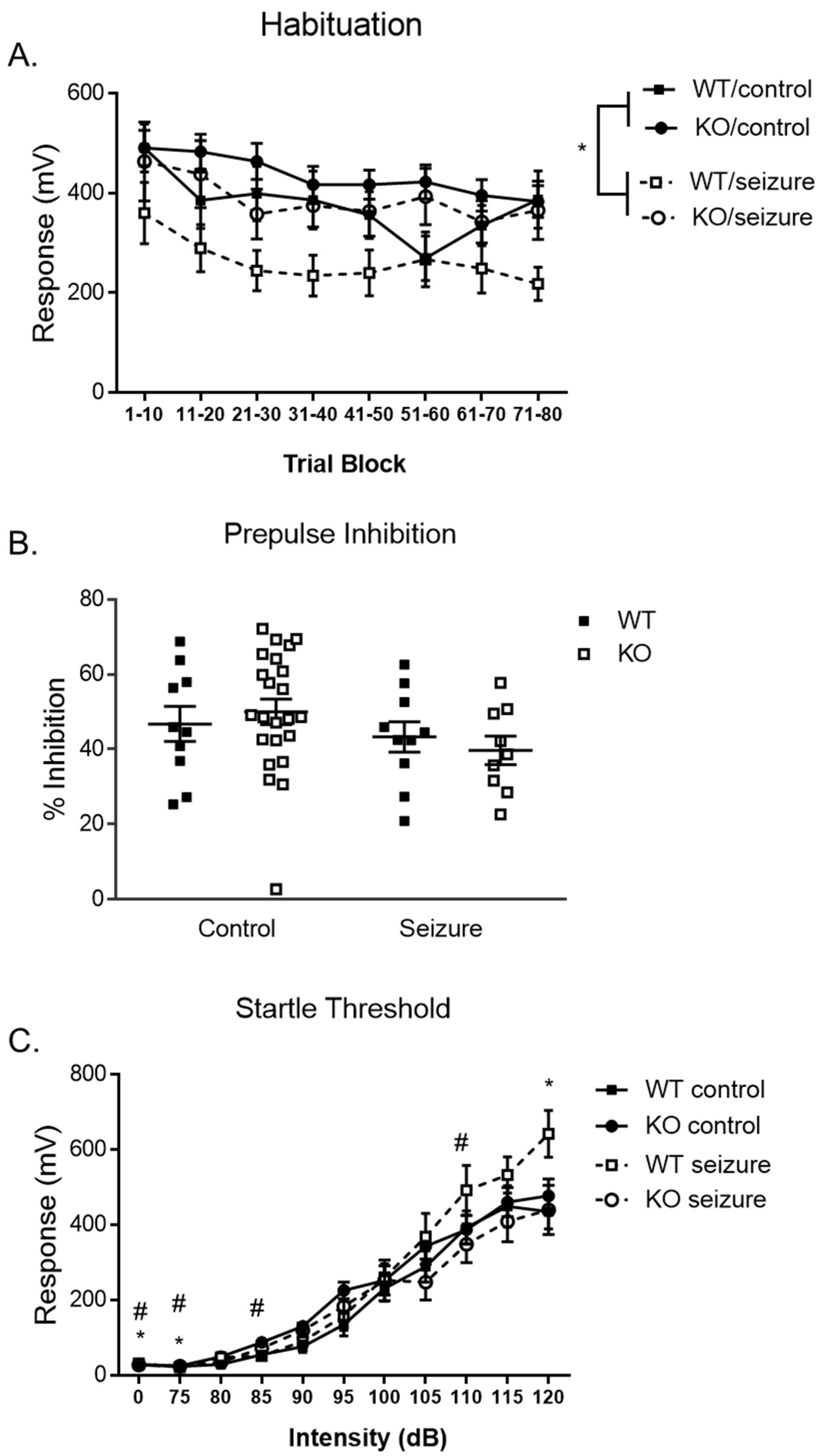
3.5. Early-Life Seizures Increase Habituation and Startle Response in the Pre-Pulse Inhibition Task
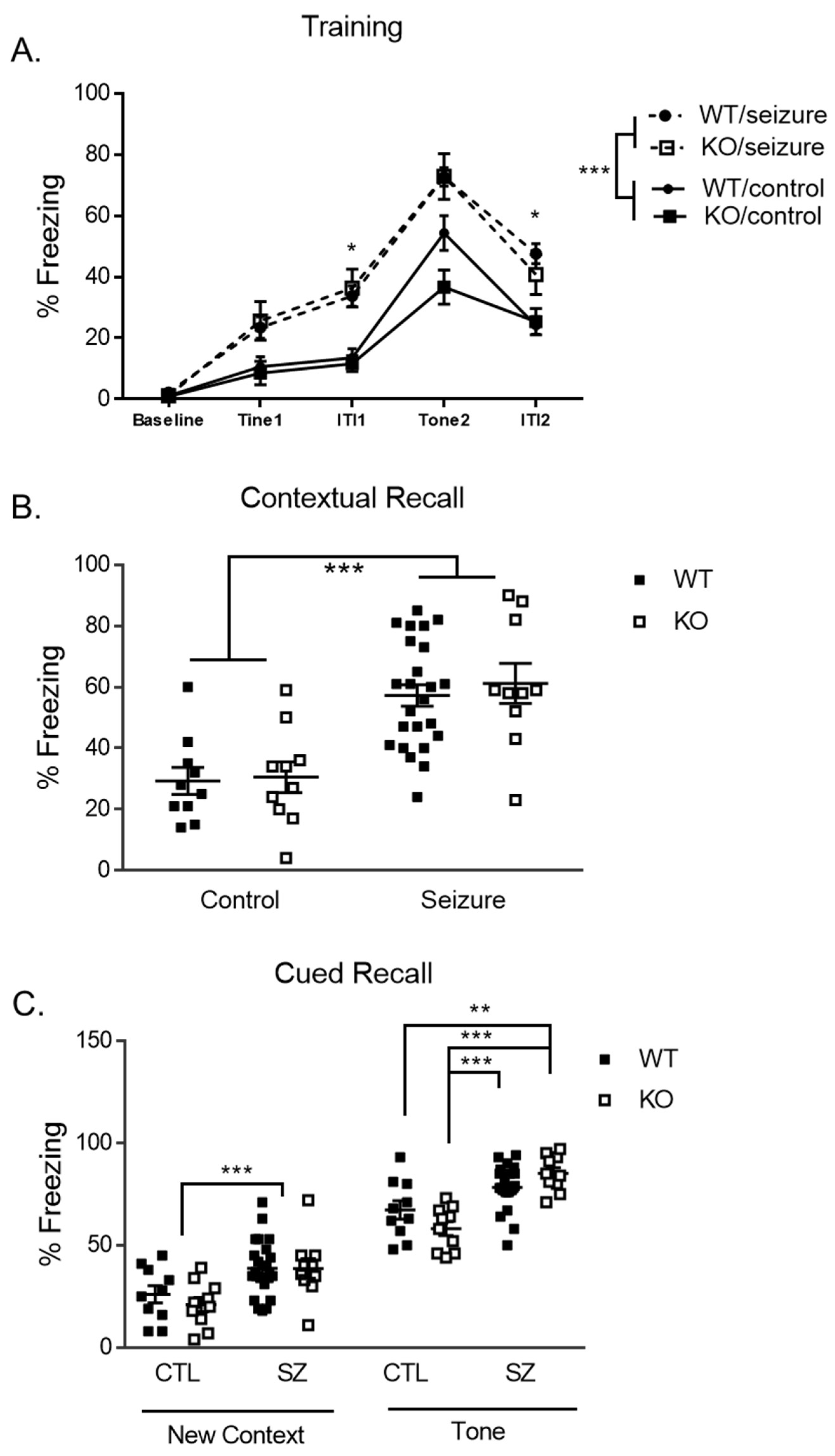
3.6. Early-Life Seizures Increase Contextual Recall While Tone-Cued Recall Is Increased within Fmr1 KO Mice
3.7. Early-Life Seizures Do Not Alter mTOR Signaling, Neural Inflammation, Scaffolding, or Ion Channel Protein Levels in Our Model Long-Term
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, J.; Rivero-Arias, O.; Angelov, A.; Kim, E.; Fotheringham, I.; Leal, J. Epidemiology of fragile X syndrome: A systematic review and meta-analysis. Am. J. Med. Genet. A 2014, 164, 1648–1658. [Google Scholar] [CrossRef]
- Westmark, C.J. Parental Reports on Early Autism Behaviors in Their Children with Fragile X Syndrome as a Function of Infant Feeding. Nutrients 2021, 13, 2888. [Google Scholar] [CrossRef]
- Boyle, L.; Kaufmann, W.E. The behavioral phenotype of FMR1 mutations. In American Journal of Medical Genetics Part C: Seminars in Medical Genetics; Wiley Subscription Services, Inc.: Hoboken, NJ, USA, 2010; Volume 154, pp. 469–476. [Google Scholar]
- Lozano, R.; Azarang, A.; Wilaisakditipakorn, T.; Hagerman, R.J. Fragile X syndrome: A review of clinical management. Intractable Rare Dis. Res. 2016, 5, 145–157. [Google Scholar] [CrossRef]
- Lubs, H.A. A marker X chromosome. Am. J. Hum. Genet. 1969, 21, 231–244. [Google Scholar] [PubMed]
- Berry-Kravis, E. Epilepsy in fragile X syndrome. Dev. Med. Child. Neurol. 2002, 44, 724–728. [Google Scholar] [CrossRef]
- Bailey, D.B.; Raspa, M.; Bishop, E.; Holiday, D. No Change in the Age of Diagnosis for Fragile X Syndrome: Findings From a National Parent Survey. Pediatrics 2009, 124, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Bernard, P.B.; Benke, T.A. Early life seizures: Evidence for chronic deficits linked to autism and intellectual disability across species and models. Exp. Neurol. 2015, 263, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.L.; Ben-Ari, Y. Seizures in the Developing Brain: Perhaps Not So Benign after All. Neuron 1998, 21, 1231–1234. [Google Scholar] [CrossRef]
- Kleen, J.K.; Sesqué, A.; Wu, E.X.; Miller, F.A.; Hernan, A.E.; Holmes, G.L.; Scott, R.C. Early-life seizures produce lasting alterations in the structure and function of the prefrontal cortex. Epilepsy Behav. 2011, 22, 214–219. [Google Scholar] [CrossRef]
- Smith, G.; Ahmed, N.; Arbuckle, E.; Lugo, J.N. Early-life status epilepticus induces long-term deficits in anxiety and spatial learning in mice. Int. J. Epilepsy 2017, 4, 36–45. [Google Scholar] [CrossRef]
- Nolan, S.O.; Hodges, S.L.; Lugo, J.N. High-throughput analysis of vocalizations reveals sex-specific changes in Fmr1 mutant pups. Genes. Brain Behav. 2020, 19, e12611. [Google Scholar] [CrossRef] [PubMed]
- Lipton, J.O.; Sahin, M. The neurology of mTOR. Neuron 2014, 84, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Brewster, A.L.; Clark, M.E.; Regnier-Golanov, A.; Sunnen, C.N.; Patil, V.V.; D’Arcangelo, G.; Anderson, A.E. mTOR inhibition suppresses established epilepsy in a mouse model of cortical dysplasia. Epilepsia 2015, 56, 636–646. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Anderson, A.E. mTOR-dependent alterations of Kv1.1 subunit expression in the neuronal subset-specific Pten knockout mouse model of cortical dysplasia with epilepsy. Sci. Rep. 2018, 8, 3568. [Google Scholar] [CrossRef]
- Lugo, J.N.; Smith, G.D.; Arbuckle, E.P.; White, J.; Holley, A.J.; Floruta, C.M.; Ahmed, N.; Gomez, M.C.; Okonkwo, O. Deletion of PTEN produces autism-like behavioral deficits and alterations in synaptic proteins. Front. Mol. Neurosci. 2014, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Sawicka, K.; Pyronneau, A.; Chao, M.; Bennett, M.V.; Zukin, R.S. Elevated ERK/p90 ribosomal S6 kinase activity underlies audiogenic seizure susceptibility in fragile X mice. Proc. Natl. Acad. Sci. USA 2016, 113, E6290–E6297. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Kaphzan, H.; Alvarez-Dieppa, A.C.; Murphy, J.P.; Pierre, P.; Klann, E. Genetic removal of p70 S6 kinase 1 corrects molecular, synaptic, and behavioral phenotypes in fragile X syndrome mice. Neuron 2012, 76, 325–337. [Google Scholar] [CrossRef]
- McIlwain, K.L.; Merriweather, M.Y.; Yuva-Paylor, L.A.; Paylor, R. The use of behavioral test batteries: Effects of training history. Physiol. Behav. 2001, 73, 705–717. [Google Scholar] [CrossRef]
- Lugo, J.N.; Swann, J.W.; Anderson, A.E. Early-life seizures result in deficits in social behavior and learning. Exp. Neurol. 2014, 256, 74–80. [Google Scholar] [CrossRef]
- Hodges, S.L.; Reynolds, C.D.; Nolan, S.O.; Huebschman, J.L.; Okoh, J.T.; Binder, M.S.; Lugo, J.N. A single early-life seizure results in long-term behavioral changes in the adult Fmr1 knockout mouse. Epilepsy Res. 2019, 157, 106193. [Google Scholar] [CrossRef]
- Tabolacci, E.; Pomponi, M.G.; Remondini, L.; Pietrobono, R.; Orteschi, D.; Nobile, V.; Pucci, C.; Musto, E.; Pane, M.; Mercuri, E.M.; et al. Co-Occurrence of Fragile X Syndrome with a Second Genetic Condition: Three Independent Cases of Double Diagnosis. Genes 2021, 12, 1909. [Google Scholar] [CrossRef] [PubMed]
- Dolan, B.M.; Duron, S.G.; Campbell, D.A.; Vollrath, B.; Rao, B.S.; Ko, H.-Y.; Lin, G.G.; Govindarajan, A.; Choi, S.-Y.; Tonegawa, S. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc. Natl. Acad. Sci. USA 2013, 110, 5671–5676. [Google Scholar] [CrossRef] [PubMed]
- Cogram, P.; Deacon, R.M.; Warner-Schmidt, J.L.; Von Schimmelmann, M.J.; Abrahams, B.S.; During, M.J. Gaboxadol normalizes behavioral abnormalities in a mouse model of fragile X syndrome. Front. Behav. Neurosci. 2019, 13, 141. [Google Scholar] [CrossRef]
- 25. Castelhano, A.S.S.; Cassane GD, S.T.; Scorza, F.A.; Cysneiros, R.M. Altered anxiety-related and abnormal social behaviors in rats exposed to early life seizures. Front. Behav. Neurosci. 2013, 7, 36. [Google Scholar] [CrossRef]
- Castelhano, A.S.S.; Cassane, G.d.S.T.; Scorza, F.A.; Cysneiros, R.M. Early life seizures in female rats lead to anxiety-related behavior and abnormal social behavior characterized by reduced motivation to novelty and deficit in social discrimination. J. Neural Transm. 2015, 122, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.J.; Hagerman, P.J. Fragile X Syndrome: Diagnosis, Treatment, and Research; Taylor & Francis: New York, NY, USA, 2002. [Google Scholar]
- Restivo, L.; Ferrari, F.; Passino, E.; Sgobio, C.; Bock, J.; Oostra, B.A.; Bagni, C.; Ammassari-Teule, M. Enriched environment promotes behavioral and morphological recovery in a mouse model for the fragile X syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 11557–11562. [Google Scholar] [CrossRef]
- Heulens, I.; D’Hulst, C.; Van Dam, D.; De Deyn, P.P.; Kooy, R.F. Pharmacological treatment of fragile X syndrome with GABAergic drugs in a knockout mouse model. Behav. Brain Res. 2012, 229, 244–249. [Google Scholar] [CrossRef]
- Liu, Z.-H.; Chuang, D.-M.; Smith, C.B. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. Int. J. Neuropsychopharmacol. 2011, 14, 618–630. [Google Scholar] [CrossRef]
- Peier, A.M.; McIlwain, K.L.; Kenneson, A.; Warren, S.T.; Paylor, R.; Nelson, D.L. (Over) correction of FMR1 deficiency with YAC transgenics: Behavioral and physical features. Hum. Mol. Genet. 2000, 9, 1145–1159. [Google Scholar] [CrossRef]
- Yuskaitis, C.J.; Mines, M.A.; King, M.K.; Sweatt, J.D.; Miller, C.A.; Jope, R.S. Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of Fragile X syndrome. Biochem. Pharmacol. 2010, 79, 632–646. [Google Scholar] [CrossRef]
- Yan, Q.; Asafo-Adjei, P.; Arnold, H.; Brown, R.; Bauchwitz, R. A phenotypic and molecular characterization of the fmr1-tm1Cgr fragile X mouse. Genes Brain Behav. 2004, 3, 337–359. [Google Scholar] [CrossRef]
- Spencer, C.M.; Alekseyenko, O.; Hamilton, S.M.; Thomas, A.M.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Modifying behavioral phenotypes in Fmr1KO mice: Genetic background differences reveal autistic-like responses. Autism Res. 2011, 4, 40–56. [Google Scholar] [CrossRef]
- Cordeiro, L.; Ballinger, E.; Hagerman, R.; Hessl, D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: Prevalence and characterization. J. Neurodev. Disord. 2011, 3, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; d’Amato, F.R.; Crusio, W.E. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Alekseyenko, O.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes. Brain Behav. 2005, 4, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Waltereit, R.; Japs, B.; Schneider, M.; de Vries, P.J.; Bartsch, D. Epilepsy and Tsc2 haploinsufficiency lead to autistic-like social deficit behaviors in rats. Behav. Genet. 2011, 41, 364–372. [Google Scholar] [CrossRef]
- Ding, Q.; Sethna, F.; Wang, H. Behavioral analysis of male and female Fmr1 knockout mice on C57BL/6 background. Behav. Brain Res. 2014, 271, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.A.; Tejada-Simon, M.V. Pharmacological Rescue of Hippocampal Fear Learning Deficits in Fragile X Syndrome. Mol. Neurobiol. 2018, 55, 5951–5961. [Google Scholar] [CrossRef]
- Van Dam, D.; D’Hooge, R.; Hauben, E.; Reyniers, E.; Gantois, I.; Bakker, C.E.; Oostra, B.A.; Kooy, R.F.; De Deyn, P.P. Spatial learning, contextual fear conditioning and conditioned emotional response in Fmr1 knockout mice. Behav. Brain Res. 2000, 117, 127–136. [Google Scholar] [CrossRef]
- Dobkin, C.; Rabe, A.; Dumas, R.; El Idrissi, A.; Haubenstock, H.; Brown, W.T. Fmr1 knockout mouse has a distinctive strain-specific learning impairmen. Neuroscience 2000, 100, 423–429. [Google Scholar] [CrossRef]
- Paradee, W.; Melikian, H.; Rasmussen, D.; Kenneson, A.; Conn, P.; Warren, S. Fragile X mouse: Strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience 1999, 94, 185–192. [Google Scholar] [CrossRef]
- Hagerman, R.J.; Berry-Kravis, E.; Hazlett, H.C.; Bailey, D.B.; Moine, H.; Kooy, R.F.; Tassone, F.; Gantois, I.; Sonenberg, N.; Mandel, J.L. Fragile X syndrome. Nat. Rev. Dis. Primers 2017, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- McLennan, Y.; Polussa, J.; Tassone, F.; Hagerman, R. Fragile x syndrome. Curr. Genom. 2011, 12, 216–224. [Google Scholar] [CrossRef] [PubMed]
- de Vrij, F.M.; Levenga, J.; Van der Linde, H.C.; Koekkoek, S.K.; De Zeeuw, C.I.; Nelson, D.L.; Oostra, B.A.; Willemsen, R. Rescue of behavioral phenotype and neuronal protrusion morphology in Fmr1 KO mice. Neurobiol. Dis. 2008, 31, 127–132. [Google Scholar] [CrossRef]
- Thomas, A.M.; Bui, N.; Perkins, J.R.; Yuva-Paylor, L.A.; Paylor, R. Group I metabotropic glutamate receptor antagonists alter select behaviors in a mouse model for fragile X syndrome. Psychopharmacology 2012, 219, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Veeraragavan, S.; Bui, N.; Perkins, J.R.; Yuva-Paylor, L.A.; Carpenter, R.L.; Paylor, R. Modulation of behavioral phenotypes by a muscarinic M1 antagonist in a mouse model of fragile X syndrome. Psychopharmacology 2011, 217, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Labbate, G.P.; da Silva, A.V.; Barbosa-Silva, R.C. Effect of severe neonatal seizures on prepulse inhibition and hippocampal volume of rats tested in early adulthood. Neurosci. Lett. 2014, 568, 62–66. [Google Scholar] [CrossRef]
- Koch, M.; Ebert, U. Deficient sensorimotor gating following seizures in amygdala-kindled rats. Biol. Psychiatry 1998, 44, 290–297. [Google Scholar] [CrossRef]
- Hoeffer, C.A.; Sanchez, E.; Hagerman, R.J.; Mu, Y.; Nguyen, D.V.; Wong, H.; Whelan, A.M.; Zukin, R.S.; Klann, E.; Tassone, F. Altered mTOR signaling and enhanced CYFIP2 expression levels in subjects with fragile X syndrome. Genes Brain Behav. 2012, 11, 332–341. [Google Scholar] [CrossRef]
- Testa, G.; Olimpico, F.; Pancrazi, L.; Borello, U.; Cattaneo, A.; Caleo, M.; Costa, M.; Mainardi, M. Cortical Seizures in FoxG1+/− Mice are Accompanied by Akt/S6 Overactivation, Excitation/Inhibition Imbalance and Impaired Synaptic Transmission. Int. J. Mol. Sci. 2019, 20, 4127. [Google Scholar] [CrossRef]
- Tokuda, S.; Mahaffey, C.L.; Monks, B.; Faulkner, C.R.; Birnbaum, M.J.; Danzer, S.C.; Frankel, W.N. A novel Akt3 mutation associated with enhanced kinase activity and seizure susceptibility in mice. Hum. Mol. Genet. 2010, 20, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.D.; Smith, G.; Jefferson, T.; Lugo, J.N. The effect of early life status epilepticus on ultrasonic vocalizations in mice. Epilepsia 2016, 57, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Hoeffer, C.A.; Takayasu, Y.; Miyawaki, T.; McBride, S.M.; Klann, E.; Zukin, R.S. Dysregulation of mTOR signaling in fragile X syndrome. J. Neurosci. 2010, 30, 694–702. [Google Scholar] [CrossRef]
- Sidhu, H.; Dansie, L.E.; Hickmott, P.W.; Ethell, D.W.; Ethell, I.M. Genetic removal of matrix metalloproteinase 9 rescues the symptoms of fragile X syndrome in a mouse model. J. Neurosci. 2014, 34, 9867–9879. [Google Scholar] [CrossRef] [PubMed]
- Saré, R.M.; Song, A.; Loutaev, I.; Cook, A.; Maita, I.; Lemons, A.; Sheeler, C.; Smith, C.B. Negative Effects of Chronic Rapamycin Treatment on Behavior in a Mouse Model of Fragile X Syndrome. Front. Mol. Neurosci. 2017, 10, 452. [Google Scholar] [CrossRef]



| Target | Group | |||
|---|---|---|---|---|
| Protein Levels | WT/CTL | WT/SZ | KO/CTL | KO/SZ |
| pS6/Total S6 | 100 ± 1.6 | 95.04 ± 2.0 | 98.56 ± 6.1 | 104.56 ± 2.5 |
| pAKT/Total Akt | 100 ± 6.7 | 96.99 ± 6.6 | 117.50 ± 20.5 | 129.71 ± 30.2 |
| GFAP | 100 ± 2.69 | 115.97 ± 11.6 | 115.54 ± 13.2 | 119.10 ± 18.6 |
| Iba-1 | 100 ± 9.2 | 115.35 ± 18.7 | 123.20 ± 39.3 | 96.49 ± 11.4 |
| HCN1 | 100 ± 12.4 | 93.32 ± 13.7 | 82.55 ± 15.4 | 109.35 ± 30.1 |
| HCN2 | 100 ± 16.0 | 95.39 ± 13.6 | 151.06 ± 42.2 | 72.74 ± 13.2 |
| Kv4.2 | 100 ± 10.6 | 109.31 ± 6.7 | 104.31 ± 18.9 | 105.43 ± 16.7 |
| PSD-95 | 100 ± 4.0 | 124.23 ± 16.5 | 102.32 ± 11.9 | 117.25 ± 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blandin, K.J.; Narvaiz, D.A.; Sullens, D.G.; Womble, P.D.; Hodges, S.L.; Binder, M.S.; Faust, A.; Nguyen, P.H.; Pranske, Z.J.; Lugo, J.N. A Two-Hit Approach Inducing Flurothyl Seizures in Fmr1 Knockout Mice Impacts Anxiety and Repetitive Behaviors. Brain Sci. 2024, 14, 892. https://doi.org/10.3390/brainsci14090892
Blandin KJ, Narvaiz DA, Sullens DG, Womble PD, Hodges SL, Binder MS, Faust A, Nguyen PH, Pranske ZJ, Lugo JN. A Two-Hit Approach Inducing Flurothyl Seizures in Fmr1 Knockout Mice Impacts Anxiety and Repetitive Behaviors. Brain Sciences. 2024; 14(9):892. https://doi.org/10.3390/brainsci14090892
Chicago/Turabian StyleBlandin, Katherine J., David A. Narvaiz, Donald Gregory Sullens, Paige D. Womble, Samantha L. Hodges, Matthew S. Binder, Amanda Faust, Phuoc H. Nguyen, Zachary J. Pranske, and Joaquin N. Lugo. 2024. "A Two-Hit Approach Inducing Flurothyl Seizures in Fmr1 Knockout Mice Impacts Anxiety and Repetitive Behaviors" Brain Sciences 14, no. 9: 892. https://doi.org/10.3390/brainsci14090892
APA StyleBlandin, K. J., Narvaiz, D. A., Sullens, D. G., Womble, P. D., Hodges, S. L., Binder, M. S., Faust, A., Nguyen, P. H., Pranske, Z. J., & Lugo, J. N. (2024). A Two-Hit Approach Inducing Flurothyl Seizures in Fmr1 Knockout Mice Impacts Anxiety and Repetitive Behaviors. Brain Sciences, 14(9), 892. https://doi.org/10.3390/brainsci14090892







