The Neural Development of Chinese Lexical Tone Perception: A Mismatch Negativity Study Across Childhood, Adolescence, and Adulthood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Stimuli
2.3. ERP Procedure
2.4. EEG Recording and Data Analysis
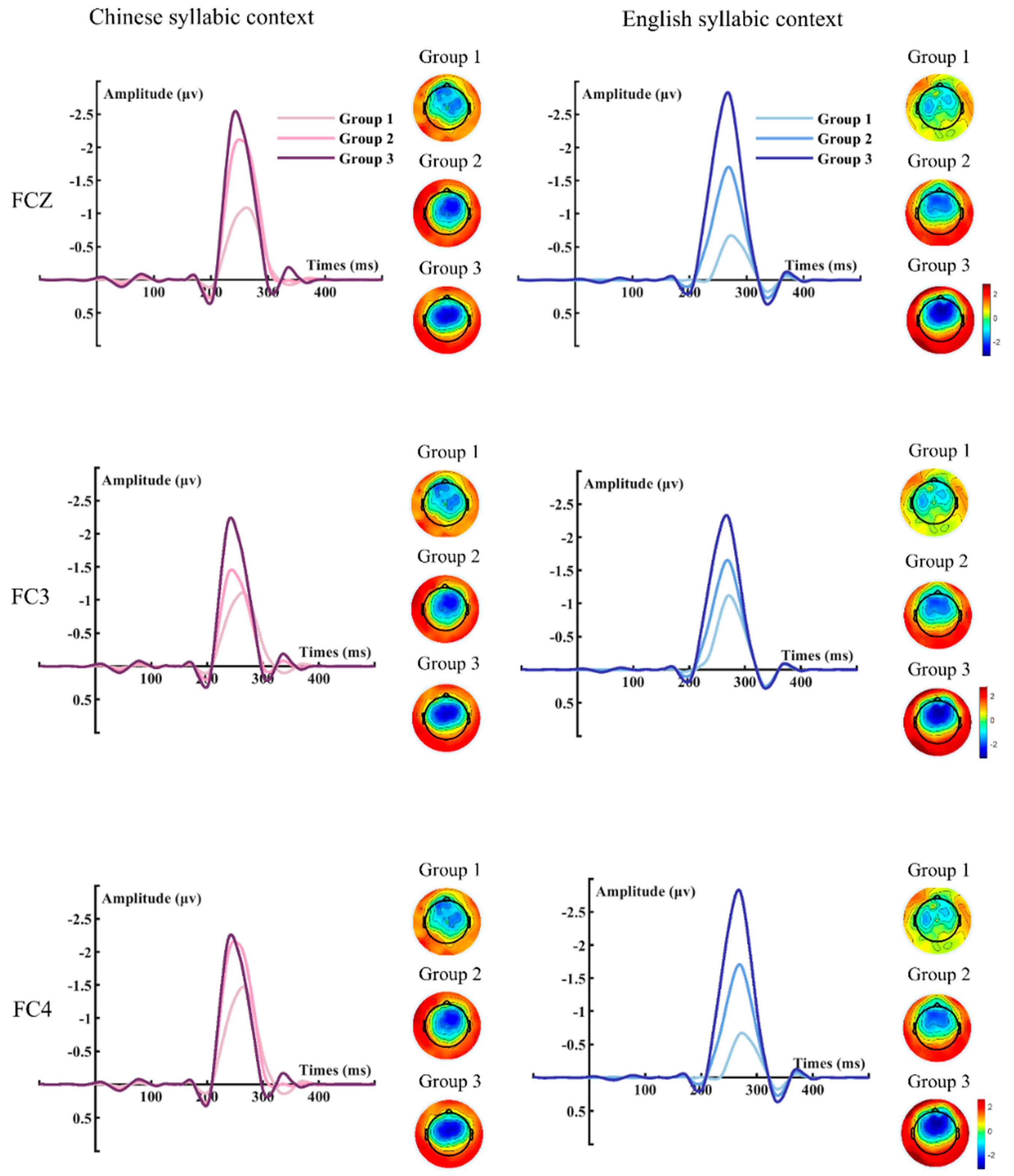
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuhl, P.K.; Stevens, E.; Hayashi, A.; Deguchi, T.; Kiritani, S.; Iverson, P. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Dev. Sci. 2006, 9, F13–F21. [Google Scholar] [CrossRef] [PubMed]
- Lovcevic, I.; Tsuji, S. The developmental pattern of native and non-native speech perception during the 1st year of life in Japanese infants. Infant Behav. Dev. 2024, 76, 101977. [Google Scholar] [CrossRef] [PubMed]
- Chládková, K.; Paillereau, N. The What and When of Universal Perception: A Review of Early Speech Sound Acquisition. Lang. Learn. 2020, 70, 1136–1182. [Google Scholar] [CrossRef]
- Best, C.C.; McRoberts, G.W. Infant perception of non-native consonant contrasts that adults assimilate in different ways. Lang. Speech 2003, 46, 183–216. [Google Scholar] [CrossRef]
- Tsao, F.M.; Liu, H.M.; Kuhl, P.K. Perception of native and non-native affricate-fricative contrasts: Cross-language tests on adults and infants. J. Acoust. Soc. Am. 2006, 120, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Kuhl, P.K.; Conboy, B.T.; Padden, D.; Nelson, T.; Pruitt, J. Early Speech Perception and Later Language Development: Implications for the “Critical Period”. Lang. Learn. Dev. 2005, 1, 237–264. [Google Scholar] [CrossRef]
- Kuhl, P.; Rivera-Gaxiola, M. Neural substrates of language acquisition. Annu. Rev. Neurosci. 2008, 31, 511–534. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.K.; Guan, Y.L. Cytoarchitectural and axonal maturation in human auditory cortex. J. Assoc. Res. Otolaryngol. 2001, 2, 297–311. [Google Scholar] [CrossRef]
- Moore, J.K.; Linthicum, F.H. The human auditory system: A timeline of development. Int. J. Audiol. 2007, 46, 460–478. [Google Scholar] [CrossRef] [PubMed]
- Werker, J.F.; Tees, R.C. Speech perception as a window for understanding plasticity and commitment in language systems of the brain. Dev. Psychobiol. 2005, 46, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Jusczyk, P.W. Toward a model of the development of speech perception. In Invariance and Variability in Speech Processes; Psychology Press: Hove, UK, 2014; pp. 1–35. [Google Scholar]
- Hong, T.; Shuai, L.; Frost, S.J.; Landi, N.; Pugh, K.R.; Shu, H. Cortical responses to Chinese phonemes in preschoolers predict their literacy skills at school age. Dev. Neuropsychol. 2018, 43, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, E.; Boets, B.; Ghesquiere, P.; Zink, I. Auditory processing and speech perception in children with specific language impairment: Relations with oral language and literacy skills. Res. Dev. Disabil. 2012, 33, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Hazan, V.; Barrett, S. The development of phonemic categorization in children aged 6–12. J. Phon. 2000, 28, 377–396. [Google Scholar] [CrossRef]
- Chen, F.; Peng, G.; Yan, N.; Wang, L. The development of categorical perception of Mandarin tones in four- to seven-year-old children. J. Child Lang. 2017, 44, 1413–1434. [Google Scholar] [CrossRef]
- Lee, C.Y.; Yen, H.L.; Yeh, P.W.; Lin, W.H.; Cheng, Y.Y.; Tzeng, Y.L.; Wu, H.C. Mismatch responses to lexical tone, initial consonant, and vowel in Mandarin-speaking preschoolers. Neuropsychologia 2012, 50, 3228–3239. [Google Scholar] [CrossRef]
- Näätänen, R. The perception of speech sounds by the human brain as reflected by the mismatch negativity (MMN) and its magnetic equivalent (MMNm). Psychophysiology 2001, 38, 1–21. [Google Scholar] [CrossRef]
- Turgeon, C.; Lazzouni, L.; Lepore, F.; Ellemberg, D. An objective auditory measure to assess speech recognition in adult cochlear implant users. Clin. Neurophysiol. 2014, 125, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Cheour, M.; Korpilahti, P.; Martynova, O.; Lang, A.H. Mismatch negativity and late discriminative negativity in investigating speech perception and learning in children and infants. Audiol. Neuro-Otol. 2001, 6, 2–11. [Google Scholar] [CrossRef]
- Schulte-Körne, G.; Deimel, W.; Bartling, J.; Remschmidt, H. Speech perception deficit in dyslexic adults as measured by mismatch negativity (MMN). Int. J. Psychophysiol. 2001, 40, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Werwach, A.; Maennel, C.; Obrig, H.; Friederici, A.D.; Schaadt, G. Longitudinal trajectories of electrophysiological mismatch responses in infant speech discrimination differ across speech features. Dev. Cogn. Neurosci. 2022, 56, 101127. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Peter, V.; Burnham, D. Development of neural discrimination of pitch across speech and music in the first year of life, a mismatch response study. Lang. Cogn. Neurosci. 2022, 37, 1153–1168. [Google Scholar] [CrossRef]
- Xi, J.; Zhang, L.; Shu, H.; Zhang, Y.; Li, P. Categorical perception of lexical tones in chinese revealed by mismatch negativity. Neuroscience 2010, 170, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Paavilainen, P.; Rinne, T.; Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: A review. Clin. Neurophysiol. 2007, 118, 2544–2590. [Google Scholar] [CrossRef] [PubMed]
- Ceponiene, R.; Lepistö, T.; Alku, P.; Aro, H.; Näätänen, R. Event-related potential indices of auditory vowel processing in 3-year-old children. Clin. Neurophysiol. 2003, 114, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Wu, H.C.; Tzeng, Y.L.; Yang, M.T.; Zhao, L.L.; Lee, C.Y. The Development of Mismatch Responses to Mandarin Lexical Tones in Early Infancy. Dev. Neuropsychol. 2013, 38, 281–300. [Google Scholar] [CrossRef]
- Csepe, V. On the origin and development of the mismatch negativity. Ear Hear 1995, 16, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Kristin, L.; Jennifer, M.; Hannah, D.; Ruth, R. Mapping development of the MMN and P3a potentials during adolescence: A longitudinal investigation of healthy individuals and individuals at-risk for psychosis. In Proceedings of the Conference Abstract: XII International Conference on Cognitive Neuroscience (ICON-XII), Brisbane, Australia, 27–31 July 2014; Volume 9. [Google Scholar] [CrossRef]
- Maurer, U.; Bucher, K.; Brem, S.; Brandeis, D. Development of the automatic mismatch response: From frontal positivity in kindergarten children to the mismatch negativity. Clin. Neurophysiol. 2003, 114, 808–817. [Google Scholar] [CrossRef]
- McGee, T.J.; King, C.; Tremblay, K.; Nicol, T.G.; Cunningham, J.; Kraus, N. Long-term habituation of the speech-elicited mismatch negativity. Psychophysiology 2001, 38, 653–658. [Google Scholar] [CrossRef]
- Kraus, N.; McGee, T.; Carrell, T.; Sharma, A.; Micco, A.; Nicol, T. Speech-evoked cortical potentials in children. J. Am. Acad. Audiol. 1993, 4, 238–248. [Google Scholar] [PubMed]
- Shafer, V.L.; Yu, Y.H.; Datta, H. Maturation of Speech Discrimination in 4-to 7-Yr-Old Children as Indexed by Event-Related Potential Mismatch Responses. Ear Hear 2010, 31, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.V.; Hardiman, M.J.; Barry, J.G. Is auditory discrimination mature by middle childhood? A study using time-frequency analysis of mismatch responses from 7 years to adulthood. Dev. Sci. 2011, 14, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Chen, Y.; Tsao, F.M. Developmental changes in mismatch responses to mandarin consonants and lexical tones from early to middle childhood. PLoS ONE 2014, 9, e95587. [Google Scholar] [CrossRef] [PubMed]
- Joanisse, M.F.; Zevin, J.D.; McCandliss, B.D. Brain mechanisms implicated in the preattentive categorization of speech sounds revealed using fMRI and a short-interval habituation trial paradigm. Cereb. Cortex 2007, 17, 2084–2093. [Google Scholar] [CrossRef] [PubMed]
- Näätänen, R.; Lehtokoski, A.; Lennes, M.; Cheour, M.; Huotilainen, M.; Iivonen, A.; Vainio, M.; Alku, P.; Ilmoniemi, R.J.; Luuk, A.; et al. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature 1997, 385, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Vandermosten, M.; Boets, B.; Luts, H.; Poelmans, H.; Golestani, N.; Wouters, J.; Ghesquière, P. Adults with dyslexia are impaired in categorizing speech and nonspeech sounds on the basis of temporal cues. Proc. Natl. Acad. Sci. USA 2010, 107, 10389–10394. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.S.; Gandour, J.; Talavage, T.; Wong, D.; Dzemidzic, M.; Tong, Y.X.; Li, X.J.; Lowe, M. Activation of the left planum temporale in pitch processing is shaped by language experience. Hum. Brain Mapp. 2006, 27, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Bent, T.; Bradlow, A.R.; Wright, B.A. The influence of linguistic experience on the cognitive processing of pitch in speech and nonspeech sounds. J. Exp. Psychol.-Hum. Percept. Perform. 2006, 32, 97–103. [Google Scholar] [CrossRef]
- Francis, A.L.; Ciocca, V.; Ng, B.K.C. On the (non)categorical perception of lexical tones. Percept. Psychophys. 2003, 65, 1029–1044. [Google Scholar] [CrossRef] [PubMed]
- Stilp, C.E.; Shorey, A.E.; King, C.J. Nonspeech sounds are not all equally good at being nonspeecha). J. Acoust. Soc. Am. 2022, 152, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, D.; Skrandies, W. Spatial analysis of evoked potentials in man—A review. Prog. Neurobiol. 1984, 23, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, S.P.; Fan, Y.B.; Huang, D.; Zhang, Y. Speech-specific categorical perception deficit in autism: An Event-Related Potential study of lexical tone processing in Mandarin-speaking children. Sci. Rep. 2017, 7, 43254. [Google Scholar] [CrossRef]
- Bishop, D.V.M.; Hardiman, M.J.; Barry, J.G. Lower-Frequency Event-Related Desynchronization: A Signature of Late Mismatch Responses to Sounds, Which Is Reduced or Absent in Children with Specific Language Impairment. J. Neurosci. 2010, 30, 15578–15584. [Google Scholar] [CrossRef]
- Rivera-Gaxiola, M.; Silva-Pereyra, J.; Klarman, L.; Garcia-Sierra, A.; Lara-Ayala, L.; Cadena-Salazar, C.; Kuhl, P. Principal Component Analyses and scalp distribution of the auditory P150-250 and N250-550 to speech contrasts in Mexican and American infants. Dev. Neuropsychol. 2007, 31, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Partanen, E.; Pakarinen, S.; Kujala, T.; Huotilainen, M. Infants’ brain responses for speech sound changes in fast multifeature MMN paradigm. Clin. Neurophysiol. 2013, 124, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Dien, J. Applying Principal Components Analysis to Event-Related Potentials: A Tutorial. Dev. Neuropsychol. 2012, 37, 497–517. [Google Scholar] [CrossRef]
- Zhang, G.H.; Ristaniemi, T.; Cong, F.Y. Objective Extraction of Evoked Event-Related Oscillation from Time-Frequency Representation of Event-Related Potentials. Neural. Plast. 2020, 2020, 8841354. [Google Scholar] [CrossRef] [PubMed]
- Cong, F.; Huang, Y.; Kalyakin, I.; Li, H.; Huttunen-Scott, T.; Lyytinen, H.; Ristaniemi, T. Frequency-response-based wavelet decomposition for extracting children’s mismatch negativity elicited by uninterrupted sound. J. Med. Biol. Eng. 2012, 32, 205–213. [Google Scholar] [CrossRef]
- Wang, X.-D.; Liu, A.-P.; Wu, Y.-Y.; Wang, P. Rapid extraction of lexical tone phonology in Chinese characters: A visual mismatch negativity study. PLoS ONE 2013, 8, e56778. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.Y.; Guo, Z.H.; Wang, X.D.; Guo, X.T.; Sun, J.W.; Wang, M.; Li, H.W.; Chen, L. Event-Related Potential Evidence for Involuntary Consciousness During Implicit Memory Retrieval. Front. Behav. Neurosci. 2022, 16, 902175. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Werner, L.A. Issues in human auditory development. J. Commun. Disord. 2007, 40, 275–283. [Google Scholar] [CrossRef]
- Coady, J.A.; Kluender, K.R.; Evans, J.L. Categorical perception of speech by children with specific language impairments. J. Speech Lang. Hear. Res. 2005, 48, 944–959. [Google Scholar] [CrossRef]
- Kraus, N.; McGee, T.; Sharma, A.; Carrell, T.; Nicol, T. mismatch negativity event-related potential elicited by speech stimuli. Ear Hear 1992, 13, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Korpilahti, P.; Lang, H.A. Auditory ERP components and mismatch negativity in dysphasic children. Electroencephalogr. Clin. Neurophysiol. 1994, 91, 256–264. [Google Scholar] [CrossRef]
- Kurtzberg, D.; Vaughan, H.G.; Kreuzer, J.A.; Flieger, K.Z. Developmental Studies and Clinical Application of Mismatch Negativity: Problems and Prospects. Ear Hear 1995, 16, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ceponiene, R.; Service, E.; Kurjenluoma, S.; Cheour, M.; Naatanen, R. Children’s performance on pseudoword repetition depends on auditory trace quality: Evidence from event-related potentials. Dev. Psychol. 1999, 35, 709–720. [Google Scholar] [CrossRef]
- Gomot, M.; Giard, M.H.; Roux, S.; Barthelemy, C.; Bruneau, N. Maturation of frontal and temporal components of mismatch negativity (MMN) in children. Neuroreport 2000, 11, 3109–3112. [Google Scholar] [CrossRef] [PubMed]
- Shafer, V.L.; Morr, M.L.; Kreuzer, J.A.; Kurtzberg, D. Maturation of mismatch negativity in school-age children. Ear Hear 2000, 21, 242–251. [Google Scholar] [CrossRef]
- Uwer, R.; von Suchodoletz, W. Stability of mismatch negativities in children. Clin. Neurophysiol. 2000, 111, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gumenyuk, V.; Korzyukov, O.; Alho, K.; Winkler, I.; Paavilainen, P.; Naatanen, R. Electric brain responses indicate preattentive processing of abstract acoustic regularities in children. Neuroreport 2003, 14, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Shestakova, A.; Huotilainen, M.; Ceponiene, R.; Cheour, M. Event-related potentials associated with second language learning in children. Clin. Neurophysiol. 2003, 114, 1507–1512. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, T.; Halonen, A.; Kaartinen, J.; Lyytinen, H. Does mismatch negativity show differences in reading-disabled children compared to normal children and children with attention deficit? Dev. Neuropsychol. 2007, 31, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Dehaenelambertz, G.; Dehaene, S. Speed and cerebral correlates of syllable discrimination in infants. Nature 1994, 370, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Morr, M.L.; Shafer, V.L.; Kreuzer, J.A.; Kurtzberg, D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear Hear 2002, 23, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Cheour, M.; Leppanen, P.H.T.; Kraus, N. Mismatch negativity (MMN) as a tool for investigating auditory discrimination and sensory memory in infants and children. Clin. Neurophysiol. 2000, 111, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Themas, L.; Lippus, P.; Padrik, M.; Kask, L.; Kreegipuu, K. Maturation of the mismatch response in pre-school children: Systematic literature review and meta-analysis. Neurosci. Biobehav. Rev. 2023, 153, 105366. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Q.; Xiao, R.; Liang, D.D. Lexical tone perception mechanism in 2- to 4-year-old Mandarin-speaking children in the pre-attention stage. Acta Psychol. Sin. 2020, 52, 730–741. [Google Scholar] [CrossRef]
- Yu, K.K.; Li, L.; Chen, Y.; Zhou, Y.C.; Wang, R.M.; Zhang, Y.; Li, P. Effects of native language experience on Mandarin lexical tone processing in proficient second language learners. Psychophysiology 2019, 56, e13448. [Google Scholar] [CrossRef]
- Yu, L.D.; Huang, D.; Wang, S.P.; Zhang, Y. Reduced Neural Specialization for Word-level Linguistic Prosody in Children with Autism. J. Autism Dev. Disord. 2023, 53, 4351–4367. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, W.; Ding, H.W.; Peng, G.; Zhang, Y. Phonological Awareness and Working Memory in Mandarin-Speaking Preschool-Aged Children with Cochlear Implants. J. Speech Lang. Hear. Res. 2022, 65, 4485–4497. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Zhang, Y.; Liu, Y.; Zhang, S.; Zhang, L.; Shu, H.; Zhang, Y. The Neural Development of Chinese Lexical Tone Perception: A Mismatch Negativity Study Across Childhood, Adolescence, and Adulthood. Brain Sci. 2025, 15, 93. https://doi.org/10.3390/brainsci15010093
Wu H, Zhang Y, Liu Y, Zhang S, Zhang L, Shu H, Zhang Y. The Neural Development of Chinese Lexical Tone Perception: A Mismatch Negativity Study Across Childhood, Adolescence, and Adulthood. Brain Sciences. 2025; 15(1):93. https://doi.org/10.3390/brainsci15010093
Chicago/Turabian StyleWu, Han, Yixiao Zhang, Yiru Liu, Shijun Zhang, Linjun Zhang, Hua Shu, and Yang Zhang. 2025. "The Neural Development of Chinese Lexical Tone Perception: A Mismatch Negativity Study Across Childhood, Adolescence, and Adulthood" Brain Sciences 15, no. 1: 93. https://doi.org/10.3390/brainsci15010093
APA StyleWu, H., Zhang, Y., Liu, Y., Zhang, S., Zhang, L., Shu, H., & Zhang, Y. (2025). The Neural Development of Chinese Lexical Tone Perception: A Mismatch Negativity Study Across Childhood, Adolescence, and Adulthood. Brain Sciences, 15(1), 93. https://doi.org/10.3390/brainsci15010093





