Monoaminergic Modulation of Learning and Cognitive Function in the Prefrontal Cortex
Abstract
1. Introduction
2. Monoamines and the Prefrontal Cortex: A Comprehensive Overview
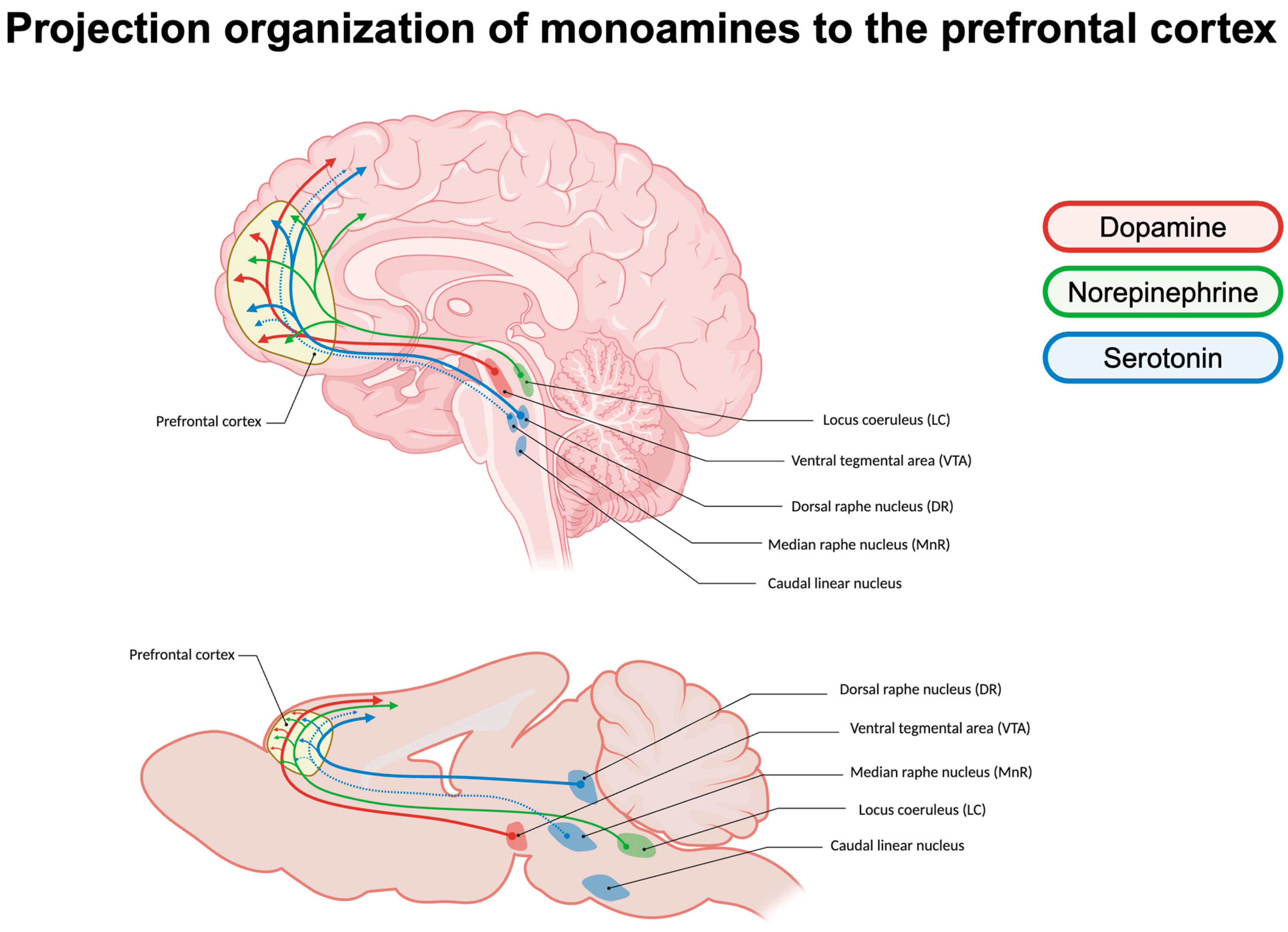
2.1. Projection Organization of Monoamines in the Prefrontal Cortex
2.1.1. Dopamine Signaling
2.1.2. Serotonin Signaling
2.1.3. Norepinephrine Signaling
2.2. Cellular Distribution of Monoamine Receptors in the Prefrontal Cortex
2.2.1. Dopamine Receptors
2.2.2. Serotonin Receptors
2.2.3. Norepinephrine Receptors
3. Monoamines and Behavior: Roles in Learning and Cognition
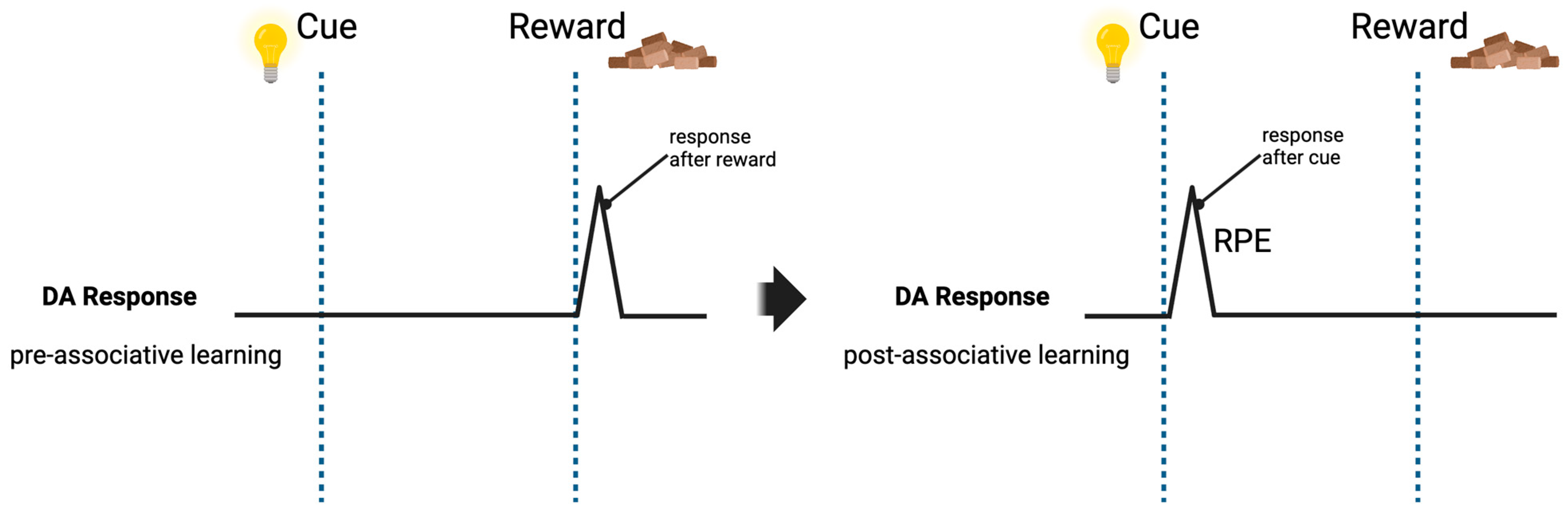
3.1. Dopamine: Reinforcement and Rule Coding
3.2. Serotonin: Aversive Learning, Cognitive Flexibility, and Social Reward Prediction
3.3. Norepinephrine: Attention, Working Memory, and Emotional Consolidation
4. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, S.V.; Chatterjee, U.; Kumar, D.; Siddiqui, A.; Goyal, N. Neuropsychology of prefrontal cortex. Indian J. Psychiatry 2008, 50, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Chen, A.; Li, Y.; Xing, X.; Lu, H. Medial prefrontal cortex in neurological diseases. Physiol. Genom. 2019, 51, 432–442. [Google Scholar] [CrossRef]
- Peters, G.J.; David, C.N.; Marcus, M.D.; Smith, D.M. The medial prefrontal cortex is critical for memory retrieval and resolving interference. Learn. Mem. 2013, 20, 201–209. [Google Scholar] [CrossRef]
- Wise, S.P. Forward frontal fields: Phylogeny and fundamental function. Trends Neurosci. 2008, 31, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Carlén, M. What constitutes the prefrontal cortex? Science 2017, 358, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.J.; Griffin, A.L. Representations of On-Going Behavior and Future Actions During a Spatial Working Memory Task by a High Firing-Rate Population of Medial Prefrontal Cortex Neurons. Front. Behav. Neurosci. 2020, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Small, D.M.; Gitelman, D.R.; Gregory, M.D.; Nobre, A.C.; Parrish, T.B.; Mesulam, M.M. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage 2003, 18, 633–641. [Google Scholar] [CrossRef]
- Seamans, J.K.; Floresco, S.B.; Phillips, A.G. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav. Neurosci. 1995, 109, 1063–1073. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. Cellular basis of working memory. Neuron 1995, 14, 477–485. [Google Scholar] [CrossRef]
- Domanski, A.P.F.; Kucewicz, M.T.; Russo, E.; Tricklebank, M.D.; Robinson, E.S.J.; Durstewitz, D.; Jones, M.W. Distinct hippocampal-prefrontal neural assemblies coordinate memory encoding, maintenance, and recall. Curr. Biol. 2023, 33, 1220–1236. [Google Scholar] [CrossRef]
- Euston, D.R.; Gruber, A.J.; McNaughton, B.L. The role of medial prefrontal cortex in memory and decision making. Neuron 2012, 76, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Wang, C.J.; Gamo, N.J.; Jin, L.E.; Mazer, J.A.; Morrison, J.H.; Wang, X.J.; Arnsten, A.F. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 2013, 77, 736–749. [Google Scholar] [CrossRef]
- Luo, L. Principles of Neurobiology, 2nd ed.; Garland Science: New York, NY, USA, 2020. [Google Scholar]
- Vugt, B.v.; Kerkoerle, T.v.; Vartak, D.; Roelfsema, P.R. The Contribution of AMPA and NMDA Receptors to Persistent Firing in the Dorsolateral Prefrontal Cortex in Working Memory. J. Neurosci. 2020, 40, 2458–2470. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jin, J.; Zhang, X.; Xu, H.; Yang, L.; Du, D.; Zeng, Q.; Tsien, J.Z.; Yu, H.; Cao, X. Forebrain NR2B Overexpression Facilitating the Prefrontal Cortex Long-Term Potentiation and Enhancing Working Memory Function in Mice. PLoS ONE 2011, 6, e20312. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Wang, M.J.; Paspalas, C.D. Neuromodulation of Thought: Flexibilities and Vulnerabilities in Prefrontal Cortical Network Synapses. Neuron 2012, 76, 223–239. [Google Scholar] [CrossRef]
- Lüscher, C.; Malenka, R.C. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 2012, 4, a005710. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Rein, B. Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: Pathophysiological implications. Mol. Psychiatry 2022, 27, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Santana, N.; Artigas, F. Laminar and Cellular Distribution of Monoamine Receptors in Rat Medial Prefrontal Cortex. Front. Neuroanat. 2017, 11, 87. [Google Scholar] [CrossRef]
- Ferguson, B.R.; Gao, W.J. PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front. Neural Circuits 2018, 12, 37. [Google Scholar] [CrossRef]
- Anastasiades, P.G.; Carter, A.G. Circuit organization of the rodent medial prefrontal cortex. Trends Neurosci. 2021, 44, 550–563. [Google Scholar] [CrossRef]
- Page, C.E.; Coutellier, L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: Evidence for over-inhibition. Neurosci. Biobehav. Rev. 2019, 105, 39–51. [Google Scholar] [CrossRef]
- Savalliya, M.a.G.; John, J. The Monoaminergic System in Humans. In Proceedings of the 12th National Science Symposium Recent Trends in Science and Technology-2020, Chongqing, China, 19 January 2020; pp. 190–203. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef] [PubMed]
- Jenni, N.L.; Larkin, J.D.; Floresco, S.B. Prefrontal Dopamine D(1) and D(2) Receptors Regulate Dissociable Aspects of Decision Making via Distinct Ventral Striatal and Amygdalar Circuits. J. Neurosci. 2017, 37, 6200–6213. [Google Scholar] [CrossRef]
- Ott, T.; Jacob, S.N.; Nieder, A. Dopamine Receptors Differentially Enhance Rule Coding in Primate Prefrontal Cortex Neurons. Neuron 2014, 84, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Weele, C.M.V.; Siciliano, C.A.; Tye, K.M. Dopamine tunes prefrontal outputs to orchestrate aversive processing. Brain Res. 2019, 1713, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.M.; Tassin, J.P.; Blanc, G.; Glowinski, J. Selective activation of the mesocortical DA system by stress. Nature 1976, 263, 242–244. [Google Scholar] [CrossRef]
- Abercrombie, E.D.; Keefe, K.A.; DiFrischia, D.S.; Zigmond, M.J. Differential Effect of Stress on In Vivo Dopamine Release in Striatum, Nucleus Accumbens, and Medial Frontal Cortex. J. Neurochem. 1989, 52, 1655–1658. [Google Scholar] [CrossRef] [PubMed]
- Lammel, S.; Ion, D.I.; Roeper, J.; Malenka, R.C. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 2011, 70, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Vander Weele, C.M.; Siciliano, C.A.; Matthews, G.A.; Namburi, P.; Izadmehr, E.M.; Espinel, I.C.; Nieh, E.H.; Schut, E.H.S.; Padilla-Coreano, N.; Burgos-Robles, A.; et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 2018, 563, 397–401. [Google Scholar] [CrossRef]
- Sesack, S.R.; Hawrylak, V.A.; Guido, M.A.; Levey, A.I. Cellular and Subcellular Localization of the Dopamine Transporter in Rat Cortex. In Advances in Pharmacology; Goldstein, D.S., Eisenhofer, G., McCarty, R., Eds.; Academic Press: Cambridge, MA, USA, 1997; Volume 42, pp. 171–174. [Google Scholar]
- Garris, P.A.; Wightman, R.M. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: An in vivo voltammetric study. J. Neurosci. 1994, 14, 442–450. [Google Scholar] [CrossRef]
- Cass, W.A.; Gerhardt, G.A. In vivo assessment of dopamine uptake in rat medial prefrontal cortex: Comparison with dorsal striatum and nucleus accumbens. J. Neurochem. 1995, 65, 201–207. [Google Scholar] [CrossRef]
- Morón, J.A.; Brockington, A.; Wise, R.A.; Rocha, B.A.; Hope, B.T. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: Evidence from knock-out mouse lines. J. Neurosci. 2002, 22, 389–395. [Google Scholar] [CrossRef]
- Devoto, P.; Flore, G.; Saba, P.; Scheggi, S.; Mulas, G.; Gambarana, C.; Spiga, S.; Gessa, G.L. Noradrenergic terminals are the primary source of α(2)-adrenoceptor mediated dopamine release in the medial prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Devoto, P.; Flore, G.; Saba, P.; Frau, R.; Gessa, G.L. Selective inhibition of dopamine-beta-hydroxylase enhances dopamine release from noradrenergic terminals in the medial prefrontal cortex. Brain Behav. 2015, 5, e00393. [Google Scholar] [CrossRef] [PubMed]
- Karoum, F.; Chrapusta, S.J.; Egan, M.F. 3-Methoxytyramine Is the Major Metabolite of Released Dopamine in the Rat Frontal Cortex: Reassessment of the Effects of Antipsychotics on the Dynamics of Dopamine Release and Metabolism in the Frontal Cortex, Nucleus Accumbens, and Striatum by a Simple Two Pool Model. J. Neurochem. 1994, 63, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Sharp, T.; Zetterström, T.; Ungerstedt, U. An In Vivo Study of Dopamine Release and Metabolism in Rat Brain Regions Using Intracerebral Dialysis. J. Neurochem. 1986, 47, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Whitaker-Azmitia, P.M.; Druse, M.; Walker, P.; Lauder, J.M. Serotonin as a developmental signal. Behav. Brain Res. 1996, 73, 19–29. [Google Scholar] [CrossRef]
- Kaumann, A.J.; Parsons, A.A.; Brown, A.M. Human arterial constrictor serotonin receptors. Cardiovasc. Res. 1993, 27, 2094–2103. [Google Scholar] [CrossRef]
- De Clerck, F.; Xhonneux, B.; Leysen, J.; Janssen, P.A.J. Evidence for functional 5-HT2 receptor sites on human blood platelets. Biochem. Pharmacol. 1984, 33, 2807–2811. [Google Scholar] [CrossRef]
- Yadav, V.K.; Oury, F.; Tanaka, K.F.; Thomas, T.; Wang, Y.; Cremers, S.; Hen, R.; Krust, A.; Chambon, P.; Karsenty, G. Leptin-dependent serotonin control of appetite: Temporal specificity, transcriptional regulation, and therapeutic implications. J. Exp. Med. 2010, 208, 41–52. [Google Scholar] [CrossRef]
- Lambe, E.K.; Fillman, S.G.; Webster, M.J.; Shannon Weickert, C. Serotonin receptor expression in human prefrontal cortex: Balancing excitation and inhibition across postnatal development. PLoS ONE 2011, 6, e22799. [Google Scholar] [CrossRef] [PubMed]
- Hornung, J.P. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003, 26, 331–343. [Google Scholar] [CrossRef]
- Walker, E.P.; Tadi, P. Neuroanatomy, Nucleus Raphe. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Celada, P.; Puig, M.V.; Artigas, F. Serotonin modulation of cortical neurons and networks. Front. Integr. Neurosci. 2013, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Mlinar, B.; Montalbano, A.; Piszczek, L.; Gross, C.; Corradetti, R. Firing Properties of Genetically Identified Dorsal Raphe Serotonergic Neurons in Brain Slices. Front. Cell Neurosci. 2016, 10, 195. [Google Scholar] [CrossRef]
- Celada, P.; Puig, M.V.; Casanovas, J.M.; Guillazo, G.; Artigas, F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: Involvement of serotonin-1A, GABA(A), and glutamate receptors. J. Neurosci. 2001, 21, 9917–9929. [Google Scholar] [CrossRef]
- Glaeser-Khan, S.; Savalia, N.K.; Cressy, J.; Feng, J.; Li, Y.; Kwan, A.C.; Kaye, A.P. Spatiotemporal Organization of Prefrontal Norepinephrine Influences Neuronal Activity. Eneuro 2024, 11, ENEURO.0252-0223.2024. [Google Scholar] [CrossRef] [PubMed]
- Tsetsenis, T.; Badyna, J.K.; Li, R.; Dani, J.A. Activation of a Locus Coeruleus to Dorsal Hippocampus Noradrenergic Circuit Facilitates Associative Learning. Front. Cell. Neurosci. 2022, 16, 887679. [Google Scholar] [CrossRef]
- Lee, M.; Mueller, A.; Moore, T. Differences in Noradrenaline Receptor Expression Across Different Neuronal Subtypes in Macaque Frontal Eye Field. Front. Neuroanat. 2020, 14, 574130. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Yang, J.-H.; Yu, A.; Glaeser-Khan, S.; Rondeau, J.A.; Feng, J.; Krystal, J.H.; Li, Y.; Kaye, A.P. Frontal Norepinephrine Represents a Threat Prediction Error Under Uncertainty. Biol. Psychiatry 2024, 96, 256–267. [Google Scholar] [CrossRef]
- Jodoj, E.; Chiang, C.; Aston-Jones, G. Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience 1998, 83, 63–79. [Google Scholar] [CrossRef]
- Sara, S.J.; Herve-Minvielle, A. Inhibitory influence of frontal cortex on locus coeruleus neurons. Proc. Natl. Acad. Sci. USA 1995, 92, 6032–6036. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, B.D.; Predale, H.K.; Plummer, N.W.; Jensen, P.; Chandler, D.J. Probing the structure and function of locus coeruleus projections to CNS motor centers. Front. Neural Circuits 2022, 16, 895481. [Google Scholar] [CrossRef] [PubMed]
- Vallone, D.; Picetti, R.; Borrelli, E. Structure and function of dopamine receptors. Neurosci. Biobehav. Rev. 2000, 24, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ott, T.; Nieder, A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Seamans, J.K.; Yang, C.R. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog. Neurobiol. 2004, 74, 1–58. [Google Scholar] [CrossRef]
- Santana, N.; Mengod, G.; Artigas, F. Quantitative Analysis of the Expression of Dopamine D1 and D2 Receptors in Pyramidal and GABAergic Neurons of the Rat Prefrontal Cortex. Cereb. Cortex 2008, 19, 849–860. [Google Scholar] [CrossRef]
- Puig, M.V.; Rose, J.; Schmidt, R.; Freund, N. Dopamine modulation of learning and memory in the prefrontal cortex: Insights from studies in primates, rodents, and birds. Front. Neural Circuits 2014, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Bordelon-Glausier, J.R.; Khan, Z.U.; Muly, E.C. Quantification of D1 and D5 dopamine receptor localization in layers I, III, and V of Macaca mulatta prefrontal cortical area 9: Coexpression in dendritic spines and axon terminals. J. Comp. Neurol. 2008, 508, 893–905. [Google Scholar] [CrossRef]
- Lidow, M.S.; Goldman-Rakic, P.S.; Gallager, D.W.; Rakic, P. Distribution of dopaminergic receptors in the primate cerebral cortex: Quantitative autoradiographic analysis using [3H]raclopride, [3H]spiperone and [3H]SCH23390. Neuroscience 1991, 40, 657–671. [Google Scholar] [CrossRef]
- Vincent, S.; Khan, Y.; Benes, F. Cellular distribution of dopamine D1 and D2 receptors in rat medial prefrontal cortex. J. Neurosci. 1993, 13, 2551–2564. [Google Scholar] [CrossRef]
- Xu, T.-X.; Yao, W.-D. D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proc. Natl. Acad. Sci. USA 2010, 107, 16366–16371. [Google Scholar] [CrossRef]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar]
- Pithadia, A.B.; Jain, S.M. 5-Hydroxytryptamine Receptor Subtypes and their Modulators with Therapeutic Potentials. J. Clin. Med. Res. 2009, 1, 72–80. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef]
- Ju, A.; Fernandez-Arroyo, B.; Wu, Y.; Jacky, D.; Beyeler, A. Expression of serotonin 1A and 2A receptors in molecular- and projection-defined neurons of the mouse insular cortex. Mol. Brain 2020, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.V.; Gulledge, A.T. Serotonin and prefrontal cortex function: Neurons, networks, and circuits. Mol. Neurobiol. 2011, 44, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zeng, L.; Zhao, S. Ligands of Adrenergic Receptors: A Structural Point of View. Biomolecules 2021, 11, 936. [Google Scholar] [CrossRef] [PubMed]
- Goldman-Rakic, P.S.; Lidow, M.S.; Gallager, D.W. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J. Neurosci. 1990, 10, 2125–2138. [Google Scholar] [CrossRef] [PubMed]
- U’PRICHARD, D.C.; Bechtel, W.D.; ROUOT, B.M.; SNYDER, S.H. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: Effect of 6-hydroxydopamine. Mol. Pharmacol. 1979, 16, 47–60. [Google Scholar]
- Aoki, C.; Venkatesan, C.; Kurose, H. Noradrenergic modulation of the prefrontal cortex as revealed by electron microscopic immunocytochemistry. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 42, pp. 777–780. [Google Scholar]
- Logue, S.F.; Gould, T.J. The neural and genetic basis of executive function: Attention, cognitive flexibility, and response inhibition. Pharmacol. Biochem. Behav. 2014, 123, 45–54. [Google Scholar] [CrossRef]
- Ramos, B.P.; Arnsten, A.F.T. Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol. Ther. 2007, 113, 523–536. [Google Scholar] [CrossRef]
- Devilbiss, D.M.; Waterhouse, B.D. Norepinephrine exhibits two distinct profiles of action on sensory cortical neuron responses to excitatory synaptic stimuli. Synapse 2000, 37, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Handler, A.; Graham, T.G.W.; Cohn, R.; Morantte, I.; Siliciano, A.F.; Zeng, J.; Li, Y.; Ruta, V. Distinct Dopamine Receptor Pathways Underlie the Temporal Sensitivity of Associative Learning. Cell 2019, 178, 60–75.e19. [Google Scholar] [CrossRef] [PubMed]
- Popescu, A.T.; Zhou, M.R.; Poo, M.-m. Phasic dopamine release in the medial prefrontal cortex enhances stimulus discrimination. Proc. Natl. Acad. Sci. USA 2016, 113, E3169–E3176. [Google Scholar] [CrossRef]
- Schultz, W. Behavioral Theories and the Neurophysiology of Reward. Annu. Rev. Psychol. 2006, 57, 87–115. [Google Scholar] [CrossRef] [PubMed]
- Grogan, J.P.; Tsivos, D.; Smith, L.; Knight, B.E.; Bogacz, R.; Whone, A.; Coulthard, E.J. Effects of dopamine on reinforcement learning and consolidation in Parkinson’s disease. eLife 2017, 6, e26801. [Google Scholar] [CrossRef]
- Schultz, W.; Apicella, P.; Ljungberg, T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 1993, 13, 900–913. [Google Scholar] [CrossRef]
- Schultz, W.; Dayan, P.; Montague, P.R. A neural substrate of prediction and reward. Science 1997, 275, 1593–1599. [Google Scholar] [CrossRef]
- Marinelli, M.; McCutcheon, J.E. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience 2014, 282, 176–197. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.C.; Ford, K.A.; Pagels, N.E.; McCutcheon, J.E.; Marinelli, M. Adolescents are more vulnerable to cocaine addiction: Behavioral and electrophysiological evidence. J. Neurosci. 2013, 33, 4913–4922. [Google Scholar] [CrossRef]
- Espejo, E.F. Selective dopamine depletion within the medial prefrontal cortex induces anxiogenic-like effects in rats placed on the elevated plus maze. Brain Res. 1997, 762, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Paraouty, N.; Rizzuto, C.R.; Sanes, D.H. Dopaminergic signaling supports auditory social learning. Sci. Rep. 2021, 11, 13117. [Google Scholar] [CrossRef]
- Rybicki, A.J.; Sowden, S.L.; Schuster, B.; Cook, J.L. Dopaminergic challenge dissociates learning from primary versus secondary sources of information. eLife 2022, 11, e74893. [Google Scholar] [CrossRef]
- Solié, C.; Girard, B.; Righetti, B.; Tapparel, M.; Bellone, C. VTA dopamine neuron activity encodes social interaction and promotes reinforcement learning through social prediction error. Nat. Neurosci. 2022, 25, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Mack, N.R.; Zhang, Y.-X.; McEachern, E.P.; Gao, W.-J. Distinct Roles for Prefrontal Dopamine D1 and D2 Neurons in Social Hierarchy. J. Neurosci. 2022, 42, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Onge, J.R.S.; Abhari, H.; Floresco, S.B. Dissociable Contributions by Prefrontal D1 and D2 Receptors to Risk-Based Decision Making. J. Neurosci. 2011, 31, 8625–8633. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T. Catecholamine regulation of the prefrontal cortex. J. Psychopharmacol. 1997, 11, 151–162. [Google Scholar] [CrossRef]
- Williams, G.V.; Castner, S.A. Under the curve: Critical issues for elucidating D1 receptor function in working memory. Neuroscience 2006, 139, 263–276. [Google Scholar] [CrossRef]
- Cools, R.; D’Esposito, M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef]
- Apitz, T.; Bunzeck, N. Dopamine controls the neural dynamics of memory signals and retrieval accuracy. Neuropsychopharmacology 2013, 38, 2409–2417. [Google Scholar] [CrossRef]
- Swart, E.K.; Sikkema-de Jong, M.T. The effects of increased dopamine-levels on attentional control during reading and reading comprehension. Curr. Psychol. 2023, 42, 11009–11025. [Google Scholar] [CrossRef]
- Vijayraghavan, S.; Wang, M.; Birnbaum, S.G.; Williams, G.V.; Arnsten, A.F.T. Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 2007, 10, 376–384. [Google Scholar] [CrossRef]
- Cools, R.; Arnsten, A.F.T. Neuromodulation of prefrontal cortex cognitive function in primates: The powerful roles of monoamines and acetylcholine. Neuropsychopharmacology 2022, 47, 309–328. [Google Scholar] [CrossRef]
- Sagheddu, C.; Cancedda, E.; Bagheri, F.; Kalaba, P.; Muntoni, A.L.; Lubec, J.; Lubec, G.; Sanna, F.; Pistis, M. The Atypical Dopamine Transporter Inhibitor CE-158 Enhances Dopamine Neurotransmission in the Prefrontal Cortex of Male Rats: A Behavioral, Electrophysiological, and Microdialysis Study. Int. J. Neuropsychopharmacol. 2023, 26, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Sagheddu, C.; Pintori, N.; Kalaba, P.; Dragačević, V.; Piras, G.; Lubec, J.; Simola, N.; De Luca, M.A.; Lubec, G.; Pistis, M. Neurophysiological and Neurochemical Effects of the Putative Cognitive Enhancer (S)-CE-123 on Mesocorticolimbic Dopamine System. Biomolecules 2020, 10, 779. [Google Scholar] [CrossRef] [PubMed]
- Nikiforuk, A.; Kalaba, P.; Ilic, M.; Korz, V.; Dragačević, V.; Wackerlig, J.; Langer, T.; Höger, H.; Golebiowska, J.; Popik, P.; et al. A Novel Dopamine Transporter Inhibitor CE-123 Improves Cognitive Flexibility and Maintains Impulsivity in Healthy Male Rats. Front. Behav. Neurosci. 2017, 11, 21–29. [Google Scholar] [CrossRef]
- Zvejniece, L.; Svalbe, B.; Vavers, E.; Makrecka-Kuka, M.; Makarova, E.; Liepins, V.; Kalvinsh, I.; Liepinsh, E.; Dambrova, M. S-phenylpiracetam, a selective DAT inhibitor, reduces body weight gain without influencing locomotor activity. Pharmacol. Biochem. Behav. 2017, 160, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Nesbit, M.O.; Ahn, S.; Zou, H.; Floresco, S.B.; Phillips, A.G. Potentiation of prefrontal cortex dopamine function by the novel cognitive enhancer d-govadine. Neuropharmacology 2024, 246, 109849. [Google Scholar] [CrossRef]
- Auger, M.L.; Meccia, J.; Phillips, A.G.; Floresco, S.B. Amelioration of cognitive impairments induced by GABA hypofunction in the male rat prefrontal cortex by direct and indirect dopamine D1 agonists SKF-81297 and d-Govadine. Neuropharmacology 2020, 162, 107844. [Google Scholar] [CrossRef] [PubMed]
- Dalton, G.L.; Floresco, S.B.; Phillips, A.G. Differential effects of d- and l-enantiomers of govadine on distinct forms of cognitive flexibility and a comparison with dopaminergic drugs. Psychopharmacology 2021, 238, 1069–1085. [Google Scholar] [CrossRef] [PubMed]
- Hindi Attar, C.; Finckh, B.; Büchel, C. The Influence of Serotonin on Fear Learning. PLoS ONE 2012, 7, e42397. [Google Scholar] [CrossRef]
- Wellman, C.L.; Izquierdo, A.; Garrett, J.E.; Martin, K.P.; Carroll, J.; Millstein, R.; Lesch, K.-P.; Murphy, D.L.; Holmes, A. Impaired Stress-Coping and Fear Extinction and Abnormal Corticolimbic Morphology in Serotonin Transporter Knock-Out Mice. J. Neurosci. 2007, 27, 684–691. [Google Scholar] [CrossRef]
- Grossman, C.D.; Bari, B.A.; Cohen, J.Y. Serotonin neurons modulate learning rate through uncertainty. Curr. Biol. 2022, 32, 586–599.e587. [Google Scholar] [CrossRef]
- Matias, S.; Lottem, E.; Dugué, G.P.; Mainen, Z.F. Activity patterns of serotonin neurons underlying cognitive flexibility. eLife 2017, 6, e20552. [Google Scholar] [CrossRef]
- Iigaya, K.; Fonseca, M.S.; Murakami, M.; Mainen, Z.F.; Dayan, P. An effect of serotonergic stimulation on learning rates for rewards apparent after long intertrial intervals. Nat. Commun. 2018, 9, 2477. [Google Scholar] [CrossRef]
- Michely, J.; Eldar, E.; Erdman, A.; Martin, I.M.; Dolan, R.J. Serotonin modulates asymmetric learning from reward and punishment in healthy human volunteers. Commun. Biol. 2022, 5, 812. [Google Scholar] [CrossRef]
- Luo, Q.; Kanen, J.W.; Bari, A.; Skandali, N.; Langley, C.; Knudsen, G.M.; Alsiö, J.; Phillips, B.U.; Sahakian, B.J.; Cardinal, R.N.; et al. Comparable roles for serotonin in rats and humans for computations underlying flexible decision-making. Neuropsychopharmacology 2024, 49, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Lapiz-Bluhm, M.D.S.; Soto-Piña, A.E.; Hensler, J.G.; Morilak, D.A. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology 2009, 202, 329–341. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanen, J.W.; Apergis-Schoute, A.M.; Yellowlees, R.; Arntz, F.E.; van der Flier, F.E.; Price, A.; Cardinal, R.N.; Christmas, D.M.; Clark, L.; Sahakian, B.J.; et al. Serotonin depletion impairs both Pavlovian and instrumental reversal learning in healthy humans. Mol. Psychiatry 2021, 26, 7200–7210. [Google Scholar] [CrossRef]
- Clarke, H.F.; Dalley, J.W.; Crofts, H.S.; Robbins, T.W.; Roberts, A.C. Cognitive inflexibility after prefrontal serotonin depletion. Science 2004, 304, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.F.; Walker, S.C.; Crofts, H.S.; Dalley, J.W.; Robbins, T.W.; Roberts, A.C. Prefrontal Serotonin Depletion Affects Reversal Learning But Not Attentional Set Shifting. J. Neurosci. 2005, 25, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Alsiö, J.; Lehmann, O.; McKenzie, C.; Theobald, D.E.; Searle, L.; Xia, J.; Dalley, J.W.; Robbins, T.W. Serotonergic Innervations of the Orbitofrontal and Medial-prefrontal Cortices are Differentially Involved in Visual Discrimination and Reversal Learning in Rats. Cereb. Cortex 2020, 31, 1090–1105. [Google Scholar] [CrossRef]
- Boulougouris, V.; Glennon, J.C.; Robbins, T.W. Dissociable Effects of Selective 5-HT2A and 5-HT2C Receptor Antagonists on Serial Spatial Reversal Learning in Rats. Neuropsychopharmacology 2008, 33, 2007–2019. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Renner, M.C.; Gonzalez, M.C.; Weisstaub, N. Role of medial prefrontal cortex serotonin 2A receptors in the control of retrieval of recognition memory in rats. J. Neurosci. 2013, 33, 15716–15725. [Google Scholar] [CrossRef]
- van Erp, A.M.; Miczek, K.A. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J. Neurosci. 2000, 20, 9320–9325. [Google Scholar] [CrossRef]
- Frey, A.-L.; McCabe, C. Effects of serotonin and dopamine depletion on neural prediction computations during social learning. Neuropsychopharmacology 2020, 45, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Janet, R.; Ligneul, R.; Losecaat-Vermeer, A.B.; Philippe, R.; Bellucci, G.; Derrington, E.; Park, S.Q.; Dreher, J.-C. Regulation of social hierarchy learning by serotonin transporter availability. Neuropsychopharmacology 2022, 47, 2205–2212. [Google Scholar] [CrossRef]
- McGaughy, J.; Ross, R.S.; Eichenbaum, H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 2008, 153, 63–71. [Google Scholar] [CrossRef]
- Berridge, C.W.; Spencer, R.C. Differential cognitive actions of norepinephrine a2 and a1 receptor signaling in the prefrontal cortex. Brain Res. 2016, 1641, 189–196. [Google Scholar] [CrossRef]
- Lapiz, M.D.S.; Morilak, D.A. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 2006, 137, 1039–1049. [Google Scholar] [CrossRef]
- De Martino, B.; Strange, B.A.; Dolan, R.J. Noradrenergic neuromodulation of human attention for emotional and neutral stimuli. Psychopharmacology 2008, 197, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ming, X.; Mulvey, M.; Mohanty, S.; Patel, V. Safety and efficacy of clonidine and clonidine extended-release in the treatment of children and adolescents with attention deficit and hyperactivity disorders. Adolesc. Health Med. Ther. 2011, 2, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Coradazzi, M.; Gulino, R.; Fieramosca, F.; Falzacappa, L.V.; Riggi, M.; Leanza, G. Selective noradrenaline depletion impairs working memory and hippocampal neurogenesis. Neurobiol. Aging 2016, 48, 93–102. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Goldman-Rakic, P.S. α2-Adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science 1985, 230, 1273–1276. [Google Scholar] [CrossRef]
- Cai, J.X.; Ma, Y.-y.; Xu, L.; Hu, X.-t. Reserpine impairs spatial working memory performance in monkeys: Reversal by the α2-adrenergic agonist clonidine. Brain Res. 1993, 614, 191–196. [Google Scholar]
- Marrs, W.; Kuperman, J.; Avedian, T.; Roth, R.H.; Jentsch, J.D. Alpha-2 Adrenoceptor Activation Inhibits Phencyclidine-Induced Deficits of Spatial Working Memory in Rats. Neuropsychopharmacology 2005, 30, 1500–1510. [Google Scholar] [CrossRef][Green Version]
- Wang, M.; Ramos, B.P.; Paspalas, C.D.; Shu, Y.; Simen, A.; Duque, A.; Vijayraghavan, S.; Brennan, A.; Dudley, A.; Nou, E.; et al. α2A-Adrenoceptors Strengthen Working Memory Networks by Inhibiting cAMP-HCN Channel Signaling in Prefrontal Cortex. Cell 2007, 129, 397–410. [Google Scholar] [CrossRef]
- Zhang, Z.; Cordeiro Matos, S.; Jego, S.; Adamantidis, A.; Séguéla, P. Norepinephrine Drives Persistent Activity in Prefrontal Cortex via Synergistic α1 and α2 Adrenoceptors. PLoS ONE 2013, 8, e66122. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Yang, S.-T.; Galvin, V.C.; Solder, J.; Luo, F.; Morozov, Y.M.; Arellano, J.; Duque, A.; Rakic, P.; Arnsten, A.F.T.; et al. Noradrenergic α1-Adrenoceptor Actions in the Primate Dorsolateral Prefrontal Cortex. J. Neurosci. 2019, 39, 2722–2734. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kojima, M.; Koyanagi, Y.; Adachi, K.; Imamura, K.; Koshikawa, N. Presynaptic and postsynaptic modulation of glutamatergic synaptic transmission by activation of α1- and β-adrenoceptors in layer V pyramidal neurons of rat cerebral cortex. Synapse 2009, 63, 269–281. [Google Scholar] [CrossRef]
- Harley, C.W. A role for norepinephrine in arousal, emotion and learning?: Limbic modulation by norepinephrine and the Kety hypothesis. Prog. Neuropsychopharmacol. Biol. Psychiatry 1987, 11, 419–458. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Real, E.; Takamiya, K.; Kang, M.G.; Ledoux, J.; Huganir, R.L.; Malinow, R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell 2007, 131, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Nakane, H.; Shimizu, N.; Hori, T. Stress-induced norepinephrine release in the rat prefrontal cortex measured by microdialysis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1994, 267, R1559–R1566. [Google Scholar] [CrossRef]
- Berridge, C.W.; Waterhouse, B.D. The locus coeruleus-noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003, 42, 33–84. [Google Scholar] [CrossRef] [PubMed]
- Giustino, T.F.; Maren, S. Noradrenergic Modulation of Fear Conditioning and Extinction. Front. Behav. Neurosci. 2018, 12, 43. [Google Scholar] [CrossRef]
- Uematsu, A.; Tan, B.Z.; Ycu, E.A.; Cuevas, J.S.; Koivumaa, J.; Junyent, F.; Kremer, E.J.; Witten, I.B.; Deisseroth, K.; Johansen, J.P. Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat. Neurosci. 2017, 20, 1602–1611. [Google Scholar] [CrossRef]
| Receptor Family | Binding Mechanism | Ligand | Behavior |
|---|---|---|---|
| D1-like (D1 and D5) | Gs coupled | Agonist | Social dominance (mice) |
| Antagonist | Impaired social learning (humans) | ||
| D2-like (D2, D3, D4) | Gi/o coupled | Agonist | Social submission (mice) |
| 5-HT2A | Gq coupled | Antagonist | Impaired reversal learning (rats) |
| 5-HT2C | Gq coupled | Antagonist | Improved reversal learning (rats) |
| α1 | Gq coupled | Agonist | (in mPFC) Improves attentional set-shifting task performance (rats) |
| α2 | Gi/o coupled | Agonist | Ameliorates spatial working memory deficits (rats, primates) |
| β | Gs coupled | Antagonist | Impairs detection of stimuli and attention independent of target valence (humans) Improves working memory (primates) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyle, N.; Betts, S.; Lu, H. Monoaminergic Modulation of Learning and Cognitive Function in the Prefrontal Cortex. Brain Sci. 2024, 14, 902. https://doi.org/10.3390/brainsci14090902
Boyle N, Betts S, Lu H. Monoaminergic Modulation of Learning and Cognitive Function in the Prefrontal Cortex. Brain Sciences. 2024; 14(9):902. https://doi.org/10.3390/brainsci14090902
Chicago/Turabian StyleBoyle, Natalie, Sarah Betts, and Hui Lu. 2024. "Monoaminergic Modulation of Learning and Cognitive Function in the Prefrontal Cortex" Brain Sciences 14, no. 9: 902. https://doi.org/10.3390/brainsci14090902
APA StyleBoyle, N., Betts, S., & Lu, H. (2024). Monoaminergic Modulation of Learning and Cognitive Function in the Prefrontal Cortex. Brain Sciences, 14(9), 902. https://doi.org/10.3390/brainsci14090902






