Differences in Cognitive Health and Brain Activity According to Mild Cognitive Impairment and Physical Activity Levels in Older Women
Abstract
1. Introduction
2. Methods
2.1. Participants
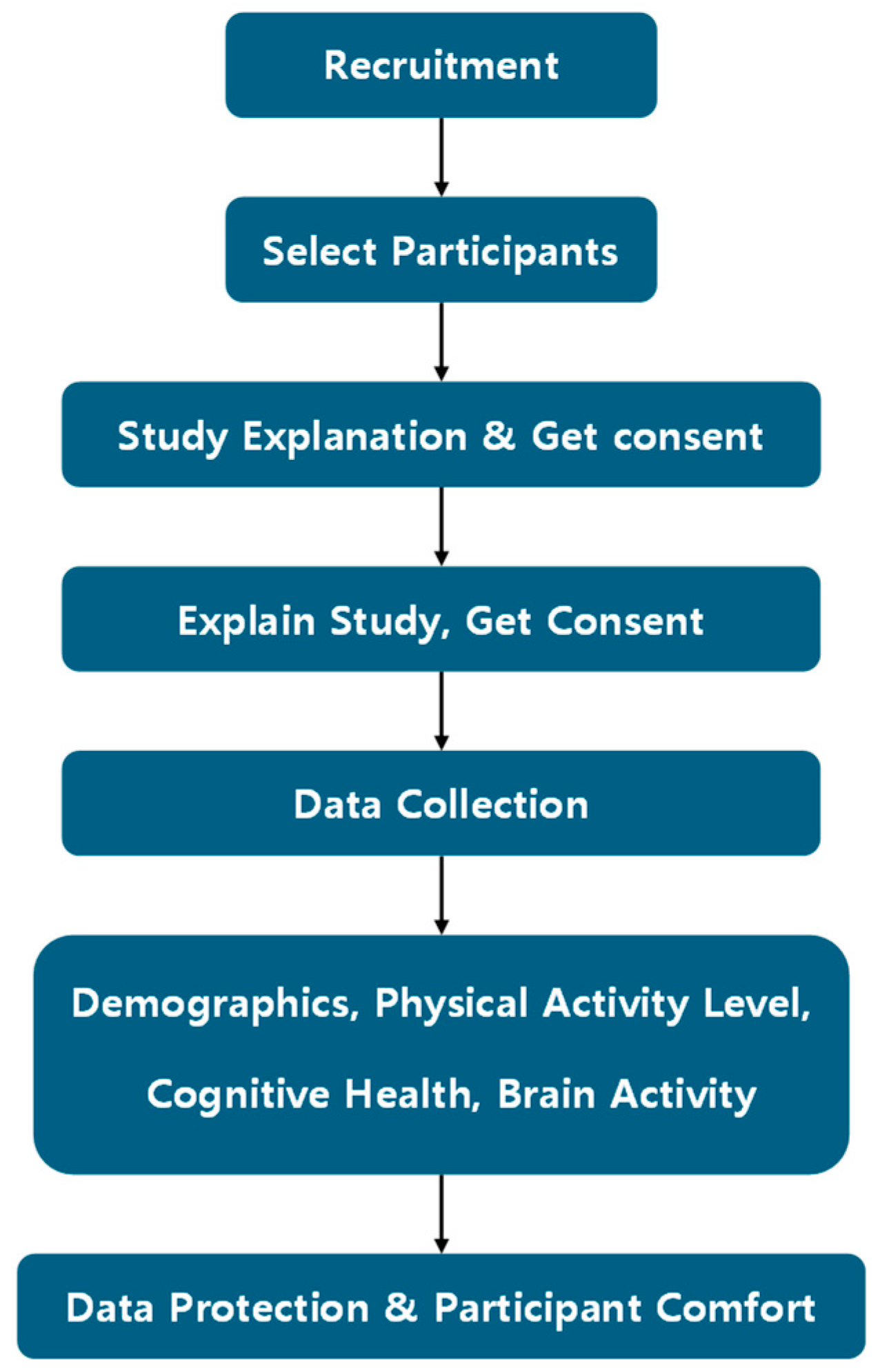
2.2. Research Procedures
2.3. Methods
2.3.1. Mild Cognitive Impairment Assessment
2.3.2. Physical Activity Scale for the Elderly-Korea (PASE-K)
2.3.3. Mini-Mental State Examination-Korea (MMSE-K)
2.3.4. Brain Activity Test
2.4. Statistical Analysis
3. Results
3.1. Differences in Demographics with and Without MCI
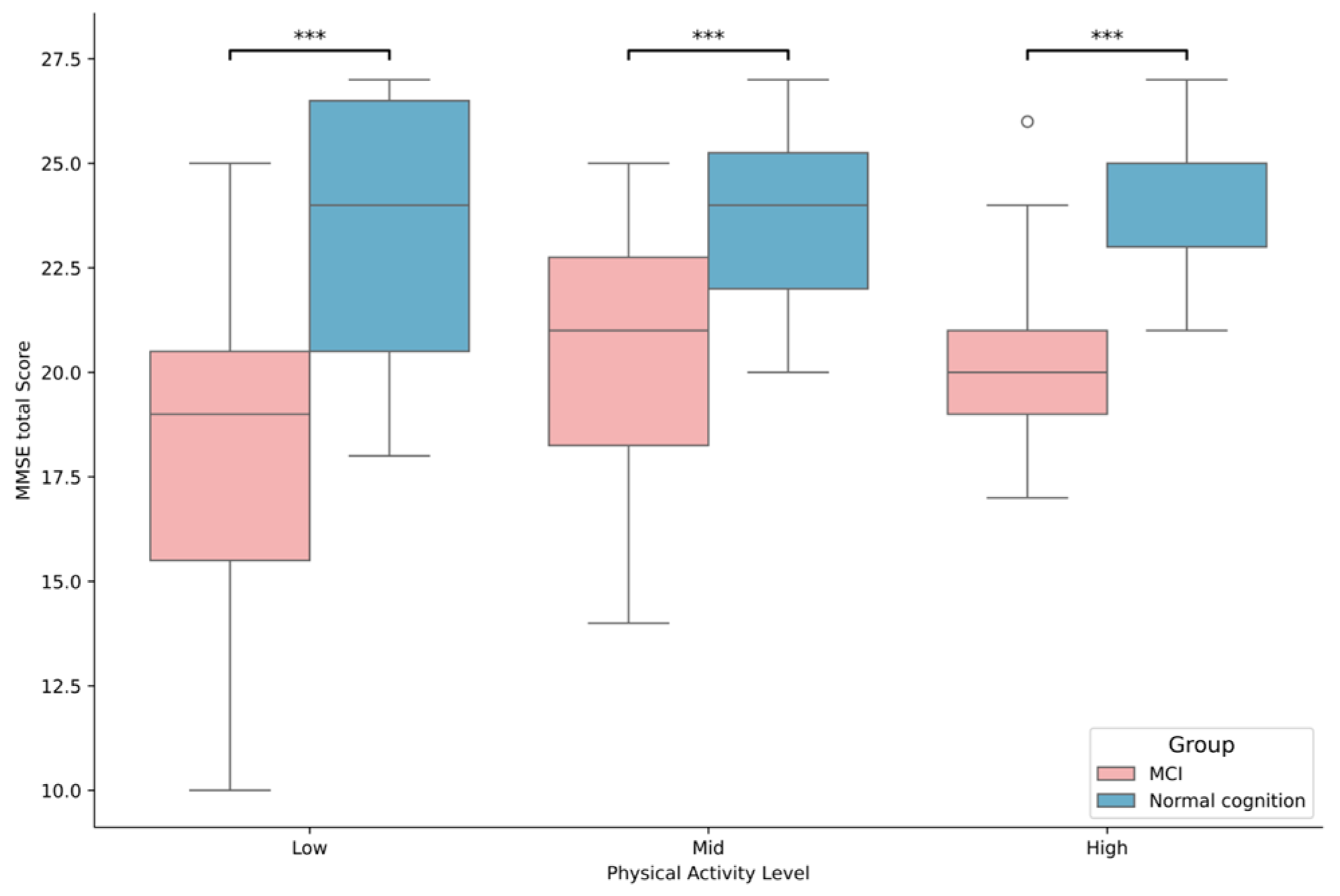
3.2. Differences in Cognitive Health Based on MCI Status and Physical Activity Levels
3.3. Differences in Brain Waves Based on MCI Status and Physical Activity Levels
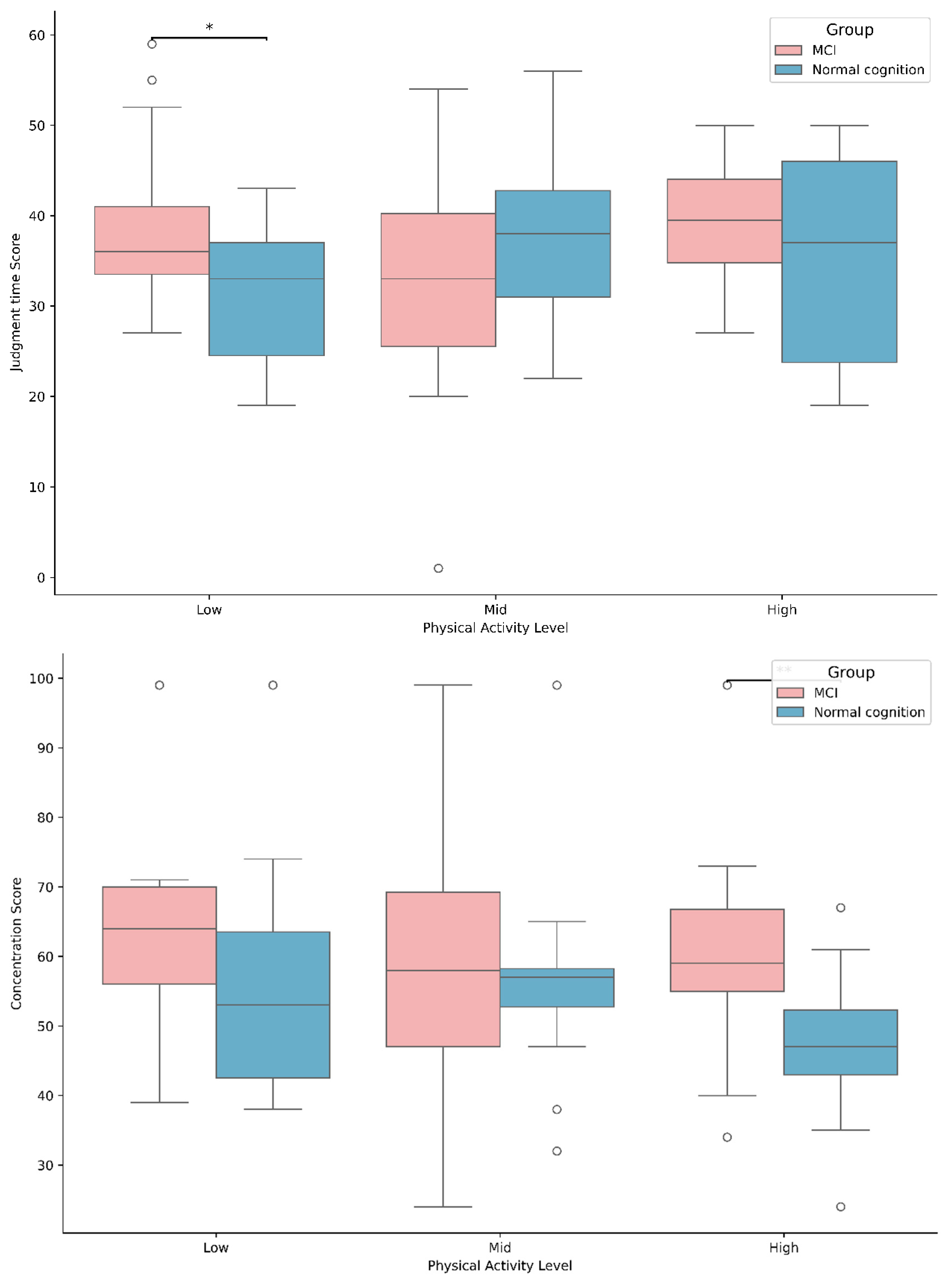
3.4. Differences in Brain Activities Based on MCI Status and Physical Activity Levels
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shon, C.; Yoon, H. Health-economic burden of dementia in South Korea. BMC Geriatr. 2021, 21, 549. [Google Scholar] [CrossRef]
- Jang, J.-W.; Park, J.H.; Kim, S.; Lee, S.-H.; Lee, S.-H.; Kim, Y.-J. Prevalence and incidence of dementia in South Korea: A nationwide analysis of the national health insurance service senior cohort. J. Clin. Neurol. 2021, 17, 249. [Google Scholar] [CrossRef] [PubMed]
- Gainotti, G. Origins, controversies and recent developments of the MCI construct. Curr. Alzheimer Res. 2010, 7, 271–279. [Google Scholar] [CrossRef]
- Bohlken, J.; Riedel-Heller, S.; Steininger, G.; Kostev, K.; Michalowsky, B. Trends in dementia and mild cognitive impairment prevalence and incidence in German general and specialist practices between 2015 and 2019. J. Alzheimer’s Dis. 2021, 79, 1683–1690. [Google Scholar] [CrossRef]
- Michaud, T.L.; Su, D.; Siahpush, M.; Murman, D.L. The risk of incident mild cognitive impairment and progression to dementia considering mild cognitive impairment subtypes. Dement. Geriatr. Cogn. Disord. Extra 2017, 7, 15–29. [Google Scholar] [CrossRef]
- Yue, J.; Han, S.-w.; Liu, X.; Wang, S.; Zhao, W.-w.; Cai, L.-n.; Cao, D.-n.; Mah, J.Z.; Hou, Y.; Cui, X. Functional brain activity in patients with amnestic mild cognitive impairment: An rs-fMRI study. Front. Neurol. 2023, 14, 1244696. [Google Scholar] [CrossRef]
- O’Donovan, G.; Lee, I.-M.; Hamer, M.; García-Garro, P.; Duran-Aniotz, C.; Ibáñez, A.; Sarmiento, O.L.; Hessel, P. The burden of mild cognitive impairment attributable to physical inactivity in Colombia. Eur. Rev. Aging Phys. Act. 2022, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Musaeus, C.S.; Engedal, K.; Høgh, P.; Jelic, V.; Mørup, M.; Naik, M.; Oeksengaard, A.-R.; Snaedal, J.; Wahlund, L.-O.; Waldemar, G. EEG theta power is an early marker of cognitive decline in dementia due to Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 64, 1359–1371. [Google Scholar] [CrossRef]
- Meghdadi, A.H.; Stevanović Karić, M.; McConnell, M.; Rupp, G.; Richard, C.; Hamilton, J.; Salat, D.; Berka, C. Resting state EEG biomarkers of cognitive decline associated with Alzheimer’s disease and mild cognitive impairment. PLoS ONE 2021, 16, e0244180. [Google Scholar] [CrossRef] [PubMed]
- Abdin, S.; Welch, R.; Byron-Daniel, J.; Meyrick, J. The effectiveness of physical activity interventions in improving well-being across office-based workplace settings: A systematic review. Public Health 2018, 160, 70–76. [Google Scholar] [CrossRef]
- Raggi, A.; Iannaccone, S.; Marcone, A.; Ginex, V.; Ortelli, P.; Nonis, A.; Giusti, M.C.; Cappa, S.F. The effects of a comprehensive rehabilitation program of Alzheimer’s disease in a hospital setting. Behav. Neurol. 2007, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Zhao, L.; Xiao, S.; Zhang, S.; Li, L.; Nie, J.; Bai, L.; Qian, S.; Yang, Y.; Phillips, M. Efficacy of comprehensive cognitive health management for Shanghai community older adults with mild cognitive impairment. Gen. Psychiatry 2022, 35, e100532. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Ten Brinke, L.F.; Bolandzadeh, N.; Nagamatsu, L.S.; Hsu, C.L.; Davis, J.C.; Miran-Khan, K.; Liu-Ambrose, T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: A 6-month randomised controlled trial. Br. J. Sports Med. 2015, 49, 248–254. [Google Scholar] [CrossRef]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, D.W.; Cho, S.-J.; Na, D.L.; Jeon, H.J.; Kim, S.-K.; Lee, Y.R.; Youn, J.-H.; Kwon, M.; Lee, J.-H. Brief screening for mild cognitive impairment in elderly outpatient clinic: Validation of the Korean version of the Montreal Cognitive Assessment. J. Geriatr. Psychiatry Neurol. 2008, 21, 104–110. [Google Scholar] [CrossRef]
- Washburn, R.A.; Smith, K.W.; Jette, A.M.; Janney, C.A. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J. Clin. Epidemiol. 1993, 46, 153–162. [Google Scholar] [CrossRef]
- Choe, M.; Kim, J.; Jeon, M.-y.; Chae, Y.-R. Evaluation of the Korean version of physical activity scale for the elderly (K-PASE). Korean J. Women Health Nurs. 2010, 16, 47–59. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, W.M.; Kim, S.H.; Kang, Y.S.; Ha, J.Y.; Lee, H.L. The Usefulness of Clock Drawing Test as Screening for Dementia On the basis of the correlation between clock drawing and MMSE-K. J. Korean Acad. Fam. Med. 1997, 18, 785–792. [Google Scholar]
- Kwon, Y.C. Korean version of mini-mental state examination (MMSE-K) Part I: Development of the test for the elderly. J. Korean Neuro-Psychiatr. Assoc. 1989, 28, 125. [Google Scholar]
- Williams-Russo, P.; Sharrock, N.E.; Mattis, S.; Szatrowski, T.P.; Charlson, M.E. Cognitive effects after epidural vs general anesthesia in older adults: A randomized trial. JAMA 1995, 274, 44–50. [Google Scholar] [CrossRef]
- Cheon, W.; Tian, J.; Park, J. Analysing Differences in Cognitive Health, Physical Fitness and Brain Activity in Older Women With and Without MCI. Geriatrics 2025, 10, 25. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B. Structural brain changes in aging: Courses, causes and cognitive consequences. Rev. Neurosci. 2010, 21, 187–222. [Google Scholar] [CrossRef]
- Bennett, D.A.; Schneider, J.A.; Tang, Y.; Arnold, S.E.; Wilson, R.S. The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. Lancet Neurol. 2006, 5, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.E.; Lusignolo, T.M.; Albert, M.; Berkman, L. Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychol. 2001, 20, 243. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, T.C.; Lansford, J.E.; Akiyama, H.; Smith, J.; Baltes, M.M.; Takahashi, K.; Fuhrer, R.; Dartigues, J.F. Differences between men and women in social relations, resource deficits, and depressive symptomatology during later life in four nations. J. Soc. Issues 2002, 58, 767–783. [Google Scholar] [CrossRef]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef]
- Valenzuela, M.J.; Sachdev, P. Brain reserve and dementia: A systematic review. Psychol. Med. 2006, 36, 441–454. [Google Scholar] [CrossRef]
- Crooks, V.C.; Lubben, J.; Petitti, D.B.; Little, D.; Chiu, V. Social network, cognitive function, and dementia incidence among elderly women. Am. J. Public Health 2008, 98, 1221–1227. [Google Scholar] [CrossRef]
- Manly, J.J.; Touradji, P.; Tang, M.-X.; Stern, Y. Literacy and memory decline among ethnically diverse elders. J. Clin. Exp. Neuropsychol. 2003, 25, 680–690. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Tangney, C.C.; Bienias, J.L.; Wilson, R.S. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology 2006, 67, 1370–1376. [Google Scholar] [CrossRef]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Gheysen, F.; Poppe, L.; DeSmet, A.; Swinnen, S.; Cardon, G.; De Bourdeaudhuij, I.; Chastin, S.; Fias, W. Physical activity to improve cognition in older adults: Can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, J.H.; Na, H.R.; Hiroyuki, S.; Kim, G.M.; Jung, M.K.; Kim, W.K.; Park, K.W. Combined intervention of physical activity, aerobic exercise, and cognitive exercise intervention to prevent cognitive decline for patients with mild cognitive impairment: A randomized controlled clinical study. J. Clin. Med. 2019, 8, 940. [Google Scholar] [CrossRef] [PubMed]
- Koščak Tivadar, B. Physical activity improves cognition: Possible explanations. Biogerontology 2017, 18, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Sutkowy, P.; Woźniak, A.; Mila-Kierzenkowska, C.; Szewczyk-Golec, K.; Wesołowski, R.; Pawłowska, M.; Nuszkiewicz, J. Physical activity vs. redox balance in the brain: Brain health, aging and diseases. Antioxidants 2021, 11, 95. [Google Scholar] [CrossRef]
- Begega, A.; Alvarez-Suarez, P.; Sampedro-Piquero, P.; Cuesta, M. Effects of physical activity on the cerebral networks. In Physical Activity and the Aging Brain; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–11. [Google Scholar]
- Guerdoux-Ninot, E.; Trouillet, R. Impact of perceived stress on cognitive performance: Moderating effect of mild cognitive impairment and Alzheimer’s disease. J. Clin. Exp. Neuropsychol. 2019, 41, 364–379. [Google Scholar] [CrossRef]
- Split, M.; Saurman, J.L.; Rodriguez, A.; Goldstein, F.C.; Vickers, K.L. 43 Evaluating the Relationship Between Social Support, Executive Function, and Communicative Effectiveness. J. Int. Neuropsychol. Soc. 2023, 29, 251–252. [Google Scholar] [CrossRef]
- Engels, A.S.; Heller, W.; Mohanty, A.; Herrington, J.D.; Banich, M.T.; Webb, A.G.; Miller, G.A. Specificity of regional brain activity in anxiety types during emotion processing. Psychophysiology 2007, 44, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Kouvonen, A.; Vahtera, J.; Oksanen, T.; Pentti, J.; Väänänen, A.K.; Heponiemi, T.; Salo, P.; Virtanen, M.; Kivimäki, M. Chronic workplace stress and insufficient physical activity: A cohort study. Occup. Environ. Med. 2013, 70, 3–8. [Google Scholar] [CrossRef] [PubMed]

| Variables | MCI (n,%) | Non MCI (n,%) | |
|---|---|---|---|
| Age | 65–75 | 14 11.22% | 38 30.27% |
| 76–85 | 38 30.27% | 23 18.36% | |
| Over 86 | 11 8.84% | 1 1.04% | |
| Education | No formal education/less than elementary school | 13 10.32% | 2 1.59% |
| Elementary school | 38 30.16% | 28 22.22% | |
| Middle school and above | 12 9.52% | 33 26.19% | |
| Residency | Community dwelling | 35 27.78% | 15 11.90% |
| Assisted living facility | 28 22.22% | 48 38.10% | |
| Number of people in the household | One | 28 22.22% | 16 12.69% |
| Two | 28 22.22% | 40 31.75% | |
| Three or more | 7 5.56% | 7 5.56% | |
| Marital status | Currently married | 42 33.33% | 53 42.06% |
| Separated or widowed | 21 16.67% | 10 7.94% | |
| Household income (KRW) | Less 1 million | 49 38.89% | 25 19.84% |
| Over 1.01 million | 14 11.11% | 38 30.16% | |
| Number of diseases | None | 11 8.84% | 13 9.76% |
| One | 21 16.67% | 19 15.08% | |
| Two | 13 9.76% | 23 18.25% | |
| Over two | 18 14.29% | 8 7.35% | |
| Details | Neurophysiological Brainwave Signatures |
|---|---|
| Instant memory | The mean cognitive intensity, averaged over each difficulty level, is expressed in microvolts per second (μV/S). The amplitude change (in height) of the cognitive gamma-peak. |
| Judgement time | The mean reaction time for each difficulty level was calculated as a mean value in seconds. |
| Concentration | (SMR + M-Beta)/Theta SMR = Sensory Motor Rhythm |
| Mental workload | SEF90% (spectral edge frequency-90%) |
| L/R brain activity | The relative prevalence of gamma power in the left and right cerebral hemispheres. |
| Variable | MCI | Non MCI | t-Value | 95% CI | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Age | 79.43 ± 5.87 | 74.08 ± 5.45 | 5.298 *** | 0.574 | 1.311 |
| Education | 3.00 ± 0.80 | 3.90 ± 1.10 | −5.263 *** | −1.304 | −0.568 |
| Housing type | 1.44 ± 0.50 | 1.76 ± 0.43 | −3.819 *** | −1.039 | −0.320 |
| Household size | 1.79 ± 1.00 | 1.89 ± 0.70 | −0.619 | −0.460 | 0.239 |
| Marital status | 2.33 ± 0.48 | 2.16 ± 0.37 | 2.305 * | 0.057 | 0.763 |
| Cost | 87.54 ± 46.08 | 159.05 ± 78.01 | −6.264 *** | −1.490 | −0.738 |
| Chronic disease | 1.86 ± 1.84 | 1.43 ± 1.00 | 1.627 | −0.062 | 0.640 |
| Variable | GPL | MCI | Non MCI | F | |
|---|---|---|---|---|---|
| Low | 67.71 ± 14.22 | 62.36 ± 11.17 | G | 2.185 | |
| Gamma | Mid | 64.56 ± 20.86 | 63.71 ± 12.93 | PL | 0.472 |
| High | 64.93 ± 17.98 | 58.54 ± 11.87 | G ∗ PL | 0.385 | |
| Low | 67.61 ± 15.91 | 60.27 ± 11.01 | G | 3.103 | |
| H-Beta | Mid | 62.67 ± 18.97 | 60.92 ± 12.42 | PL | 1.117 |
| High | 61.57 ± 17.78 | 55.96 ± 10.42 | G ∗ PL | 0.362 | |
| Low | 70.52 ± 17.81 | 64.64 ± 14.88 | G | 2.310 | |
| M-Beta | Mid | 63.83 ± 18.09 | 60.79 ± 11.69 | PL | 1.615 |
| High | 63.71 ± 15.88 | 59.68 ± 10.40 | G ∗ PL | 0.083 | |
| Low | 66.29 ± 14.51 | 66.36 ± 13.86 | G | 0.661 | |
| SMR | Mid | 65.33 ± 17.14 | 59.67 ± 10.98 | PL | 0.723 |
| High | 64.21 ± 14.66 | 63.46 ± 11.43 | G ∗ PL | 0.497 | |
| Low | 57.52 ± 13.06 | 63.27 ± 14.76 | G | 0.447 | |
| Alpha | Mid | 60.94 ± 16.42 | 57.13 ± 10.72 | PL | 0.118 |
| High | 57.79 ± 12.78 | 60.68 ± 9.56 | G ∗ PL | 1.426 | |
| Low | 51.68 ± 9.32 | 58.55 ± 10.24 # | G | 7.081 ** | |
| Theta | Mid | 53.28 ± 9.44 | 52.92 ± 7.52 | PL | 0.544 |
| High | 51.21 ± 8.32 | 57.68 ± 7.03 # | G ∗ PL | 2.199 | |
| Variable | GPL | MCI | Non MCI | F | |
|---|---|---|---|---|---|
| Low | 48.03 ± 5.29 | 49.36 ± 7.57 | G | 0.085 | |
| Instant memory | Mid | 52.11 ± 6.09 | 48.63 ± 5.13 | PL | 0.742 |
| High | 49.79 ± 8.78 | 50.86 ± 7.19 | G ∗ PL | 1.688 | |
| Low | 37.84 ± 8.51 | 31.00 ± 8.41 # | G | 0.914 | |
| Judgement time | Mid | 32.83 ± 12.12 | 37.67 ± 9.59 | PL | 0.831 |
| High | 39.00 ± 6.96 | 35.64 ± 11.24 | G ∗ PL | 3.515 * | |
| Low | 65.74 ± 15.19 | 56.09 ± 18.33 | G | 9.872 ** | |
| Concentration | Mid | 59.83 ± 19.07 | 56.21 ± 11.80 | PL | 1.839 |
| High | 60.71 ± 15.56 | 47.82 ± 9.81 # | G ∗ PL | 1.028 | |
| Low | 61.00 ± 12.63 | 54.36 ± 13.38 | G | 5.579 * | |
| Mental workload | Mid | 57.28 ± 17.97 | 54.54 ± 12.36 | PL | 2.742 |
| High | 54.93 ± 17.48 | 46.29 ± 8.08 | G ∗ PL | 0.500 | |
| Low | 50.19 ± 8.88 | 50.09 ± 9.49 | G | 0.327 | |
| L brain activity | Mid | 48.39 ± 2.68 | 48.75 ± 6.19 | PL | 3.880 * |
| High | 45.14 ± 3.72 | 46.93 ± 2.93 | G ∗ PL | 0.223 | |
| Low | 53.45 ± 3.92 | 52.18 ± 5.69 | G | 2.773 | |
| R brain activity | Mid | 52.67 ± 3.16 | 51.67 ± 6.51 | PL | 1.796 |
| High | 55.00 ± 3.59 | 53.07 ± 2.93 | G ∗ PL | 0.113 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Cheon, W. Differences in Cognitive Health and Brain Activity According to Mild Cognitive Impairment and Physical Activity Levels in Older Women. Brain Sci. 2025, 15, 1181. https://doi.org/10.3390/brainsci15111181
Tian J, Cheon W. Differences in Cognitive Health and Brain Activity According to Mild Cognitive Impairment and Physical Activity Levels in Older Women. Brain Sciences. 2025; 15(11):1181. https://doi.org/10.3390/brainsci15111181
Chicago/Turabian StyleTian, Jidong, and Wookwang Cheon. 2025. "Differences in Cognitive Health and Brain Activity According to Mild Cognitive Impairment and Physical Activity Levels in Older Women" Brain Sciences 15, no. 11: 1181. https://doi.org/10.3390/brainsci15111181
APA StyleTian, J., & Cheon, W. (2025). Differences in Cognitive Health and Brain Activity According to Mild Cognitive Impairment and Physical Activity Levels in Older Women. Brain Sciences, 15(11), 1181. https://doi.org/10.3390/brainsci15111181





