Real-Time fMRI Neurofeedback Training of Selective Attention in Older Adults
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Procedure
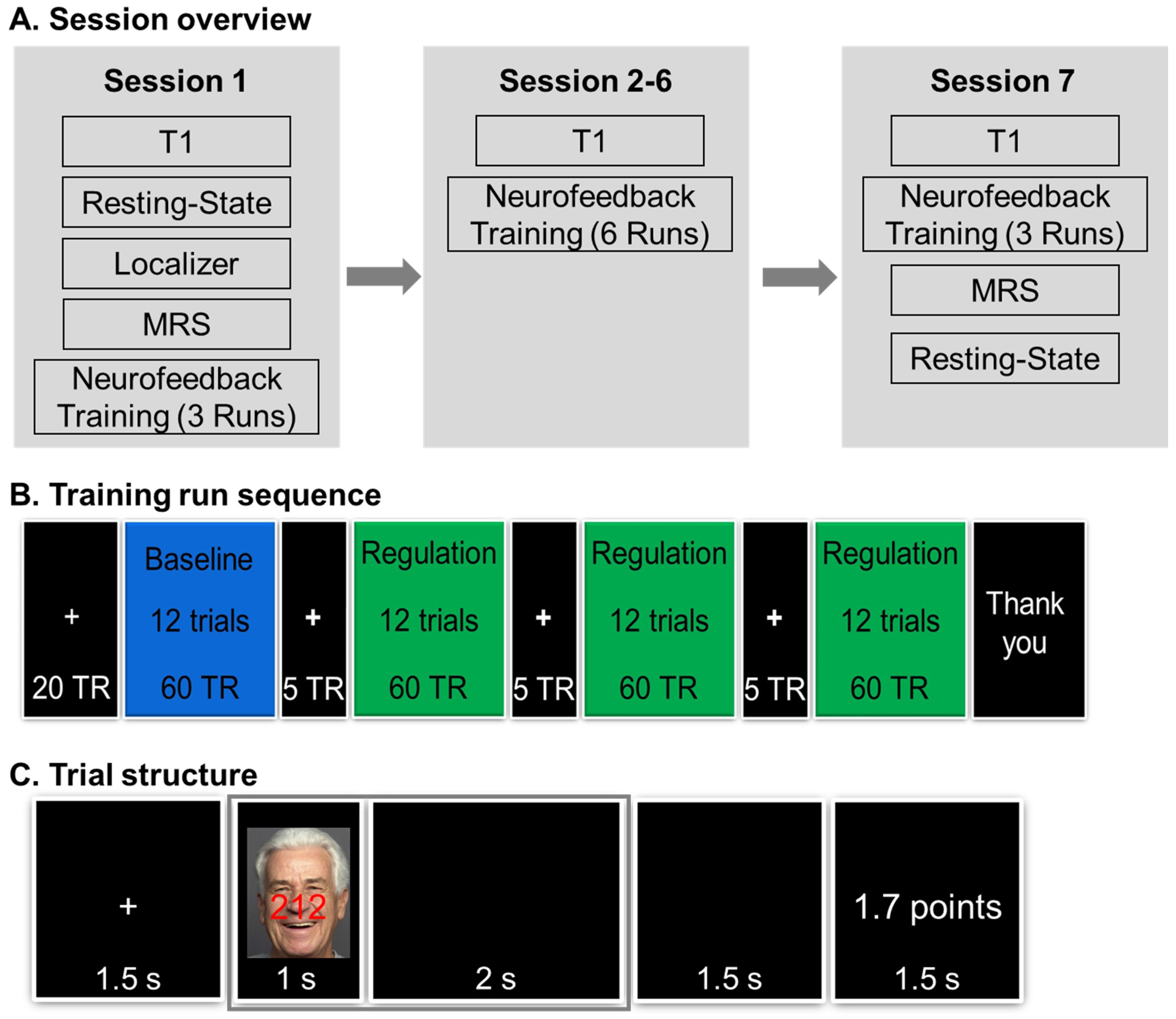
2.2.1. Pre-Training Session
2.2.2. Neurofeedback Training Sessions
2.3. Image Acquisition and Online Data Preprocessing
2.4. Neurofeedback Training Task
2.5. Online Data Analysis and Neurofeedback/Reward Points Calculation
2.6. Analysis on fMRI Data to Confirm dACC Involvement in Localizer Task and Neurofeedback Training
2.6.1. Localizer
2.6.2. Neurofeedback Training
2.7. Analysis of Neurofeedback Training Effects
2.7.1. Neurofeedback Training Success (Aim 1)
2.7.2. Behavioral Benefits from Neurofeedback Training (Aim 2)
3. Results
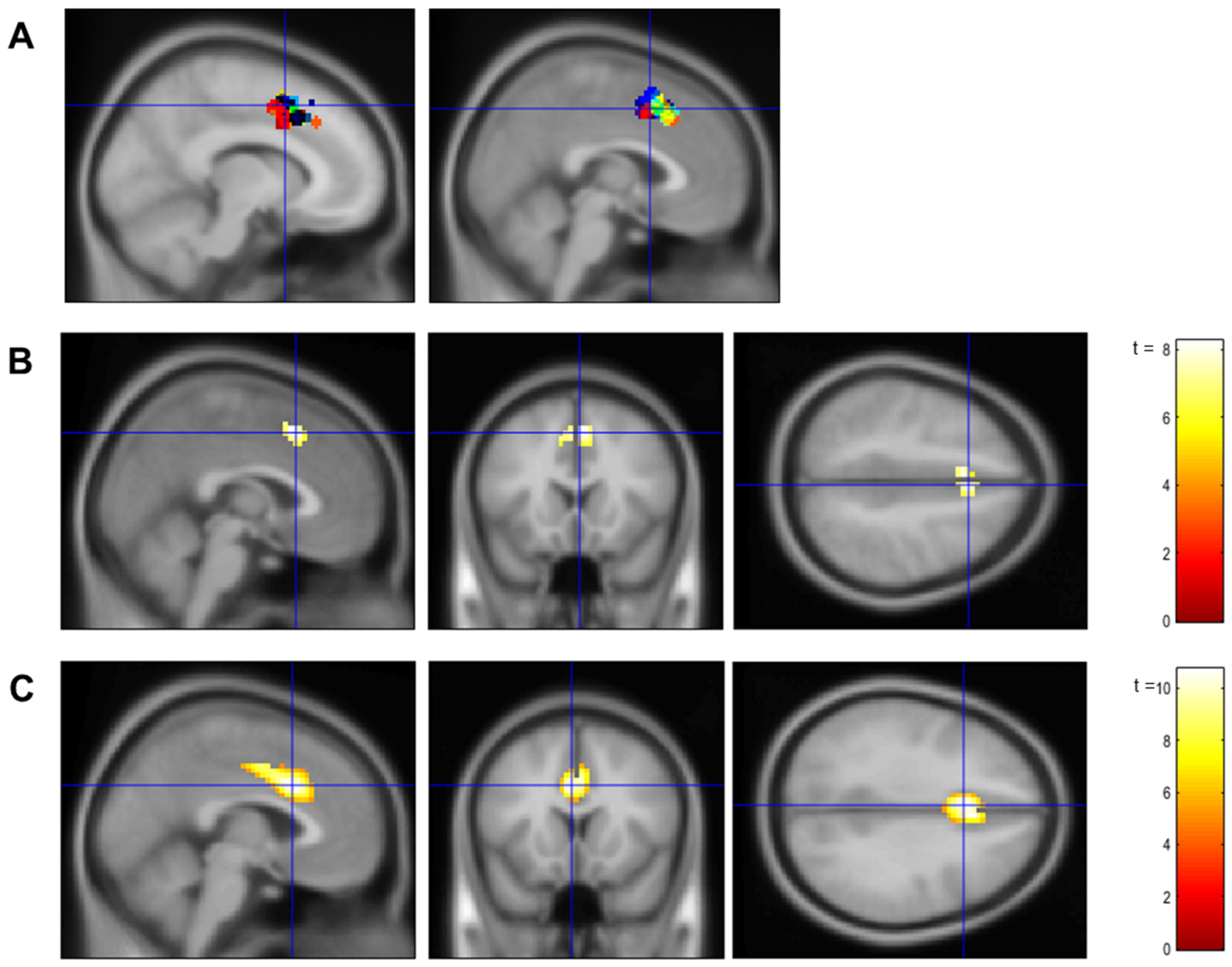
3.1. Confirmation of dACC Involvement in the Localizer Task and the Neurofeedback Training
3.2. Neurofeedback Training Effects
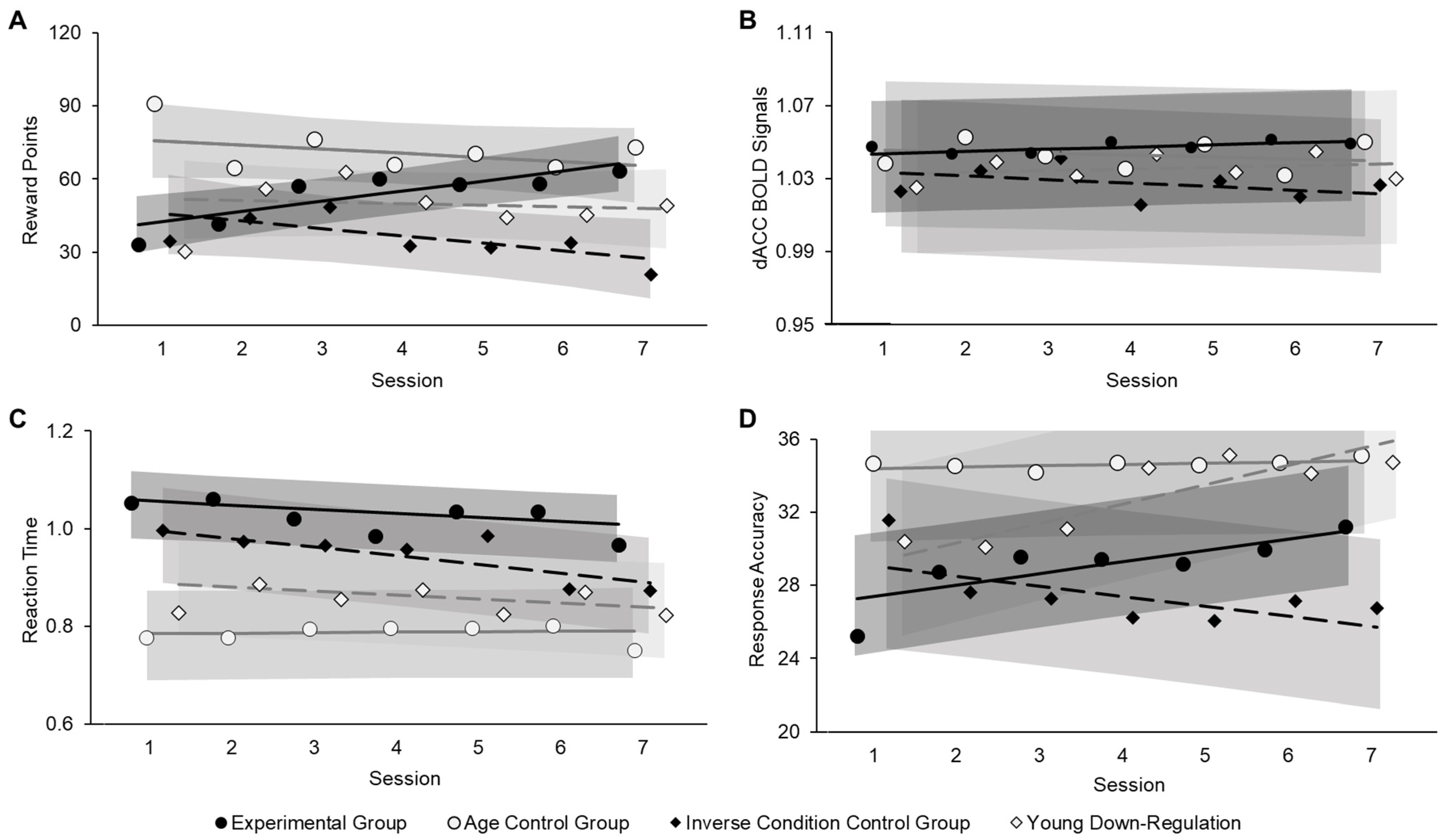
3.2.1. Neurofeedback Training Success (Aim 1)
3.2.2. Behavioral Benefits from Neurofeedback Training (Aim 2)
3.2.3. Follow-Up Brain-Behavior Analysis
3.2.4. Self-Reported Motivation (Post Hoc Analysis)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, N.L.; Cowan, N. The Cocktail Party Phenomenon Revisited: Attention and Memory in the Classic Selective Listening Procedure of Cherry (1953). J. Exp. Psychol. Gen. 1995, 124, 243–262. [Google Scholar] [CrossRef]
- Bell, T.R.; Sprague, B.N.; Ross, L.A. Supplemental material for longitudinal associations of pain and cognitive decline in community-dwelling older adults. Psychol. Aging 2022, 37, 715–730. [Google Scholar] [CrossRef]
- Madden, D.J.; Langley, L.K. Age-related changes in selective attention and perceptual load during visual search. Psychol. Aging 2003, 18, 54–67. [Google Scholar] [CrossRef]
- Brewster, B.M.; Pasqualini, M.S.; Martin, L.E. Functional brain connectivity and inhibitory control in older adults: A preliminary study. Front. Aging Neurosci. 2022, 14, 763494. [Google Scholar] [CrossRef]
- Chavarría-Elizondo, P.; del Río-Torné, C.; Maturana-Quijada, P.; Martínez-Zalacaín, I.; Juaneda, A.; del Cerro, I.; Guinea-Izquierdo, A.; Gascón-Bayarri, J.; Reñé-Ramírez, R.; Urretavizcaya, M.; et al. Altered activation of the dorsal anterior cingulate cortex during oddball performance in individuals at risk for Alzheimer’s disease. Spanish J. Psychiatry Ment. Health 2024, in press. [Google Scholar] [CrossRef]
- Kim, C.; Kroger, J.K.; Kim, J. A functional dissociation of conflict processing within anterior cingulate cortex. Hum. Brain Mapp. 2011, 32, 304–312. [Google Scholar] [CrossRef]
- Botvinick, M.; Nystrom, L.E.; Fissell, K.; Carter, C.S.; Cohen, J.D. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature 1999, 402, 179–181. [Google Scholar] [CrossRef]
- Bush, G.; Shin, L.M.; Holmes, J.; Rosen, B.R.; Vogt, B.A. The multi-source interference task: Validation study with fMRI in individual subjects. Mol. Psychiatry 2003, 8, 60–70. [Google Scholar] [CrossRef]
- Salami, A.; Rieckmann, A.; Fischer, H.; Bäckman, L. A multivariate analysis of age-related differences in functional networks supporting conflict resolution. Neuroimage 2014, 86, 150–163. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn. Sci. 2000, 4, 215–222. Available online: http://www.ncbi.nlm.nih.gov/pubmed/10827444 (accessed on 22 February 2017). [CrossRef]
- Bush, G.; Vogt, B.A.; Holmes, J.; Dale, A.M.; Greve, D.; Jenike, M.A.; Rosen, B.R. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proc. Natl. Acad. Sci. USA 2002, 99, 523–528. [Google Scholar] [CrossRef]
- Barch, D.M.; Braver, T.S.; Akbudak, E.; Conturo, T.; Ollinger, J.; Snyder, A. Anterior cingulate cortex and response conflict: Effects of response modality and processing domain. Cereb. Cortex 2001, 11, 837–848. [Google Scholar] [CrossRef]
- Clairis, N.; Lopez-Persem, A. Debates on the dorsomedial prefrontal/dorsal anterior cingulate cortex: Insights for future research. Brain 2023, 146, 4826–4844. [Google Scholar] [CrossRef]
- Blasi, G.; Taurisano, P.; Papazacharias, A.; Caforio, G.; Romano, R.; Lobianco, L.; Fazio, L.; Di Giorgio, A.; Latorre, V.; Sambataro, F.; et al. Nonlinear response of the anterior cingulate and prefrontal cortex in Schizophrenia as a function of variable attentional control. Cereb. Cortex 2010, 20, 837–845. [Google Scholar] [CrossRef]
- Duncan, J.; Owen, A.M. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000, 23, 475–483. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, Y.; Liu, Y.; Jiang, T.; Dong, H.; Zhang, Y.; Walter, M. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage 2011, 54, 2571–2581. [Google Scholar] [CrossRef]
- Bertocci, M.A.; Afriyie-Agyemang, Y.; Rozovsky, R.; Iyengar, S.; Stiffler, R.; Aslam, H.A.; Bebko, G.; Phillips, M.L. Altered patterns of central executive, default mode and salience network activity and connectivity are associated with current and future depression risk in two independent young adult samples. Mol. Psychiatry 2023, 28, 1046–1056. [Google Scholar] [CrossRef]
- Yeo, B.T.T.; Krienen, F.M.; Chee, M.W.L.; Buckner, R.L. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage 2014, 88, 212–227. [Google Scholar] [CrossRef]
- Karbach, J.; Verhaeghen, P. Making working memory work: A meta-analysis of executive-control and working memory training in older adults. Psychol. Sci. 2014, 25, 2027–2037. [Google Scholar] [CrossRef]
- Bush, G.; Shin, L.M. The Multi-Source Interference Task: An fMRI task that reliably activates the cingulo-frontal-parietal cognitive/attention network. Nat. Protoc. 2006, 1, 308–313. [Google Scholar] [CrossRef]
- Kim, H.; Chey, J.; Lee, S. Effects of multicomponent training of cognitive control on cognitive function and brain activation in older adults. Neurosci. Res. 2017, 124, 8–15. [Google Scholar] [CrossRef]
- Dudek, E.; Dodell-Feder, D. The efficacy of real-time functional magnetic resonance imaging neurofeedback for psychiatric illness: A meta-analysis of brain and behavioral outcomes. Neurosci. Biobehav. Rev. 2021, 121, 291–306. [Google Scholar] [CrossRef]
- Thibault, R.T.; MacPherson, A.; Lifshitz, M.; Roth, R.R.; Raz, A. Neurofeedback with fMRI: A critical systematic review. Neuroimage 2018, 172, 786–807. [Google Scholar] [CrossRef]
- Rana, M.; Varan, A.Q.; Davoudi, A.; Cohen, R.A.; Sitaram, R.; Ebner, N.C. Real-time fMRI in neuroscience research and its use in studying the aging brain. Front. Aging Neurosci. 2016, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Sitaram, R.; Ros, T.; Stoeckel, L.; Haller, S.; Scharnowski, F.; Lewis-Peacock, J.; Weiskopf, N.; Blefari, M.L.; Rana, M.; Oblak, E.; et al. Closed-loop brain training: The science of neurofeedback. Nat. Neurosci. 2016, 18, 86–100. [Google Scholar] [CrossRef]
- deBettencourt, M.T.; Cohen, J.D.; Lee, R.F.; Norman, K.A.; Turk-Browne, N.B. Closed-loop training of attention with real-time brain imaging. Nat. Neurosci. 2015, 18, 470–475. [Google Scholar] [CrossRef]
- Sherwood, M.S.; Weisend, M.P.; Kane, J.H.; Parker, J.G. Combining real-time fMRI neurofeedback training of the DLPFC with N-back practice results in neuroplastic effects confined to the neurofeedback target region. Front. Behav. Neurosci. 2016, 10, 138. [Google Scholar] [CrossRef]
- Yamashita, A.; Hayasaka, S.; Kawato, M.; Imamizu, H. Connectivity Neurofeedback Training Can Differentially Change Functional Connectivity and Cognitive Performance. Cereb. Cortex 2017, 27, 4960–4970. [Google Scholar] [CrossRef]
- Zhang, G.; Yao, L.; Zhang, H.; Long, Z.; Zhao, X. Improved Working Memory Performance through Self-Regulation of Dorsal Lateral Prefrontal Cortex Activation Using Real-Time fMRI. PLoS ONE 2013, 8, e73735. [Google Scholar] [CrossRef]
- Ruiz, S.; Lee, S.; Soekadar, S.R.; Caria, A.; Veit, R.; Kircher, T.; Birbaumer, N.; Sitaram, R. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum. Brain Mapp. 2013, 34, 200–212. [Google Scholar] [CrossRef]
- Zilverstand, A.; Sorger, B.; Slaats-Willemse, D.; Kan, C.C.; Goebel, R.; Buitelaar, J.K. fMRI neurofeedback training for increasing anterior cingulate cortex activation in adult attention deficit hyperactivity disorder. An exploratory randomized, single-blinded study. PLoS ONE 2017, 12, e0170795. [Google Scholar] [CrossRef] [PubMed]
- Hohenfeld, C.; Nellessen, N.; Dogan, I.; Kuhn, H.; Müller, C.; Papa, F.; Ketteler, S.; Goebel, R.; Heinecke, A.; Shah, N.J.; et al. Cognitive improvement and brain changes after real-time functional MRI neurofeedback training in healthy elderly and prodromal Alzheimer’s disease. Front. Neurol. 2017, 8, 384. [Google Scholar] [CrossRef] [PubMed]
- Hohenfeld, C.; Kuhn, H.; Müller, C.; Nellessen, N.; Ketteler, S.; Heinecke, A.; Goebel, R.; Shah, N.J.; Schulz, J.B.; Reske, M.; et al. Changes in brain activation related to visuo-spatial memory after real-time fMRI neurofeedback training in healthy elderly and Alzheimer’s disease. Behav. Brain Res. 2020, 381, 112435. [Google Scholar] [CrossRef]
- Trambaiolli, L.R.; Cassani, R.; Mehler, D.M.A.; Falk, T.H. Neurofeedback and the Aging Brain: A Systematic Review of Training Protocols for Dementia and Mild Cognitive Impairment. Front. Aging Neurosci. 2021, 13, 682683. [Google Scholar] [CrossRef]
- Milham, M.P.; Erickson, K.I.; Banich, M.T.; Kramer, A.F.; Webba, A.; Wszaleka, T.; Cohen, N.J. Attentional Control in the Aging Brain: Insights from an fMRI Study of the Stroop Task. Brain Cogn. 2002, 49, 277–296. [Google Scholar] [CrossRef]
- Pardo, J.V.; Lee, J.T.; Sheikh, S.A.; Surerus-Johnson, C.; Shah, H.; Munch, K.R.; Carlis, J.V.; Lewis, S.M.; Kuskowski, M.A.; Dysken, M.W. Where the brain grows old: Decline in anterior cingulate and medial prefrontal function with normal aging. Neuroimage 2007, 35, 1231–1237. [Google Scholar] [CrossRef]
- Lustig, C.; Shah, P.; Seidler, R.; Reuter-Lorenz, P.A. Aging, Training, and the Brain: A Review and Future Directions. Neuropsychol. Rev. 2009, 19, 504–522. [Google Scholar] [CrossRef]
- Thibault, R.T.; Lifshitz, M.; Raz, A. The self-regulating brain and neurofeedback: Experimental science and clinical promise. Cortex 2016, 74, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Spencer, M.; Folstein, M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol. Behav. Neurol. 1988, 1, 111–117. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Dumville, J.C.; Hahn, S.; Miles, J.N.V.; Torgerson, D.J. The use of unequal randomisation ratios in clinical trials: A review. Contemp. Clin. Trials 2006, 27, 1–12. [Google Scholar] [CrossRef]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Bauer, P.J.; Carlozzi, N.E.; Slotkin, J.; Blitz, D.; Wallner-Allen, K.; et al. Cognition assessment using the NIH Toolbox. Neurology 2013, 80 (Suppl. S3), S54–S64. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Brett, M.; Anton, J.L.; Valabregue, R.; Poline, J.B. Region of interest analysis using an SPM toolbox. In Proceedings of the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, 2–6 June 2002; p. 497. [Google Scholar]
- RJenkinson, M.; Beckmann, C.F.; Behrens, T.E.J.; Woolrich, M.W.; Smith, S.M. New advances in the Clinica software platform for clinical neuroimaging studies. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Mugler, J.P.; Brookeman, J.R. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn. Reson. Med. 1990, 15, 152–157. [Google Scholar] [CrossRef]
- Griswold, M.A.; Jakob, P.M.; Heidemann, R.M.; Nittka, M.; Jellus, V.; Wang, J.; Kiefer, B.; Haase, A. Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn. Reson. Med. 2002, 47, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Gupta, N.; Da Rocha, J.L.D.; Lee, S.; Sitaram, R. A toolbox for real-time subject-independent and subject-dependent classification of brain states from fMRI signals. Front. Neurosci. 2013, 7, 170. [Google Scholar] [CrossRef]
- Pavuluri, M.N.; O’Connor, M.M.; Harral, E.; Sweeney, J.A. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol. Psychiatry 2007, 62, 158–167. [Google Scholar] [CrossRef]
- Tracy, J.L.; Robins, R.W. The automaticity of emotion recognition. Emotion 2008, 8, 81–95. [Google Scholar] [CrossRef]
- Ebner, N.C.; Johnson, M.K. Age-group differences in interference from young and older emotional faces. Cogn. Emot. 2010, 24, 1095–1116. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Albinet, C.T.; Boucard, G.; Bouquet, C.A.; Audiffren, M. Processing speed and executive functions in cognitive aging: How to disentangle their mutual relationship? Brain Cogn. 2012, 79, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Moldes, S.; Cleeremans, A. Delineating implicit and explicit processes in neurofeedback learning. Neurosci. Biobehav. Rev. 2020, 118, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Laver, G.D. Adult Aging Effects on Semantic and Episodic Priming in Word Recognition. Psychol. Aging 2009, 24, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Morrow, D.G.; Miller, L.M.; Ridolfo, H.E.; Mangor, C.; Fischer, U.M.; Kokayerff, N.K. Expertise and age differences in pilot decision making. Aging, Neuropsychol. Cogn. 2008, 16, 33–35. [Google Scholar] [CrossRef]
- Howard, J.H.; Howard, D.V. Aging mind and brain: Is implicit learning spared in healthy aging? Front. Psychol. 2013, 4, 817. [Google Scholar] [CrossRef]
- Rieckmann, A.; Bäckman, L. Implicit learning in aging: Extant patterns and new directions. Neuropsychol. Rev. 2009, 19, 490–503. [Google Scholar] [CrossRef]
- Quirk, G.J.; Mueller, D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008, 33, 56–72. [Google Scholar] [CrossRef]
- Banca, P.; Morris, L.S.; Mitchell, S.; Harrison, N.A.; Potenza, M.N.; Voon, V. Novelty, conditioning and attentional bias to sexual rewards. J. Psychiatr. Res. 2016, 72, 91–101. [Google Scholar] [CrossRef]
- Gröne, M.; Dyck, M.; Koush, Y.; Bergert, S.; Mathiak, K.A.; Alawi, E.M.; Elliott, M. Upregulation of the Rostral Anterior Cingulate Cortex can Alter the Perception of Emotions: fMRI-Based Neurofeedback at 3 and 7 T. Brain Topogr. 2015, 28, 197–207. [Google Scholar] [CrossRef]
- Lam, S.L.; Criaud, M.; Lukito, S.; Westwood, S.J.; Agbedjro, D.; Kowalczyk, O.S.; Curran, S.; Barret, N.; Abbott, C.; Liang, H.; et al. Double-Blind, Sham-Controlled Randomized Trial Testing the Efficacy of fMRI Neurofeedback on Clinical and Cognitive Measures in Children With ADHD. Am. J. Psychiatry 2022, 179, 947–958. [Google Scholar] [CrossRef]
- Brockett, A.T.; Roesch, M.R. Anterior cingulate cortex and adaptive control of brain and behavior. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2021; pp. 283–309. [Google Scholar]
- Etkin, A.; Egner, T.; Kalisch, R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn. Sci. 2011, 15, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Venezia, J.H.; Matchin, W.; Saberi, K.; Hickok, G. An fMRI Study of Audiovisual Speech Perception Reveals Multisensory Interactions in Auditory Cortex. PLoS ONE 2013, 8, e68959. [Google Scholar] [CrossRef] [PubMed]
- Esteban, O.; Markiewicz, C.J.; Blair, R.W.; Moodie, C.A.; Isik, A.I.; Erramuzpe, A.; Kent, J.D.; Goncalves, M.; DuPre, E.; Snyder, M.; et al. fMRIPrep: A robust preprocessing pipeline for functional MRI. Nat. Methods 2019, 16, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Gorgolewski, K.; Burns, C.D.; Madison, C.; Clark, D.; Halchenko, Y.O.; Waskom, M.L.; Ghosh, S.S. Nipype: A flexible, lightweight and extensible neuroimaging data processing framework in Python. Front. Neuroinform. 2011, 5, 12318. [Google Scholar] [CrossRef] [PubMed]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 bias correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef]
- Avants, B.B.; Epstein, C.L.; Grossman, M.; Gee, J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Brady, M.; Smith, S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans. Med. Imaging 2001, 20, 45–57. [Google Scholar] [CrossRef]
- Reuter, M.; Rosas, H.D.; Fischl, B. Highly accurate inverse consistent registration: A robust approach. Neuroimage 2010, 53, 1181–1196. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Greve, D.N.; Fischl, B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 2009, 48, 63–72. [Google Scholar] [CrossRef]
- Jenkinson, M.; Bannister, P.; Brady, M.; Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002, 17, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.W.; Hyde, J.S. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997, 10, 171–178. [Google Scholar] [CrossRef]
| Experimental Group (N = 18) | Age Control Group (N = 10) | Inverse Condition Control Group (N = 9) | Young Down-Regulation Group (N = 9) | |
|---|---|---|---|---|
| Cognition | ||||
| Flanker Inhibitory Control and Attention Test a,c | 7.49 (0.95) | 8.23 (0.58) | 7.97 (0.79) | 9.16 (0.63) |
| Dimensional Change Card Sort Test a,b,c | 7.39 (1.08) | 8.49 (1.08) | 8.44 (1.05) | 9.21 (0.65) |
| List Sorting Working Memory Test c | 15.75 (2.21) | 16.78 (1.86) | 17.43 (2.44) | 21.75 (2.55) |
| Pattern Comparison Processing Speed Test a,c | 39.06 (10.48) | 48.78 (9.30) | 45.57 (6.71) | 55.38 (5.40) |
| Affect | ||||
| Positive Affect c | 3.66 (0.73) | 3.14 (0.68) | 3.90 (0.89) | 2.73 (0.93) |
| Negative Affect c | 1.14 (0.16) | 1.24 (0.27) | 1.14 (0.16) | 1.46 (0.38) |
| Health | ||||
| Physical Health | 8.00 (1.04) | 9.00 (1.20) | 8.25 (1.49) | 8.44 (1.33) |
| Mental Health | 8.57 (0.85) | 8.88 (1.13) | 8.50 (2.33) | 8.44 (0.88) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.; Rana, M.; Liu, P.; Polk, R.; Heemskerk, A.; Weisberg, S.M.; Bowers, D.; Sitaram, R.; Ebner, N.C. Real-Time fMRI Neurofeedback Training of Selective Attention in Older Adults. Brain Sci. 2024, 14, 931. https://doi.org/10.3390/brainsci14090931
Lin T, Rana M, Liu P, Polk R, Heemskerk A, Weisberg SM, Bowers D, Sitaram R, Ebner NC. Real-Time fMRI Neurofeedback Training of Selective Attention in Older Adults. Brain Sciences. 2024; 14(9):931. https://doi.org/10.3390/brainsci14090931
Chicago/Turabian StyleLin, Tian, Mohit Rana, Peiwei Liu, Rebecca Polk, Amber Heemskerk, Steven M. Weisberg, Dawn Bowers, Ranganatha Sitaram, and Natalie C. Ebner. 2024. "Real-Time fMRI Neurofeedback Training of Selective Attention in Older Adults" Brain Sciences 14, no. 9: 931. https://doi.org/10.3390/brainsci14090931
APA StyleLin, T., Rana, M., Liu, P., Polk, R., Heemskerk, A., Weisberg, S. M., Bowers, D., Sitaram, R., & Ebner, N. C. (2024). Real-Time fMRI Neurofeedback Training of Selective Attention in Older Adults. Brain Sciences, 14(9), 931. https://doi.org/10.3390/brainsci14090931







