Towards a New Dawn for Neuro-Oncology: Nanomedicine at the Service of Drug Delivery for Primary and Secondary Brain Tumours
Abstract
:1. Introduction
2. Evolution of Treatment Modalities for Primary and Secondary Brain Tumours
2.1. Surgical Planning and Prediction Models
2.2. Current Strategies for Radiation Therapy
2.3. Resistance of Tumour Cells to Chemotherapy
3. Emerging Treatment Modalities Based on Nanomedicine
3.1. Overcoming the Blood–Brain Barrier Using Nanoparticles
3.2. Towards Nanosolutions
4. Materials and Methods
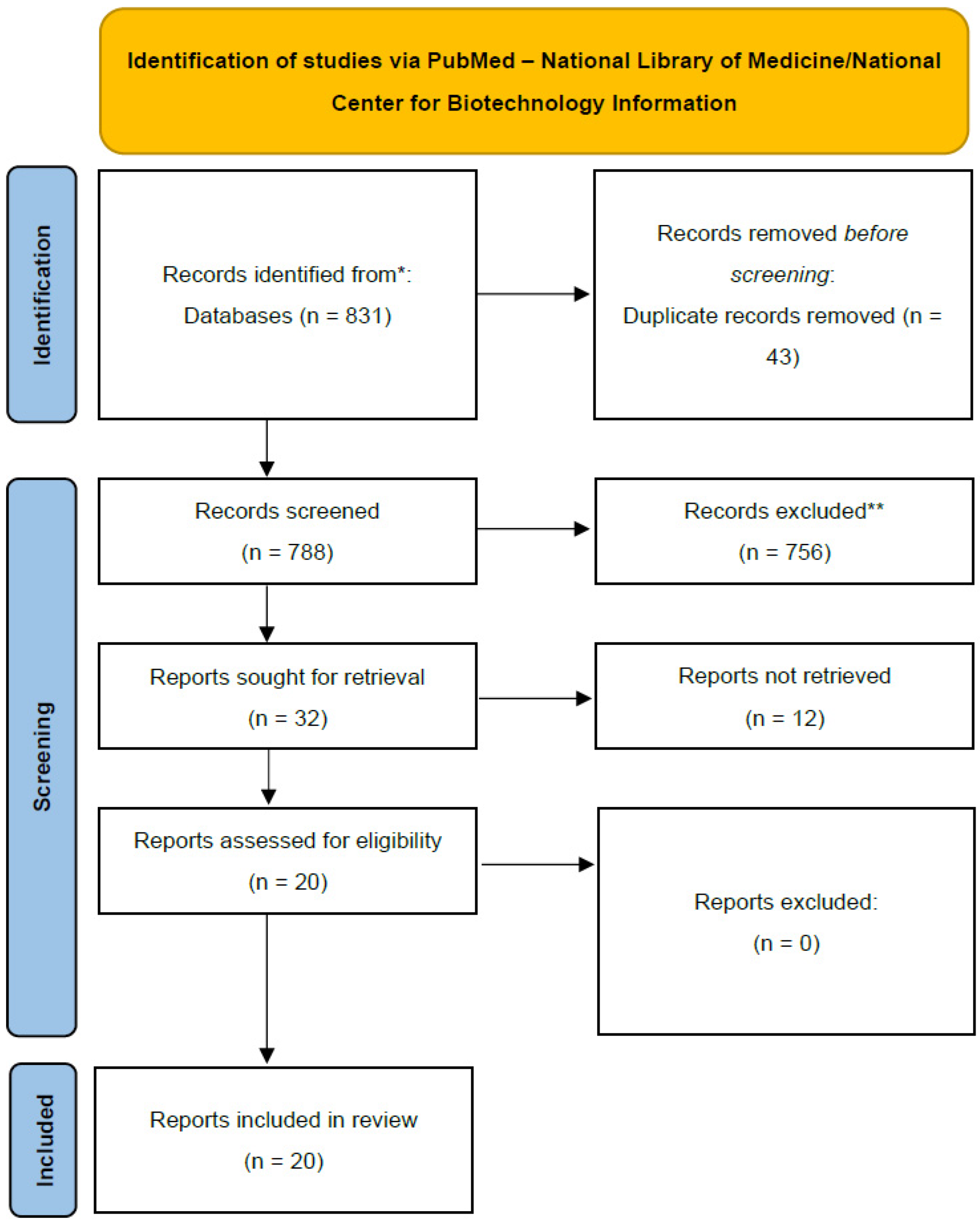
Scoping Review Methodology
5. Results
5.1. The Blood–Brain Barrier and Chemotherapeutic Drug Delivery via NPs
5.1.1. Polymeric NPs
5.1.2. Lipid NPs
5.1.3. Magnetic NPs
5.1.4. Combining MNPs with the Technique of Focused Ultrasound to Increase BBB Permeability and Drug Delivery via NPs
5.1.5. Silica NPs
5.1.6. Ultrasound-Modulated Chemotherapy: The Case of Zirconium NPs
5.1.7. NPs as Radiosensitisers
5.2. Immunotherapy
5.2.1. Nanoscale Immunoconjugates (NICs)
5.2.2. Co-Encapsulating Paclitaxel with Immune Checkpoint Inhibitors
5.2.3. Immunotherapy with Multiplexing Targeting
5.2.4. Immunotherapy Plus siRNA
5.3. Radio-Immunotherapy
5.4. Anti-Angiogenic Therapy
6. Discussion
- (a)
- The scenarios presented illustrate the different stages of readiness, with some solutions that are already being tested in patients and others that are far too premature despite promising laboratory results.
- (b)
- This scoping review outlines some commonalities between primary and secondary brain tumours, commonalities which can be exploited by scientists to identify innovative solutions and change the way we diagnose and treat patients with brain tumours. Furthermore, it highlights the bottlenecks of current management, from barriers to vehiculate contrast agents and drugs across the BBB and BTB to the issue of the tumour microenvironment’s immune privilege [92,144,145], from metabolic plasticity for brain metastases [70,154] to the issue of nanotoxicity.
- (c)
- We found a rising interest regarding the link between different types of primary tumours and ways to target common aspects of their biology. For instance, regarding the association between malignant melanoma (MM) and GBM, we counted fifteen studies with a total of 220 patients who all showed an association between these two tumour types [121]. Analysing those studies in detail, several mechanisms to support this linkage and possible targets for therapeutic solutions were noted, such as telomerase reverse-transcriptase promoter mutations [122,123,124,125,126,127], protein tyrosine phosphate receptor type D gene mutations occurring at high rates [128], and BRAF mutations [129,130,131,132]. Interestingly, all of them have been tested using various immunotherapy strategies [134,135,136], indicating that this area requires closer inspection and research, especially due to the aggressive nature of brain tumours.
Limitations of the Study
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACGs | Annonaceous acetogenins |
| AGuIX | Activation by the guidance of irradiation by X-ray |
| AMF | Alternating magnetic field |
| BBB | Blood–brain barrier |
| BTB | Blood–tumour barrier |
| CCL2 | Chemokine (CC motif) ligand 2 |
| CCR2 | CC chemokine receptor 2 |
| CNS | Central nervous system |
| CPPs | Cell-penetrating peptides |
| CTLs | Cytotoxic T-lymphocytes |
| CTLA-4 | Cytotoxic T-lymphocyte-associated antigen 4 |
| DAMPs | Damage-associated molecular patterns |
| DCA | Dichloroacetate |
| DOX | Doxorubicin |
| ECM | Extracellular matrix |
| EMF | External magnetic field |
| EOR | Extent of resection |
| EPR | Enhanced permeability and retention |
| fMRI | Functional magnetic resonance imaging |
| FUS | Focused ultrasound |
| GBM | Glioblastoma |
| HA | Hyaluronic acid |
| HACE | Hyaluronic acid–ceramide |
| HA-NPs | Hyaluronic acid nanoparticles |
| HBP | Hyperbranched polymers |
| HER | Human epidermal growth factor receptor |
| HFn | Ferritin nanoparticles |
| HGGs | High-grade gliomas |
| iCT | Intraoperative computed tomography |
| IN | Intraoperative neurophysiology |
| IoUS | Intraoperative ultrasound |
| Ipi | Ipilimumab |
| LGG | Low-grade gliomas |
| mAbs | Humanised monoclonal antibodies |
| MGMT | O-methylguanine-DNA methyltransferase |
| MM | Malignant melanoma |
| MNPs | Magnetic nanoparticles |
| MOFs | Metal–organic frameworks |
| MRI | Magnetic resonance imaging |
| MRP1 | Multi-drug resistant protein 1 |
| MSC | Mesenchymal stem cell |
| MSNs | Mesoporous silica nanoparticles |
| NICs | Nanoscale immunoconjugates |
| NMRSA | Proton magnetic resonance spectroscopic imaging |
| NPs | Nanoparticles |
| NRP-1 | Neurolipin-1 |
| o-HA | Hyaluronic acid oligomers |
| OMV | Outer membrane vesicle |
| OS | Overall survival |
| PD-1 | Programmed cell death-1 |
| PEI | Polyethyleneimine |
| PEO-PBO | Poly(ethylene oxide)-b-poly(butylene oxide) |
| PFS | Progression-free survival |
| P-gp | P-glycoprotein |
| PTX | Paclitaxel |
| RT | Radiotherapy |
| SCLC | Small-cell lung cancer |
| SLNs | Solid lipid nanoparticles |
| SRS | Stereotactic radiosurgery |
| TMZ | Temozolomide |
| TfR1 | Transferrin receptor 1 |
| Tregs | Regulatory T-cells |
| TME | Tumour microenvironment |
| TPP | Triphenylphosphonium |
| TTPs | Tumour-targeting peptides |
| TZ | Trastuzumab |
| USLPs | Ultra-small Silica NPs with large pores |
| usNLCs | Ultra-small nanostructure lipid carriers |
| VEGFR-2 | Vascular endothelial growth factor 2 |
| WHO | World Health Organisation |
References
- Ganau, L.; Paris, M.; Ligarotti, G.K.; Ganau, M. Management of Gliomas: Overview of the Latest Technological Advancements and Related Behavioral Drawbacks. Behav. Neurol. 2015, 2015, 862634. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro. Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Stienen, M.N.; Freyschlag, C.F.; Schaller, K.; Meling, T.; EANS Young Neurosurgeons and EANS Training Committee. Procedures performed during neurosurgery residency in Europe. Acta Neurochir. 2020, 162, 2303–2311. [Google Scholar] [CrossRef]
- Hatoum, R.; Chen, J.S.; Lavergne, P.; Shlobin, N.A.; Wang, A.; Elkaim, L.M.; Dodin, P.; Couturier, C.P.; Ibrahim, G.M.; Fallah, A.; et al. Extent of Tumor Resection and Survival in Pediatric Patients With High-Grade Gliomas: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2022, 5, e2226551. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Chang, S.; Berger, M.S. Low-Grade Gliomas in Adults: A Review. J. Neurosurg. 2011, 115, 948–965. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.B.; Liu, C.Y.; Apuzzo, M.L. Neurosurgery in the realm of 10(-9), Part 2: Applications of nanotechnology to neurosurgery--present and future. Neurosurgery 2008, 62, 269–285. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A Multivariate Analysis of 416 Patients with Glioblastoma Multiforme: Prognosis, Extent of Resection, and Survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Talacchi, A.; Turazzi, S.; Locatelli, F.; Sala, F.; Beltramello, A.; Alessandrini, F.; Manganotti, P.; Lanteri, P.; Gambin, R.; Ganau, M.; et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: A 171-patient series. J. Neurooncol 2010, 100, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Young, R.M.; Jamshidi, A.; Davis, G.L.; Sherman, J.H. Current Trends in the Surgical Management and Treatment of Adult Glioblastoma. Ann. Transl. Med. 2015, 3, 121. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Bélanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Duffau, H. Surgery of low-grade gliomas: Towards a ‘functional neurooncology’. Curr. Opin. Oncol. 2009, 21, 543–549. [Google Scholar] [CrossRef]
- Soffietti, R.; Baumert, B.G.; Bello, L.; Von Deimling, A.; Duffau, H.; Frénay, M.; Grisold, W.; Grant, R.; Graus, F.; Hoang-Xuan, K.; et al. Guidelines on management of low-grade gliomas: Report of an EFNS–EANO* Task Force. Eur. J. Neurol. 2010, 17, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Nitta, M.; Muragaki, Y.; Maruyama, T.; Ikuta, S.; Komori, T.; Maebayashi, K.; Iseki, H.; Tamura, M.; Saito, T.; Okamoto, S.; et al. Proposed therapeutic strategy for adult low-grade glioma based on aggressive tumor resection. Neurosurg. Focus. 2015, 38, E7. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Ligarotti, G.K.; Apostolopoulos, V. Real-time intraoperative ultrasound in brain surgery: Neuronavigation and use of contrast-enhanced image fusion. Quant. Imaging Med. Surg. 2019, 9, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.M.V.; Certo, F.; Di Gregorio, S.; Maione, M.; Garozzo, M.; Peschillo, S.; Altieri, R. Recurrent High-Grade Glioma Surgery: A Multimodal Intraoperative Protocol toSafely Increase Extent of Tumor Resection and Analysis of Its Impact on Patient Outcome. Neurosurg. Focus 2021, 50, E20. [Google Scholar] [CrossRef]
- Marcus, H.J.; Williams, S.; Hughes-Hallett, A.; Camp, S.J.; Nandi, D.; Thorne, L. Predicting surgical outcome in patients with glioblastoma multiforme using pre-operative magnetic resonance imaging: Development and preliminary validation of a grading system. Neurosurg. Rev. 2017, 40, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Ganau, L.; Ligarotti, G.K.I.; Ganau, M. Predicting complexity of tumor removal and postoperative outcome in patients with high-grade gliomas. Neurosurg. Rev. 2017, 41, 371–373. [Google Scholar] [CrossRef]
- Saraswathy, S.; Crawford, F.W.; Lamborn, K.R.; Pirzkall, A.; Chang, S.; Cha, S.; Nelson, S.J. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J. Neurooncol 2008, 91, 69–81. [Google Scholar] [CrossRef]
- Yi, Z.; Luo, Z.; Barth, N.D.; Meng, X.; Liu, H.; Bu, W.; All, A.; Vendrell, M.; Liu, X. In Vivo Tumor Visualization through MRI Off-On Switching of NaGdF4 -CaCO3 Nanoconjugates. Adv. Mater. 2019, 31, e1901851. [Google Scholar] [CrossRef]
- Marko, N.F.; Weil, R.J.; Schroeder, J.L.; Lang, F.F.; Suki, D.; Sawaya, R.E. Extent of resection of glioblastoma revisited: Personalized survival modeling facilitates more accurate survival prediction and supports a Maximum-Safe-Resection approach to surgery. J. Clin. Oncol. 2014, 32, 774–782. [Google Scholar] [CrossRef]
- Sanai, N.; Berger, M.S. GLIOMA EXTENT OF RESECTION AND ITS IMPACT ON PATIENT OUTCOME. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef]
- D’Arco, F.; Khan, F.; Mankad, K.; Ganau, M.; Caro-Dominguez, P.; Bisdas, S. Differential diagnosis of posterior fossa tumours in children: New insights. Pediatr. Radiol. 2018, 48, 1955–1963. [Google Scholar] [CrossRef]
- Leksell, L. The stereotaxic method and radiosurgery of the brain. Acta Chir. Scand. 1951, 102, 316–319. [Google Scholar]
- Ganau, M.; Foroni, R.I.; Gerosa, M.; Zivelonghi, E.; Longhi, M.; Nicolato, A. Radiosurgical options in neuro-oncology: A review on current tenets and future opportunities. Part I: Therapeutic strategies. Tumori 2014, 100, 459–465. [Google Scholar] [CrossRef]
- Molina-Romero, O.I.; Segura-Hernandez, A.; Fonnegra-Caballero, A.; Diez-Palma, J.C.; Cortés-Muñoz, F.; Fonnegra-Pardo, J.R. Gamma Knife radiosurgery—12 years of experience in a high-complexity center of a middle-income country. Surg. Neurol. Int. 2022, 13, 582. [Google Scholar] [CrossRef] [PubMed]
- Monaco, E.A.; Grandhi, R.; Niranjan, A.; Lunsford, L.D. The past, present and future of Gamma Knife radiosurgery for brain tumors: The Pittsburgh experience. Expert. Rev. Neurother. 2012, 12, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Apra, C.; Peyre, M.; Kalamarides, M. Current treatment options for meningioma. Expert. Rev. Neurother. 2018, 18, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Boukobza, M.; Cebula, H.; Pop, R.; Kouakou, F.; Sadoun, A.; Coca, H.A.; Polivka, M.; Diemidio, P.; Ganau, M.; George, B.; et al. Cystic meningioma: Radiological, histological, and surgical particularities in 43 patients. Acta Neurochir. 2016, 158, 1955–1964. [Google Scholar] [CrossRef]
- Gong, X.; Ding, J.; Knisely, J.P.S.; Wang, E.; Pan, L.; Wang, B.; Zhang, N.; Wu, H.; Dai, J.; Yu, T.; et al. Dose-staged Gamma Knife radiosurgery for meningiomas: A retrospective study in a single center. Front. Neurol. 2022, 13, 893480. [Google Scholar] [CrossRef] [PubMed]
- Imber, B.S.; Kanungo, I.; Braunstein, S.; Barani, I.J.; Fogh, S.E.; Nakamura, J.L.; Berger, M.S.; Chang, E.F.; Molinaro, A.M.; Cabrera, J.R.; et al. Indications and efficacy of gamma knife stereotactic radiosurgery for recurrent glioblastoma: 2 decades of institutional experience. Neurosurgery 2016, 80, 129–139. [Google Scholar] [CrossRef]
- Oh, B.C.; Liu, C.Y.; Wang, M.Y.; Pagnini, P.G.; Yu, C.; Apuzzo, M.L.J. STEREOTACTIC RADIOSURGERY. Neurosurgery 2007, 60, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Foroni, R.I.; Gerosa, M.; Ricciardi, G.K.; Longhi, M.; Nicolato, A. Radiosurgical Options in Neuro-Oncology: A review on current tenets and future opportunities. Part II: Adjuvant Radiobiological Tools. Tumori 2015, 101, 57–63. [Google Scholar] [CrossRef]
- Suh, J.H.; Stea, B.; Nabid, A.; Kresl, J.J.; Fortin, A.; Mercier, J.-P.; Senzer, N.; Chang, E.L.; Boyd, A.P.; Cagnoni, P.J.; et al. Phase III study of Efaproxiral as an adjunct to Whole-Brain radiation therapy for brain metastases. J. Clin. Oncol. 2005, 24, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Pourmadadi, M.; Shamsabadipour, A.; Bhatti, A.; Forouzanfar, M.; Rajabnejad, M.; Behzadmehr, R.; Rahdar, A.; Medina, D.I.; Díez-Pascual, A.M. Therapeutic performance of temozolomide-loaded nanomaterials: A state-of-the-art. J. Drug Deliv. Sci. Technol. 2023, 85, 104568. [Google Scholar] [CrossRef]
- Fuchs, D.; Bley, C.R.; Morandi, L.; Tonon, C.; Weyland, M.S.; Nytko, K.J. Triple combination of lomustine, temozolomide and irradiation reduces canine glioma cell survival in vitro. Veter. Med. Sci. 2023, 9, 1573–1583. [Google Scholar] [CrossRef]
- Lee, S.W.; Cho, H.Y.; Na, G.; Yoo, M.R.; Seo, S.K.; Hur, D.Y.; Han, J.; Lee, C.K.; Choi, I. CD40 stimulation induces vincristine resistance via AKT activation and MRP1 expression in a human multiple myeloma cell line. Immunol. Lett. 2012, 144, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug Transport across the Blood–Brain Barrier. J. Cereb. Blood Flow. Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef]
- Pardridge, W.M. The Blood-Brain Barrier: Bottleneck in Brain Drug Development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, P.; Yao, P.; Sun, Y.; Chen, X.; Zhao, Z.; Han, B.-J. Recent Advances in Drug Delivery Systems for Targeting Brain Tumors. Drug Deliv. 2023, 30, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Palazzo, C.; Évrard, B.; Piel, G. Nanocarriers for the Treatment of Glioblastoma Multiforme: Current State-of-the-Art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.P.; Liu, C.Y.; Apuzzo, M.L.J. Toward the Emergence of nanoneurosurgery: Part III—Nanomedicine: Targeted nanotherapy, nanosurgery, and Progress toward the realization of nanoneurosurgery. Neurosurgery 2006, 58, 1009–1026. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M. Tackling gliomas with nanoformulated antineoplastic drugs: Suitability of hyaluronic acid nanoparticles. Clin. Transl. Oncol. 2013, 16, 220–223. [Google Scholar] [CrossRef]
- Ganau, M.; Syrmos, N.C.; D’Arco, F.; Ganau, L.; Chibbaro, S.; Prisco, L.; Gki, L.; Ambu, R.; Soddu, A. Enhancing contrast agents and radiotracers performance through hyaluronic acid-coating in neuroradiology and nuclear medicine. Hell. J. Nucl. Med. 2017, 20, 166–168. [Google Scholar] [CrossRef]
- Delpech, B.; Maingonnat, C.; Girard, N.; Chauzy, C.; Olivier, A.; Maunoury, R.; Tayot, J.; Creissard, P. Hyaluronan and hyaluronectin in the extracellular matrix of human brain tumour stroma. Eur. J. Cancer 1993, 29, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kim, S.; Jin, S.; Ryu, H.; Jin, Y.; Jung, T.; Kim, I.; Jung, S. Cisplatin-incorporated hyaluronic acid nanoparticles based on ion-complex formation. J. Pharm. Sci. 2007, 97, 1268–1276. [Google Scholar] [CrossRef]
- Boeckman, H.J.; Trego, K.S.; Turchi, J.J. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer Res. 2005, 3, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-J.; Yoon, H.Y.; Koo, H.; Ko, S.-H.; Shim, J.-S.; Cho, J.-H.; Park, J.H.; Kim, K.; Kwon, I.C.; Kim, D.-D. Hyaluronic acid-ceramide-based optical/MR dual imaging nanoprobe for cancer diagnosis. J. Control. Release 2012, 162, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Gilg, A.G.; Tye, S.L.; Tolliver, L.B.; Wheeler, W.G.; Visconti, R.P.; Duncan, J.D.; Kostova, F.V.; Bolds, L.N.; Toole, B.P.; Maria, B.L. Targeting hyaluronan interactions in malignant gliomas and their Drug-Resistant multipotent progenitors. Clin. Cancer Res. 2008, 14, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Ganau, M.; Bosco, A.; Palma, A.; Corvaglia, S.; Parisse, P.; Fruk, L.; Beltrami, A.P.; Cesselli, D.; Casalis, L.; Scoles, G. A DNA-based nano-immunoassay for the label-free detection of glial fibrillary acidic protein in multicell lysates. Nanomedicine 2015, 11, 293–300. [Google Scholar] [CrossRef]
- Yuan, H.; Wilson, C.M.; Xia, J.; Doyle, S.L.; Li, S.; Fales, A.M.; Liu, Y.; Ozaki, E.; Mulfaul, K.; Hanna, G.; et al. Plasmonics-enhanced and optically modulated delivery of gold nanostars into brain tumor. Nanoscale 2014, 6, 4078–4082. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Feng, J.J.; Cheok, S.; Shah, I.; Dicharry, H.; Cote, D.J.; Briggs, R.G.; Guerra, G.A.; Peterson, R.; Salhia, B.; et al. Incidence of brain metastasis according to patient race and primary cancer origin: A systematic review. J. Neurooncol. 2024, 169, 457–467. [Google Scholar] [CrossRef]
- Miller, D.S.; Bauer, B.; Hartz, A.M.S. Modulation of P-Glycoprotein at the Blood-Brain Barrier: Opportunities to Improve Central Nervous System Pharmacotherapy. Pharmacol. Rev. 2008, 60, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Iwashita, Y.; Wakayama, E.; Nishino, I.; Nishikaji, T.; Kondoh, M. Tight Junction Modulating Bioprobes for Drug Delivery System to the Brain: A Review. Pharmaceutics 2020, 12, 1236. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Liu, J.; Li, Y.; Li, W. Pluronic F127-Complexed PEGylated Poly(glutamic acid)-Cisplatin Nanomedicine for Enhanced Glioblastoma Therapy. Macromol. Rapid Commun. 2024, 45, e2400662. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Wang, Z.; Lu, L.; Ma, H.; Li, H.; Fu, J.; Li, M.; Han, M.; Guo, Y.; Wang, X. Enhanced tumor accumulation and therapeutic efficacy of liposomal drugs through over-threshold dosing. J. Nanobiotechnology 2022, 20, 137. [Google Scholar] [CrossRef] [PubMed]
- Ahammadsahib, K.I.; Hollingworth, R.M.; McGovren, J.P.; Hui, Y.-h; McLaughlin, J.L. Mode of action of bullatacin: A potent antitumor and pesticidal Annonaceous acetogenin. Life Sci. 1993, 53, 1113–1120. [Google Scholar] [CrossRef]
- Ao, H.; Fu, Y.; Wang, X. A comparative study of PEO-PBO content on the targeting and anti-glioma activity of annonaceous acetogenins-loaded nanomicelles. Colloids Surf. B Biointerfaces 2024, 244, 114176. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in Vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.D.; et al. The Entry of Nanoparticles into Solid Tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Maier-Hauff, K.; Frank, U.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and Safety of Intratumoral Thermotherapy Using Magnetic Iron-Oxide Nanoparticles Combined with External Beam Radiotherapy on Patients with Recurrent Glioblastoma Multiforme. J. Neurooncol. 2010, 103, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.; Van Den Bent, M.J.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Bélanger, K.; et al. Effects of Radiotherapy with Concomitant and Adjuvant Temozolomide versus Radiotherapy Alone on Survival in Glioblastoma in a Randomised Phase III Study: 5-Year Analysis of the EORTC-NCIC Trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Gubian, A.; Ganau, M.; Cebula, H.; Todeschi, J.; Scibilia, A.; Noel, G.; Spatola, G.; Chaussemy, D.; Nannavecchia, B.; Gallinaro, P.; et al. Intracranial Solitary Fibrous Tumors: A Heterogeneous Entity with an Uncertain Clinical Behavior. World Neurosurg. 2019, 126, e48–e56. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Hua, M.-Y.; Yang, H.; Huang, C.-Y.; Chu, P.-C.; Wu, J.; Tseng, I.-F.; Wang, J.; Yen, T.; Chen, P.-Y.; et al. Magnetic Resonance Monitoring of Focused Ultrasound/Magnetic Nanoparticle Targeting Delivery of Therapeutic Agents to the Brain. Proc. Natl. Acad. Sci. USA 2010, 107, 15205–15210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Stevens, M.F.G.; Bradshaw, T.D. Temozolomide: Mechanisms of Action, Repair and Resistance. Curr. Mol. Pharmacol. 2012, 5, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.; Corsa, P.; Raguso, A.; Perrone, A.; Cossa, S.; Munafò, T.; Sanpaolo, G.; Donno, E.; Clemente, M.A.; Piombino, M.; et al. Temozolomide and Radiotherapy versus Radiotherapy Alone in High Grade Gliomas: A Very Long Term Comparative Study and Literature Review. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Ahmed-Cox, A.; Raza, A.; Moniruzzaman, M.; Akhter, D.T.; Fletcher, N.L.; Kavallaris, M.; Thurecht, K.J.; Popat, A. Efficient Delivery of Temozolomide Using Ultrasmall Large-Pore Silica Nanoparticles for Glioblastoma. J. Control. Release 2023, 357, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, M.; Song, H.; Yu, C. Silica-Based Nanoparticles for Biomedical Applications: From Nanocarriers to Biomodulators. Acc. Chem. Res. 2020, 53, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical Translation of Silica Nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Li, C.; Gu, J.-M.; Qian, J.; Zhu, J.; Wang, J.; Li, Y.; Jiang, J.; Chen, H.; Luo, C. Accurately Controlled Delivery of Temozolomide by Biocompatible UIO-66-NH2 through Ultrasound to Enhance the Antitumor Efficacy and Attenuate the Toxicity for Treatment of Malignant Glioma. Int. J. Nanomed. 2021, 16, 6905–6922. [Google Scholar] [CrossRef] [PubMed]
- Ashokan, A.; Sarkar, S.; Kamran, M.Z.; Surnar, B.; Kalathil, A.A.; Spencer, A.; Dhar, S. Simultaneous targeting of peripheral and brain tumors with a therapeutic nanoparticle to disrupt metabolic adaptability at both sites. Proc. Natl. Acad. Sci. USA 2024, 121, e2318119121. [Google Scholar] [CrossRef] [PubMed]
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.-L.; Quesada, J.-L.; et al. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 2021, 160, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Nunes, S.; Cova, T.; Branco, F.; Dyrks, M.; Koksch, B.; Vale, N.; Sousa, J.; Pais, A.; Vitorino, C. Charge-switchable cell-penetrating peptides for rerouting nanoparticles to glioblastoma treatment. Colloids Surfaces B Biointerfaces 2024, 241, 113983. [Google Scholar] [CrossRef]
- Joshy, K.S.; Sharma, C.P.; Kalarikkal, N.; Sandeep, K.P.; Thomas, S.; Pothen, L.A. Evaluation of in-vitro cytotoxicity and cellular uptake efficiency of zidovudine-loaded solid lipid nanoparticles modified with Aloe Vera in glioma cells. Mater. Sci. Eng. C 2016, 66, 40–50. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current State of Immunotherapy for Glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.; McDermott, D.F.; Weber, R.; Sosman, J.A.; Haanen, J.B.A.G.; González, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.S.; Garbe, C.; Lebbe, C.; Baurain, J.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, D.A.; Chang, A.L.; Dey, M.; Balyasnikova, I.V.; Kim, C.K.; Tobias, A.L.; Cheng, Y.; Kim, J.W.; Qiao, J.; Zhang, L.; et al. Durable Therapeutic Efficacy Utilizing Combinatorial Blockade against IDO, CTLA-4, and PD-L1 in Mice with Brain Tumors. Clin. Cancer Res. 2014, 20, 5290–5301. [Google Scholar] [CrossRef] [PubMed]
- Belcaid, Z.; Phallen, J.; Zeng, J.; See, A.P.; Mathios, D.; Gottschalk, C.; Nicholas, S.E.; Kellett, M.; Ruzevick, J.; Jackson, C.M.; et al. Focal Radiation Therapy Combined with 4-1BB Activation and CTLA-4 Blockade Yields Long-Term Survival and a Protective Antigen-Specific Memory Response in a Murine Glioma Model. PLoS ONE 2014, 9, e101764. [Google Scholar] [CrossRef]
- Galstyan, A.; Markman, J.L.; Shatalova, E.S.; Chiechi, A.; Korman, A.J.; Patil, R.; Klymyshyn, D.; Tourtellotte, W.G.; Israel, L.; Braubach, O.; et al. Blood–Brain Barrier Permeable Nano Immunoconjugates Induce Local Immune Responses for Glioma Therapy. Nat. Commun. 2019, 10, 3850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Xu, X.; Juan, D.; Chen, X.; Xue, Y.; Zhang, J.; Yang, X.; Chen, X.; Xie, J.; Ju, S. Redox-Responsive Polymer Micelles Co-Encapsulating Immune Checkpoint Inhibitors and Chemotherapeutic Agents for Glioblastoma Therapy. Nat. Commun. 2024, 15, 1118. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Fan, J.; You, Y.; Du, Y.; Liu, D.; Xu, X.; Wang, J.; Zhu, L.; Chen, M.; Gao, S.; et al. Synergistic Effect of Tumor Chemo-Immunotherapy Induced by Leukocyte-Hitchhiking Thermal-Sensitive Micelles. Nat. Commun. 2021, 12, 4755. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, G.; Chen, Y.; Wang, H.; Hua, Y.; Cai, Z. Immunogenic Cell Death in Cancer Therapy: Present and Emerging Inducers. J. Cell Mol. Med. 2019, 23, 4854–4865. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N. Engl. J. Med. 2014, 372, 311–319. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Powles, T.; Eder, J.P.; Fine, G.D.; Braiteh, F.S.; Loriot, Y.; Cruz, C.; Bellmunt, J.; Burris, H.A.; Petrylak, D.P.; Teng, S.-L.; et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014, 515, 558–562. [Google Scholar] [CrossRef]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and Long-Term safety in patients with advanced melanoma receiving Nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti–PD-1 Therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, V.; Postow, M.A.; Tuck, D.; Wolchok, J.D. Immune-priming of the tumor microenvironment by radiotherapy. Am. J. Clin. Oncol. 2013, 38, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.J.; Rutten, J.; Huveneers, H.a.M.; Twijnstra, A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002, 94, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Eichler, A.F.; Loeffler, J.S. Multidisciplinary management of brain metastases. Oncologist 2007, 12, 884–898. [Google Scholar] [CrossRef] [PubMed]
- Cagney, D.; Martin, A.; Catalano, P.J.; Brown, P.D.; Alexander, B.M.; Lin, N.U.; Aizer, A.A. Implications of Screening for Brain Metastases in Patients with Breast Cancer and Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 1001. [Google Scholar] [CrossRef]
- McTyre, E.; Scott, J.G.; Chinnaiyan, P. Whole Brain Radiotherapy for Brain Metastasis. Surg. Neurol. Int. 2013, 4, 236. [Google Scholar] [CrossRef]
- Hsieh, A.C.; Moasser, M.M. Targeting HER Proteins in Cancer Therapy and the Role of the Non-Target HER3. Br. J. Cancer 2007, 97, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Abdel-Fatah, T.M.A.; Moseley, P.M.; Nolan, C.C.; Durham, A.C.; Rakha, E.A.; Chan, S.; Ellis, I.O.; Green, A. Characterisation of HER Heterodimers in Breast Cancer Using in Situ Proximity Ligation Assay. Breast Cancer Res. Treat. 2014, 144, 273–285. [Google Scholar] [CrossRef]
- Green, A.; Barros, F.; Abdel-Fatah, T.M.; Moseley, P.M.; Nolan, C.C.; Durham, A.C.; Rakha, E.A.; Chan, S.; Ellis, I.O. HER2/HER3 Heterodimers and P21 Expression Are Capable of Predicting Adjuvant Trastuzumab Response in HER2+ Breast Cancer. Breast Cancer Res. Treat. 2014, 145, 33–44. [Google Scholar] [CrossRef]
- Lim, M.; Fletcher, N.L.; Saunus, J.M.; Reed, A.E.M.; Chittoory, H.; Simpson, P.T.; Thurecht, K.J.; Lakhani, S.R. Targeted Hyperbranched Nanoparticles for Delivery of Doxorubicin in Breast Cancer Brain Metastasis. Mol. Pharm. 2023, 20, 6169–6183. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kizilbash, S.H.; Laramy, J.K.; Gampa, G.; Parrish, K.E.; Sarkaria, J.N.; Elmquist, W.F. Barriers to effective drug treatment for brain metastases: A multifactorial problem in the delivery of precision medicine. Pharm. Res. 2018, 35, 177. [Google Scholar] [CrossRef] [PubMed]
- Rathi, S.; Griffith, J.I.; Zhang, W.; Zhang, W.; Oh, J.; Talele, S.; Sarkaria, J.N.; Elmquist, W.F. The influence of the blood–brain barrier in the treatment of brain tumours. J. Intern. Med. 2022, 292, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Fiandra, L.; Mazzucchelli, S.; Truffi, M.; Bellini, M.; Sorrentino, L.; Corsi, F. In Vitro Permeation of FITC-loaded Ferritins Across a Rat Blood-brain Barrier: A Model to Study the Delivery of Nanoformulated Molecules. J. Vis. Exp. 2016, 114, e54279. [Google Scholar] [CrossRef]
- Fan, K.; Jia, X.; Zhou, M.; Wang, K.; Conde, J.; He, J.; Tian, J.; Yan, X. Ferritin nanocarrier traverses the blood brain barrier and kills glioma. ACS Nano 2018, 12, 4105–4115. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Fu, Y.; Huang, S.; Guo, C.; Li, L.; Wang, L.; Zhang, Z.; Zhang, L. Target delivering paclitaxel by ferritin heavy chain nanocages for glioma treatment. J. Control. Release 2019, 323, 191–202. [Google Scholar] [CrossRef]
- Sevieri, M.; Mazzucchelli, S.; Barbieri, L.; Garbujo, S.; Carelli, S.; Bonizzi, A.; Rey, F.; Recordati, C.; Recchia, M.; Allevi, R.; et al. Ferritin nanoconjugates guide trastuzumab brain delivery to promote an antitumor response in murine HER2 + breast cancer brain metastasis. Pharmacol. Res. 2023, 196, 106934. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fan, X.; Deng, H.; Brezski, R.J.; Rycyzyn, M.; Jordan, R.E.; Strohl, W.R.; Zou, Q.; Zhang, N.; An, Z. Trastuzumab Triggers Phagocytic Killing of High HER2 Cancer Cells In Vitro and In Vivo by Interaction with Fcγ Receptors on Macrophages. J. Immunol. 2015, 194, 4379–4386. [Google Scholar] [CrossRef]
- Richards, J.O.; Karki, S.; Lazar, G.A.; Chen, H.; Dang, W.; Desjarlais, J.R. Optimization of antibody binding to FcγRIIa enhances macrophage phagocytosis of tumor cells. Mol. Cancer Ther. 2008, 7, 2517–2527. [Google Scholar] [CrossRef] [PubMed]
- Ngamcherdtrakul, W.; Bejan, D.S.; Cruz-Muñoz, W.; Reda, M.; Zaidan, H.Y.; Siriwon, N.; Marshall, S.; Wang, R.; Nelson, M.A.; Rehwaldt, J.P.C.; et al. Targeted nanoparticle for co-delivery of HER2 siRNA and a taxane to mirror the standard treatment of HER2+ Breast cancer: Efficacy in breast tumor and brain metastasis. Small 2022, 18, e2107550. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-J.; Wang, M.; Shi, S.; Hu, X.; Xu, P. A Therapeutic sheep in metastatic wolf’s clothing: Trojan Horse Approach for Cancer Brain Metastases Treatment. Nanomicro Lett. 2022, 14, 114. [Google Scholar] [CrossRef]
- Birch-Machin, M.A. The role of mitochondria in ageing and carcinogenesis. Clin. Exp. Dermatol. 2006, 31, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Blasiak, J. Role of mitochondria in carcinogenesis. Acta Biochim. Pol. 2014, 61, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Gazdar, A.F.; Bunn, P.A.; Minna, J.D. Small-cell lung cancer: What we know, what we need to know and the path forward. Nat. Rev. Cancer 2017, 17, 725–737, Erratum in Nat. Rev. Cancer 2017, 17, 765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Han, M.; Zhang, J.; Abdalla, M.; Sun, P.; Yang, Z.; Zhang, C.; Liu, Y.; Chen, C.; Jiang, X. Syphilis mimetic nanoparticles for cuproptosis-based synergistic cancer therapy via reprogramming copper metabolism. Int. J. Pharm. 2023, 640, 123025. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, D.; Zhou, S.; Dong, N.; Ji, Y.; An, P.; Wang, J.; Luo, Y.; Luo, J. Regulatory roles of copper metabolism and cuproptosis in human cancers. Front. Oncol. 2023, 13, 1123420. [Google Scholar] [CrossRef]
- Hartwig, C.; Zlatic, S.A.; Wallin, M.; Vrailas-Mortimer, A.; Fahrni, C.J.; Faundez, V. Trafficking mechanisms of P-type ATPase copper transporters. Curr. Opin. Cell Biol. 2019, 59, 24–33. [Google Scholar] [CrossRef]
- Xie, J.; Yang, Y.; Gao, Y.; He, J. Cuproptosis: Mechanisms and links with cancers. Mol. Cancer 2023, 22, 46. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.-C.A.; Pětrošová, H.; Ebady, R.; Lithgow, K.V.; Rojas, P.; Zhang, Y.; Kim, Y.-E.; Kim, Y.-R.; Odisho, T.; Gupta, N.; et al. Identification of Tp0751 (Pallilysin) as a Treponema pallidum Vascular Adhesin by Heterologous Expression in the Lyme disease Spirochete. Sci. Rep. 2017, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Todeschi, J.; Dannhoff, G.; Chibbaro, S.; Segbedji, F.; Spatola, G.; Mallereau, C.-H.; Noel, G.; Schott, R.; Lhermitte, B.; Cebula, H.; et al. Second Cancer affecting the central nervous system: Systematic literature review exploring the link between malignant melanoma and glioblastoma. World Neurosurg. 2023, 179, 178–184. [Google Scholar] [CrossRef]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef]
- Nonoguchi, N.; Ohta, T.; Oh, J.-E.; Kim, Y.-H.; Kleihues, P.; Ohgaki, H. TERT promoter mutations in primary and secondary glioblastomas. Acta Neuropathol. 2013, 126, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly recurrent TERT promoter mutations in human melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef]
- Qi, F.; Yin, Z.; Wang, G.; Zeng, S. Clinical and Prognostic Significance of O6-Methylguanine-DNA Methyltransferase Promoter Methylation in Patients with Melanoma: A Systematic Meta-Analysis. Ann. Dermatol. 2018, 30, 129. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.A.; Kim, J.-S.; Cronin, J.C.; Sibenaller, Z.; Ryken, T.; Rosenberg, S.A.; Ressom, H.; Jean, W.; Bigner, D.; Yan, H.; et al. Mutational inactivation of PTPRD in glioblastoma multiforme and malignant melanoma. Cancer Res. 2008, 68, 10300–10306. [Google Scholar] [CrossRef]
- Schindler, G.; Capper, D.; Meyer, J.; Janzarik, W.; Omran, H.; Herold-Mende, C.; Schmieder, K.; Wesseling, P.; Mawrin, C.; Hasselblatt, M.; et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011, 121, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Horbinski, C. ToBRAFoR Not ToBRAF: Is that even a question anymore? J. Neuropathol. Exp. Neurol. 2012, 72, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Scarbrough, P.M.; Akushevich, I.; Wrensch, M.; Il’yasova, D. Exploring the association between melanoma and glioma risks. Ann. Epidemiol. 2014, 24, 469–474. [Google Scholar] [CrossRef]
- Sambade, M.; Deal, A.; Schorzman, A.; Luft, J.C.; Bowerman, C.; Chu, K.; Karginova, O.; Van Swearingen, A.; Zamboni, W.; DeSimone, J.; et al. Efficacy and Pharmacokinetics of a Modified acid-labile docetaxel-PRINT ® Nanoparticle Formulation Against non-small-cell Lung Cancer Brain Metastases. Nanomedicine 2016, 11, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Choi, Y.J.; Sung, K.J.; Yoo, S.; Sung, Y.H.; Kim, J.K.; Choi, C.; Yun, M.; Lee, E.Y.; Jin, Y.S.; et al. Efficacy of nano-particulated, water-soluble erlotinib against intracranial metastases of EGFR-mutant lung cancer. Mol. Oncol. 2018, 12, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Negrini, S.; De Palma, R.; Filaci, G. Anti-cancer Immunotherapies Targeting Telomerase. Cancers 2020, 12, 2260. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Dong, J.; Zhang, Z.Y. Protein tyrosine phosphatases as emerging targets for cancer immunotherapy. Br. J. Pharmacol. 2023; early view. [Google Scholar] [CrossRef]
- Koya, R.C.; Mok, S.; Otte, N.; Blacketor, K.J.; Comin-Anduix, B.; Tumeh, P.C.; Minasyan, A.; Graham, N.A.; Graeber, T.G.; Chodon, T.; et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res. 2012, 72, 3928–3937. [Google Scholar] [CrossRef]
- Kreuter, J.; Shamenkov, D.; Petrov, V.; Ramge, P.; Cychutek, K.; Koch-Brandt, C.; Alyautdin, R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 2002, 10, 317–325. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.; Dalrymple, T.; Husiev, Y.; Bronkhorst, H.; Forn-Cuní, G.; Lopes-Bastos, B.; Snaar-Jagalska, E.; Bonnet, S. Cyclic Ruthenium-Peptide Prodrugs Penetrate the Blood-Brain Barrier and Attack Glioblastoma upon Light Activation in Orthotopic Zebrafish Tumor Models. ACS Cent. Sci. 2024, 10, 2294–2311. [Google Scholar] [CrossRef]
- Yaremenko, A.V.; Khan, M.M.; Zhen, X.; Tang, Y.; Tao, W. Clinical advances of mRNA vaccines for cancer immunotherapy. Med 2025, 6, 100562. [Google Scholar] [CrossRef] [PubMed]
- Cesarini, V.; Scopa, C.; Silvestris, D.A.; Scafidi, A.; Petrera, V.; Del Baldo, G.; Gallo, A. Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules 2020, 25, 4267. [Google Scholar] [CrossRef]
- Ni, K.; Lan, G.; Chan, C.; Quigley, B.; Lu, K.; Aung, T.; Guo, N.; La Riviere, P.; Weichselbaum, R.R.; Lin, W. Nanoscale metal-organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun. 2018, 9, 2351. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Godfrey, D.I.; Trapani, J.A. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2001, 2, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Woo, S.-R.; Zha, Y.; Spaapen, R.; Zheng, Y.; Corrales, L.; Spranger, S. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 2013, 25, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D. A century of radiation therapy and adaptive immunity. Front. Immunol. 2017, 8, 431. [Google Scholar] [CrossRef]
- Ganau, M.; Gallinaro, P.; Cebula, H.; Scibilia, A.; Todeschi, J.; Gubian, A.; Nannavecchia, B.; Signorelli, F.; Pop, R.; Coca, H.A.; et al. Intracranial Metastases from Prostate Carcinoma: Classification, Management, and Prognostication. World Neurosurg. 2020, 134, e559–e565. [Google Scholar] [CrossRef] [PubMed]
- Kiess, A.P.; Wolchok, J.D.; Barker, C.A.; Postow, M.A.; Tabar, V.; Huse, J.T.; Chan, T.A.; Yamada, Y.; Beal, K. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: Safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, F.; Cao, Y.; Zhang, F.; Sun, L.; Yang, C.; Xie, X.; Wu, Z.; Sun, M.; Ma, F.; et al. An Engineered Nanoplatform with Tropism Toward Irradiated Glioblastoma Augments Its Radioimmunotherapy Efficacy. Adv. Mater. 2024, 36, e2314197. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Liu, T.-Y.; Chen, Y.-C.; Chen, M.-H. Combining Augmented Radiotherapy and Immunotherapy through a Nano-Gold and Bacterial Outer-Membrane Vesicle Complex for the Treatment of Glioblastoma. Nanomaterials 2021, 11, 1661. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, P.; Li, F.; Jin, X.; Li, J.; Chen, W.; Li, Q. Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and synergistic effects on tumor cells. Theranostics 2018, 8, 1824–1849. [Google Scholar] [CrossRef]
- Blezinger, P.; Wang, J.; Gondo, M.; Quezada, A.; Mehrens, D.; French, M.; Singhal, A.; Sullivan, S.; Rolland, A.; Ralston, R.; et al. Systemic Inhibition of Tumor Growth and Tumor Metastases by Intramuscular Administration of the Endostatin Gene. Nat. Biotechnol. 1999, 17, 343–348. [Google Scholar] [CrossRef]
- Lu, L.; Chen, H.; Wang, L.; Zhao, L.; Cheng, Y.; Wang, A.; Wang, F.; Zhang, X. A Dual Receptor Targeting- and BBB Penetrating- Peptide Functionalized Polyethyleneimine Nanocomplex for Secretory Endostatin Gene Delivery to Malignant Glioma. Int. J. Nanomed. 2020, 15, 8875–8892. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.V.; Serganova, I.S.; Kruchevsky, N.; Leftin, A.; Shestov, A.A.; Thaler, H.T.; Sukenick, G.; Locasale, J.W.; Blasberg, R.G.; Koutcher, J.A.; et al. Metabolic plasticity of metastatic breast cancer cells: Adaptation to changes in the microenvironment. Neoplasia 2015, 17, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Himič, V.; Syrmos, N.; Ligarotti, G.K.I.; Ganau, M. Latest Insights on Genomic and Epigenomic Mechanisms of Nanotoxicity. In Impact of Engineered Nanomaterials in Genomics and Epigenomics; Sahu, S.C., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]

| Treatment Modality Using NPs: Primary Brain Tumours | Reference | Administration Model | Strategy Described in the Study |
|---|---|---|---|
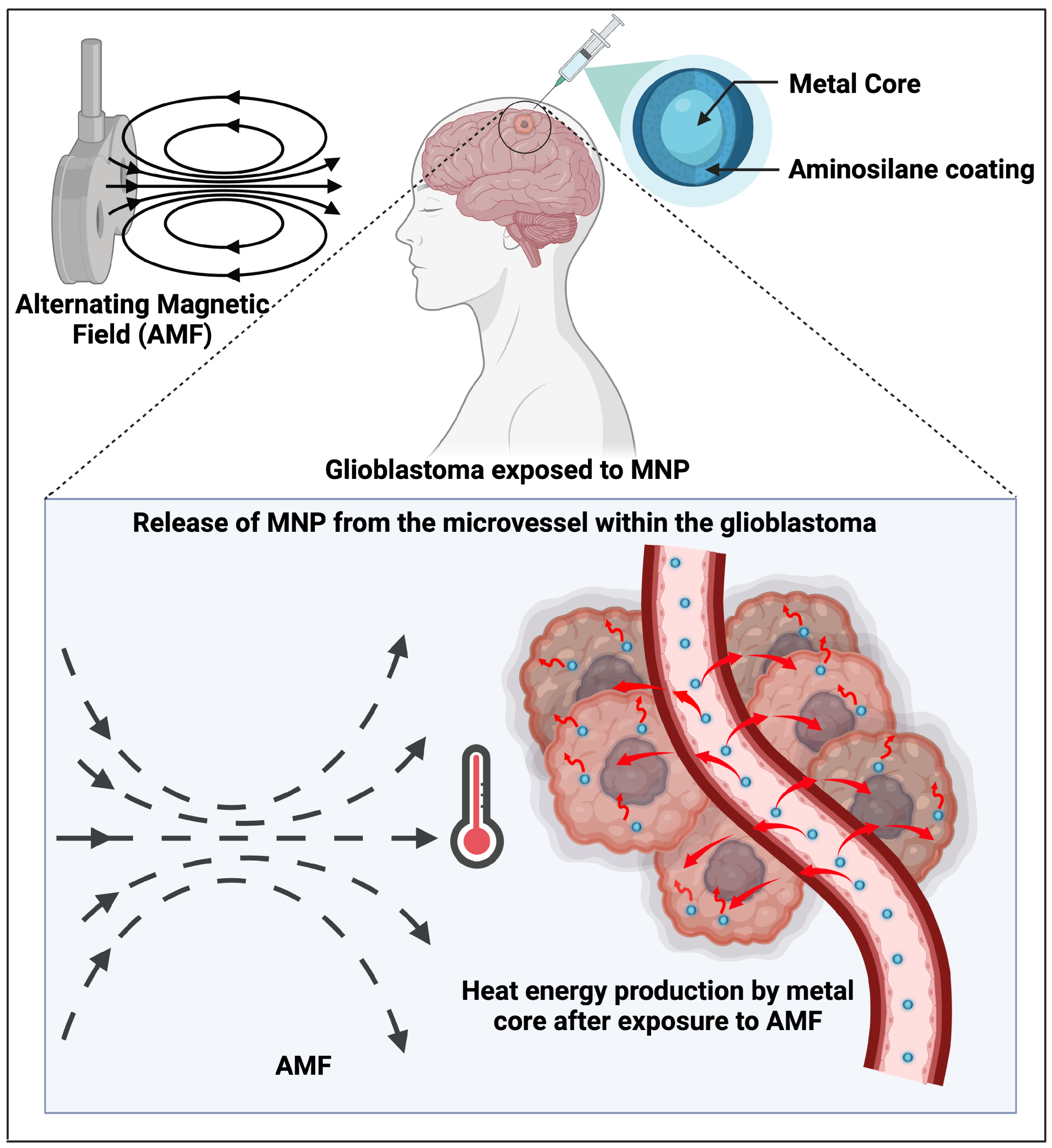
| Chemotherapy | Maier-Hauff et al. [60] | HT | Applied Intratumoural Thermotherapy using iron oxide (magnetite) NPs and alternating magnetic field (AMF). |
| Liu et al. [63] | EM—in vivo (cultured C6 tumour cells) and in vitro | Combined FUS and MNPs (encapsulated iron oxide (Fe3O4) within poly [aniline-co-N-(1-one-butyric acid)] aniline (SPAnH) as a surface layer). | |
| Janjua et al. [66] | EM—in vivo (U87 and GL261 glioblastoma cell lines) and in vitro | Developed novel ultra-small (30 nm) Silica Nanoparticles for the delivery of TMZ across the BBB. | |
| Wan et al. [69] | EM—in vivo (Glioma cells of U251, BMSCs, HUVECs, SHG44 and U87 lines) and in vitro | Used NPs within a Zirconium-based framework to deliver TMZ with the concurrent use of ultrasound, | |
| Chang et al. [54] | EM—in vivo (GL261 glioma cells) and in vitro | Conjugated cisplatin with Pluronic F127-complexed PEGylated poly(glutamic acid) to produce an NP called PLG-PEG/PF127-CDDP. | |
| Ao et al. [57] | EM—in vivo (U87 MG cell line) | ACG-loaded nanomicelles in three different feeding ratios, ACGs/EB5-NCs, ACGs/EB10-NCs, and ACGs/EB20-NCs, were delivered using Poly(ethylene oxide)-b-poly(butylene oxide) (PEO-PBO), as an amphiphilic polymeric carrier toward U87 MG tumour-bearing mice. The NPs had the following sizes: 148.8 ± 0.5 nm, 32.7 ± 4.1 nm, and 27.1 ± 0.3 nm, corresponding to ACGs/EB5-NCs, ACGs/EB10-NCs and ACGs/EB20-NCs, respectively. | |
| Immunotherapy | Galstyan et al. [80] | EM—in vivo (Mouse glioblastoma cell line GL261 implanted intracranially in 8 weeks old female C57BL/6J mice) | Abx against CTLA-4 and PD-1 was covalently bonded to a drug carrier called the poly (Beta-L-malic acid) PMLA backbone. |
| Zhang et al. [81] | EM—in vivo (orthotopic GBM-bearing mice) | Loaded antibodies against PD-1 (as termed by the study aPD-L1) into redox-responsive micelles and combined it with Paclitaxel (PTX). | |
| Radio-immunotherapy | Wang et al. [149] | EM—2 murine models with orthotopic GBM tumours used | Encapsulated PD-L1 antibodies (alphaPD-L1) and diselenide-bridged mesoporous silica nanoparticles (MSNs) within a mesenchymal stem cell (MSC) membrane. CC chemokine receptor 2 (CCR2) was also overexpressed on the MSC membrane. Glioma tumour cells were concurrently irradiated, which allowed radiation-induced tropism of NPs towards chemokine (CC motif) ligand 2 (CCL2). |
| Chen et al. [150] | EM—E. coli cells and GL261 mouse glioma cells, C8D1A mouse astrocytes, B.end3 mouse endothelial cell lines and RAW264.7 mouse macrophages | Combined gold NPs (AuNP) with an outer membrane vesicle (OMV) derived from E.Coli to create the Au-OMV complex. The complex increased ROS generation in GL261 glioma cells by 2.5-fold when they were treated with RT compared to just the Au-OMV complex alone. | |
| Anti-angiogenic therapy | Lu et al. [153] | EM—in vivo (Orthotopic U87-mCherry-luc glioma-bearing nude mice) and in vitro | Penetrated peptide-modified polyethyleneimine (PEI) nanocomplex with TAT-AT7 on the surface to improve binding and crossing BBB. The nanocomplex was loaded with the pVAXI-EN plasmid (secretory endostatin gene)—the total complex was termed PPTA/pVAXI-En. |
| Treatment Modality Using NPs: Secondary Brain Tumours | Reference | Administration Model | Strategy Described in the Study | |
|---|---|---|---|---|
| Chemotherapy | Breast | Lim et al. [100] | EM—n vivo (brain metastases bearing mouse model) and in vitro (BT474 cells breast cancer cell lines) | Loaded hyperbranched polymers (HBPs) with Doxorubicin (DOX) and labelled the NP with anti-HER3/anti-PEG bispecific-antibody fragments (HER3-HBP-DOX) group. |
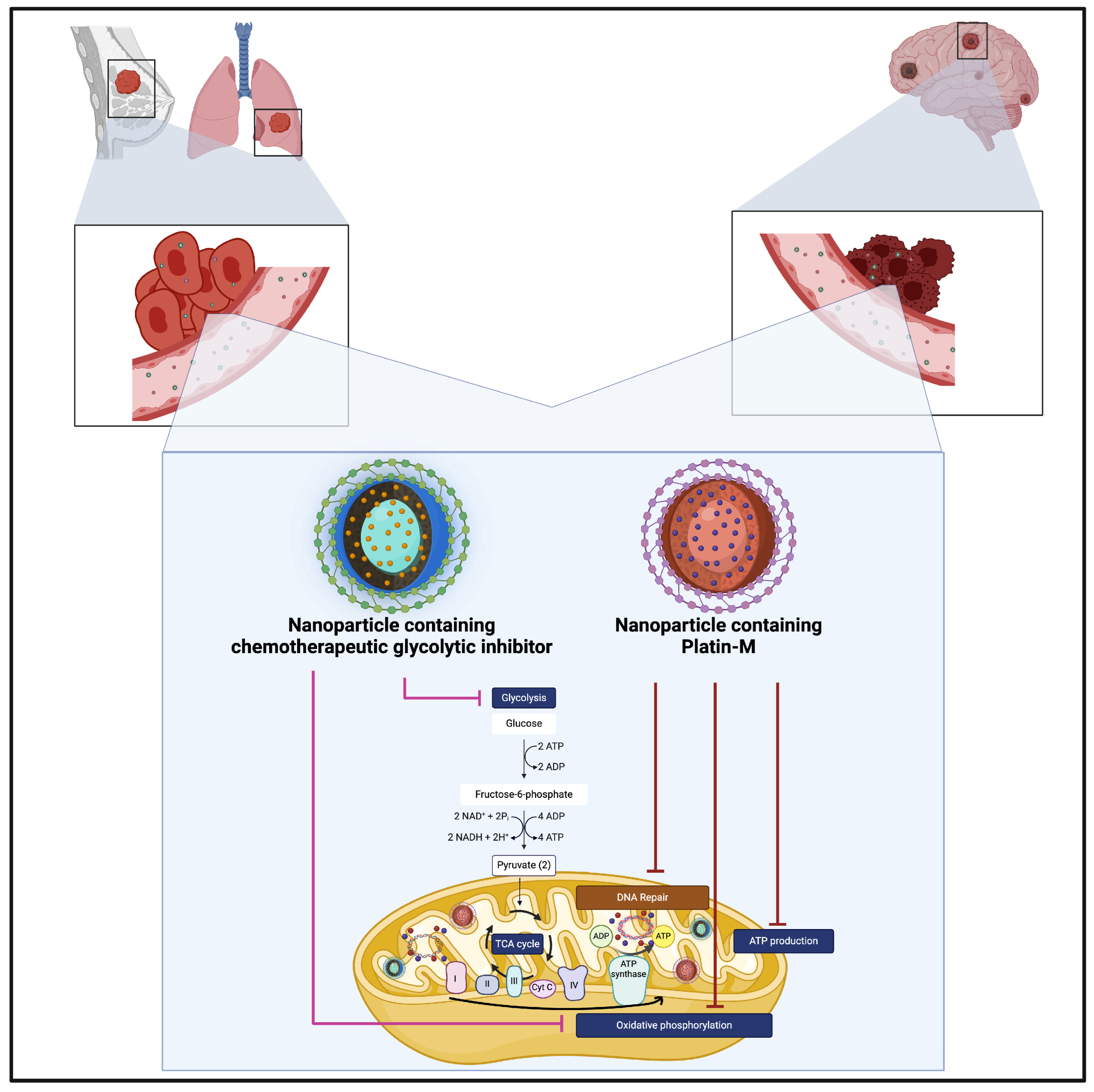
| Breast | Ashokan et al. [70] | EM—MDA-MB-231 breast cancer cell line, MDA-MB-231-BR and Breast cancer cell line HCC1806 used. | Loaded NP with a combination of Platin-M (cisplatin prodrug) and a glycolysis inhibitor to simultaneously target the primary tumour site and tumour cells that had metastasised to the brain (the potential advantages of using glycolysis inhibitors were highlighted by [112,113]). | |
| Breast | Liu et al. [111] | EM—in vivo (brain metastases breast cancer model) | “Trojan Horse strategy,”—a polymeric NP had a coating derived from the MDA-MB-231/Br cell membrane and was loaded with Doxorubicin. Collectively called DOX-PLGA@CM. | |
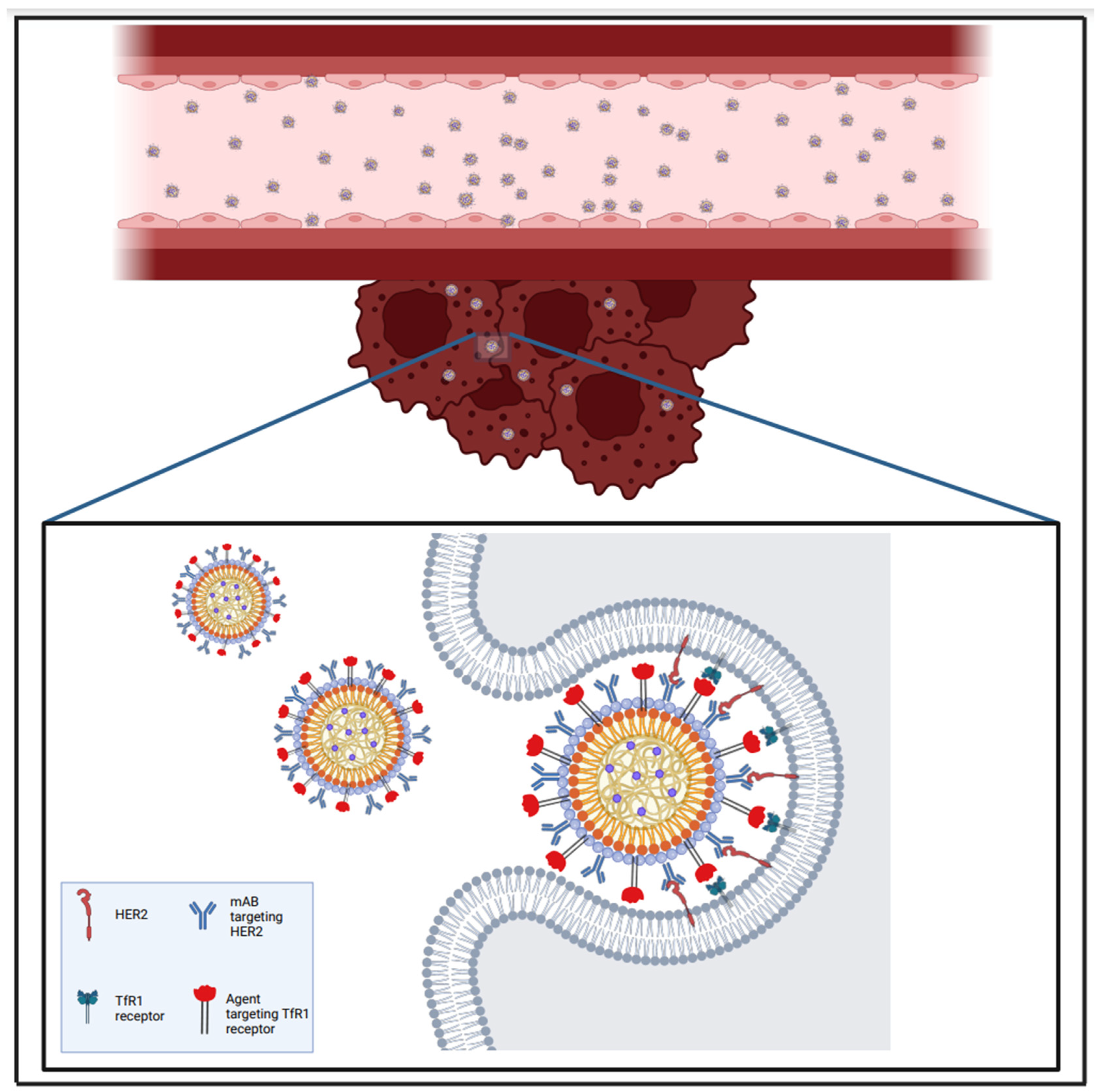
| Immunotherapy | Breast | Sevieri et al. [107] | EM—in vitro (using D2F2/E2-Luc cells) and in vivo (murine breast tumour cell line D2F2/E2, that expressed human HER2 receptor) | Combined Transtazumab with Ferritin NPs and Docetaxel (H-TZ + Dtx) for targeted drug delivery within the tumour microenvironment and for aiding the composition of a protective microenvironment against tumour cells. |
| Immunotherapy + siRNA | Breast | Ngamcherdtrakul et al. [110] | EM—in vivo (drug-resistant orthotopic HER2+ HCC1954 tumour mouse model and HER2+ BT474 tumours within mice brains) | Co-delivery of Docetaxel and HER2 targeting siRNA via a trastuzumab-conjugated NP towards the HER2 + HCC1954 drug-resistant tumour mouse cell line. |
| Chemotherapy | Lung | Sambade et al. [132] | EM—in vivo (intracranial A549 tumours in nude mice) | Docetaxel and acid-labile C2-dimethyl-Si-Docetaxel (C2-Docetaxel) were carried in “Particle Replication in Nonwetting Templates (PRINT(®)) PLGA” NPs. Within A549 tumours in nude mice, median survival was seen to have increased by 35% when PRINT-C2-Docetaxel was used. |
| siRNA delivery | Lung | Zhang et al. [115] | EM—in vivo (mice bearing SCLC tumour metastasis model) and in vitro studies | Designed an NP capable of targeting tumour cells which had metastasised to the brain from small cell lung cancer (SCLC)—the incidence of brain metastases from SCLC is 40–50% in advanced stages of SCLC and 10% in early stages [114]. Called TP-M-Cu-MOF/siATP7a, the NP was loaded with siRNA targeting the ATP7a gene, which is important in modulating the efflux of copper intracellularly. The NP had a coating made of the TP0751-peptide-decorated stem cell membrane, which was syphilis-derived as Pallidum can traverse the BBB [120], and had a copper-based framework. Overall, the NP took advantage of cupropoptosis to inhibit tumour cell growth [116,117,118,119]. |
| EGFR-tyrosine kinase inhibitors | Lung | Kim et al. [133] | EM—in vivo (Human NSCLC cell lines (HCC827 and H1975) and HCC827-luc cells implanted into xenograft mouse models | NUFS-sErt—a water-soluble NP designed using fat and supercritical fluid which delivered Osimertinib (a third-generation EGFR–tyrosine kinase inhibitor) for the treatment of EGFR-mutant lung cancer. This was carried out to counteract the problem of poor solubility of Osimertinib, which has been shown to have significantly higher brain penetration [138]. Significant tumour growth inhibition was seen when NUFS-sErt was inserted into the brain ventricle in intracranial xenograft model. |
| Radio-immunotherapy | Lung, Breast, Melanoma and Colon | Verry et al. [71] | HT—Phase I | Phase I NANO-RAD trial showing the use of a gadolinium-based NP in combination with radiotherapy for the treatment of brain metastases from breast, lung, melanoma and colon cancer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khilar, S.; Dembinska-Kenner, A.; Hall, H.; Syrmos, N.; Ligarotti, G.K.I.; Plaha, P.; Apostolopoulos, V.; Chibbaro, S.; Barbagallo, G.M.V.; Ganau, M. Towards a New Dawn for Neuro-Oncology: Nanomedicine at the Service of Drug Delivery for Primary and Secondary Brain Tumours. Brain Sci. 2025, 15, 136. https://doi.org/10.3390/brainsci15020136
Khilar S, Dembinska-Kenner A, Hall H, Syrmos N, Ligarotti GKI, Plaha P, Apostolopoulos V, Chibbaro S, Barbagallo GMV, Ganau M. Towards a New Dawn for Neuro-Oncology: Nanomedicine at the Service of Drug Delivery for Primary and Secondary Brain Tumours. Brain Sciences. 2025; 15(2):136. https://doi.org/10.3390/brainsci15020136
Chicago/Turabian StyleKhilar, Smita, Antonina Dembinska-Kenner, Helen Hall, Nikolaos Syrmos, Gianfranco K. I. Ligarotti, Puneet Plaha, Vasileios Apostolopoulos, Salvatore Chibbaro, Giuseppe Maria Vincenzo Barbagallo, and Mario Ganau. 2025. "Towards a New Dawn for Neuro-Oncology: Nanomedicine at the Service of Drug Delivery for Primary and Secondary Brain Tumours" Brain Sciences 15, no. 2: 136. https://doi.org/10.3390/brainsci15020136
APA StyleKhilar, S., Dembinska-Kenner, A., Hall, H., Syrmos, N., Ligarotti, G. K. I., Plaha, P., Apostolopoulos, V., Chibbaro, S., Barbagallo, G. M. V., & Ganau, M. (2025). Towards a New Dawn for Neuro-Oncology: Nanomedicine at the Service of Drug Delivery for Primary and Secondary Brain Tumours. Brain Sciences, 15(2), 136. https://doi.org/10.3390/brainsci15020136







