Comparing the Rates of Further Resection After Intraoperative MRI Visualisation of Residual Tumour Between Brain Tumour Subtypes: A 17-Year Single-Centre Experience
Abstract
1. Introduction
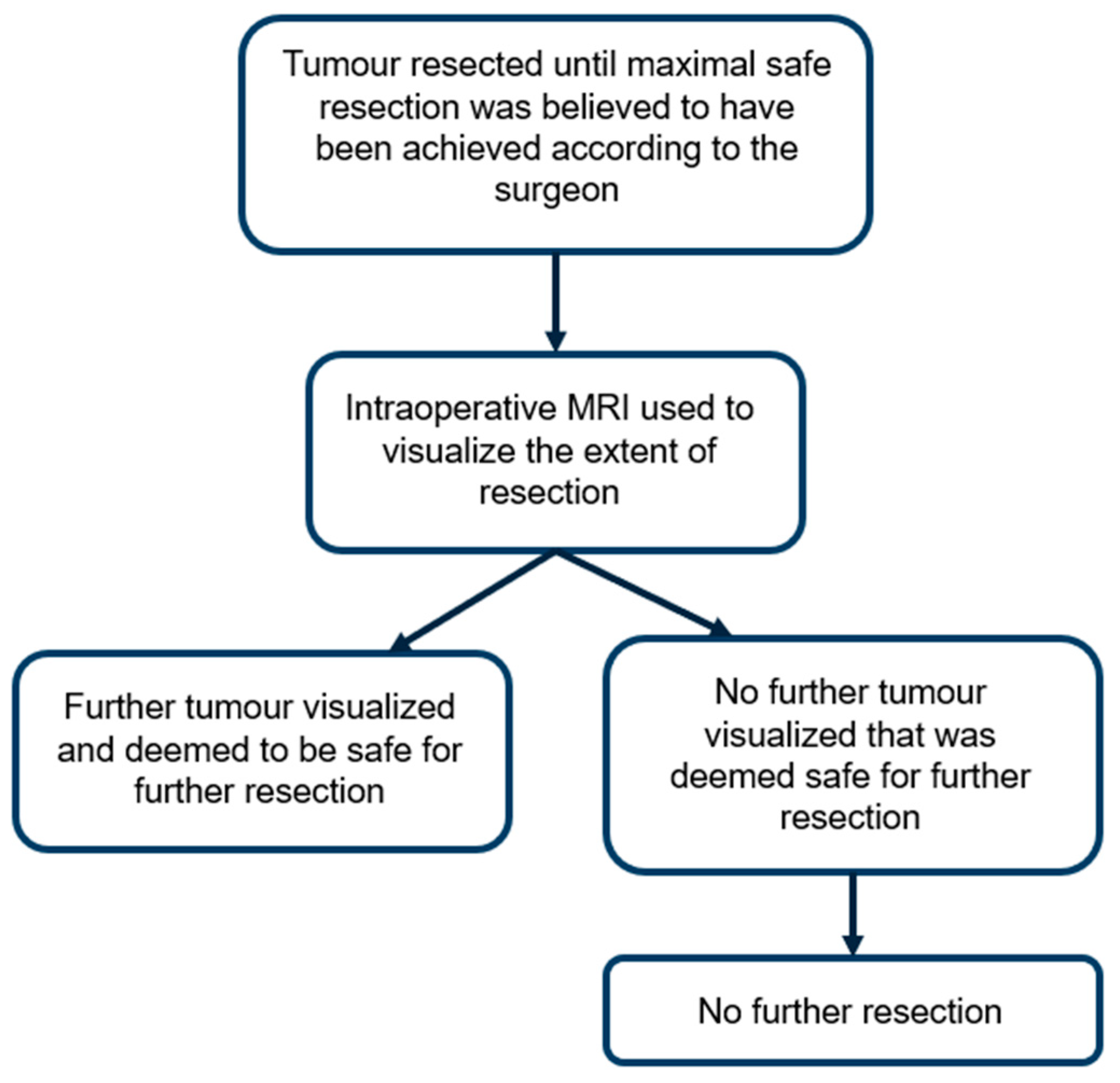
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Black, P.M.; Moriarty, T.; Alexander, E., 3rd; Stieg, P.; Woodard, E.J.; Gleason, P.L.; Martin, C.H.; Kikinis, R.; Schwartz, R.B.; Jolesz, F.A. Development and implementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery 1997, 41, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Banu, M.A.; Canoll, P.; Bruce, J.N. Rationale and Clinical Implications of Fluorescein-Guided Supramarginal Resection in Newly Diagnosed High-Grade Glioma. Front. Oncol. 2021, 11, 666734. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Hou, W.; Dong, G.; Wei, Z.; Zhou, H.; Duan, Y. The relationship between intra-operative ultrasonography and pathological grade in cerebral glioma. J. Int. Med. Res. 2008, 36, 1426–1434. [Google Scholar] [CrossRef]
- Gasser, T.; Ganslandt, O.; Sandalcioglu, E.; Stolke, D.; Fahlbusch, R.; Nimsky, C. Intraoperative functional MRI: Implementation and preliminary experience. Neuroimage 2005, 26, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Avula, S.; Jaspan, T.; Pizer, B.; Pettorini, B.; Garlick, D.; Hennigan, D.; Mallucci, C. Comparison of intraoperative and post-operative 3-T MRI performed at 24–72 h following brain tumour resection in children. Neuroradiology 2021, 63, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Ginat, D.T.; Swearingen, B.; Curry, W.; Cahill, D.; Madsen, J.; Schaefer, P.W. 3 Tesla intraoperative MRI for brain tumor surgery. J. Magn. Reson. Imaging 2014, 39, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Polley, M.-Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas: Clinical article. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Chang, E.F.; Lamborn, K.R.; Chang, S.M.; Prados, M.D.; Cha, S.; Tihan, T.; VandenBerg, S.; McDermott, M.W.; Berger, M.S. Role of Extent of Resection in the Long-Term Outcome of Low-Grade Hemispheric Gliomas. J. Clin. Oncol. 2008, 26, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Brochier, S.; Galland, F.; Kujas, M.; Parker, F.; Gaillard, S.; Raftopoulos, C.; Young, J.; Alexopoulou, O.; Maiter, D.; Chanson, P. Factors predicting relapse of nonfunctioning pituitary macroadenomas after neurosurgery: A study of 142 patients. Eur. J. Endocrinol. 2010, 163, 193–200. [Google Scholar] [CrossRef]
- Sastry, R.; Bi, W.L.; Pieper, S.; Frisken, S.; Kapur, T.; Wells, W., 3rd; Golby, A.J. Applications of Ultrasound in the Resection of Brain Tumors. J. Neuroimaging 2017, 27, 5–15. [Google Scholar] [CrossRef]
- Gerard, I.J.; Kersten-Oertel, M.; Petrecca, K.; Sirhan, D.; Hall, J.A.; Collins, D.L. Brain shift in neuronavigation of brain tumors: A review. Med. Image Anal. 2017, 35, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Foroglou, N.; Zamani, A.; Black, P. Intra-operative MRI (iop-MR) for brain tumour surgery. Br. J. Neurosurg. 2009, 23, 14–22. [Google Scholar] [CrossRef]
- Sunshine, J.L.; Tarr, R.W.; Lanzieri, C.F.; Landis, D.M.; Selman, W.R.; Lewin, J.S. Hyperacute stroke: Ultrafast MR imaging to triage patients prior to therapy. Radiology 1999, 212, 325–332. [Google Scholar] [CrossRef]
- Mamata, Y.; Mamata, H.; Nabavi, A.; Kacher, D.F.; Pergolizzi, R.S.; Schwartz, R.B.; Kikinis, R.; Jolesz, F.A.; Maier, S.E. Intraoperative diffusion imaging on a 0.5 Tesla interventional scanner. J. Magn. Reson. Imaging 2001, 13, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Nimsky, C.; Ganslandt, O.; Hastreiter, P.; Wang, R.; Benner, T.; Sorensen, A.G.; Fahlbusch, R. Intraoperative diffusion-tensor MR imaging: Shifting of white matter tracts during neurosurgical procedures—Initial experience. Radiology 2005, 234, 218–225. [Google Scholar] [CrossRef]
- Giordano, M.; Gallieni, M.; Metwali, H.; Fahlbusch, R.; Samii, M.; Samii, A. Can Intraoperative Magnetic Resonance Imaging Be Helpful in the Surgical Resection of Parasellar Meningiomas? A Case Series. World Neurosurg. 2019, 132, e577–e584. [Google Scholar] [CrossRef] [PubMed]
- Hatiboglu, M.A.; Weinberg, J.S.; Suki, D.; Rao, G.; Prabhu, S.S.; Shah, K.; Jackson, E.; Sawaya, R. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: A prospective volumetric analysis. Neurosurgery 2009, 64, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Kremer, P.; Tronnier, V.; Steiner, H.H.; Metzner, R.; Ebinger, F.; Rating, D.; Hartmann, M.; Seitz, A.; Unterberg, A.; Wirtz, C.R. Intraoperative MRI for interventional neurosurgical procedures and tumor resection control in children. Childs Nerv. Syst. 2006, 22, 674–678. [Google Scholar] [CrossRef]
- Kuhnt, D.; Ganslandt, O.; Schlaffer, S.M.; Buchfelder, M.; Nimsky, C. Quantification of glioma removal by intraoperative high-field magnetic resonance imaging: An update. Neurosurgery 2011, 69, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Senft, C.; Bink, A.; Franz, K.; Vatter, H.; Gasser, T.; Seifert, V. Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol. 2011, 12, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.H.; Stieg, P.E.; Anand, V.K. Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Neurosurgery 2006, 58 (Suppl. S1), ONS44-51. [Google Scholar] [CrossRef]
- Ferraro, N.; Barbarite, E.; Albert, T.R.; Berchmans, E.; Shah, A.H.; Bregy, A.; Ivan, M.E.; Brown, T.; Komotar, R.J. The role of 5-aminolevulinic acid in brain tumor surgery: A systematic review. Neurosurg. Rev. 2016, 39, 545–555. [Google Scholar] [CrossRef]
- Maugeri, R.; Villa, A.; Pino, M.; Imperato, A.; Giammalva, G.R.; Costantino, G.; Graziano, F.; Gulì, C.; Meli, F.; Francaviglia, N.; et al. With a Little Help from My Friends: The Role of Intraoperative Fluorescent Dyes in the Surgical Management of High-Grade Gliomas. Brain Sci. 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Smith, E.J.; Barreau, A.; Nyaeme, M.; Cramer, S.W.; Najafali, D.; Krist, D.T.; Arnold, P.M.; Hassaneen, W. Comparison of fluorescein sodium, 5-ALA, and intraoperative MRI for resection of high-grade gliomas: A systematic review and network meta-analysis. J. Clin. Neurosci. 2022, 98, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Gandhe, R.U.; Bhave, C.P. Intraoperative magnetic resonance imaging for neurosurgery—An anaesthesiologist’s challenge. Indian J. Anaesth. 2018, 62, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Ramina, R.; Coelho Neto, M.; Giacomelli, A.; Barros, E., Jr.; Vosgerau, R.; Nascimento, A.; Coelho, G. Optimizing costs of intraoperative magnetic resonance imaging. A series of 29 glioma cases. Acta Neurochir. 2010, 152, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Sarkar, R.; Brandel, M.G.; Wali, A.R.; Rennert, R.C.; Ramos, C.L.; Padwal, J.; Steinberg, J.A.; Santiago-Dieppa, D.R.; Cheung, V.; et al. Cost-effectiveness of Intraoperative MRI for Treatment of High-Grade Gliomas. Radiology 2019, 291, 689–697. [Google Scholar] [CrossRef]
- Chowdhury, T.; Zeiler, F.A.; Singh, N.; Gray, K.D.R.; Qadri, A.; Beiko, J.; Cappellani, R.B.M.; West, M.B. Awake Craniotomy Under 3-Tesla Intraoperative Magnetic Resonance Imaging: A Retrospective Descriptive Report and Canadian Institutional Experience. J. Neurosurg. Anesthesiol. 2022, 34, e46–e51. [Google Scholar] [CrossRef]


| Variable | All Types | Glioma | PitNET | Metastases | Meningioma |
|---|---|---|---|---|---|
| 486 | 270 | 194 | 14 | 8 | |
| Age | |||||
| 55 or less (n [%]) | 213 (44%) | 157 (58%) | 52 (27%) | 1 (7%) | 3 (38%) |
| Over 55 (n [%]) | 273 (56%) | 113 (42%) | 142 (73%) | 13 (93%) | 5 (62%) |
| Gender | |||||
| Female (n [%]) | 252 (52%) | 130 (48%) | 107 (55%) | 7 (50%) | 8 (100%) |
| Male (n [%]) | 234 (48%) | 140 (52%) | 87 (45%) | 7 (50%) | 0 (0%) |
| Awake craniotomy | 5 | 5 | - | - | - |
| WHO grade | |||||
| 1 | 214 | 16 | 194 | - | 4 |
| 2 | 79 | 79 | - | - | - |
| 3 | 93 | 93 | - | - | - |
| 4 | 77 | 77 | - | - | - |
| NOS | 15 | 5 | - | 10 | - |
| Case Type | 2007–2012 | 2018–2023 | p-Value | Overall |
|---|---|---|---|---|
| PitNET | 25/86 (29%) | 10/33 (30%) | 0.956 | 35/119 (29%) |
| HGG | 27/61 (44%) | 14/26 (54%) | 0.412 | 41/87 (47%) |
| LGG | 19/50 (38%) | 14/23 (61%) | 0.07 | 33/73 (45%) |
| Meningioma | 0/6 | 0/0 | - | 0/6 |
| Metastasis | 0/11 | 0/0 | - | 0/11 |
| Overall | 71/214 (33%) | 38/82 (46%) | 0.036 | 109/296 (37%) |
| Year | Minutes Per Case (SD) * |
|---|---|
| 2017 | 315 (98.8) |
| 2018 | 306 (105) |
| 2019 | 294 (115) |
| 2020 | 294 (102) |
| 2021 | 291 (91.7) |
| 2022 | 281 (90.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madani, D.; Fonseka, R.D.; Kim, S.J.; Tang, P.; Muralidharan, K.; Chang, N.; Wong, J. Comparing the Rates of Further Resection After Intraoperative MRI Visualisation of Residual Tumour Between Brain Tumour Subtypes: A 17-Year Single-Centre Experience. Brain Sci. 2025, 15, 45. https://doi.org/10.3390/brainsci15010045
Madani D, Fonseka RD, Kim SJ, Tang P, Muralidharan K, Chang N, Wong J. Comparing the Rates of Further Resection After Intraoperative MRI Visualisation of Residual Tumour Between Brain Tumour Subtypes: A 17-Year Single-Centre Experience. Brain Sciences. 2025; 15(1):45. https://doi.org/10.3390/brainsci15010045
Chicago/Turabian StyleMadani, Daniel, R. Dineth Fonseka, Sihyong Jake Kim, Patrick Tang, Krishna Muralidharan, Nicholas Chang, and Johnny Wong. 2025. "Comparing the Rates of Further Resection After Intraoperative MRI Visualisation of Residual Tumour Between Brain Tumour Subtypes: A 17-Year Single-Centre Experience" Brain Sciences 15, no. 1: 45. https://doi.org/10.3390/brainsci15010045
APA StyleMadani, D., Fonseka, R. D., Kim, S. J., Tang, P., Muralidharan, K., Chang, N., & Wong, J. (2025). Comparing the Rates of Further Resection After Intraoperative MRI Visualisation of Residual Tumour Between Brain Tumour Subtypes: A 17-Year Single-Centre Experience. Brain Sciences, 15(1), 45. https://doi.org/10.3390/brainsci15010045






