Visual Neurorestoration: An Expert Review of Current Strategies for Restoring Vision in Humans
Abstract
1. Introduction
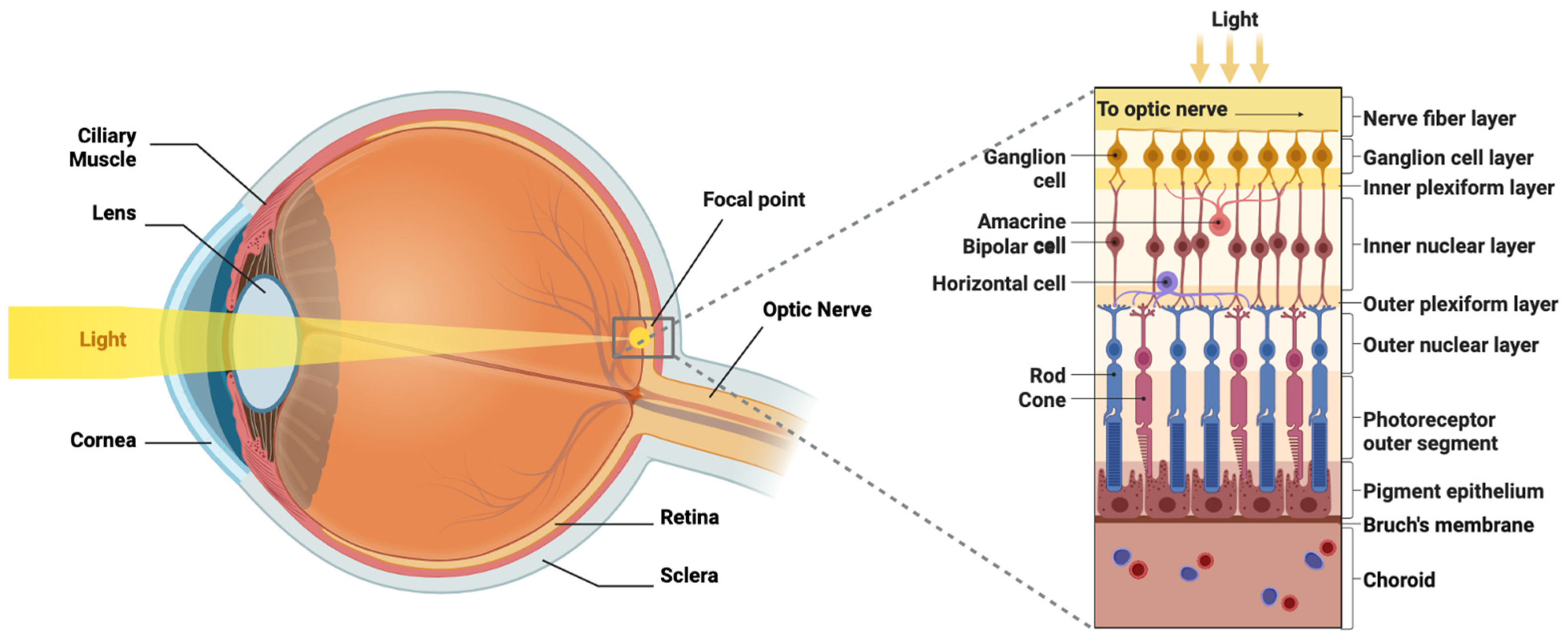
2. Visual Pathway Anatomy
3. Regenerative Therapies
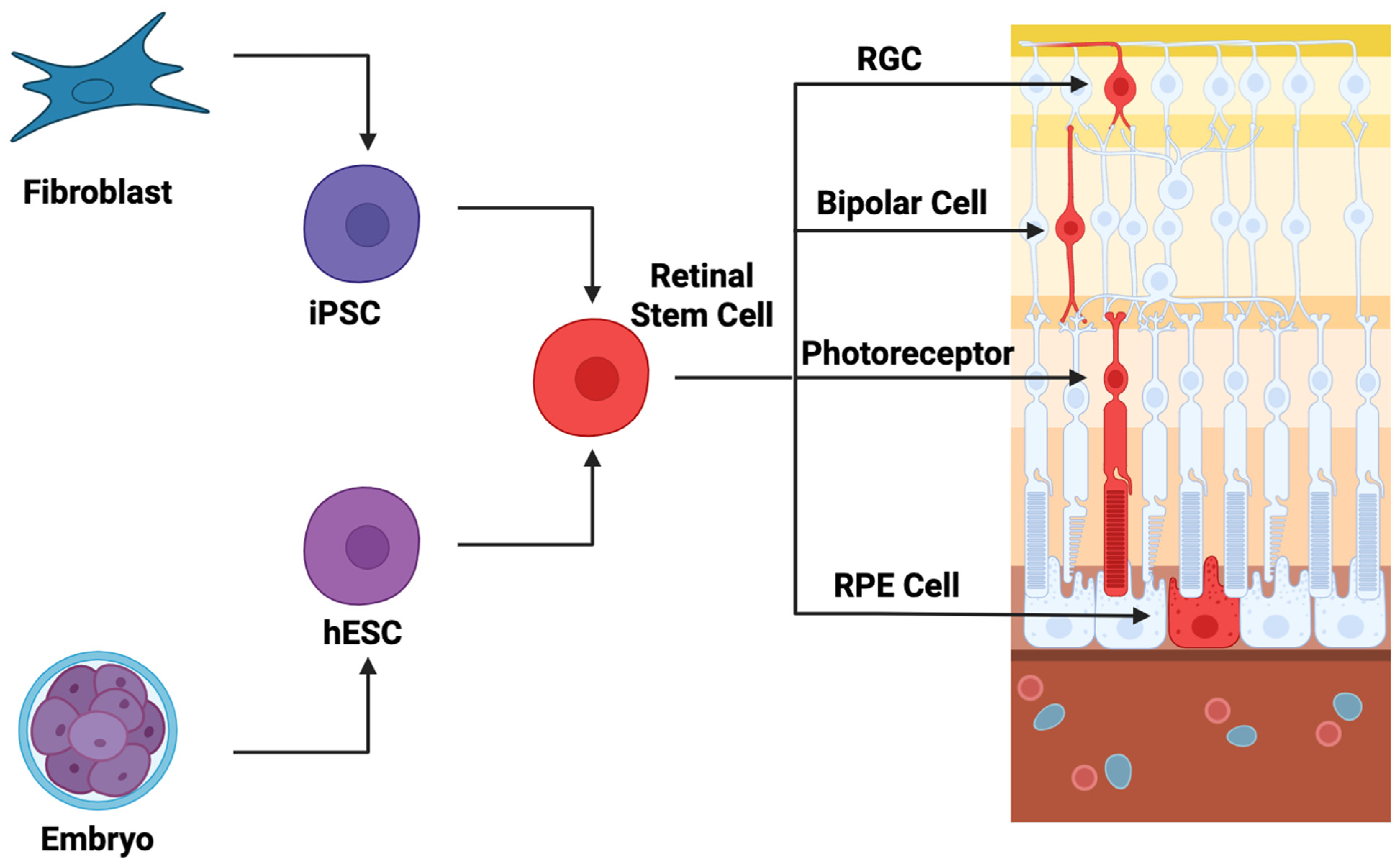
3.1. Cellular Therapeutics
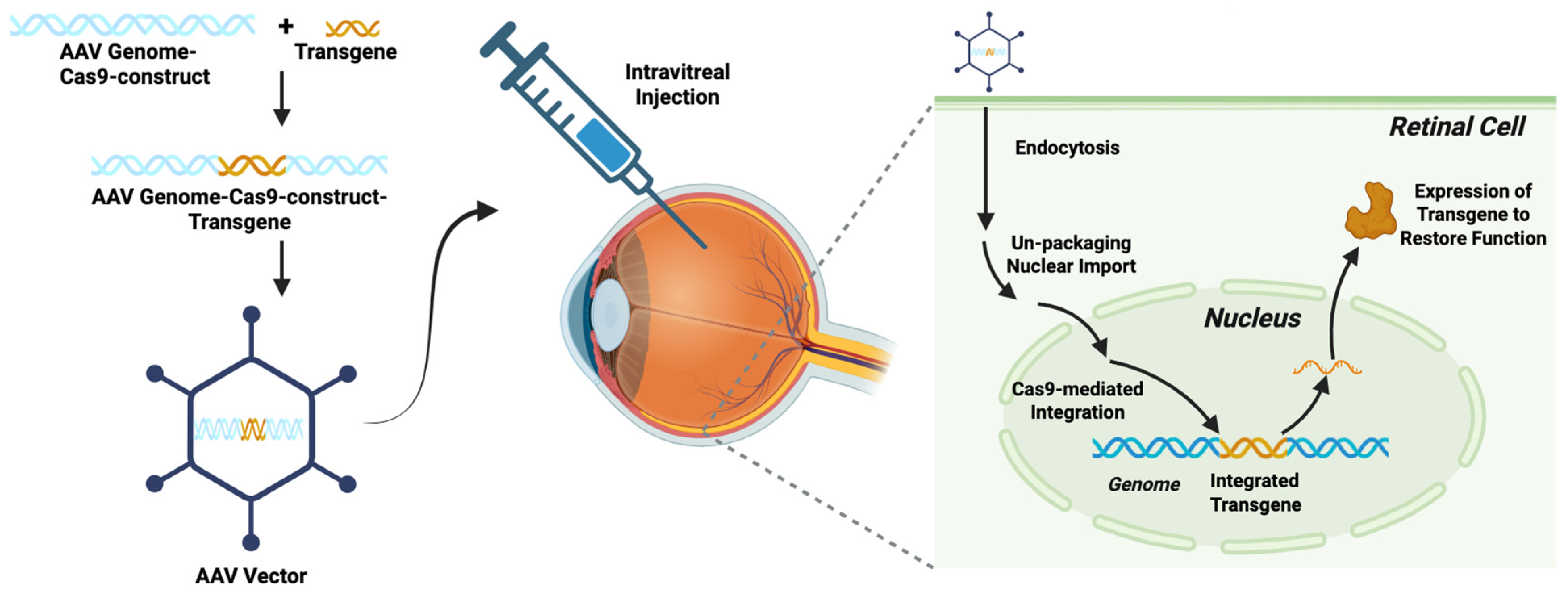
3.2. Genetic Engineering
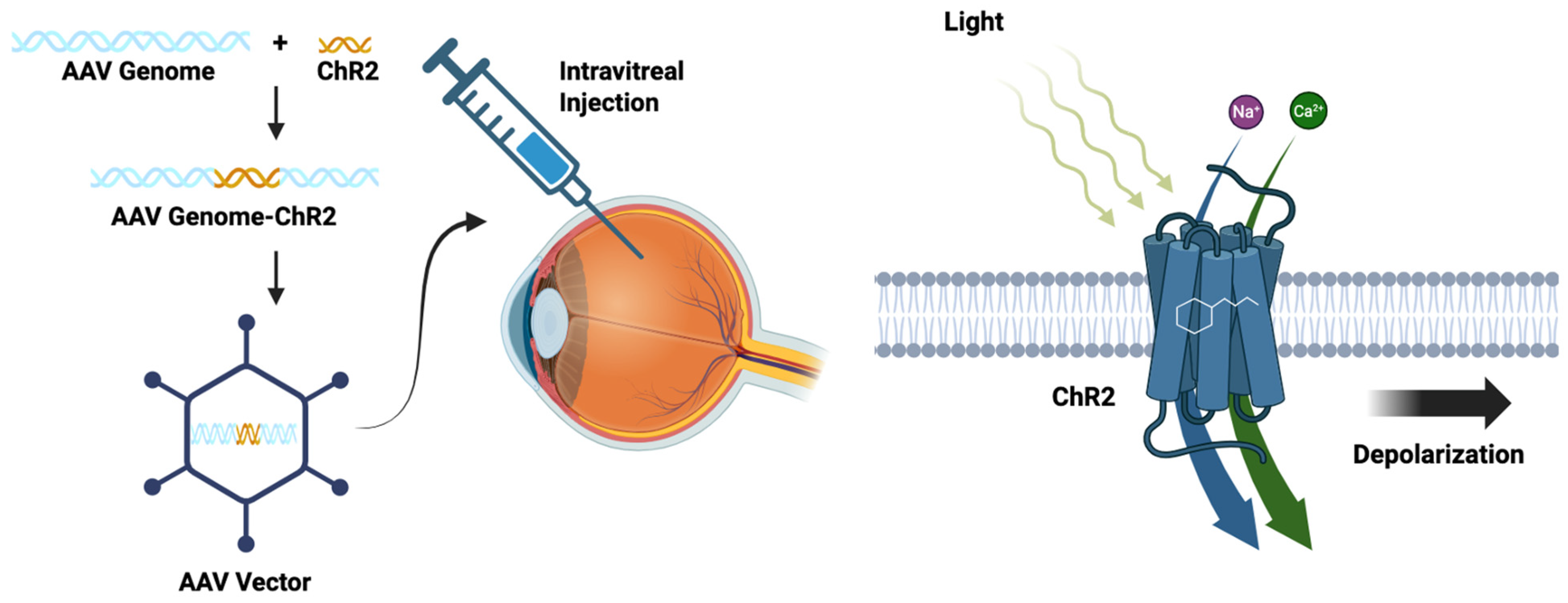
3.3. Optogenetics
3.4. Whole-Eye Transplant
4. Visual Prosthetics
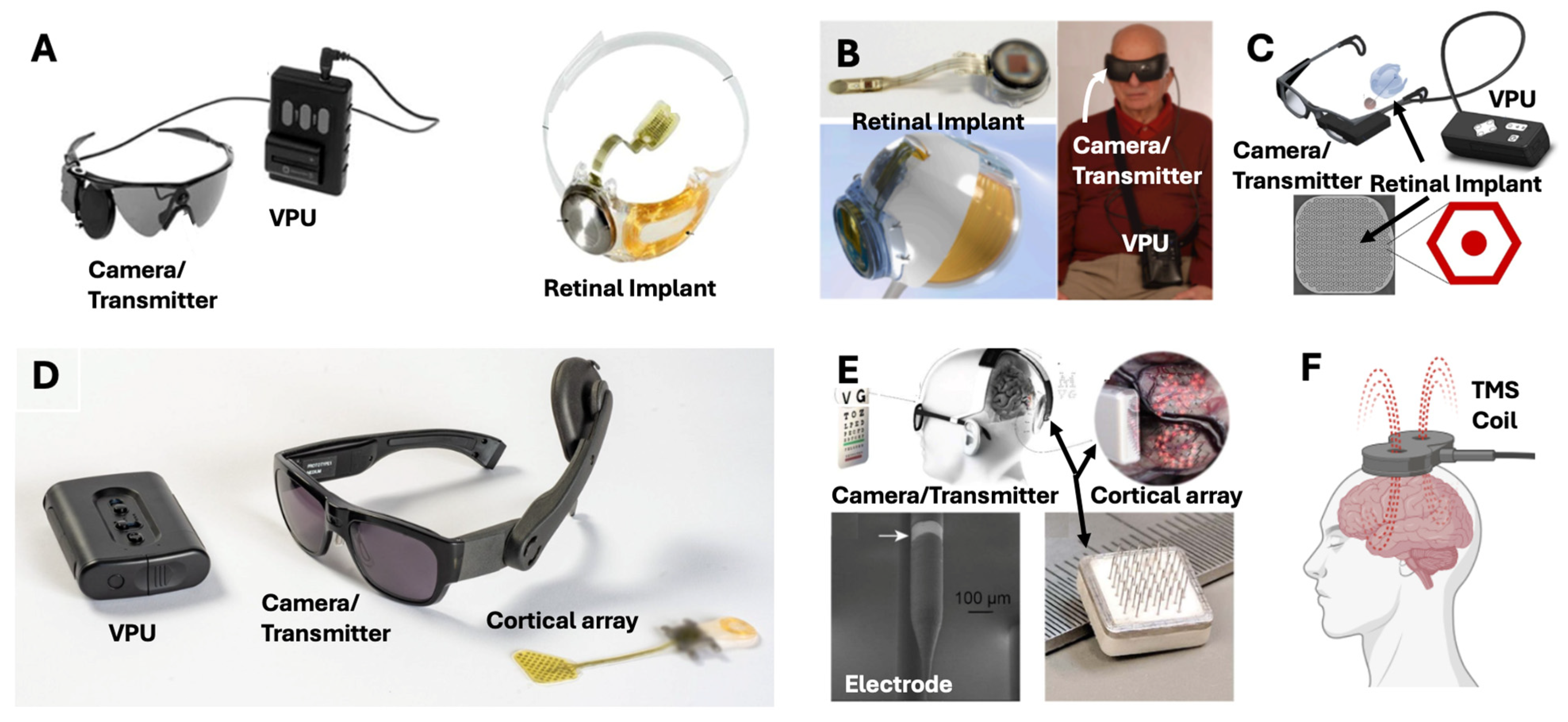
4.1. Retinal Implants
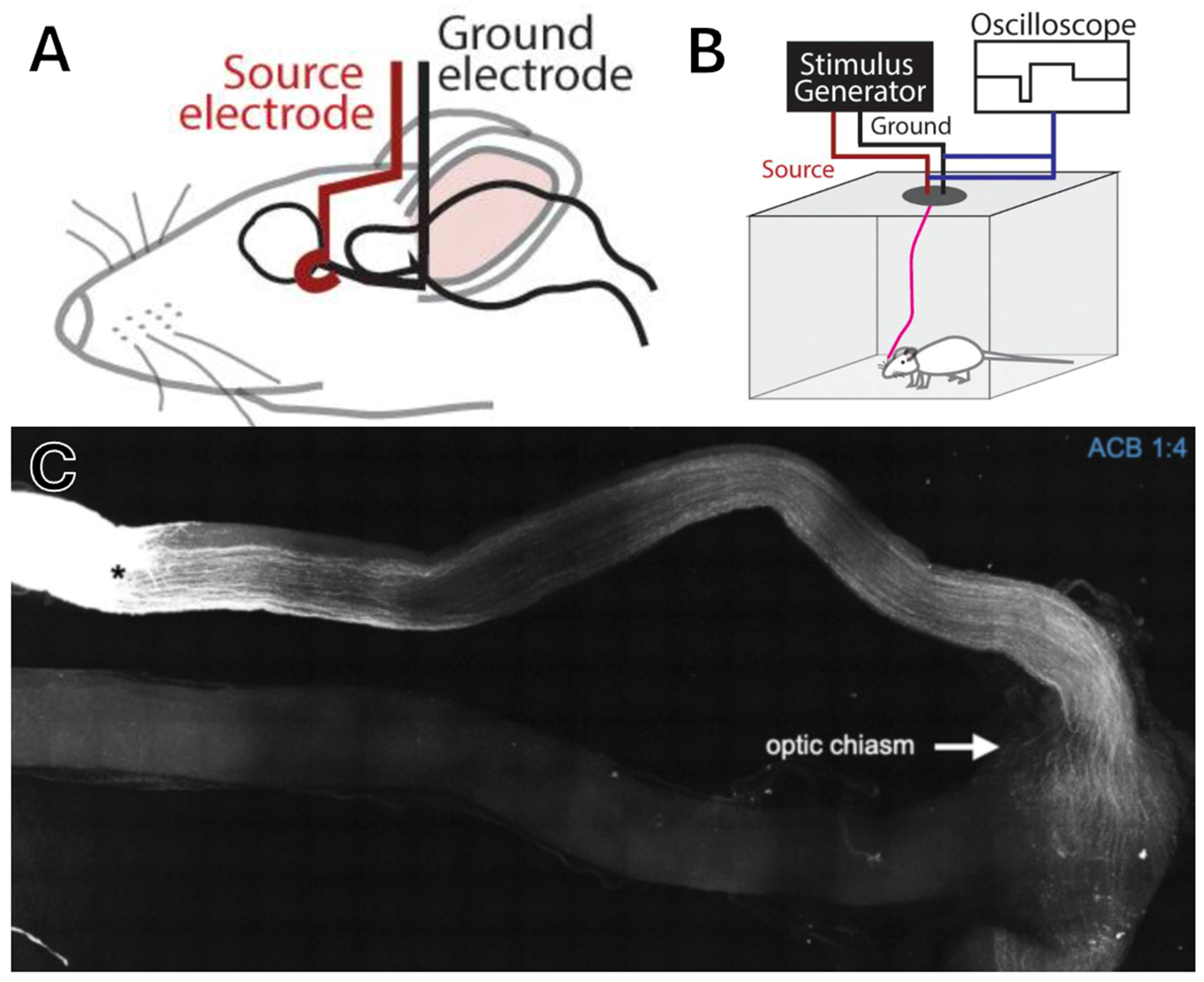
4.2. Optic Nerve Implants
4.3. Thalamic Implants
4.4. Cortical Implants
5. Non-Invasive Neuromodulation
6. Challenges and Future Directions
7. Limitations
8. Conclusion
9. Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bourne, R.R.A.; Flaxman, S.R.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; Leasher, J.; Limburg, H.; et al. Magnitude, Temporal Trends, and Projections of the Global Prevalence of Blindness and Distance and near Vision Impairment: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2017, 5, e888–e897. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Blindness and Vision Impairment Collaborators; Vision Loss Expert Group of the Global Burden of Disease Study. Causes of Blindness and Vision Impairment in 2020 and Trends over 30 Years, and Prevalence of Avoidable Blindness in Relation to VISION 2020: The Right to Sight: An Analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Ah Tong, B.A.M.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The Lancet Global Health Commission on Global Eye Health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Wittenborn, J.S.; Zhang, P.; Sublett, F.; Lamuda, P.A.; Lundeen, E.A.; Saaddine, J. The Economic Burden of Vision Loss and Blindness in the United States. Ophthalmology 2022, 129, 369–378. [Google Scholar] [CrossRef]
- Congdon, N.; O’Colmain, B.; Klaver, C.C.W.; Klein, R.; Muñoz, B.; Friedman, D.S.; Kempen, J.; Taylor, H.R.; Mitchell, P.; Eye Diseases Prevalence Research Group. Causes and Prevalence of Visual Impairment among Adults in the United States. Arch. Ophthalmol. 2004, 122, 477–485. [Google Scholar] [CrossRef]
- Rosen, E. The Invention of Eyeglasses. J. Hist. Med. Allied Sci. 1956, XI, 13–46. [Google Scholar] [CrossRef]
- Visser, N.; Bauer, N.J.C.; Nuijts, R.M.M.A. Toric Intraocular Lenses: Historical Overview, Patient Selection, IOL Calculation, Surgical Techniques, Clinical Outcomes, and Complications. J. Cataract. Refract. Surg. 2013, 39, 624–637. [Google Scholar] [CrossRef]
- Wagner, I.V.; Stewart, M.W.; Dorairaj, S.K. Updates on the Diagnosis and Management of Glaucoma. Mayo Clin. Proc. Innov. Qual. Outcomes 2022, 6, 618–635. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.; Tsubota, K.; Negishi, K.; Kurihara, T. Updates on the Current Treatments for Diabetic Retinopathy and Possibility of Future Oral Therapy. J. Clin. Med. 2021, 10, 4666. [Google Scholar] [CrossRef]
- Georgiou, M.; Fujinami, K.; Michaelides, M. Inherited Retinal Diseases: Therapeutics, Clinical Trials and End Points-A Review. Clin. Exp. Ophthalmol. 2021, 49, 270–288. [Google Scholar] [CrossRef]
- Gallego, C.; Gonçalves, M.A.F.V.; Wijnholds, J. Novel Therapeutic Approaches for the Treatment of Retinal Degenerative Diseases: Focus on CRISPR/Cas-Based Gene Editing. Front. Neurosci. 2020, 14, 838. [Google Scholar] [CrossRef]
- Drag, S.; Dotiwala, F.; Upadhyay, A.K. Gene Therapy for Retinal Degenerative Diseases: Progress, Challenges, and Future Directions. Investig. Ophthalmol. Vis. Sci. 2023, 64, 39. [Google Scholar] [CrossRef] [PubMed]
- Poboży, K.; Poboży, T.; Domański, P.; Derczyński, M.; Konarski, W.; Domańska-Poboża, J. Evolution of Light-Sensitive Proteins in Optogenetic Approaches for Vision Restoration: A Comprehensive Review. Biomedicines 2025, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Gokoffski, K.K.; Washington, K.M.; Chuck, R.S. Clinical and Scientific Considerations for Whole Eye Transplantation: An Ophthalmologist’s Perspective. Transl. Vis. Sci. Technol. 2025, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Vardalakis, N.; Wagner, F.B. Neuroprosthetics: From Sensorimotor to Cognitive Disorders. Commun. Biol. 2023, 6, 14. [Google Scholar] [CrossRef]
- Chuang, A.T.; Margo, C.E.; Greenberg, P.B. Retinal Implants: A Systematic Review. Br. J. Ophthalmol. 2014, 98, 852–856. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Kamei, M.; Fujikado, T.; Yonezawa, E.; Ozawa, M.; Cecilia-Gonzalez, C.; Ustariz-Gonzalez, O.; Quiroz-Mercado, H.; Tano, Y. Artificial Vision by Direct Optic Nerve Electrode (AV-DONE) Implantation in a Blind Patient with Retinitis Pigmentosa. J. Artif. Organs 2009, 12, 206–209. [Google Scholar] [CrossRef]
- Liu, X.; Chen, P.; Ding, X.; Liu, A.; Li, P.; Sun, C.; Guan, H. A Narrative Review of Cortical Visual Prosthesis Systems: The Latest Progress and Significance of Nanotechnology for the Future. Ann. Transl. Med. 2022, 10, 716. [Google Scholar] [CrossRef]
- Navarro, P.A.; Contreras-Lopez, W.O.; Tello, A.; Cardenas, P.L.; Vargas, M.D.; Martinez, L.C.; Yepes-Nuñez, J.J. Effectiveness and Safety of Non-Invasive Neuromodulation for Vision Restoration: A Systematic Review and Meta-Analysis. Neuroophthalmology 2024, 48, 93–110. [Google Scholar] [CrossRef]
- Chen, X.; Xu, N.; Li, J.; Zhao, M.; Huang, L. Stem Cell Therapy for Inherited Retinal Diseases: A Systematic Review and Meta-Analysis. Stem Cell Res. Ther. 2023, 14, 286. [Google Scholar] [CrossRef]
- Murphy, R.; Martin, K.R. Genetic Engineering and the Eye. Eye 2025, 39, 57–68. [Google Scholar] [CrossRef]
- Sakai, D.; Tomita, H.; Maeda, A. Optogenetic Therapy for Visual Restoration. Int. J. Mol. Sci. 2022, 23, 15041. [Google Scholar] [CrossRef] [PubMed]
- Sharf, T.; Kalakuntla, T.; Lee, D.J.; Gokoffski, K.K. Electrical Devices for Visual Restoration. Surv. Ophthalmol. 2022, 67, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Galetta, S.L. Anatomy and Physiology of the Afferent Visual System. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2011; Volume 102, pp. 3–19. ISBN 978-0-444-52903-9. [Google Scholar]
- Mehta, J.; Ang, M. Cornea and Sclera. In Adler’s Physiology of the Eye; Levin, L.A., Kaufman, P.L., Hartnett, M.E., Adler, F.H., Eds.; Elsevier: Philadelphia, PA, USA, 2025; pp. 69–123. ISBN 978-0-323-83406-3. [Google Scholar]
- Donaldson, P. The Lens. In Adler’s Physiology of the Eye; Levin, L.A., Kaufman, P.L., Hartnett, M.E., Adler, F.H., Eds.; Elsevier: Philadelphia, PA, USA, 2025; pp. 124–163. ISBN 978-0-323-83406-3. [Google Scholar]
- Andley, U.P. Crystallins in the Eye: Function and Pathology. Prog. Retin. Eye Res. 2007, 26, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Stamer, W.D.; Kaufman, P.; Delamere, N. Production and Flow of Aqueous Humor. In Adler’s Physiology of the Eye; Levin, L.A., Kaufman, P.L., Hartnett, M.E., Adler, F.H., Eds.; Elsevier: Philadelphia, PA, USA, 2025; pp. 245–283. ISBN 978-0-323-83406-3. [Google Scholar]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The Pathophysiology and Treatment of Glaucoma: A Review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Lim, R. The Surgical Management of Glaucoma: A Review. Clin. Exp. Ophthalmol. 2022, 50, 213–231. [Google Scholar] [CrossRef]
- Chew, L.; Sebag, J. Vitreous. In Adler’s Physiology of the Eye; Levin, L.A., Kaufman, P.L., Hartnett, M.E., Adler, F.H., Eds.; Elsevier: Philadelphia, PA, USA, 2025; pp. 164–188. ISBN 978-0-323-83406-3. [Google Scholar]
- Bowes Rickman, C.; Strauss, O. The Function of the Retinal Pigment Epithelium. In Adler’s Physiology of the Eye; Levin, L.A., Kaufman, P.L., Hartnett, M.E., Adler, F.H., Eds.; Elsevier: Philadelphia, PA, USA, 2025; pp. 339–346. ISBN 978-0-323-83406-3. [Google Scholar]
- MacLeish, P.; Makino, C. Photoresponses of Rods and Cones. In Adler’s Physiology of the Eye; Levin, L.A., Kaufman, P.L., Hartnett, M.E., Adler, F.H., Eds.; Elsevier: Philadelphia, PA, USA, 2025; pp. 432–450. ISBN 978-0-323-83406-3. [Google Scholar]
- Palczewski, K.; Kumasaka, T.; Hori, T.; Behnke, C.A.; Motoshima, H.; Fox, B.A.; Le Trong, I.; Teller, D.C.; Okada, T.; Stenkamp, R.E.; et al. Crystal Structure of Rhodopsin: A G Protein-Coupled Receptor. Science 2000, 289, 739–745. [Google Scholar] [CrossRef]
- Marc, R.; Jones, B. The Synaptic Organization of the Retina. In Adler’s Physiology of the Eye; Levin, L.A., Kaufman, P.L., Hartnett, M.E., Adler, F.H., Eds.; Elsevier: Philadelphia, PA, USA, 2025; pp. 464–479. ISBN 978-0-323-83406-3. [Google Scholar]
- Backus, B.T.; Fleet, D.J.; Parker, A.J.; Heeger, D.J. Human Cortical Activity Correlates with Stereoscopic Depth Perception. J. Neurophysiol. 2001, 86, 2054–2068. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Disner, G.R.; Pinto, F.J.; Lima, C.; Lopes-Ferreira, M. Revisiting Retinal Degeneration Hallmarks: Insights from Molecular Markers and Therapy Perspectives. Int. J. Mol. Sci. 2023, 24, 13079. [Google Scholar] [CrossRef]
- Wärntges, S.; Michelson, G. Detailed Illustration of the Visual Field Representation along the Visual Pathway to the Primary Visual Cortex: A Graphical Summary. Ophthalmic Res. 2014, 51, 37–41. [Google Scholar] [CrossRef]
- Benner, J.D.; Sunness, J.S.; Ziegler, M.D.; Soltanian, J. Limited Macular Translocation for Atrophic Maculopathy. Arch. Ophthalmol. 2002, 120, 586–591. [Google Scholar] [CrossRef]
- Cahill, M.T.; Mruthyunjaya, P.; Bowes Rickman, C.; Toth, C.A. Recurrence of Retinal Pigment Epithelial Changes after Macular Translocation with 360 Degrees Peripheral Retinectomy for Geographic Atrophy. Arch. Ophthalmol. 2005, 123, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Caramoy, A.; Liakopoulos, S.; Menrath, E.; Kirchhof, B. Autologous Translocation of Choroid and Retinal Pigment Epithelium in Geographic Atrophy: Long-Term Functional and Anatomical Outcome. Br. J. Ophthalmol. 2010, 94, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.; Krebs, I.; Hilgers, R.-D.; Abri, A.; Stolba, U.; Assadoulina, A.; Kellner, L.; Stanzel, B.V.; Jahn, C.; Feichtinger, H. Outcome of Transplantation of Autologous Retinal Pigment Epithelium in Age-Related Macular Degeneration: A Prospective Trial. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4151–4160. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.T.L.; Nguyen, Q.T.; Phan, T.T.K.; Nguyen, G.H.; Le, P.T.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem Cell-Based Therapy for Human Diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Radu, M.; Brănișteanu, D.C.; Pirvulescu, R.A.; Dumitrescu, O.M.; Ionescu, M.A.; Zemba, M. Exploring Stem-Cell-Based Therapies for Retinal Regeneration. Life 2024, 14, 668. [Google Scholar] [CrossRef]
- Lamba, D.A.; Karl, M.O.; Ware, C.B.; Reh, T.A. Efficient Generation of Retinal Progenitor Cells from Human Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2006, 103, 12769–12774. [Google Scholar] [CrossRef]
- Banin, E.; Obolensky, A.; Idelson, M.; Hemo, I.; Reinhardtz, E.; Pikarsky, E.; Ben-Hur, T.; Reubinoff, B. Retinal Incorporation and Differentiation of Neural Precursors Derived from Human Embryonic Stem Cells. Stem Cells 2006, 24, 246–257. [Google Scholar] [CrossRef]
- Klimanskaya, I.; Hipp, J.; Rezai, K.A.; West, M.; Atala, A.; Lanza, R. Derivation and Comparative Assessment of Retinal Pigment Epithelium from Human Embryonic Stem Cells Using Transcriptomics. Cloning Stem Cells 2004, 6, 217–245. [Google Scholar] [CrossRef]
- Vugler, A.; Carr, A.-J.; Lawrence, J.; Chen, L.L.; Burrell, K.; Wright, A.; Lundh, P.; Semo, M.; Ahmado, A.; Gias, C.; et al. Elucidating the Phenomenon of HESC-Derived RPE: Anatomy of Cell Genesis, Expansion and Retinal Transplantation. Exp. Neurol. 2008, 214, 347–361. [Google Scholar] [CrossRef]
- Osakada, F.; Ikeda, H.; Mandai, M.; Wataya, T.; Watanabe, K.; Yoshimura, N.; Akaike, A.; Sasai, Y.; Takahashi, M. Toward the Generation of Rod and Cone Photoreceptors from Mouse, Monkey and Human Embryonic Stem Cells. Nat. Biotechnol. 2008, 26, 215–224. [Google Scholar] [CrossRef]
- Hirami, Y.; Osakada, F.; Takahashi, K.; Okita, K.; Yamanaka, S.; Ikeda, H.; Yoshimura, N.; Takahashi, M. Generation of Retinal Cells from Mouse and Human Induced Pluripotent Stem Cells. Neurosci. Lett. 2009, 458, 126–131. [Google Scholar] [CrossRef]
- Meyer, J.S.; Shearer, R.L.; Capowski, E.E.; Wright, L.S.; Wallace, K.A.; McMillan, E.L.; Zhang, S.-C.; Gamm, D.M. Modeling Early Retinal Development with Human Embryonic and Induced Pluripotent Stem Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 16698–16703. [Google Scholar] [CrossRef]
- Lamba, D.A.; McUsic, A.; Hirata, R.K.; Wang, P.-R.; Russell, D.; Reh, T.A. Generation, Purification and Transplantation of Photoreceptors Derived from Human Induced Pluripotent Stem Cells. PLoS ONE 2010, 5, e8763. [Google Scholar] [CrossRef]
- Nakano, T.; Ando, S.; Takata, N.; Kawada, M.; Muguruma, K.; Sekiguchi, K.; Saito, K.; Yonemura, S.; Eiraku, M.; Sasai, Y. Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell 2012, 10, 771–785. [Google Scholar] [CrossRef]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-Organizing Optic-Cup Morphogenesis in Three-Dimensional Culture. Nature 2011, 472, 51–56. [Google Scholar] [CrossRef]
- Zhong, X.; Gutierrez, C.; Xue, T.; Hampton, C.; Vergara, M.N.; Cao, L.-H.; Peters, A.; Park, T.S.; Zambidis, E.T.; Meyer, J.S.; et al. Generation of Three-Dimensional Retinal Tissue with Functional Photoreceptors from Human iPSCs. Nat. Commun. 2014, 5, 4047. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.P.; Hewitt, A.W.; Davidson, K.C.; Pébay, A.; Wong, R.C.B. Methods of Retinal Ganglion Cell Differentiation From Pluripotent Stem Cells. Transl. Vis. Sci. Technol. 2014, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Riazifar, H.; Jia, Y.; Chen, J.; Lynch, G.; Huang, T. Chemically Induced Specification of Retinal Ganglion Cells from Human Embryonic and Induced Pluripotent Stem Cells. Stem Cells Transl. Med. 2014, 3, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yokoi, T.; Tamalu, F.; Watanabe, S.-I.; Nishina, S.; Azuma, N. Generation of Retinal Ganglion Cells with Functional Axons from Human Induced Pluripotent Stem Cells. Sci. Rep. 2015, 5, 8344. [Google Scholar] [CrossRef]
- Sluch, V.M.; Davis, C.O.; Ranganathan, V.; Kerr, J.M.; Krick, K.; Martin, R.; Berlinicke, C.A.; Marsh-Armstrong, N.; Diamond, J.S.; Mao, H.-Q.; et al. Differentiation of Human ESCs to Retinal Ganglion Cells Using a CRISPR Engineered Reporter Cell Line. Sci. Rep. 2015, 5, 16595. [Google Scholar] [CrossRef]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.-P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Patients with Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: Follow-up of Two Open-Label Phase 1/2 Studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Tan, G.; Hosseini, H.; Nagiel, A. Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium for the Treatment of Macular Degeneration: An Assessment at 4 Years. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFc1–ORSFc9. [Google Scholar] [CrossRef] [PubMed]
- Song, W.K.; Park, K.-M.; Kim, H.-J.; Lee, J.H.; Choi, J.; Chong, S.Y.; Shim, S.H.; Del Priore, L.V.; Lanza, R. Treatment of Macular Degeneration Using Embryonic Stem Cell-Derived Retinal Pigment Epithelium: Preliminary Results in Asian Patients. Stem Cell Reports 2015, 4, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Lee, M.J.; Choi, J.; Jung, S.Y.; Chong, S.Y.; Sung, J.H.; Shim, S.H.; Song, W.K. Long-Term Safety and Tolerability of Subretinal Transplantation of Embryonic Stem Cell-Derived Retinal Pigment Epithelium in Asian Stargardt Disease Patients. Br. J. Ophthalmol. 2021, 105, 829–837. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 Clinical Study of an Embryonic Stem Cell-Derived Retinal Pigment Epithelium Patch in Age-Related Macular Degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- Mehat, M.S.; Sundaram, V.; Ripamonti, C.; Robson, A.G.; Smith, A.J.; Borooah, S.; Robinson, M.; Rosenthal, A.N.; Innes, W.; Weleber, R.G.; et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 2018, 125, 1765–1775. [Google Scholar] [CrossRef]
- Li, S.-Y.; Liu, Y.; Wang, L.; Wang, F.; Zhao, T.-T.; Li, Q.-Y.; Xu, H.-W.; Meng, X.-H.; Hao, J.; Zhou, Q.; et al. A Phase I Clinical Trial of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells for Early-Stage Stargardt Macular Degeneration: 5-Years’ Follow-Up. Cell Prolif. 2021, 54, e13100. [Google Scholar] [CrossRef]
- Brant Fernandes, R.A.; Lojudice, F.H.; Zago Ribeiro, L.; Santos da Cruz, N.F.; Polizelli, M.U.; Cristovam, P.C.; Innocenti, F.; Morimoto, L.; Magalhães, O.; Ferraz Sallum, J.M.; et al. TRANSPLANTATION OF SUBRETINAL STEM CELL-DERIVED RETINAL PIGMENT EPITHELIUM FOR STARGARDT DISEASE: A Phase I Clinical Trial. Retina 2023, 43, 263–274. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A Bioengineered Retinal Pigment Epithelial Monolayer for Advanced, Dry Age-Related Macular Degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; Ingram, A.; Dang, W.; et al. One-Year Follow-Up in a Phase 1/2a Clinical Trial of an Allogeneic RPE Cell Bioengineered Implant for Advanced Dry Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 13. [Google Scholar] [CrossRef]
- Kashani, A.H.; Lebkowski, J.S.; Hinton, D.R.; Zhu, D.; Faynus, M.A.; Chen, S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chan, C.; et al. Survival of an HLA-Mismatched, Bioengineered RPE Implant in Dry Age-Related Macular Degeneration. Stem Cell Reports 2022, 17, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Humayun, M.S.; Clegg, D.O.; Dayan, M.S.; Kashani, A.H.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; et al. Long-Term Follow-up of a Phase 1/2a Clinical Trial of a Stem Cell-Derived Bioengineered Retinal Pigment Epithelium Implant for Geographic Atrophy. Ophthalmology 2024, 131, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef]
- Park, S.S.; Bauer, G.; Abedi, M.; Pontow, S.; Panorgias, A.; Jonnal, R.; Zawadzki, R.J.; Werner, J.S.; Nolta, J. Intravitreal Autologous Bone Marrow CD34+ Cell Therapy for Ischemic and Degenerative Retinal Disorders: Preliminary Phase 1 Clinical Trial Findings. Investig. Ophthalmol. Vis. Sci. 2014, 56, 81–89. [Google Scholar] [CrossRef]
- Oner, A.; Gonen, Z.B.; Sevim, D.G.; Smim Kahraman, N.; Unlu, M. Suprachoroidal Adipose Tissue-Derived Mesenchymal Stem Cell Implantation in Patients with Dry-Type Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: 6-Month Follow-Up Results of a Phase 2 Study. Cell. Reprogram. 2018, 20, 329–336. [Google Scholar] [CrossRef]
- Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Scalinci, S.Z.; Nebbioso, M. Regenerative Therapy by Suprachoroidal Cell Autograft in Dry Age-Related Macular Degeneration: Preliminary In Vivo Report. J. Vis. Exp. 2018, 12, 56469. [Google Scholar] [CrossRef]
- Tuekprakhon, A.; Sangkitporn, S.; Trinavarat, A.; Pawestri, A.R.; Vamvanij, V.; Ruangchainikom, M.; Luksanapruksa, P.; Pongpaksupasin, P.; Khorchai, A.; Dambua, A.; et al. Intravitreal Autologous Mesenchymal Stem Cell Transplantation: A Non-Randomized Phase I Clinical Trial in Patients with Retinitis Pigmentosa. Stem Cell Res. Ther. 2021, 12, 52. [Google Scholar] [CrossRef]
- Nittala, M.G.; Uji, A.; Velaga, S.B.; Hariri, A.H.; Naor, J.; Birch, D.G.; Spencer, R.; Leng, T.; Capela, A.; Tsukamoto, A.; et al. Effect of Human Central Nervous System Stem Cell Subretinal Transplantation on Progression of Geographic Atrophy Secondary to Nonneovascular Age-Related Macular Degeneration. Ophthalmol. Retina 2021, 5, 32–40. [Google Scholar] [CrossRef]
- Lanigan, T.M.; Kopera, H.C.; Saunders, T.L. Principles of Genetic Engineering. Genes 2020, 11, 291. [Google Scholar] [CrossRef]
- Sander, J.D.; Joung, J.K. CRISPR-Cas Systems for Editing, Regulating and Targeting Genomes. Nat. Biotechnol. 2014, 32, 347–355. [Google Scholar] [CrossRef]
- Pacesa, M.; Pelea, O.; Jinek, M. Past, Present, and Future of CRISPR Genome Editing Technologies. Cell 2024, 187, 1076–1100. [Google Scholar] [CrossRef]
- Wang, J.Y.; Doudna, J.A. CRISPR Technology: A Decade of Genome Editing Is Only the Beginning. Science 2023, 379, eadd8643. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, Y.; Qi, H.; Cui, W.; Zhang, L.; Fu, X.; He, X.; Liu, M.; Li, P.-F.; Yu, T. CRISPR/Cas9 Therapeutics: Progress and Prospects. Signal Transduct. Target. Ther. 2023, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral Vector Platforms within the Gene Therapy Landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-H.; Tso, A.; Breazzano, M.P.; Jenny, L.A.; Levi, S.R.; Tsang, S.H.; Quinn, P.M.J. Culture of Human Retinal Explants for Ex Vivo Assessment of AAV Gene Delivery. Methods Mol. Biol. 2023, 2560, 303–311. [Google Scholar] [CrossRef]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and Safety of Voretigene Neparvovec (AAV2-hRPE65v2) in Patients with RPE65-Mediated Inherited Retinal Dystrophy: A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Kahle, N.A.; Peters, T.; Zobor, D.; Kuehlewein, L.; Kohl, S.; Zhour, A.; Werner, A.; Seitz, I.P.; Sothilingam, V.; Michalakis, S.; et al. Development of Methodology and Study Protocol: Safety and Efficacy of a Single Subretinal Injection of rAAV.hCNGA3 in Patients with CNGA3-Linked Achromatopsia Investigated in an Exploratory Dose-Escalation Trial. Hum. Gene Ther. Clin. Dev. 2018, 29, 121–131. [Google Scholar] [CrossRef]
- Fischer, M.D.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Ochakovski, G.A.; Klein, R.; Schoen, C.; et al. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial. JAMA Ophthalmol. 2020, 138, 643–651. [Google Scholar] [CrossRef]
- Reichel, F.F.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Sothilingam, V.; Kuehlewein, L.; Kahle, N.; et al. Three-Year Results of Phase I Retinal Gene Therapy Trial for CNGA3-Mutated Achromatopsia: Results of a Non Randomised Controlled Trial. Br. J. Ophthalmol. 2022, 106, 1567–1572. [Google Scholar] [CrossRef]
- Michaelides, M.; Hirji, N.; Wong, S.C.; Besirli, C.G.; Zaman, S.; Kumaran, N.; Georgiadis, A.; Smith, A.J.; Ripamonti, C.; Gottlob, I.; et al. First-in-Human Gene Therapy Trial of AAV8-hCARp.hCNGB3 in Adults and Children With CNGB3-Associated Achromatopsia. Am. J. Ophthalmol. 2023, 253, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.F.; Borchert, G.A. Gene Therapy for Achromatopsia. Int. J. Mol. Sci. 2024, 25, 9739. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Yu, S.; Li, H.; Chen, S.; Luo, J.; Wang, H.; Guan, Y.; Zhang, H.; Yin, S.; et al. Gene Replacement Therapy in Bietti Crystalline Corneoretinal Dystrophy: An Open-Label, Single-Arm, Exploratory Trial. Signal Transduct. Target. Ther. 2024, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.; Cui, S.; Wang, G.; Liu, Y.; Qu, G.; Jiang, L.; Liu, Y.; Li, X. Safety and Vision Outcomes Following Gene Therapy for Bietti Crystalline Dystrophy: A Nonrandomized Clinical Trial. JAMA Ophthalmol. 2025, 143, 126–133. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.M.; Black, G.C.M.; et al. Retinal Gene Therapy in Patients with Choroideremia: Initial Findings from a Phase 1/2 Clinical Trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef]
- Dimopoulos, I.S.; Hoang, S.C.; Radziwon, A.; Binczyk, N.M.; Seabra, M.C.; MacLaren, R.E.; Somani, R.; Tennant, M.T.S.; MacDonald, I.M. Two-Year Results After AAV2-Mediated Gene Therapy for Choroideremia: The Alberta Experience. Am. J. Ophthalmol. 2018, 193, 130–142. [Google Scholar] [CrossRef]
- Fischer, M.D.; Ochakovski, G.A.; Beier, B.; Seitz, I.P.; Vaheb, Y.; Kortuem, C.; Reichel, F.F.L.; Kuehlewein, L.; Kahle, N.A.; Peters, T.; et al. Changes in Retinal Sensitivity After Gene Therapy in Choroideremia. Retina 2020, 40, 160–168. [Google Scholar] [CrossRef]
- Aleman, T.S.; Huckfeldt, R.M.; Serrano, L.W.; Pearson, D.J.; Vergilio, G.K.; McCague, S.; Marshall, K.A.; Ashtari, M.; Doan, T.M.; Weigel-DiFranco, C.A.; et al. Adeno-Associated Virus Serotype 2-hCHM Subretinal Delivery to the Macula in Choroideremia: Two-Year Interim Results of an Ongoing Phase I/II Gene Therapy Trial. Ophthalmology 2022, 129, 1177–1191. [Google Scholar] [CrossRef]
- Silson, E.H.; Baker, C.I.; Aleman, T.S.; Maguire, A.M.; Bennett, J.; Ashtari, M. Motion-Selective Areas V5/MT and MST Appear Resistant to Deterioration in Choroideremia. Neuroimage Clin. 2023, 38, 103384. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Fischer, M.D.; Gow, J.A.; Lam, B.L.; Sankila, E.-M.K.; Girach, A.; Panda, S.; Yoon, D.; Zhao, G.; Pennesi, M.E. Subretinal Timrepigene Emparvovec in Adult Men with Choroideremia: A Randomized Phase 3 Trial. Nat. Med. 2023, 29, 2464–2472. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Audo, I.; Fischer, M.D.; Huckfeldt, R.M.; Lam, B.L.; Pennesi, M.E.; Sisk, R.; Gow, J.A.; Li, J.; Zhu, K.; et al. An Open-Label Phase II Study Assessing the Safety of Bilateral, Sequential Administration of Retinal Gene Therapy in Participants with Choroideremia: The GEMINI Study. Hum. Gene Ther. 2024, 35, 564–575. [Google Scholar] [CrossRef]
- Ghazi, N.G.; Abboud, E.B.; Nowilaty, S.R.; Alkuraya, H.; Alhommadi, A.; Cai, H.; Hou, R.; Deng, W.-T.; Boye, S.L.; Almaghamsi, A.; et al. Treatment of Retinitis Pigmentosa Due to MERTK Mutations by Ocular Subretinal Injection of Adeno-Associated Virus Gene Vector: Results of a Phase I Trial. Hum. Genet. 2016, 135, 327–343. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial Results from a First-in-Human Gene Therapy Trial on X-Linked Retinitis Pigmentosa Caused by Mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef] [PubMed]
- von Krusenstiern, L.; Liu, J.; Liao, E.; Gow, J.A.; Chen, G.; Ong, T.; Lotery, A.J.; Jalil, A.; Lam, B.L.; MacLaren, R.E.; et al. Changes in Retinal Sensitivity Associated With Cotoretigene Toliparvovec in X-Linked Retinitis Pigmentosa With RPGR Gene Variations. JAMA Ophthalmol. 2023, 141, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.L.; Pennesi, M.E.; Kay, C.N.; Panda, S.; Gow, J.A.; Zhao, G.; MacLaren, R.E.; XIRIUS Study Group. Assessment of Visual Function with Cotoretigene Toliparvovec in X-Linked Retinitis Pigmentosa in the Randomized XIRIUS Phase 2/3 Study. Ophthalmology 2024, 131, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, M.; Besirli, C.G.; Yang, Y.; DE Guimaraes, T.A.C.; Wong, S.C.; Huckfeldt, R.M.; Comander, J.I.; Sahel, J.-A.; Shah, S.M.; Tee, J.J.L.; et al. Phase 1/2 AAV5-hRKp.RPGR (Botaretigene Sparoparvovec) Gene Therapy: Safety and Efficacy in RPGR-Associated X-Linked Retinitis Pigmentosa. Am. J. Ophthalmol. 2024, 267, 122–134. [Google Scholar] [CrossRef]
- Yang, P.; Birch, D.; Lauer, A.; Sisk, R.; Anand, R.; Pennesi, M.E.; Iannaccone, A.; Yaghy, A.; Scaria, A.; Jung, J.A.; et al. Subretinal Gene Therapy Drug AGTC-501 for XLRP Phase 1/2 Multicenter Study (HORIZON): 24-Month Safety and Efficacy Results. Am. J. Ophthalmol. 2025, 271, 268–285. [Google Scholar] [CrossRef]
- Parker, M.A.; Erker, L.R.; Audo, I.; Choi, D.; Mohand-Said, S.; Sestakauskas, K.; Benoit, P.; Appelqvist, T.; Krahmer, M.; Ségaut-Prévost, C.; et al. Three-Year Safety Results of SAR422459 (EIAV-ABCA4) Gene Therapy in Patients With ABCA4-Associated Stargardt Disease: An Open-Label Dose-Escalation Phase I/IIa Clinical Trial, Cohorts 1-5. Am. J. Ophthalmol. 2022, 240, 285–301. [Google Scholar] [CrossRef]
- Bowles, D.E.; McPhee, S.W.J.; Li, C.; Gray, S.J.; Samulski, J.J.; Camp, A.S.; Li, J.; Wang, B.; Monahan, P.E.; Rabinowitz, J.E.; et al. Phase 1 Gene Therapy for Duchenne Muscular Dystrophy Using a Translational Optimized AAV Vector. Mol. Ther. 2012, 20, 443–455. [Google Scholar] [CrossRef]
- Cukras, C.; Wiley, H.E.; Jeffrey, B.G.; Sen, H.N.; Turriff, A.; Zeng, Y.; Vijayasarathy, C.; Marangoni, D.; Ziccardi, L.; Kjellstrom, S.; et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol. Ther. 2018, 26, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Vijayasarathy, C.; Cukras, C.A.; Wiley, H.E.; Sen, H.N.; Zeng, Y.; Wei, L.L.; Sieving, P.A. Immune Function in X-Linked Retinoschisis Subjects in an AAV8-RS1 Phase I/IIa Gene Therapy Trial. Mol. Ther. 2021, 29, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Yang, P.; Birch, D.G.; Weng, C.Y.; Moore, A.T.; Iannaccone, A.; Comander, J.I.; Jayasundera, T.; Chulay, J.; XLRS-001 Study Group. Intravitreal Delivery of rAAV2tYF-CB-hRS1 Vector for Gene Augmentation Therapy in Patients with X-Linked Retinoschisis: 1-Year Clinical Results. Ophthalmol. Retina 2022, 6, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, I.; Heredero Berzal, A.; Koster, C.; Ten Asbroek, A.L.M.A.; Bergen, A.A.; Boon, C.J.F. The Road towards Gene Therapy for X-Linked Juvenile Retinoschisis: A Systematic Review of Preclinical Gene Therapy in Cell-Based and Rodent Models of XLRS. Int. J. Mol. Sci. 2024, 25, 1267. [Google Scholar] [CrossRef]
- Lam, B.L.; Feuer, W.J.; Abukhalil, F.; Porciatti, V.; Hauswirth, W.W.; Guy, J. Leber Hereditary Optic Neuropathy Gene Therapy Clinical Trial Recruitment: Year 1. Arch. Ophthalmol. 2010, 128, 1129–1135. [Google Scholar] [CrossRef]
- Koilkonda, R.D.; Yu, H.; Chou, T.-H.; Feuer, W.J.; Ruggeri, M.; Porciatti, V.; Tse, D.; Hauswirth, W.W.; Chiodo, V.; Boye, S.L.; et al. Safety and Effects of the Vector for the Leber Hereditary Optic Neuropathy Gene Therapy Clinical Trial. JAMA Ophthalmol. 2014, 132, 409–420. [Google Scholar] [CrossRef]
- Feuer, W.J.; Schiffman, J.C.; Davis, J.L.; Porciatti, V.; Gonzalez, P.; Koilkonda, R.D.; Yuan, H.; Lalwani, A.; Lam, B.L.; Guy, J. Gene Therapy for Leber Hereditary Optic Neuropathy: Initial Results. Ophthalmology 2016, 123, 558–570. [Google Scholar] [CrossRef]
- Guy, J.; Feuer, W.J.; Davis, J.L.; Porciatti, V.; Gonzalez, P.J.; Koilkonda, R.D.; Yuan, H.; Hauswirth, W.W.; Lam, B.L. Gene Therapy for Leber Hereditary Optic Neuropathy: Low- and Medium-Dose Visual Results. Ophthalmology 2017, 124, 1621–1634. [Google Scholar] [CrossRef]
- Vignal, C.; Uretsky, S.; Fitoussi, S.; Galy, A.; Blouin, L.; Girmens, J.-F.; Bidot, S.; Thomasson, N.; Bouquet, C.; Valero, S.; et al. Safety of rAAV2/2-ND4 Gene Therapy for Leber Hereditary Optic Neuropathy. Ophthalmology 2018, 125, 945–947. [Google Scholar] [CrossRef]
- Bouquet, C.; Vignal Clermont, C.; Galy, A.; Fitoussi, S.; Blouin, L.; Munk, M.R.; Valero, S.; Meunier, S.; Katz, B.; Sahel, J.A.; et al. Immune Response and Intraocular Inflammation in Patients With Leber Hereditary Optic Neuropathy Treated With Intravitreal Injection of Recombinant Adeno-Associated Virus 2 Carrying the ND4 Gene: A Secondary Analysis of a Phase 1/2 Clinical Trial. JAMA Ophthalmol. 2019, 137, 399–406. [Google Scholar] [CrossRef]
- Yu-Wai-Man, P.; Newman, N.J.; Carelli, V.; Moster, M.L.; Biousse, V.; Sadun, A.A.; Klopstock, T.; Vignal-Clermont, C.; Sergott, R.C.; Rudolph, G.; et al. Bilateral Visual Improvement with Unilateral Gene Therapy Injection for Leber Hereditary Optic Neuropathy. Sci. Transl. Med. 2020, 12, eaaz7423. [Google Scholar] [CrossRef]
- Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Moster, M.L.; Biousse, V.; Vignal-Clermont, C.; Sergott, R.C.; Klopstock, T.; Sadun, A.A.; Barboni, P.; et al. Efficacy and Safety of Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy Treated within 6 Months of Disease Onset. Ophthalmology 2021, 128, 649–660. [Google Scholar] [CrossRef]
- Moster, M.L.; Sergott, R.C.; Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Bryan, M.S.; Smits, G.; Biousse, V.; Vignal-Clermont, C.; Klopstock, T.; et al. Cross-Sectional Analysis of Baseline Visual Parameters in Subjects Recruited Into the RESCUE and REVERSE ND4-LHON Gene Therapy Studies. J. Neuroophthalmol. 2021, 41, 298–308. [Google Scholar] [CrossRef]
- Biousse, V.; Newman, N.J.; Yu-Wai-Man, P.; Carelli, V.; Moster, M.L.; Vignal-Clermont, C.; Klopstock, T.; Sadun, A.A.; Sergott, R.C.; Hage, R.; et al. Long-Term Follow-Up After Unilateral Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy: The RESTORE Study. J. Neuroophthalmol. 2021, 41, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.L.; Feuer, W.J.; Porciatti, V.; Davis, J.L.; Zheng, D.D.; Vanner, E.A.; Savatovsky, E.J.; Alba, D.E.; Guy, J. Leber Hereditary Optic Neuropathy Gene Therapy: Longitudinal Relationships Among Visual Function and Anatomical Measures. Am. J. Ophthalmol. 2024, 257, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Yu-Wai-Man, P.; Newman, N.J.; Biousse, V.; Carelli, V.; Moster, M.L.; Vignal-Clermont, C.; Klopstock, T.; Sadun, A.A.; Sergott, R.C.; Hage, R.; et al. Five-Year Outcomes of Lenadogene Nolparvovec Gene Therapy in Leber Hereditary Optic Neuropathy. JAMA Ophthalmol. 2025, 143, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sergott, R.C.; Carelli, V.; Newman, N.J.; Biousse, V.; Yu-Wai-Man, P.; Vignal-Clermont, C.; Josse, C.; Taiel, M.; Sahel, J.-A.; Barboni, P.; et al. Predictors of Final Visual Outcome in Patients With Leber Hereditary Optic Neuropathy Treated With Lenadogene Nolparvovec Gene Therapy. Investig. Ophthalmol. Vis. Sci. 2025, 66, 42. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.; Qi, J.; Ruan, K.; Li, B.; Dan, Y.; Zhang, Y. The rAAV2-ND1 Gene Therapy for Leber Hereditary Optic Neuropathy. Graefes Arch. Clin. Exp. Ophthalmol. 2025, 263, 2017–2024. [Google Scholar] [CrossRef]
- Chen, B.S.; Perot, S.; Taiel, M.; Yu-Wai-Man, P.; Horton, M.; LHON Study Group. Rasch Analysis of the NEI-VFQ-25: Vision-Related Quality of Life in Leber Hereditary Optic Neuropathy after Lenadogene Nolparvovec Gene Therapy. BMJ Open Ophthalmol. 2025, 10, e002164. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Wallsh, J.O.; Gallemore, R.P. Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells 2021, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, E.P.; Lai, C.-M.; Magno, A.L.; Wikstrom, M.E.; French, M.A.; Pierce, C.M.; Schwartz, S.D.; Blumenkranz, M.S.; Chalberg, T.W.; Degli-Esposti, M.A.; et al. Gene Therapy with Recombinant Adeno-Associated Vectors for Neovascular Age-Related Macular Degeneration: 1 Year Follow-up of a Phase 1 Randomised Clinical Trial. Lancet 2015, 386, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Constable, I.J.; Pierce, C.M.; Lai, C.-M.; Magno, A.L.; Degli-Esposti, M.A.; French, M.A.; McAllister, I.L.; Butler, S.; Barone, S.B.; Schwartz, S.D.; et al. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal rAAV.sFLT-1 for Wet Age-Related Macular Degeneration. EBioMedicine 2016, 14, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, E.P.; Magno, A.L.; Lai, C.-M.; Pierce, C.M.; Degli-Esposti, M.A.; Blumenkranz, M.S.; Constable, I.J. Three-Year Follow-Up of Phase 1 and 2a rAAV.sFLT-1 Subretinal Gene Therapy Trials for Exudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2019, 204, 113–123. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Avery, R.; Brown, D.M.; Heier, J.S.; Ho, A.C.; Huddleston, S.M.; Jaffe, G.J.; Khanani, A.M.; Pakola, S.; Pieramici, D.J.; et al. Gene Therapy for Neovascular Age-Related Macular Degeneration by Subretinal Delivery of RGX-314: A Phase 1/2a Dose-Escalation Study. Lancet 2024, 403, 1563–1573. [Google Scholar] [CrossRef]
- Pivotal 1 Study of ABBV-RGX-314 (Also Known as RGX-314) Gene Therapy Administered Via Subretinal Delivery One Time in Participants With Namd. Available online: https://www.abbvieclinicaltrials.com/study/?id=RGX-314-2104 (accessed on 10 June 2025).
- Regenexbio Announces Positive Interim Data from Phase II Aaviate Trial of ABBV-RGX-314 for Treatment of Wet AMD. Available online: https://www.ophthalmologytimes.com/view/regenexbio-announces-positive-interim-data-from-phase-ii-aaviate-trial-of-abbv-rgx-314-for-treatment-of-wet-amd (accessed on 10 June 2025).
- RGX-314 Gene Therapy Administered in the Suprachoroidal Space for Participants With Diabetic Retinopathy (DR) Without Center Involved-Diabetic Macular Edema (CI-DME). Available online: https://www.abbvieclinicaltrials.com/study/?id=RGX-314-2202 (accessed on 10 June 2025).
- Greenlee, T.E.; Malhotra, N.A.; Iyer, A.I.; Conti, T.F.; Chen, A.X.; Singh, R.P. Association of Socioeconomic Health Care Disparities With Use of Anti-Vascular Endothelial Growth Factor and Visual Acuity Outcomes in Patients With Diabetic Macular Edema. Ophthalmic Surg. Lasers Imaging Retin. 2022, 53, 380–391. [Google Scholar] [CrossRef]
- Desai, D.; Dugel, P.U. Complement Cascade Inhibition in Geographic Atrophy: A Review. Eye 2022, 36, 294–302. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Feuer, W.J. Lessons from Recent Phase III Trial Failures: Don’t Design Phase III Trials Based on Retrospective Subgroup Analyses from Phase II Trials. Ophthalmology 2018, 125, 1488–1491. [Google Scholar] [CrossRef]
- Heier, J.S.; Cohen, M.N.; Chao, D.L.; Pepio, A.; Shiraga, Y.; Capuano, G.; Rogers, A.; Ackert, J.; Sen, H.N.; Csaky, K. Phase 1 Study of JNJ-81201887 Gene Therapy in Geographic Atrophy Secondary to Age-Related Macular Degeneration. Ophthalmology 2024, 131, 1377–1388. [Google Scholar] [CrossRef]
- Rowe, L.W.; Ciulla, T.A. Gene Therapy for Non-Hereditary Retinal Disease: Age-Related Macular Degeneration, Diabetic Retinopathy, and Beyond. Genes. 2024, 15, 720. [Google Scholar] [CrossRef]
- Chern, K.J.; Nettesheim, E.R.; Reid, C.A.; Li, N.W.; Marcoe, G.J.; Lipinski, D.M. Prostaglandin-Based rAAV-Mediated Glaucoma Gene Therapy in Brown Norway Rats. Commun. Biol. 2022, 5, 1169. [Google Scholar] [CrossRef]
- Wu, J.; Bell, O.H.; Copland, D.A.; Young, A.; Pooley, J.R.; Maswood, R.; Evans, R.S.; Khaw, P.T.; Ali, R.R.; Dick, A.D.; et al. Gene Therapy for Glaucoma by Ciliary Body Aquaporin 1 Disruption Using CRISPR-Cas9. Mol. Ther. 2020, 28, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Martínez, T.; González, M.V.; Roehl, I.; Wright, N.; Pañeda, C.; Jiménez, A.I. In Vitro and in Vivo Efficacy of SYL040012, a Novel siRNA Compound for Treatment of Glaucoma. Mol. Ther. 2014, 22, 81–91. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.; Crosbie, D.E.; Cassidy, P.S.; Sherwood, J.M.; Flügel-Koch, C.; Lütjen-Drecoll, E.; Humphries, M.M.; Reina-Torres, E.; Wallace, D.; Kiang, A.-S.; et al. Therapeutic Potential of AAV-Mediated MMP-3 Secretion from Corneal Endothelium in Treating Glaucoma. Hum. Mol. Genet. 2017, 26, 1230–1246. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Zode, G.; Kasetti, R.B.; Ran, F.A.; Yan, W.; Sharma, T.P.; Bugge, K.; Searby, C.C.; Fingert, J.H.; Zhang, F.; et al. CRISPR-Cas9-Based Treatment of Myocilin-Associated Glaucoma. Proc. Natl. Acad. Sci. USA 2017, 114, 11199–11204. [Google Scholar] [CrossRef]
- Rayana, N.P.; Sugali, C.K.; Dai, J.; Peng, M.; Liu, S.; Zhang, Y.; Wan, J.; Mao, W. Using CRISPR Interference as a Therapeutic Approach to Treat TGFβ2-Induced Ocular Hypertension and Glaucoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 7. [Google Scholar] [CrossRef]
- Reichel, F.F.; Dauletbekov, D.L.; Klein, R.; Peters, T.; Ochakovski, G.A.; Seitz, I.P.; Wilhelm, B.; Ueffing, M.; Biel, M.; Wissinger, B.; et al. AAV8 Can Induce Innate and Adaptive Immune Response in the Primate Eye. Mol. Ther. 2017, 25, 2648–2660. [Google Scholar] [CrossRef]
- Li, Q.; Miller, R.; Han, P.-Y.; Pang, J.; Dinculescu, A.; Chiodo, V.; Hauswirth, W.W. Intraocular Route of AAV2 Vector Administration Defines Humoral Immune Response and Therapeutic Potential. Mol. Vis. 2008, 14, 1760–1769. [Google Scholar]
- Dong, J.Y.; Fan, P.D.; Frizzell, R.A. Quantitative Analysis of the Packaging Capacity of Recombinant Adeno-Associated Virus. Hum. Gene Ther. 1996, 7, 2101–2112. [Google Scholar] [CrossRef]
- Hauswirth, W.W.; Aleman, T.S.; Kaushal, S.; Cideciyan, A.V.; Schwartz, S.B.; Wang, L.; Conlon, T.J.; Boye, S.L.; Flotte, T.R.; Byrne, B.J.; et al. Treatment of Leber Congenital Amaurosis Due to RPE65 Mutations by Ocular Subretinal Injection of Adeno-Associated Virus Gene Vector: Short-Term Results of a Phase I Trial. Hum. Gene Ther. 2008, 19, 979–990. [Google Scholar] [CrossRef]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and Efficacy of Gene Transfer for Leber’s Congenital Amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef]
- Dalkara, D.; Kolstad, K.D.; Caporale, N.; Visel, M.; Klimczak, R.R.; Schaffer, D.V.; Flannery, J.G. Inner Limiting Membrane Barriers to AAV-Mediated Retinal Transduction from the Vitreous. Mol. Ther. 2009, 17, 2096–2102. [Google Scholar] [CrossRef]
- Shen, J.; Kim, J.; Tzeng, S.Y.; Ding, K.; Hafiz, Z.; Long, D.; Wang, J.; Green, J.J.; Campochiaro, P.A. Suprachoroidal Gene Transfer with Nonviral Nanoparticles. Sci. Adv. 2020, 6, eaba1606. [Google Scholar] [CrossRef]
- Emiliani, V.; Entcheva, E.; Hedrich, R.; Hegemann, P.; Konrad, K.R.; Lüscher, C.; Mahn, M.; Pan, Z.-H.; Sims, R.R.; Vierock, J.; et al. Optogenetics for Light Control of Biological Systems. Nat. Rev. Methods Primers 2022, 2, 55. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Liang, W.; Li, Y.; Zou, Z.; Xie, Y.; Liao, Y.; Yu, L.; Lin, Q.; Huang, M.; et al. The Roles of Optogenetics and Technology in Neurobiology: A Review. Front. Aging Neurosci. 2022, 14, 867863. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, A.; Mohamed, A.M.; Phan, H.T.; Weber, W. Lighting the Way: Recent Developments and Applications in Molecular Optogenetics. Curr. Opin. Biotechnol. 2024, 87, 103126. [Google Scholar] [CrossRef] [PubMed]
- Mathony, J.; Hoffmann, M.D.; Niopek, D. Optogenetics and CRISPR: A New Relationship Built to Last. Methods Mol. Biol. 2020, 2173, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a Directly Light-Gated Cation-Selective Membrane Channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef]
- Bi, A.; Cui, J.; Ma, Y.-P.; Olshevskaya, E.; Pu, M.; Dizhoor, A.M.; Pan, Z.-H. Ectopic Expression of a Microbial-Type Rhodopsin Restores Visual Responses in Mice with Photoreceptor Degeneration. Neuron 2006, 50, 23–33. [Google Scholar] [CrossRef]
- Busskamp, V.; Duebel, J.; Balya, D.; Fradot, M.; Viney, T.J.; Siegert, S.; Groner, A.C.; Cabuy, E.; Forster, V.; Seeliger, M.; et al. Genetic Reactivation of Cone Photoreceptors Restores Visual Responses in Retinitis Pigmentosa. Science 2010, 329, 413–417. [Google Scholar] [CrossRef]
- Lagali, P.S.; Balya, D.; Awatramani, G.B.; Münch, T.A.; Kim, D.S.; Busskamp, V.; Cepko, C.L.; Roska, B. Light-Activated Channels Targeted to ON Bipolar Cells Restore Visual Function in Retinal Degeneration. Nat. Neurosci. 2008, 11, 667–675. [Google Scholar] [CrossRef]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal Remodeling in Human Retinitis Pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Sahel, J.-A.; Boulanger-Scemama, E.; Pagot, C.; Arleo, A.; Galluppi, F.; Martel, J.N.; Esposti, S.D.; Delaux, A.; de Saint Aubert, J.-B.; de Montleau, C.; et al. Partial Recovery of Visual Function in a Blind Patient after Optogenetic Therapy. Nat. Med. 2021, 27, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- AbbVie. Phase I/IIa, Open-Label, Dose-Escalation Study of Safety and Tolerability of Intravitreal RST-001 in Patients With Advanced Retinitis Pigmentosa (RP). 2024. Available online: https://www.clinicaltrials.gov/ (accessed on 1 June 2025).
- Bionic Sight LLC. Phase 1/2, Safety and Efficacy Trial of BS01, a Recombinant Adeno-Associated Virus Vector Expressing ChronosFP in Patients With Retinitis Pigmentosa. 2025. Available online: https://www.clinicaltrials.gov/ (accessed on 1 June 2025).
- Nanoscope Therapeutics Inc. A Phase 2b Randomized, Double-Masked, Sham-Controlled, Study to Evaluate the Efficacy and Safety of Intravitreal Injection of MCO-010 Optogenetic Therapy in Adults With Retinitis Pigmentosa [RESTORE]. 2024. Available online: https://www.clinicaltrials.gov/ (accessed on 1 June 2025).
- Nanoscope Therapeutics Inc. A Phase 2a, Open Label Multicenter Clinical Trial to Evaluate the Safety and Effects of a Single Intravitreal Injection of vMCO-010 Optogenetic Therapy in Subjects With Stargardt Disease. 2023. Available online: https://www.clinicaltrials.gov/ (accessed on 1 June 2025).
- Li, Y.; Komatsu, C.; He, L.; Miller, M.R.; Noori, J.; van der Merwe, Y.; Ho, L.C.; Rosner, I.A.; Barnett, J.M.; Jabbari, K.; et al. Surgical Techniques and Outcome Assessment of a Novel Vascularized Orthotopic Rodent Whole Eye Transplantation Model. PLoS ONE 2025, 20, e0311392. [Google Scholar] [CrossRef] [PubMed]
- Ceradini, D.J.; Tran, D.L.; Dedania, V.S.; Gelb, B.E.; Cohen, O.D.; Flores, R.L.; Levine, J.P.; Saadeh, P.B.; Staffenberg, D.A.; Ben Youss, Z.; et al. Combined Whole Eye and Face Transplant: Microsurgical Strategy and 1-Year Clinical Course. JAMA 2024, 332, 1551–1558. [Google Scholar] [CrossRef]
- THEA Awardees|ARPA-H. Available online: https://arpa-h.gov/explore-funding/programs/thea/awardees (accessed on 27 September 2025).
- Sun, F.; Park, K.K.; Belin, S.; Wang, D.; Lu, T.; Chen, G.; Zhang, K.; Yeung, C.; Feng, G.; Yankner, B.A.; et al. Sustained Axon Regeneration Induced by Co-Deletion of PTEN and SOCS3. Nature 2011, 480, 372–375. [Google Scholar] [CrossRef]
- Yin, Y.; Henzl, M.T.; Lorber, B.; Nakazawa, T.; Thomas, T.T.; Jiang, F.; Langer, R.; Benowitz, L.I. Oncomodulin Is a Macrophage-Derived Signal for Axon Regeneration in Retinal Ganglion Cells. Nat. Neurosci. 2006, 9, 843–852. [Google Scholar] [CrossRef]
- Yin, Y.; Cui, Q.; Gilbert, H.-Y.; Yang, Y.; Yang, Z.; Berlinicke, C.; Li, Z.; Zaverucha-do-Valle, C.; He, H.; Petkova, V.; et al. Oncomodulin Links Inflammation to Optic Nerve Regeneration. Proc. Natl. Acad. Sci. USA 2009, 106, 19587–19592. [Google Scholar] [CrossRef]
- Sapieha, P.S.; Peltier, M.; Rendahl, K.G.; Manning, W.C.; Di Polo, A. Fibroblast Growth Factor-2 Gene Delivery Stimulates Axon Growth by Adult Retinal Ganglion Cells after Acute Optic Nerve Injury. Mol. Cell Neurosci. 2003, 24, 656–672. [Google Scholar] [CrossRef]
- Lorber, B.; Howe, M.L.; Benowitz, L.I.; Irwin, N. Mst3b, an Ste20-like Kinase, Regulates Axon Regeneration in Mature CNS and PNS Pathways. Nat. Neurosci. 2009, 12, 1407–1414. [Google Scholar] [CrossRef]
- Harder, J.M.; Ding, Q.; Fernandes, K.A.; Cherry, J.D.; Gan, L.; Libby, R.T. BCL2L1 (BCL-X) Promotes Survival of Adult and Developing Retinal Ganglion Cells. Mol. Cell Neurosci. 2012, 51, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Weise, J.; Isenmann, S.; Klöcker, N.; Kügler, S.; Hirsch, S.; Gravel, C.; Bähr, M. Adenovirus-Mediated Expression of Ciliary Neurotrophic Factor (CNTF) Rescues Axotomized Rat Retinal Ganglion Cells but Does Not Support Axonal Regeneration in Vivo. Neurobiol. Dis. 2000, 7, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Di Polo, A.; Aigner, L.J.; Dunn, R.J.; Bray, G.M.; Aguayo, A.J. Prolonged Delivery of Brain-Derived Neurotrophic Factor by Adenovirus-Infected Müller Cells Temporarily Rescues Injured Retinal Ganglion Cells. Proc. Natl. Acad. Sci. USA 1998, 95, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Bray, G.M.; Aguayo, A.J. Neurotrophin-4/5 (NT-4/5) Increases Adult Rat Retinal Ganglion Cell Survival and Neurite Outgrowth in Vitro. J. Neurobiol. 1994, 25, 953–959. [Google Scholar] [CrossRef]
- Carmignoto, G.; Maffei, L.; Candeo, P.; Canella, R.; Comelli, C. Effect of NGF on the Survival of Rat Retinal Ganglion Cells Following Optic Nerve Section. J. Neurosci. 1989, 9, 1263–1272. [Google Scholar] [CrossRef]
- Kermer, P.; Klöcker, N.; Labes, M.; Bähr, M. Insulin-like Growth Factor-I Protects Axotomized Rat Retinal Ganglion Cells from Secondary Death via PI3-K-Dependent Akt Phosphorylation and Inhibition of Caspase-3 In Vivo. J. Neurosci. 2000, 20, 2–8. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, J.; Matheson, C.R.; Urich, J.L. Glial Cell Line-Derived Neurotrophic Factor (GDNF) Promotes the Survival of Axotomized Retinal Ganglion Cells in Adult Rats: Comparison to and Combination with Brain-Derived Neurotrophic Factor (BDNF). J. Neurobiol. 1999, 38, 382–390. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Ball, A.K. Neurturin Enhances the Survival of Axotomized Retinal Ganglion Cells in Vivo: Combined Effects with Glial Cell Line-Derived Neurotrophic Factor and Brain-Derived Neurotrophic Factor. Neuroscience 2002, 110, 555–567. [Google Scholar] [CrossRef]
- Kim, T.; Iseri, E.; Peng, M.G.; Medvidovic, S.; Silliman, T.; Pahlavan, P.; Niu, G.; Huang, C.; Simonyan, A.; Pahnahad, J.; et al. Electric Field Stimulation Directs Target-Specific Axon Regeneration and Partial Restoration of Vision after Optic Nerve Crush Injury. PLoS ONE 2025, 20, e0315562. [Google Scholar] [CrossRef]
- Ellenberg, D.; Shi, J.; Jain, S.; Chang, J.-H.; Ripps, H.; Brady, S.; Melhem, E.R.; Lakkis, F.; Adamis, A.; Chen, D.-F.; et al. Impediments to Eye Transplantation: Ocular Viability Following Optic-Nerve Transection or Enucleation. Br. J. Ophthalmol. 2009, 93, 1134–1140. [Google Scholar] [CrossRef]
- Pineles, S.L.; Chang, M.Y.; Oltra, E.L.; Pihlblad, M.S.; Davila-Gonzalez, J.P.; Sauer, T.C.; Velez, F.G. Anterior Segment Ischemia: Etiology, Assessment, and Management. Eye 2018, 32, 173–178. [Google Scholar] [CrossRef]
- Au, N.P.B.; Ma, C.H.E. Neuroinflammation, Microglia and Implications for Retinal Ganglion Cell Survival and Axon Regeneration in Traumatic Optic Neuropathy. Front. Immunol. 2022, 13, 860070. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Miller, J.H. Regeneration beyond the Glial Scar. Nat. Rev. Neurosci. 2004, 5, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Farvardin, M.; Afarid, M.; Attarzadeh, A.; Johari, M.K.; Mehryar, M.; Nowroozzadeh, M.H.; Rahat, F.; Peyvandi, H.; Farvardin, R.; Nami, M. The Argus-II Retinal Prosthesis Implantation; From the Global to Local Successful Experience. Front. Neurosci. 2018, 12, 584. [Google Scholar] [CrossRef] [PubMed]
- Muqit, M.M.K.; Velikay-Parel, M.; Weber, M.; Dupeyron, G.; Audemard, D.; Corcostegui, B.; Sahel, J.; Le Mer, Y. Six-Month Safety and Efficacy of the Intelligent Retinal Implant System II Device in Retinitis Pigmentosa. Ophthalmology 2019, 126, 637–639. [Google Scholar] [CrossRef]
- Muqit, M.M.K.; Le Mer, Y.; Olmos de Koo, L.; Holz, F.G.; Sahel, J.A.; Palanker, D. Prosthetic Visual Acuity with the PRIMA Subretinal Microchip in Patients with Atrophic Age-Related Macular Degeneration at 4 Years Follow-Up. Ophthalmol. Sci. 2024, 4, 100510. [Google Scholar] [CrossRef]
- Orion. Available online: https://www.cortigent.com/orion (accessed on 10 June 2025).
- Rosenfeld, J.V.; Wong, Y.T.; Yan, E.; Szlawski, J.; Mohan, A.; Clark, J.C.; Rosa, M.; Lowery, A. Tissue Response to a Chronically Implantable Wireless Intracortical Visual Prosthesis (Gennaris Array). J. Neural Eng. 2020, 17, 046001. [Google Scholar] [CrossRef]
- Ostad-Ahmadi, Z.; Daemi, A.; Modabberi, M.-R.; Mostafaie, A. Safety, Effectiveness, and Cost-Effectiveness of Argus II in Patients with Retinitis Pigmentosa: A Systematic Review. Int. J. Ophthalmol. 2021, 14, 310–316. [Google Scholar] [CrossRef]
- Humayun, M.S.; Dorn, J.D.; da Cruz, L.; Dagnelie, G.; Sahel, J.-A.; Stanga, P.E.; Cideciyan, A.V.; Duncan, J.L.; Eliott, D.; Filley, E.; et al. Interim Results from the International Trial of Second Sight’s Visual Prosthesis. Ophthalmology 2012, 119, 779–788. [Google Scholar] [CrossRef]
- Duncan, J.L.; Richards, T.P.; Arditi, A.; da Cruz, L.; Dagnelie, G.; Dorn, J.D.; Ho, A.C.; Olmos de Koo, L.C.; Barale, P.-O.; Stanga, P.E.; et al. Improvements in Vision-Related Quality of Life in Blind Patients Implanted with the Argus II Epiretinal Prosthesis. Clin. Exp. Optom. 2017, 100, 144–150. [Google Scholar] [CrossRef]
- Second Sight’s Implant Technology Gets a Second Chance—IEEE Spectrum. Available online: https://spectrum.ieee.org/bionic-eye (accessed on 10 June 2025).
- Argus II. Available online: https://www.cortigent.com/argus-ii (accessed on 30 August 2025).
- Ramirez, K.A.; Drew-Bear, L.E.; Vega-Garces, M.; Betancourt-Belandria, H.; Arevalo, J.F. An Update on Visual Prosthesis. Int. J. Retin. Vitr. 2023, 9, 73. [Google Scholar] [CrossRef]
- Science Corporation. Restoration of Central Vision With the PRIMA System in Patients With Atrophic Age-Related Macular Degeneration. 2024. Available online: https://www.clinicaltrials.gov/ (accessed on 1 June 2025).
- Pezaris, J.S.; Reid, R.C. Demonstration of Artificial Visual Percepts Generated through Thalamic Microstimulation. Proc. Natl. Acad. Sci. USA 2007, 104, 7670–7675. [Google Scholar] [CrossRef] [PubMed]
- Panetsos, F.; Sanchez-Jimenez, A.; Rodrigo-Diaz, E.; Diaz-Guemes, I.; Sanchez, F.M. Consistent Phosphenes Generated by Electrical Microstimulation of the Visual Thalamus. An Experimental Approach for Thalamic Visual Neuroprostheses. Front. Neurosci. 2011, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Brindley, G.S. Effects of Electrical Stimulation of the Visual Cortex. Hum. Neurobiol. 1982, 1, 281–283. [Google Scholar] [PubMed]
- Brindley, G.S.; Lewin, W.S. The Sensations Produced by Electrical Stimulation of the Visual Cortex. J. Physiol. 1968, 196, 479–493. [Google Scholar] [CrossRef]
- Dobelle, W.H.; Mladejovsky, M.G.; Girvin, J.P. Artifical Vision for the Blind: Electrical Stimulation of Visual Cortex Offers Hope for a Functional Prosthesis. Science 1974, 183, 440–444. [Google Scholar] [CrossRef]
- Dobelle, W.H.; Mladejovsky, M.G. Phosphenes Produced by Electrical Stimulation of Human Occipital Cortex, and Their Application to the Development of a Prosthesis for the Blind. J. Physiol. 1974, 243, 553–576. [Google Scholar] [CrossRef]
- Dobelle, W.H.; Mladejovsky, M.G.; Evans, J.R.; Roberts, T.S.; Girvin, J.P. “Braille” Reading by a Blind Volunteer by Visual Cortex Stimulation. Nature 1976, 259, 111–112. [Google Scholar] [CrossRef]
- Beauchamp, M.S.; Oswalt, D.; Sun, P.; Foster, B.L.; Magnotti, J.F.; Niketeghad, S.; Pouratian, N.; Bosking, W.H.; Yoshor, D. Dynamic Stimulation of Visual Cortex Produces Form Vision in Sighted and Blind Humans. Cell 2020, 181, 774–783.e5. [Google Scholar] [CrossRef]
- Wang, V.; Kuriyan, A.E. Optoelectronic Devices for Vision Restoration. Curr. Ophthalmol. Rep. 2020, 8, 69–77. [Google Scholar] [CrossRef]
- Barry, M.; Salas, M.; Patel, U.; Wuyyuru, V.; Niketeghad, S.; Bosking, W.; Yoshor, D.; Dorn, J.; Pouratian, N. Video-Mode Percepts Are Smaller than Sums of Single-Electrode Phosphenes with the Orion® Visual Cortical Prosthesis. Investig. Ophthalmol. Vis. Sci. 2020, 61, 927. [Google Scholar]
- Fernández, E.; Alfaro, A.; Soto-Sánchez, C.; Gonzalez-Lopez, P.; Lozano, A.M.; Peña, S.; Grima, M.D.; Rodil, A.; Gómez, B.; Chen, X.; et al. Visual Percepts Evoked with an Intracortical 96-Channel Microelectrode Array Inserted in Human Occipital Cortex. J. Clin. Invest. 2021, 131, e151331. [Google Scholar] [CrossRef]
- Schmidt, E.M.; Bak, M.J.; Hambrecht, F.T.; Kufta, C.V.; O’Rourke, D.K.; Vallabhanath, P. Feasibility of a Visual Prosthesis for the Blind Based on Intracortical Micro Stimulation of the Visual Cortex. Brain 1996, 119 Pt 2, 507–522. [Google Scholar] [CrossRef]
- Sabé, M.; Hyde, J.; Cramer, C.; Eberhard, A.; Crippa, A.; Brunoni, A.R.; Aleman, A.; Kaiser, S.; Baldwin, D.S.; Garner, M.; et al. Transcranial Magnetic Stimulation and Transcranial Direct Current Stimulation Across Mental Disorders: A Systematic Review and Dose-Response Meta-Analysis. JAMA Netw. Open 2024, 7, e2412616. [Google Scholar] [CrossRef]
- Cheeran, B.; Koch, G.; Stagg, C.J.; Baig, F.; Teo, J. Transcranial Magnetic Stimulation: From Neurophysiology to Pharmacology, Molecular Biology and Genomics. Neuroscientist 2010, 16, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Amassian, V.E.; Cracco, R.Q.; Maccabee, P.J.; Cracco, J.B.; Rudell, A.P.; Eberle, L. Transcranial Magnetic Stimulation in Study of the Visual Pathway. J. Clin. Neurophysiol. 1998, 15, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.; Mansouri, B.; Koski, L.; Hess, R.F. Brain Plasticity in the Adult: Modulation of Function in Amblyopia with rTMS. Curr. Biol. 2008, 18, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Clavagnier, S.; Thompson, B.; Hess, R.F. Long Lasting Effects of Daily Theta Burst rTMS Sessions in the Human Amblyopic Cortex. Brain Stimul. 2013, 6, 860–867. [Google Scholar] [CrossRef]
- Tuna, A.R.; Pinto, N.; Brardo, F.M.; Fernandes, A.; Nunes, A.F.; Pato, M.V. Transcranial Magnetic Stimulation in Adults With Amblyopia. J. Neuroophthalmol. 2020, 40, 185–192. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained Excitability Elevations Induced by Transcranial DC Motor Cortex Stimulation in Humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Battleday, R.M.; Muller, T.; Clayton, M.S.; Cohen Kadosh, R. Mapping the Mechanisms of Transcranial Alternating Current Stimulation: A Pathway from Network Effects to Cognition. Front. Psychiatry 2014, 5, 162. [Google Scholar] [CrossRef]
- Terney, D.; Chaieb, L.; Moliadze, V.; Antal, A.; Paulus, W. Increasing Human Brain Excitability by Transcranial High-Frequency Random Noise Stimulation. J. Neurosci. 2008, 28, 14147–14155. [Google Scholar] [CrossRef] [PubMed]
- Reed, T.; Cohen Kadosh, R. Transcranial Electrical Stimulation (tES) Mechanisms and Its Effects on Cortical Excitability and Connectivity. J. Inherit. Metab. Dis. 2018, 41, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, D.P.; Byblow, W.D.; Hess, R.F.; Thompson, B. Anodal Transcranial Direct Current Stimulation Transiently Improves Contrast Sensitivity and Normalizes Visual Cortex Activation in Individuals with Amblyopia. Neurorehabilit. Neural Repair 2013, 27, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, J.; Spiegel, D.P.; Chen, Z.; Chan, L.; Luo, G.; Yuan, J.; Deng, D.; Yu, M.; Thompson, B. The Effect of Transcranial Direct Current Stimulation on Contrast Sensitivity and Visual Evoked Potential Amplitude in Adults with Amblyopia. Sci. Rep. 2016, 6, 19280. [Google Scholar] [CrossRef]
- Olma, M.C.; Dargie, R.A.; Behrens, J.R.; Kraft, A.; Irlbacher, K.; Fahle, M.; Brandt, S.A. Long-Term Effects of Serial Anodal tDCS on Motion Perception in Subjects with Occipital Stroke Measured in the Unaffected Visual Hemifield. Front. Hum. Neurosci. 2013, 7, 314. [Google Scholar] [CrossRef]
- Plow, E.B.; Obretenova, S.N.; Fregni, F.; Pascual-Leone, A.; Merabet, L.B. Comparison of Visual Field Training for Hemianopia with Active versus Sham Transcranial Direct Cortical Stimulation. Neurorehabilit. Neural Repair. 2012, 26, 616–626. [Google Scholar] [CrossRef]
- Bello, U.M.; Wang, J.; Park, A.S.Y.; Tan, K.W.S.; Cheung, B.W.S.; Thompson, B.; Cheong, A.M.Y. Can Visual Cortex Non-Invasive Brain Stimulation Improve Normal Visual Function? A Systematic Review and Meta-Analysis. Front. Neurosci. 2023, 17, 1119200. [Google Scholar] [CrossRef]
- Kriegseis, A.; Hennighausen, E.; Rösler, F.; Röder, B. Reduced EEG Alpha Activity over Parieto-Occipital Brain Areas in Congenitally Blind Adults. Clin. Neurophysiol. 2006, 117, 1560–1573. [Google Scholar] [CrossRef]
- Bottari, D.; Troje, N.F.; Ley, P.; Hense, M.; Kekunnaya, R.; Röder, B. Sight Restoration after Congenital Blindness Does Not Reinstate Alpha Oscillatory Activity in Humans. Sci. Rep. 2016, 6, 24683. [Google Scholar] [CrossRef]
- Birch, E.E.; Cheng, C.; Stager, D.R.; Weakley, D.R.; Stager, D.R. The Critical Period for Surgical Treatment of Dense Congenital Bilateral Cataracts. J. AAPOS 2009, 13, 67–71. [Google Scholar] [CrossRef]
- Zaehle, T.; Rach, S.; Herrmann, C.S. Transcranial Alternating Current Stimulation Enhances Individual Alpha Activity in Human EEG. PLoS ONE 2010, 5, e13766. [Google Scholar] [CrossRef]
- De Koninck, B.P.; Brazeau, D.; Guay, S.; Herrero Babiloni, A.; De Beaumont, L. Transcranial Alternating Current Stimulation to Modulate Alpha Activity: A Systematic Review. Neuromodulation 2023, 26, 1549–1584. [Google Scholar] [CrossRef] [PubMed]
- Middag-van Spanje, M.; Nijboer, T.C.W.; Schepers, J.; van Heugten, C.; Sack, A.T.; Schuhmann, T. Alpha Transcranial Alternating Current Stimulation as Add-on to Neglect Training: A Randomized Trial. Brain Commun. 2024, 6, fcae287. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Iwasaki, Y.; Makabe, K.; Kamao, H.; Mandai, M.; Shiina, T.; Ogasawara, K.; Hirami, Y.; Kurimoto, Y.; Takahashi, M. Successful Transplantation of Retinal Pigment Epithelial Cells from MHC Homozygote iPSCs in MHC-Matched Models. Stem Cell Rep. 2016, 7, 635–648. [Google Scholar] [CrossRef]
- Gowrishankar, S.; Smith, M.E.; Creber, N.; Muzaffar, J.; Borsetto, D. Immunosuppression in Stem Cell Clinical Trials of Neural and Retinal Cell Types: A Systematic Review. PLoS ONE 2024, 19, e0304073. [Google Scholar] [CrossRef]
- Nakatsuji, N.; Nakajima, F.; Tokunaga, K. HLA-Haplotype Banking and iPS Cells. Nat. Biotechnol. 2008, 26, 739–740. [Google Scholar] [CrossRef]
- Gupta, S.; Lytvynchuk, L.; Ardan, T.; Studenovska, H.; Sharma, R.; Faura, G.; Eide, L.; Shanker Verma, R.; Znaor, L.; Erceg, S.; et al. Progress in Stem Cells-Based Replacement Therapy for Retinal Pigment Epithelium: In Vitro Differentiation to In Vivo Delivery. Stem Cells Transl. Med. 2023, 12, 536–552. [Google Scholar] [CrossRef]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Li, Y.; Stoddard, J.; Stankewicz, C.; Wan, Q.; et al. Clinical-Grade Stem Cell-Derived Retinal Pigment Epithelium Patch Rescues Retinal Degeneration in Rodents and Pigs. Sci. Transl. Med. 2019, 11, eaat5580. [Google Scholar] [CrossRef]
- Grisé, K.N.; Coles, B.L.K.; Bautista, N.X.; van der Kooy, D. Activation of Adult Mammalian Retinal Stem Cells in Vivo via Antagonism of BMP and sFRP2. Stem Cell Res. Ther. 2021, 12, 560. [Google Scholar] [CrossRef]
- Wu, Y.-R.; Hashiguchi, T.; Sho, J.; Chiou, S.-H.; Takahashi, M.; Mandai, M. Transplanted Mouse Embryonic Stem Cell-Derived Retinal Ganglion Cells Integrate and Form Synapses in a Retinal Ganglion Cell-Depleted Mouse Model. Investig. Ophthalmol. Vis. Sci. 2021, 62, 26. [Google Scholar] [CrossRef]
- Anderson, D.R. Ultrastructure of Human and Monkey Lamina Cribrosa and Optic Nerve Head. Arch. Ophthalmol. 1969, 82, 800–814. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.R. The Optic Nerve Head in Glaucoma: Role of Astrocytes in Tissue Remodeling. Prog. Retin. Eye Res. 2000, 19, 297–321. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.R.; Benowitz, L.I.; Goldberg, J.L.; He, Z. Axon Regeneration in the Mammalian Optic Nerve. Annu. Rev. Vis. Sci. 2020, 6, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Biber, J.; Gandor, C.; Becirovic, E.; Michalakis, S. Retina-Directed Gene Therapy: Achievements and Remaining Challenges. Pharmacol. Ther. 2025, 271, 108862. [Google Scholar] [CrossRef]
- Calcedo, R.; Vandenberghe, L.H.; Gao, G.; Lin, J.; Wilson, J.M. Worldwide Epidemiology of Neutralizing Antibodies to Adeno-Associated Viruses. J. Infect. Dis. 2009, 199, 381–390. [Google Scholar] [CrossRef]
- Vrellaku, B.; Sethw Hassan, I.; Howitt, R.; Webster, C.P.; Harriss, E.; McBlane, F.; Betts, C.; Schettini, J.; Lion, M.; Mindur, J.E.; et al. A Systematic Review of Immunosuppressive Protocols Used in AAV Gene Therapy for Monogenic Disorders. Mol. Ther. 2024, 32, 3220–3259. [Google Scholar] [CrossRef]
- Wiley, L.A.; Boyce, T.M.; Meyering, E.E.; Ochoa, D.; Sheehan, K.M.; Stone, E.M.; Mullins, R.F.; Tucker, B.A.; Han, I.C. The Degree of Adeno-Associated Virus-Induced Retinal Inflammation Varies Based on Serotype and Route of Delivery: Intravitreal, Subretinal, or Suprachoroidal. Hum. Gene Ther. 2023, 34, 530–539. [Google Scholar] [CrossRef]
- Zolotukhin, S.; Vandenberghe, L.H. AAV Capsid Design: A Goldilocks Challenge. Trends Mol. Med. 2022, 28, 183–193. [Google Scholar] [CrossRef]
- Wang, Y.; Rajala, A.; Cao, B.; Ranjo-Bishop, M.; Agbaga, M.-P.; Mao, C.; Rajala, R.V.S. Cell-Specific Promoters Enable Lipid-Based Nanoparticles to Deliver Genes to Specific Cells of the Retina In Vivo. Theranostics 2016, 6, 1514–1527. [Google Scholar] [CrossRef]
- Patel, S.; Ryals, R.C.; Weller, K.K.; Pennesi, M.E.; Sahay, G. Lipid Nanoparticles for Delivery of Messenger RNA to the Back of the Eye. J. Controll. Release 2019, 303, 91–100. [Google Scholar] [CrossRef]
- Bansal, H.; Pyari, G.; Roy, S. Theoretical Prediction of Broadband Ambient Light Optogenetic Vision Restoration with ChRmine and Its Mutants. Sci. Rep. 2024, 14, 11642. [Google Scholar] [CrossRef]
- Watanabe, Y.; Sugano, E.; Tabata, K.; Hatakeyama, A.; Sakajiri, T.; Fukuda, T.; Ozaki, T.; Suzuki, T.; Sayama, T.; Tomita, H. Development of an Optogenetic Gene Sensitive to Daylight and Its Implications in Vision Restoration. NPJ Regen. Med. 2021, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Katada, Y.; Yoshida, K.; Serizawa, N.; Lee, D.; Kobayashi, K.; Negishi, K.; Okano, H.; Kandori, H.; Tsubota, K.; Kurihara, T. Highly Sensitive Visual Restoration and Protection via Ectopic Expression of Chimeric Rhodopsin in Mice. iScience 2023, 26, 107716. [Google Scholar] [CrossRef] [PubMed]
- Bourne, D.; Li, Y.; Komatsu, C.; Miller, M.R.; Davidson, E.H.; He, L.; Rosner, I.A.; Tang, H.; Chen, W.; Solari, M.G.; et al. Whole-Eye Transplantation: A Look into the Past and Vision for the Future. Eye 2017, 31, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.Y.; Mina, M.; Sahyoun, J.-Y.; Kalevar, A.; Tran, S.D. Retinal Prostheses: Engineering and Clinical Perspectives for Vision Restoration. Sensors 2023, 23, 5782. [Google Scholar] [CrossRef]
- Wang, C.; Fang, C.; Zou, Y.; Yang, J.; Sawan, M. Artificial Intelligence Techniques for Retinal Prostheses: A Comprehensive Review and Future Direction. J. Neural Eng. 2023, 20, 011003. [Google Scholar] [CrossRef]
- Christie, B.P.; Ashmont, K.R.; House, P.A.; Greger, B. Approaches to a Cortical Vision Prosthesis: Implications of Electrode Size and Placement. J. Neural Eng. 2016, 13, 025003. [Google Scholar] [CrossRef]
- Cha, K.; Horch, K.; Normann, R.A. Simulation of a Phosphene-Based Visual Field: Visual Acuity in a Pixelized Vision System. Ann. Biomed. Eng. 1992, 20, 439–449. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Martinez-Cantin, R.; Guerrero, J.J. Semantic and Structural Image Segmentation for Prosthetic Vision. PLoS ONE 2020, 15, e0227677. [Google Scholar] [CrossRef]
- Perera, M.P.N.; Bailey, N.W.; Murphy, O.W.; Mallawaarachchi, S.; Sullivan, C.; Hill, A.T.; Fitzgerald, P.B. Home-Based Individualized Alpha Transcranial Alternating Current Stimulation Improves Symptoms of Obsessive-Compulsive Disorder: Preliminary Evidence from a Randomized, Sham-Controlled Clinical Trial. Depress. Anxiety 2023, 2023, 9958884. [Google Scholar] [CrossRef]
- Tervo, A.E.; Nieminen, J.O.; Lioumis, P.; Metsomaa, J.; Souza, V.H.; Sinisalo, H.; Stenroos, M.; Sarvas, J.; Ilmoniemi, R.J. Closed-Loop Optimization of Transcranial Magnetic Stimulation with Electroencephalography Feedback. Brain Stimul. 2022, 15, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S.; Rami Reddy, M.V.S.R.; Lennox, C.; Wolinsky, E.; McCullumsmith, R.E.; Singh, T. Harnessing Neuroimaging-Guided Transcranial Magnetic Stimulation for Precision Therapy in Substance Use Disorders. Mol. Psychiatry 2025, 30, 3804–3816. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Jun, S.C. Multi-Scale Computational Models for Electrical Brain Stimulation. Front. Hum. Neurosci. 2017, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Fins, J.J. Deep Brain Stimulation, Free Markets and the Scientific Commons: Is It Time to Revisit the Bayh-Dole Act of 1980? Neuromodulation 2010, 13, 153–159. [Google Scholar] [CrossRef]
- Heller, M.A.; Eisenberg, R.S. Can Patents Deter Innovation? The Anticommons in Biomedical Research. Science 1998, 280, 698–701. [Google Scholar] [CrossRef]
- Humanitarian Device Exemption (HDE). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfhde/hde.cfm?id=h110002 (accessed on 27 September 2025).
- Fins, J.J.; Mayberg, H.S.; Nuttin, B.; Kubu, C.S.; Galert, T.; Sturm, V.; Stoppenbrink, K.; Merkel, R.; Schlaepfer, T.E. Misuse of the FDA’s Humanitarian Device Exemption in Deep Brain Stimulation for Obsessive-Compulsive Disorder. Health Aff. 2011, 30, 302–311. [Google Scholar] [CrossRef]
- Pinckard-Dover, H.; Ward, H.; Foote, K.D. The Decline of Deep Brain Stimulation for Obsessive-Compulsive Disorder Following FDA Humanitarian Device Exemption Approval. Front. Surg. 2021, 8, 642503. [Google Scholar] [CrossRef]
- Search Orphan Drug Designations and Approvals. Available online: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=466414 (accessed on 27 September 2025).
- Mikami, K. Orphans in the Market: The History of Orphan Drug Policy. Soc. Hist. Med. 2019, 32, 609–630. [Google Scholar] [CrossRef]
- Assen, L.S.; Jongsma, K.R.; Isasi, R.; Tryfonidou, M.A.; Bredenoord, A.L. Recognizing the Ethical Implications of Stem Cell Research: A Call for Broadening the Scope. Stem Cell Rep. 2021, 16, 1656–1661. [Google Scholar] [CrossRef]
- Moradi, S.; Mahdizadeh, H.; Šarić, T.; Kim, J.; Harati, J.; Shahsavarani, H.; Greber, B.; Moore, J.B. Research and Therapy with Induced Pluripotent Stem Cells (iPSCs): Social, Legal, and Ethical Considerations. Stem Cell Res. Ther. 2019, 10, 341. [Google Scholar] [CrossRef]
- Ansah, E.O. Ethical Challenges and Controversies in the Practice and Advancement of Gene Therapy. Adv. Cell Gene Ther. 2022, 2022, 1015996. [Google Scholar] [CrossRef]
- Sivak, W.N.; Davidson, E.H.; Komatsu, C.; Li, Y.; Miller, M.R.; Schuman, J.S.; Solari, M.G.; Magill, G.; Washington, K.M. Ethical Considerations of Whole-Eye Transplantation. J. Clin. Ethics 2016, 27, 64–67. [Google Scholar] [CrossRef]
- Mohan, R.; Reckelbus, M.; Borry, P. Regional Disparities in Access to Gene Therapies in the European Union, the United States, Japan, and China. Per Med. 2025, 22, 267–273. [Google Scholar] [CrossRef]
| Modality | Examples | Conditions Treated | Visual Outcome |
|---|---|---|---|
| Stem Cell Therapy | hESCs, iPSCs, mesenchymal | AMD, Stargardt’s Disease | BCVA ≥ 0–20 words |
| Gene Therapy | AAV vectors delivering transgenes | Achromatopsia, Bietti’s crystalline dystrophy, choroideremia, autosomal RP, X-linked RP, Stargardt disease, Usher syndrome, X-linked retinoschisis, and LHON, AMD, diabetic retinopathy, glaucoma | BCVA ≥ 0–20 words |
| Optogenetics | AAV vectors delivering ChrimsonR, ChR2, ChronosFP, MCO1 | RP, Stargardt’s disease | Identification of objects in a blind patient |
| Whole-Eye Transplant | Eye/partial face transplant | Severe burn injury | No visual restoration |
| Retinal Neuroprosthetics | Argus II, IRIS II, PRIMA | RP, AMD | Object/picture recognition, motion detection |
| Optic Nerve Neuroprosthetics | AV-DONE | RP | Visual percepts elicited in blind patients |
| Thalamic Neuroprosthetics | Micro-electrode stimulation of LGN in non-human primates | N/A | Visual percepts elicited |
| Cortical Neuroprosthetics | Orion, Gennaris, Utah Array | Complete blindness | Visual percepts elicited, shapes recognized in blind patients |
| Non-Invasive Neuromodulation | rTMS, tDCS, tACS, tRNS | Amblyopia, hemianopsia post-stroke, normal vision | Improvements in contrast sensitivity, motion perception, visual field function, crowding in peripheral vision, and neglect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaleri, J.; Lin, M.; Wu, K.; Gilbert, Z.; Huang, C.; Lo, Y.T.; Garimella, V.; Dallas, J.C.; Briggs, R.G.; Borja, A.J.; et al. Visual Neurorestoration: An Expert Review of Current Strategies for Restoring Vision in Humans. Brain Sci. 2025, 15, 1170. https://doi.org/10.3390/brainsci15111170
Cavaleri J, Lin M, Wu K, Gilbert Z, Huang C, Lo YT, Garimella V, Dallas JC, Briggs RG, Borja AJ, et al. Visual Neurorestoration: An Expert Review of Current Strategies for Restoring Vision in Humans. Brain Sciences. 2025; 15(11):1170. https://doi.org/10.3390/brainsci15111170
Chicago/Turabian StyleCavaleri, Jonathon, Michelle Lin, Kevin Wu, Zachary Gilbert, Connie Huang, Yu Tung Lo, Vahini Garimella, Jonathan C. Dallas, Robert G. Briggs, Austin J. Borja, and et al. 2025. "Visual Neurorestoration: An Expert Review of Current Strategies for Restoring Vision in Humans" Brain Sciences 15, no. 11: 1170. https://doi.org/10.3390/brainsci15111170
APA StyleCavaleri, J., Lin, M., Wu, K., Gilbert, Z., Huang, C., Lo, Y. T., Garimella, V., Dallas, J. C., Briggs, R. G., Borja, A. J., Lee, J. E., Ng, P. R., Gokoffski, K. K., & Lee, D. J. (2025). Visual Neurorestoration: An Expert Review of Current Strategies for Restoring Vision in Humans. Brain Sciences, 15(11), 1170. https://doi.org/10.3390/brainsci15111170








