Functional MRI Assessment of Brain Activity Patterns Associated with Reading in Medulloblastoma Survivors
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Reading Scores
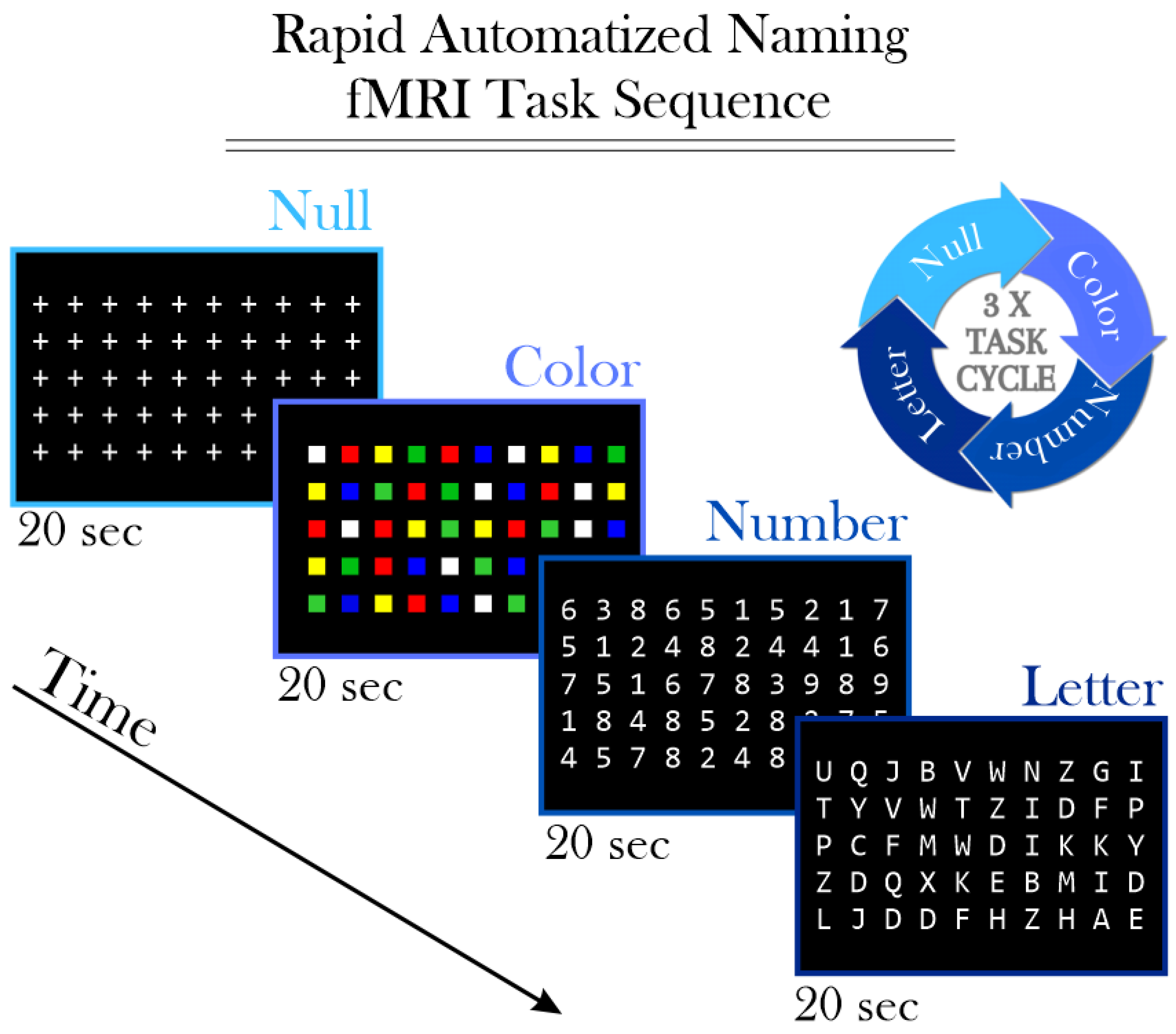
2.3. Functional Magnetic Resonance Imaging (fMRI)
2.4. Leave-One-Out Cross-Validation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carta, R.; Del Baldo, G.; Miele, E.; Po, A.; Besharat, Z.M.; Nazio, F.; Colafati, G.S.; Piccirilli, E.; Agolini, E.; Rinelli, M.; et al. Cancer Predisposition Syndromes and Medulloblastoma in the Molecular Era. Front. Oncol. 2020, 10, 566822. [Google Scholar] [CrossRef] [PubMed]
- Kombogiorgas, D. The Medulloblastoma Book. In Capitolo 1: Introduction to Medulloblastoma; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 1–12. [Google Scholar]
- Gupta, N.; Banerjee, A.; Haas-Kogan, D. Chapter 5: Embryonal tumors. In Pediatric CNS Tumors; Springer: Berlin/Heidelberg, Germany, 2016; pp. 93–104. [Google Scholar]
- Gibson, P.; Tong, Y.; Robinson, G.; Thompson, M.C.; Currle, D.S.; Eden, C.; Kranenburg, T.A.; Hogg, T.; Poppleton, H.; Martin, J.; et al. Subtypes of medulloblastoma have distinct developmental origins. Nature 2010, 468, 1095–1099. [Google Scholar] [CrossRef] [PubMed]
- McAfee, S.S.; Liu, Y.; Sillitoe, R.V.; Heck, D.H. Cerebellar Coordination of Neuronal Communication in Cerebral Cortex. Front. Syst. Neurosci. 2022, 15, 781527. [Google Scholar] [CrossRef]
- van der Heijden, M.E. Converging and Diverging Cerebellar Pathways for Motor and Social Behaviors in Mice. Cerebellum 2024. [Google Scholar] [CrossRef] [PubMed]
- Schakelaar, M.Y.; Monnikhof, M.; Crnko, S.; Pijnappel, E.W.; Meeldijk, J.; Broeke, T.T.; Bovenschen, N. Cellular immunotherapy for medulloblastoma. Neuro-Oncol. 2023, 25, 617–627. [Google Scholar] [CrossRef]
- Stripay, J.L.; Merchant, T.E.; Roussel, M.F.; Tinkle, C.L. Preclinical Models of Craniospinal Irradiation for Medulloblastoma. Cancers 2020, 12, 133. [Google Scholar] [CrossRef]
- Zou, P.; Conklin, H.M.; Scoggins, M.A.; Li, Y.; Li, X.; Jones, M.M.; Palmer, S.L.; Gajjar, A.; Ogg, R.J. Functional MRI in medulloblastoma survivors supports prophylactic reading intervention during tumor treatment. Brain Imaging Behav. 2016, 10, 258–271. [Google Scholar] [CrossRef]
- Shaik, S.; Maegawa, S.; Gopalakrishnan, V. Medulloblastoma: Novel insights into emerging therapeutic targets. Expert Opin. Ther. Targets 2021, 25, 615–619. [Google Scholar] [CrossRef]
- Palmer, S.L.; Armstrong, C.; Onar-Thomas, A.; Wu, S.; Wallace, D.; Bonner, M.J.; Schreiber, J.; Swain, M.; Chapieski, L.; Mabbott, D.; et al. Processing Speed, Attention, and Working Memory After Treatment for Medulloblastoma: An International, Prospective, and Longitudinal Study. J. Clin. Oncol. 2013, 31, 3494–3500. [Google Scholar] [CrossRef]
- Beaujean, A.A.; Woodhouse, N. Wechsler Intelligence Scale for Children (WISC) in the Wiley Encyclopedia of Personality Individual Differences; Carducci, B.J., Nave, C.S., Carducci, B.J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Davis, J.L.; Matthews, R.N. NEPSY-II Review: Korkman, M., Kirk, U., & Kemp, S. (2007). NEPSY—Second Edition (NEPSY-II). San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 2010, 28, 175–182. [Google Scholar] [CrossRef]
- Lumniczky, K.; Szatmári, T.; Sáfrány, G. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front. Immunol. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed]
- Cramer, C.K.; Cummings, T.L.; Andrews, R.N.; Strowd, R.; Rapp, S.R.; Shaw, E.G.; Chan, M.D.; Lesser, G.J. Treatment of Radiation-Induced Cognitive Decline in Adult Brain Tumor Patients. Curr. Treat. Options Oncol. 2019, 20, 42. [Google Scholar] [CrossRef] [PubMed]
- Frange, P.; Alapetite, C.; Gaboriaud, G.; Bours, D.; Zucker, J.M.; Zerah, M.; Brisse, H.; Chevignard, M.; Mosseri, V.; Bouffet, E.; et al. From childhood to adulthood: Long-term outcome of medulloblastoma patients. The Institut Curie experience (1980–2000). J. Neuro-Oncol. 2009, 95, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.; Baqai, M.W.S.; Shamim, M.S. Quality of Life in Paediatric Survivors of Medulloblastoma. JPMA J. Pak. Med. Assoc. 2022, 72, 2339–2340. [Google Scholar]
- de Spéville, E.D.; Kieffer, V.; Dufour, C.; Grill, J.; Noulhiane, M.; Hertz-Pannier, L.; Chevignard, M. Neuropsychological consequences of childhood medulloblastoma and possible interventions: A review. Neurochirurgie 2021, 67, 90–98. [Google Scholar] [CrossRef]
- Knight, S.J.; Conklin, H.M.; Palmer, S.L.; Schreiber, J.E.; Armstrong, C.L.; Wallace, D.; Bonner, M.; Swain, M.A.; Evankovich, K.D.; Mabbott, D.J.; et al. Working memory abilities among children treated for medulloblastoma: Parent report and child performance. J. Pediatr. Psychol. 2014, 39, 501–511. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reichle, E.D.; Rayner, K. Cognitive Processing and Models of Reading. In Models of the Visual System; Hung, G.K., Ciuffreda, K.J., Eds.; Topics in Biomedical Engineering International Book Series; Springer: Boston, MA, USA, 2002. [Google Scholar] [CrossRef]
- Woolnough, O.; Donos, C.; Rollo, P.S.; Forseth, K.J.; Lakretz, Y.; Crone, N.E.; Fischer-Baum, S.; Dehaene, S.; Tandon, N. Spatiotemporal dynamics of orthographic and lexical processing in the ventral visual pathway. Nat. Hum. Behav. 2021, 5, 389–398. [Google Scholar] [CrossRef]
- Taylor, J.; Davis, M.H.; Rastle, K. Mapping visual symbols onto spoken language along the ventral visual stream. Proc. Natl. Acad. Sci. USA 2019, 116, 17723–17728. [Google Scholar] [CrossRef]
- Thomas, M.; David, R.; Frank, T. Temporal Limitations in Object Processing Across the Human Ventral Visual Pathway. J. Neurophysiol. 2007, 98, 382–393. [Google Scholar] [CrossRef]
- Thesen, T.; McDonald, C.R.; Carlson, C.; Doyle, W.; Cash, S.; Sherfey, J.; Felsovalyi, O.; Girard, H.; Barr, W.; Devinsky, O.; et al. Sequential then interactive processing of letters and words in the left fusiform gyrus. Nat. Commun. 2012, 3, 1284. [Google Scholar] [CrossRef]
- Moore, M.W.; Durisko, C.; Perfetti, C.A.; Fiez, J.A. Learning to read an alphabet of human faces produces left-lateralized training effects in the fusiform gyrus. J. Cogn. Neurosci. 2014, 26, 896–913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolf, M.; Bowers, P.G. The double-deficit hypothesis for the developmental dyslexias. J. Educ. Psychol. 1999, 91, 415–438. [Google Scholar] [CrossRef]
- Lovett, M.W.; Steinbach, K.A.; Frijters, J.C. Remediating the Core Deficits of Developmental Reading Disability: A Double-Deficit Perspective. J. Learn. Disabil. 2000, 33, 334–358. [Google Scholar] [CrossRef]
- Misra, M.; Katzir, T.; Wolf, M.; Poldrack, R.A. Neural Systems for rapid automatized naming in skilled readers: Unraveling the RAN-reading relationship. Sci. Stud. Read. 2004, 8, 241–256. [Google Scholar] [CrossRef]
- Dalboni da Rocha, J.L.; Coutinho, G.; Bramati, I.; Moll, F.T.; Sitaram, R. Multilevel diffusion tensor imaging classification technique for characterizing neurobehavioral disorders. Brain Imaging Behav. 2020, 14, 641–652. [Google Scholar] [CrossRef]
- Dalboni da Rocha, J.L.; Bramati, I.; Coutinho, G.; Tovar Moll, F.; Sitaram, R. Fractional Anisotropy changes in Parahippocampal Cingulum due to Alzheimer’s Disease. Sci. Rep. 2020, 10, 2660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Woodcock, R.W.; McGrew, K.S.; Mather, N. Woodcock-Johnson Tests of Achivement, 3rd ed.; Riverside Publishing: Rolling Meadows, IL, USA, 2001. [Google Scholar]
- Norton, E.S.; Wolf, M. Rapid automatized naming (RAN) and reading fluency: Implications for understanding and treatment of reading disabilities. Annu. Rev. Psychol. 2012, 63, 427–452. [Google Scholar] [CrossRef]
- Caria, A.; da Rocha, J.L.D.; Gallitto, G.; Birbaumer, N.; Sitaram, R.; Murguialday, A.R. Brain–Machine Interface Induced Morpho-Functional Remodeling of the Neural Motor System in Severe Chronic Stroke. Neurotherapeutics 2020, 17, 635–650. [Google Scholar] [CrossRef]
- Ruiz, S.; Lee, S.; Dalboni da Rocha, J.L.; Ramos-Murguialday, A.; Pasqualotto, E.; Soares, E.; García, E.; Fetz, E.; Birbaumer, N.; Sitaram, R. Motor Intentions Decoded from fMRI Signals. Brain Sci. 2024, 14, 643. [Google Scholar] [CrossRef]
- Rana, M.; Gupta, N.; Dalboni Da Rocha, J.L.; Lee, S.; Sitaram, R. A toolbox for real-time subject-independent and subject-dependent classification of brain states from fMRI signals. Front. Neurosci. 2013, 7, 170. [Google Scholar] [CrossRef]
- Liberati, G.; Veit, R.; Kim, S.; Birbaumer, N.; Von Arnim, C.; Jenner, A.; Lulé, D.; Ludolph, A.C.; Raffone, A.; Belardinelli, M.O.; et al. Development of a Binary fMRI-BCI for Alzheimer Patients: A Semantic Conditioning Paradigm Using Affective Unconditioned Stimuli. In Proceedings of the 2013 Humaine Association Conference on Affective Computing and Intelligent Interaction, Geneva, Switzerland, 2–5 September 2013; pp. 838–842. [Google Scholar] [CrossRef]
- Zurita, M.; Montalba, C.; Labbé, T.; Cruz, J.P.; da Rocha, J.D.; Tejos, C.; Ciampi, E.; Cárcamo, C.; Sitaram, R.; Uribe, S. Characterization of relapsing-remitting multiple sclerosis patients using support vector machine classifications of functional and diffusion MRI data. NeuroImage Clin. 2018, 20, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Tourville, J.; Carper, R.; Salamon, G. Cortical Parcellation Protocol. 2010. Available online: http://neuromorphometrics.com/ParcellationProtocol_2010-04-05.PDF (accessed on 3 September 2024).
- Vapnik, V.; Lerner, A. Pattern recognition using generalized portrait method. Autom. Remote Control 1963, 24, 774–780. [Google Scholar]
- Friedman, J.; Hastie, T.; Tibshirani, R. The Elements of Statistical Learning; Springer series in statistics; Springer: New York, NY, USA, 2001. [Google Scholar]
- Kass, R.E.; Raftery, A.E. A general Monte Carlo method for estimating Bayesian p-values. Biometrika 1996, 83, 447–452. [Google Scholar]
- Lai, J.; Zou, P.; Dalboni da Rocha, J.L.; Heitzer, A.M.; Patni, T.; Li, Y.; Scoggins, M.A.; Sharma, A.; Wang, W.C.; Helton, K.J.; et al. Hydroxyurea maintains working memory function in pediatric sickle cell disease. PLoS ONE 2024, 19, e0296196. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.W.; Mulhern, R.K. Neurocognitive interventions for children and adolscents surviving cancer. J. Pediatr. Psychol. 2005, 30, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Conklin, H.M.; Schreiber, J.E.; Fournier-Goodnight, A.S. Cognitive Late Effects and Their Management. In Brain Tumors in Children; Gajjar, A., Reaman, G., Racadio, J., Smith, F., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Kramer, J.; Moore, I.M. Late effects of cancer therapy on the central nervous system. Semin. Oncol. Nurs. 1989, 5, 22–28. [Google Scholar] [CrossRef]
- Palmer, S.L.; Gajjar, A.; Reddick, W.E.; Glass, J.O.; Kun, L.E.; Wu, S.; Xiong, X.; Mulhern, R.K. Predicting intellectual outcome among children treated with 35–40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology 2003, 17, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.S.; Hong, J.H.; Stalder, A.; Sun, J.R.; Withers, H.R.; McBride, W.H. Delayed molecular responses to brain irradiation. Int. J. Radiat. Biol. 1997, 72, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Tu, K.J.; Stewart, C.E.; Williams, N.T.; Ma, Y.; Luo, L.; Ghosh, D.; Weidenhammer, L.B.; Floyd, S.R.; Fan, Y.; Kirsch, D.G.; et al. Single-fraction Radiation Treatment Dose Response in a Genetically Engineered Mouse Model of Medulloblastoma. Radiat. Res. 2023, 200, 587–592. [Google Scholar] [CrossRef]
- Dehaene, S.; Cohen, L.; Morais, J.; Kolinsky, R. Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci. 2015, 16, 234–244. [Google Scholar] [CrossRef]
- Cummine, J.; Chouinard, B.; Szepesvari, E.; Georgiou, G.K. An examination of the rapid automatized naming–reading relationship using functional magnetic resonance imaging. Neuroscience 2015, 305, 49–66. [Google Scholar] [CrossRef]
- Lee, S.; Halder, S.; Kübler, A.; Birbaumer, N.; Sitaram, R. Effective functional mapping of fMRI data with support-vector machines. Hum. Brain Mapp. 2010, 31, 1502–1511. [Google Scholar] [CrossRef]



| Task | Description |
|---|---|
| Letter-Word Identification | Assesses the ability to identify and read isolated letters and words accurately. |
| Reading Fluency | Measures the speed and accuracy of reading simple sentences within a limited time frame. |
| Passage Comprehension | Evaluates the ability to comprehend the meaning of written passages by filling in missing words. |
| Word Attack | Tests the skill of decoding and pronouncing unfamiliar pseudo-words, focusing on phonetic decoding abilities. |
| Spelling of Sounds | Assesses the ability to spell words by dictation, focusing on phonetic spelling skills. |
| Sound Awareness | Measures phonological awareness, including tasks such as rhyming, segmenting, and manipulating sounds. |
| Reading Vocabulary | Evaluates the understanding and knowledge of word meanings through synonyms, antonyms, and analogies. |
| Visit | Accuracy | Sensitivity | Specificity | p-Value |
|---|---|---|---|---|
| TP1 | 57% | 60% | 54% | 118/1000 |
| TP2 | 75% | 69% | 81% | 1/1000 (*) |
| TP3 | 79% | 81% | 76% | 3/1000 (*) |
| Neurocognitive Test | Size (H/L) | Accuracy | Sensitivity | Specificity | Balanced Accuracy | p-Value |
|---|---|---|---|---|---|---|
| Reading Fluency | 28/61 | 68.5% | 42.9% | 80.3% | 61.6% | 12/1000 (*) |
| Passage Comprehension | 45/47 | 50.0% | 44.4% | 55.3% | 49.9% | 788/1000 |
| Word Attack | 51/41 | 65.2% | 64.7% | 65.9% | 65.3% | 16/1000 (*) |
| Spelling of Sounds | 48/44 | 51.1% | 50.0% | 52.3% | 51.2% | 719/1000 |
| Sound Awareness | 51/41 | 67.4% | 70.6% | 63.4% | 67.0% | 4/1000 (*) |
| Reading Vocabulary | 53/39 | 55.4% | 64.2% | 43.6% | 53.9% | 437/1000 |
| Letter-Word Identification | 50/42 | 51.1% | 52.0% | 50.0% | 51.0% | 719/1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalboni da Rocha, J.L.; Zou Stinnett, P.; Scoggins, M.A.; McAfee, S.S.; Conklin, H.M.; Gajjar, A.; Sitaram, R. Functional MRI Assessment of Brain Activity Patterns Associated with Reading in Medulloblastoma Survivors. Brain Sci. 2024, 14, 904. https://doi.org/10.3390/brainsci14090904
Dalboni da Rocha JL, Zou Stinnett P, Scoggins MA, McAfee SS, Conklin HM, Gajjar A, Sitaram R. Functional MRI Assessment of Brain Activity Patterns Associated with Reading in Medulloblastoma Survivors. Brain Sciences. 2024; 14(9):904. https://doi.org/10.3390/brainsci14090904
Chicago/Turabian StyleDalboni da Rocha, Josue L., Ping Zou Stinnett, Matthew A. Scoggins, Samuel S. McAfee, Heather M. Conklin, Amar Gajjar, and Ranganatha Sitaram. 2024. "Functional MRI Assessment of Brain Activity Patterns Associated with Reading in Medulloblastoma Survivors" Brain Sciences 14, no. 9: 904. https://doi.org/10.3390/brainsci14090904






