The Relationship Between Soleus H-Reflex Following Standing GVS and Postural Control Responses on Firm and Foam Surfaces: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
2.3. Preparation for Soleus H-Reflex Measurement
2.4. Standing GVSH Measurement
2.5. Body Control Response Measurement
2.6. Data Extraction and Processing
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacKinnon, C.D. Sensorimotor anatomy of gait, balance, and falls. Handb. Clin. Neurol. 2018, 159, 3–26. [Google Scholar] [PubMed]
- Tanaka, H.; Nakamura, J.; Siozaki, T.; Ueta, K.; Morioka, S.; Shomoto, K.; Okada, Y. Posture influences on vestibulospinal tract excitability. Exp. Brain Res. 2021, 239, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Shiozaki, T.; Okada, Y.; Nakamura, J.; Ueta, K.; Tanaka, H.; Moritani, M.; Kitahara, T. Relationships between changes in lateral vestibulospinal tract excitability and postural control by dynamic balance intervention in healthy individuals: A preliminary study. Front. Hum. Neurosci. 2023, 17, 1109690. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.M.; Inglis, J.T. Modulation of the soleus H-reflex in prone human subjects using galvanic vestibular stimulation. Clin. Neurophysiol. 2001, 112, 2159–2163. [Google Scholar] [CrossRef]
- Okada, Y.; Shiozaki, T.; Nakamura, J.; Azumi, Y.; Inazato, M.; Ono, M.; Kondo, H.; Sugitani, M.; Matsugi, A. Influence of the intensity of galvanic vestibular stimulation and cutaneous stimulation on the soleus H-reflex in healthy individuals. NeuroReport 2018, 29, 1135–1139. [Google Scholar] [CrossRef]
- Nakamura, J.; Okada, Y.; Shiozaki, T.; Tanaka, H.; Ueta, K.; Ikuno, K.; Morioka, S.; Shomoto, K. Reliability and laterality of the soleus H-reflex following galvanic vestibular stimulation in healthy individuals. Neurosci. Lett. 2021, 755, 135910. [Google Scholar] [CrossRef]
- Kim, J.; Curthoys, I.S. Responses of primary vestibular neurons to galvanic vestibular stimulation (GVS) in the anaesthetised guinea pig. Brain Res. Bull. 2004, 64, 265–271. [Google Scholar] [CrossRef]
- Matsugi, A.; Douchi, S.; Hasada, R.; Mori, N.; Okada, Y.; Yoshida, N.; Nishishita, S.; Hosomi, K.; Saitoh, Y. Cerebellar repetitive transcranial magnetic stimulation and noisy galvanic vestibular stimulation change vestibulospinal function. Front. Neurosci. 2020, 14, 388. [Google Scholar] [CrossRef]
- Matsugi, A.; Ueta, Y.; Oku, K.; Okuno, K.; Tamaru, Y.; Nomura, S.; Tanaka, H.; Mori, N. Effect of gaze-stabilization exercises on vestibular function during postural control. NeuroReport 2017, 28, 439–443. [Google Scholar] [CrossRef]
- Tax, C.M.; Bom, A.P.; Taylor, R.L.; Todd, N.; Cho, K.K.; Fitzpatrick, R.C.; Welgampola, M.S. The galvanic whole-body sway response in health and disease. Clin. Neurophysiol. 2013, 124, 2036–2045. [Google Scholar] [CrossRef]
- Mitsutake, T.; Sakamoto, M.; Ueta, K.; Horikawa, E. Standing postural stability during galvanic vestibular stimulation is associated with the motor function of the hemiplegic lower extremity post-stroke. Top. Stroke Rehabil. 2020, 27, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Russo, V.; Spitoni, G.F.; Ciancarelli, I.; Paolucci, S.; Manzari, L.; Morone, G. Efficacy of vestibular rehabilitation in patients with neurologic disorders: A systematic review. Arch. Phys. Med. Rehabil. 2021, 102, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.D.; Herdman, S.J.; Whitney, S.L.; Anson, E.R.; Carender, W.J.; Hoppes, C.W.; Cass, S.P.; Christy, J.B.; Cohen, H.S.; Fife, T.D.; et al. Vestibular rehabilitation for peripheral vestibular hypofunction: An updated clinical practice guideline from the academy of neurologic physical therapy of the American physical therapy association. J. Neurol. Phys. Ther. 2022, 46, 118–177. [Google Scholar] [CrossRef] [PubMed]
- Mitsutake, T.; Taniguchi, T.; Nakazono, H.; Yoshizuka, H.; Sakamoto, M. Effects of noisy galvanic vestibular stimulation on the muscle activity and joint movements in different standing postures conditions. Front. Hum. Neurosci. 2022, 16, 891669. [Google Scholar] [CrossRef]
- Mitsutake, T.; Nakazono, H.; Shiozaki, T.; Taniguchi, T.; Yoshizuka, H.; Sakamoto, M. Neural interference effects on lateral vestibulospinal tract excitability by noisy galvanic vestibular stimulation. Clin. Neurophysiol. 2024, 168, 153–160. [Google Scholar] [CrossRef]
- Koceja, D.M.; Trimble, M.H.; Earles, D.R. Inhibition of the soleus H-reflex in standing man. Brain Res. 1993, 629, 155–158. [Google Scholar] [CrossRef]
- Pierrot-Deseilligny, E. Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. J. Neurosci. Methods 1997, 74, 189–199. [Google Scholar] [CrossRef]
- Taube, W.; Gruber, M.; Gollhofer, A. Spinal and supraspinal adaptations associated with balance training and their functional relevance. Acta Physiol. 2008, 193, 101–116. [Google Scholar] [CrossRef]
- Alrowayeh, H.N.; Sabbahi, M.A.; Etnyre, B. Similarities and differences of the soleus and gastrocnemius H-reflexes during varied body postures, foot positions, and muscle function: Multifactor designs for repeated measures. BMC Neurol. 2011, 11, 65. [Google Scholar] [CrossRef]
- Hanna-Boutros, B.; Sangari, S.; Giboin, L.S.; El Mendili, M.M.; Lackmy-Vallée, A.; Marchand-Pauvert, V.; Knikou, M. Corticospinal and reciprocal inhibition actions on human soleus motoneuron activity during standing and walking. Physiol. Rep. 2015, 3, e12276. [Google Scholar] [CrossRef]
- Chen, Y.S.; Tseng, W.C.; Chen, C.H.; Bezerra, P.; Ye, X. Acute effects of kinesiology tape tension on soleus muscle h-reflex modulations during lying and standing postures. PLoS ONE 2020, 15, e0236587. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.M.; Inglis, J.T. Interaction effects of galvanic vestibular stimulation and head position on the soleus H reflex in humans. Clin. Neurophysiol. 2002, 113, 1709–1714. [Google Scholar] [CrossRef]
- Fitzpatrick, R.C.; Day, B.L. Probing the human vestibular system with galvanic stimulation. J. Appl. Physiol. 2004, 96, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Murley, G.S.; Landorf, K.B.; Menz, H.B.; Bird, A.R. Effect of foot posture, foot orthoses and footwear on lower limb muscle activity during walking and running: A systematic review. Gait Posture 2009, 29, 172–187. [Google Scholar] [CrossRef]
- Huang, M.; Yick, K.L.; Ng, S.P.; Yip, J.; Cheung, R.T. The effect of support surface and footwear condition on postural sway and lower limb muscle action of the older women. PLoS ONE 2020, 15, e0234140. [Google Scholar] [CrossRef]
- Goel, R.; Kofman, I.; Jeevarajan, J.; De Dios, Y.; Cohen, H.S.; Bloomberg, J.J.; Mulavara, A.P. Using low levels of stochastic vestibular stimulation to improve balance function. PLoS ONE 2015, 10, e0136335. [Google Scholar] [CrossRef]
- McLaren, R.; Smith, P.F.; Taylor, R.L.; Niazi, I.K.; Taylor, D. Scoping out noisy galvanic vestibular stimulation: A review of the parameters used to improve postural control. Front. Neurosci. 2023, 17, 1156796. [Google Scholar] [CrossRef]
- McCall, A.A.; Miller, D.M.; Yates, B.J. Descending influences on vestibulospinal and vestibulosympathetic reflexes. Front. Neurol. 2017, 8, 112. [Google Scholar] [CrossRef]
- Inukai, Y.; Otsuru, N.; Masaki, M.; Saito, K.; Miyaguchi, S.; Kojima, S.; Onishi, H. Effect of noisy galvanic vestibular stimulation on center of pressure sway of static standing posture. Brain Stimul. 2018, 11, 85–93. [Google Scholar] [CrossRef]
- Wilson, V.J.; Yoshida, M. Comparison of effects of stimulation of Deiters’ nucleus and medial longitudinal fasciculus on neck, forelimb, and hindlimb motoneurons. J. Neurophysiol. 1969, 32, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Hongo, T.; Lund, S. The vestibulospinal tract. Effects on alpha-motoneurones in the lumbosacral spinal cord in the cat. Exp. Brain Res. 1970, 10, 94–120. [Google Scholar] [CrossRef] [PubMed]
- Kernell, D.; Hensbergen, E.; Lind, A.; Eerbeek, O. Relation between fibre composition and daily duration of spontaneous activity in ankle muscles of the cat. Arch. Ital. Biol. 1998, 136, 191–203. [Google Scholar] [PubMed]
- Huang, C.Y.; Cherng, R.J.; Yang, Z.R.; Chen, Y.T.; Hwang, I.S. Modulation of soleus H reflex due to stance pattern and haptic stabilization of posture. J. Electromyogr. Kinesiol. 2009, 19, 492–499. [Google Scholar] [CrossRef]
- Tokuno, C.D.; Carpenter, M.G.; Thorstensson, A.; Garland, S.J.; Cresswell, A.G. Control of the triceps surae during the postural sway of quiet standing. Acta Physiol. 2007, 191, 229–236. [Google Scholar] [CrossRef]
- Rudolph, K.S.; Axe, M.J.; Buchanan, T.S.; Scholz, J.P.; Snyder-Mackler, L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg. Sports Traumatol. Arthrosc. 2001, 9, 62–71. [Google Scholar] [CrossRef]
- Martino, G.; Beck, O.N.; Ting, L.H. Voluntary muscle coactivation in quiet standing elicits reciprocal rather than coactive agonist-antagonist control of reactive balance. J. Neurophysiol. 2023, 129, 1378–1388. [Google Scholar] [CrossRef]

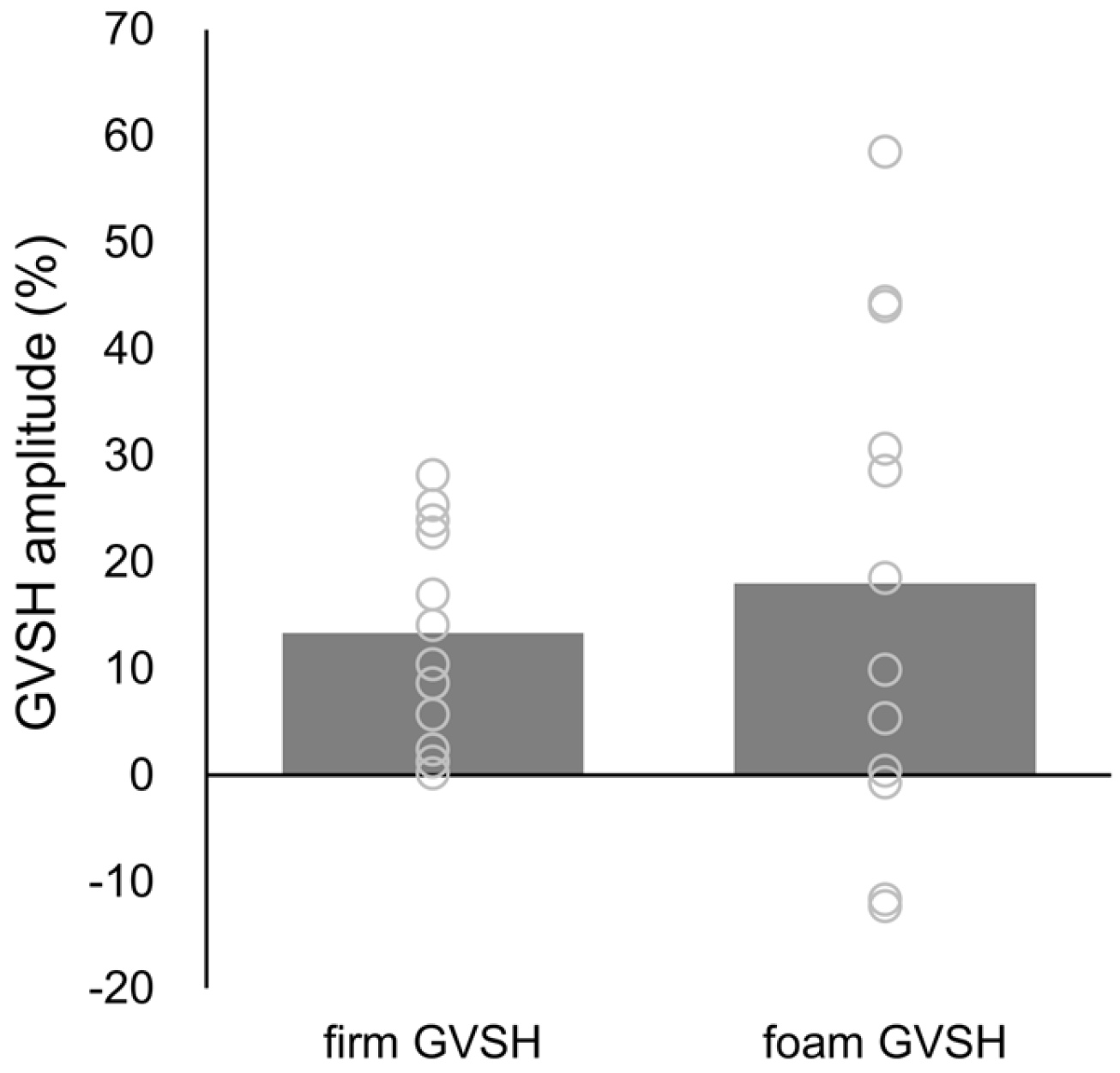

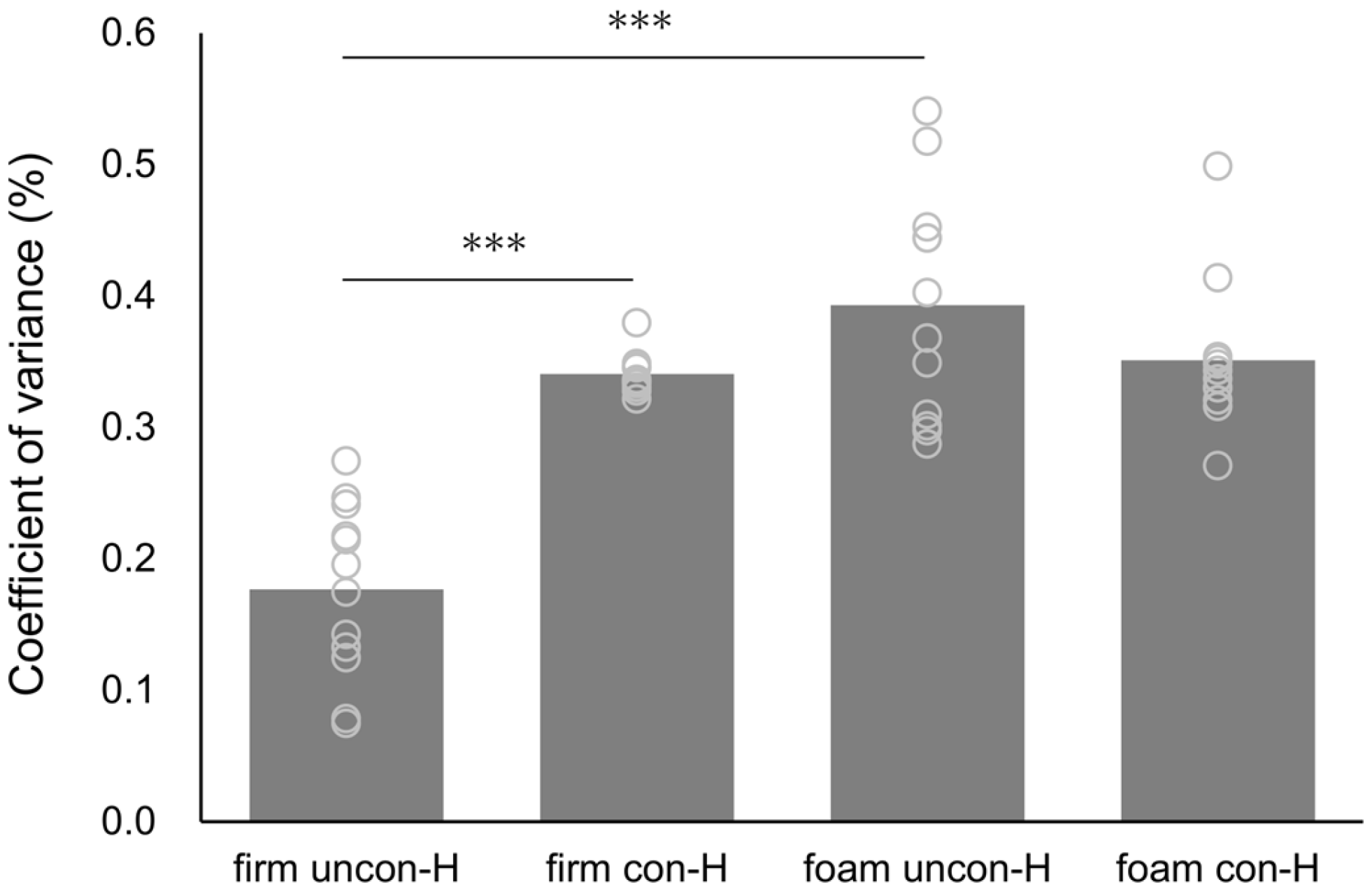
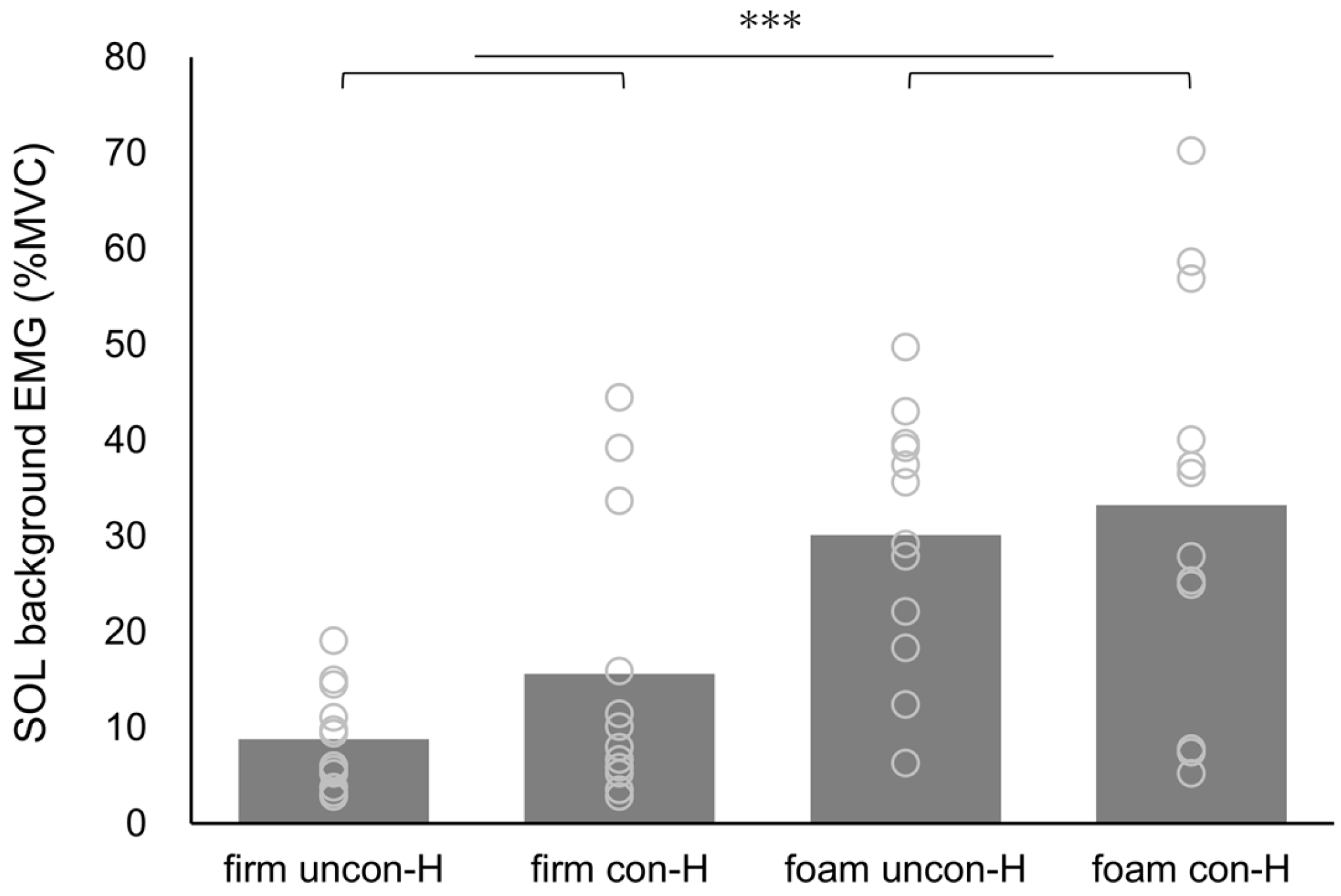
| Firm | Foam | Surface | Stimulation | Surface × Stimulation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| F-Value | p-Value | ηp2 | F-Value | p-Value | ηp2 | F-Value | p-Value | ηp2 | |||
| H-wave CV | |||||||||||
| uncon-H | 0.18 ± 0.07 | 0.39 ± 0.09 | 53.870 | <0.001 | 0.830 | 12.161 | 0.005 | 0.525 | 36.934 | <0.001 | 0.771 |
| con-H | 0.34 ± 0.01 | 0.35 ± 0.06 | |||||||||
| SOL background EMG | |||||||||||
| uncon-H | 8.83 ± 5.27 | 30.12 ± 13.14 | 20.390 | <0.001 | 0.650 | 2.633 | 0.133 | 0.193 | 0.428 | 0.527 | 0.037 |
| con-H | 15.61 ± 14.82 | 33.25 ± 21.12 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsutake, T.; Taniguchi, T.; Nakazono, H.; Shiozaki, T.; Yoshizuka, H.; Sakamoto, M. The Relationship Between Soleus H-Reflex Following Standing GVS and Postural Control Responses on Firm and Foam Surfaces: An Exploratory Study. Brain Sci. 2025, 15, 115. https://doi.org/10.3390/brainsci15020115
Mitsutake T, Taniguchi T, Nakazono H, Shiozaki T, Yoshizuka H, Sakamoto M. The Relationship Between Soleus H-Reflex Following Standing GVS and Postural Control Responses on Firm and Foam Surfaces: An Exploratory Study. Brain Sciences. 2025; 15(2):115. https://doi.org/10.3390/brainsci15020115
Chicago/Turabian StyleMitsutake, Tsubasa, Takanori Taniguchi, Hisato Nakazono, Tomoyuki Shiozaki, Hisayoshi Yoshizuka, and Maiko Sakamoto. 2025. "The Relationship Between Soleus H-Reflex Following Standing GVS and Postural Control Responses on Firm and Foam Surfaces: An Exploratory Study" Brain Sciences 15, no. 2: 115. https://doi.org/10.3390/brainsci15020115
APA StyleMitsutake, T., Taniguchi, T., Nakazono, H., Shiozaki, T., Yoshizuka, H., & Sakamoto, M. (2025). The Relationship Between Soleus H-Reflex Following Standing GVS and Postural Control Responses on Firm and Foam Surfaces: An Exploratory Study. Brain Sciences, 15(2), 115. https://doi.org/10.3390/brainsci15020115







