Skin Lesions as Signs of Neuroenhancement in Sport
Abstract
1. Introduction
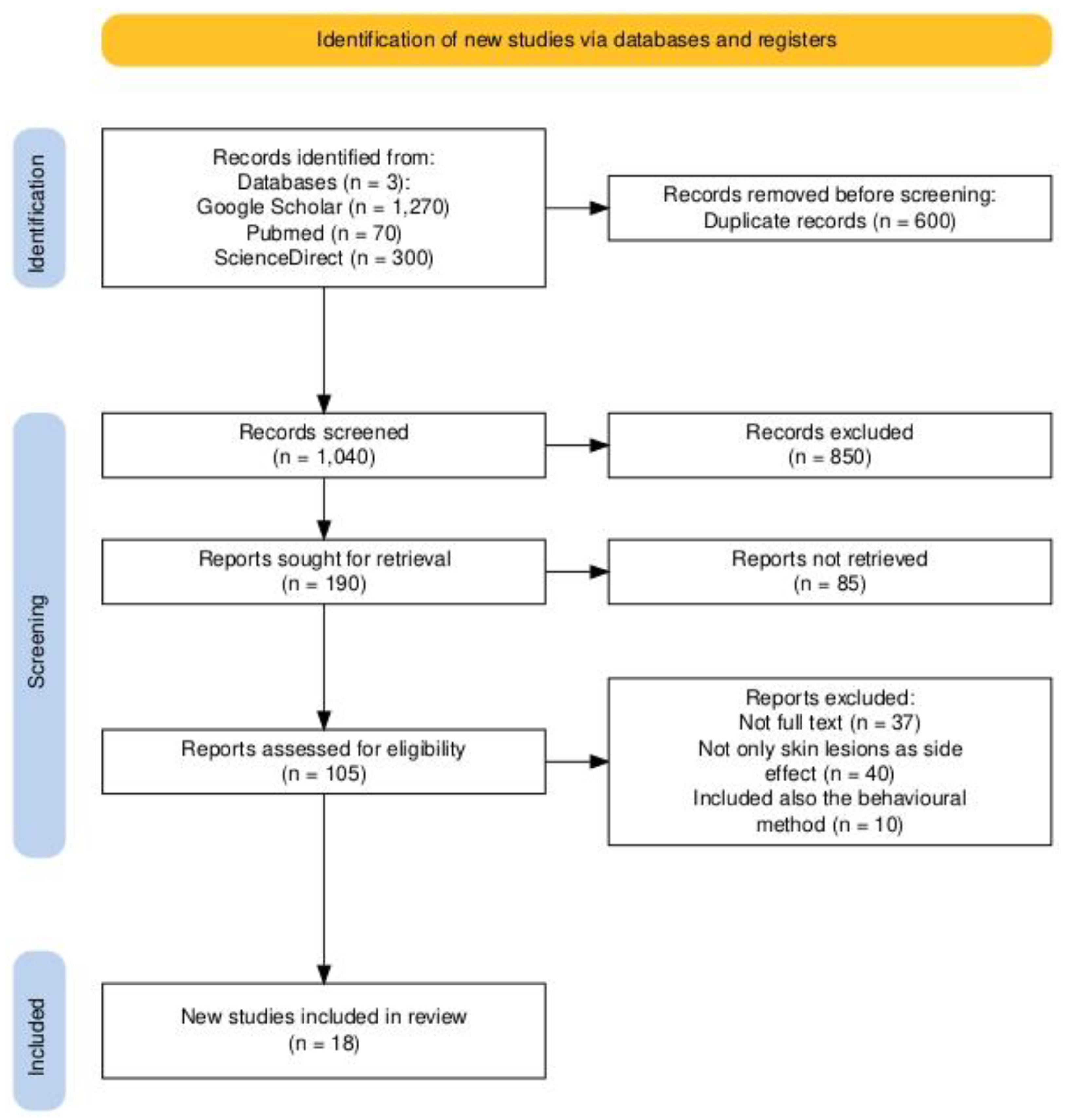
2. Materials and Methods
Selection Procedure and Inclusion Criteria
- Articles published in English.
- Publications from 2010 to 31 December 2024.
- Studies focused on skin lesions as adverse reactions to pharmacological and non-pharmacological neuroenhancement methods.
- Articles not published in English.
- Studies were published before 2010.
- Review articles, systematic reviews, or meta-analyses.
- Studies where skin lesions were not the primary focus of adverse reactions, explicitly excluding articles that predominantly reported cardiological, neurological, or gastroenterological side effects. The rationale for this exclusion was to maintain a focused scope on dermatological signs, which may serve as subtle yet valuable indicators of neurodoping in athletes.
3. Results
3.1. Studies Investigating Skin Lesions Associated with the Use of Rivastigmine
3.2. Studies Investigating Skin Lesions Associated with the Use of Memantine
3.3. Studies Investigating Skin Lesions Associated with Using Donepezil
3.4. Studies Investigating Skin Lesions Associated with Transcranial Direct Electrical Stimulation
4. Discussion
5. Limitations
6. Conclusions
7. Future Lines of Research
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| WADA | World Anti-Doping Agency |
| TDCS | Transcranial direct current stimulation |
References
- Apppleby, B.S.; Arciniegas, D.B.; Goodkin, K.; Inouye, S.K.; Lykestos, C.; McKeith, I.G.; Miller, B.L.; Moser, D.J.; Nopoulos, O.C.; Rosen, H.J.; et al. Neurocognitive Disorders in Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR), 5th ed.; Text Revision; The American Psychiatric Association, American Psychiatric Association Publishing: Washington, DC, USA, 2022; pp. 669–672. [Google Scholar]
- Jangwan, N.S.; Ashraf, G.M.; Ram, V.; Singh, V.; Alghamdi, B.S.; Abuzenadah, A.M.; Singh, M.F. Brain augmentation and neuroscience technologies: Current applications, challenges, ethics and future prospects. Front. Syst. Neurosci. 2022, 16, 1000495. [Google Scholar] [CrossRef]
- Conrad, E.C.; Humphries, S.; Chatterjee, A. Attitudes toward cognitive enhancement: The role of metaphor and context. AJOB Neurosci. 2019, 10, 35–47. [Google Scholar] [CrossRef]
- Partridge, B.; Lucke, J.; Hall, W. A comparison of attitudes toward cognitive enhancement and legalized doping in sport in a community sample of Australian adults. AJOB Prim. Res. 2012, 3, 81–86. [Google Scholar] [CrossRef]
- Smith, A.C.T.; Stavros, C.; Westberg, K. Cognitive enhancing drugs in sport: Current and future concerns. Subst. Use Misuse 2020, 55, 2064–2075. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Navarro-Triviño, F.J.; Ruiz-Villaverde, R. Allergic contact dermatitis caused by 2-ethylhexyl acrylate in a rivastigmine transdermal therapeutic system. Contact Dermat. 2020, 83, 143–145. [Google Scholar] [CrossRef]
- Golüke, N.M.S.; van Strien, A.M.; Dautzenberg, P.J.L.; Jessurun, N.; Keijsers, C.J.P.W. Skin lesions after oral acetylcholinesterase inhibitor therapy: A case report. J. Am. Geriatr. Soc. 2014, 62, 2012–2013. [Google Scholar] [CrossRef]
- Imbernón-Moya, A.; Podlipnik, S.; Burgos, F.; Vargas-Laguna, E.; Aguilar-Martínez, A.; Fernández-Cogolludo, E.; Gallego-Valdes, M.A. Acquired localized hypertrichosis induced by rivastigmine. Case Rep. Dermatol. Med. 2016, 2016, 7296572. [Google Scholar] [CrossRef]
- Grieco, T.; Rossi, M.; Faina, V.; De Marco, I.; Pigatto, P.; Calvieri, S. An atypical cutaneous reaction to rivastigmine transdermal patch. J. Allergy 2011, 2011, 752098. [Google Scholar] [CrossRef]
- Makris, M.; Koulouris, S.; Koti, I.; Aggelides, X.; Kalogeromitros, D. Maculopapular eruption to rivastigmine’s transdermal patch application and successful oral desensitization. Allergy 2010, 65, 925–926. [Google Scholar] [CrossRef]
- Allain-Veyrac, G.; Lebreton, A.; Collonnier, C.; Jolliet, P. First case of symmetric drug-related intertriginous and flexural exanthema (SDRIFE) due to rivastigmine? Am. J. Clin. Dermatol. 2011, 12, 210–213. [Google Scholar] [CrossRef]
- Alva, G.; Cummings, J.L.; Galvin, J.E.; Meng, X.; Velting, D.M. Skin reactions at the application site of rivastigmine patch (4.6 mg/24 h, 9.5 mg/24 h or 13.3 mg/24 h): A qualitative analysis of clinical studies in patients with Alzheimer’s disease. Int. J. Clin. Pract. 2015, 69, 518–530. [Google Scholar] [CrossRef]
- Naharci, M.I.; Tasci, I. Angioedema caused by Rivastigmine Patch: A rare case. J. Clin. Psychopharmacol. 2018, 38, 281–282. [Google Scholar] [CrossRef]
- Chouchana, M.; Pinel, S.; Colboc, H.; Soria, A.; Buard, G.; Delage, C.; Bloch, V.; Lilamand, M. Donepezil as a safe alternative treatment after maculo-papular eruption related to rivastigmine in Lewy body disease: A case report and pharmacovigilance data. Psychogeriatrics 2024, 24, 1180–1183. [Google Scholar] [CrossRef]
- Mancano, M.A. ISMP adverse drug reactions: Propofol-related infusion syndrome (PRIS); Ivermectine-induced Stevens-Johnson syndrome; Stevens-Johnson syndrome/toxic epidermal necrolysis from fexofenadine; Memantine-related drug eruption. Hosp. Pharm. 2018, 53, 220–222. [Google Scholar] [CrossRef]
- Saito, R.; Sawada, Y.; Yamaguchi, T.; Ohmori, S.; Haruyama, S.; Yoshioka, M.; Okada, E.; Nakamura, M. Drug eruption caused by Memantine. Ann. Allergy Asthma Immunol. 2017, 119, 89–90. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Mathew, P.; Blau, A. A randomised, double-blind study to assess the skin irritation and sensitisation potential of a once-weekly donepezil transdermal delivery system in healthy volunteers. Alzheimer Dis. Assoc. Disord. 2023, 37, 290–295. [Google Scholar] [CrossRef]
- Hussain, K.; Hepburn, N.C.; Scharrer, K.; Zdybsky, J.; Schofield, J. Donepezil-induced linear IgA disease. Clin. Exp. Dermatol. 2012, 37, 72–73. [Google Scholar] [CrossRef]
- Kortteenniemi, A.; Lehto, S.M.; Javadi, A.-H. Delayed, distant skin lesions after transcranial direct current stimulation. Brain Stimul. 2019, 12, 204–206. [Google Scholar] [CrossRef]
- Lu, H.; Lam, L.C.W. Cathodal skin lesions induced by transcranial direct current stimulation (tDCS). Neuromodulation Technol. Neural Interface 2019, 22, 989–991. [Google Scholar] [CrossRef]
- Frank, E.; Wilfurth, S.; Landgrebe, M.; Eichhammer, P.; Hajak, G.; Langguth, B. Anodal skin lesions after treatment with transcranial direct current stimulation. Brain Stimul. 2010, 3, 58–59. [Google Scholar] [CrossRef]
- Maas, R.P.; van de Warrenburg, B.P.; Schutter, D.J. Cathodal skin lesions in a tattoo following transcranial direct current stimulation. Brain Stimul. 2021, 14, 284–286. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Wen, J.; Li, X. Skin burn after a single session of transcranial direct current stimulation (t DCS). Brain Stimul. 2015, 8, 165–166. [Google Scholar] [CrossRef]
- Patel, P.H.; Gupta, V. StatPearls [Internet]–Rivastigmine. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557438/ (accessed on 21 January 2025).
- Folch, J.; Busquets, O.; Ettcheto, M.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesús, G.; Beas-Zarate, C.; et al. Memantine for the treatment of dementia: A review on its current and future applications. J. Alzheimer’s Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Menniti, F.S.; Traynelis, S.F. NMDA receptors in the central nervous system. Methods Mol. Biol. 2017, 1677, 1–80. [Google Scholar] [CrossRef]
- Birks, J.S.; Harvey, R.J. Donepezil for dementia due to Alzheimer’s disease. Cochrane Database Syst. Rev. 2018, 2018, CD001190. [Google Scholar] [CrossRef]
- Stagg, C.J.; Antal, A.; Nitsche, M.A. Physiology of transcranial direct current stimulation. J. ECT 2018, 34, 144–152. [Google Scholar] [CrossRef]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. Impact of concurrent task performance on transcranial direct current stimulation (tDCS)-Induced changes in cortical physiology and working memory. Cortex 2018, 113, 37–57. [Google Scholar] [CrossRef]
- Rizvi, A.; Bell, K.; Yang, D.; Montenegro, M.P.; Kim, H.; Bao, S.; Wright, D.L.; Buchanan, J.J.; Lei, Y. Effects of transcranial direct current stimulation over human motor cortex on cognitive-motor and sensory-motor functions. Sci. Rep. 2023, 13, 20968. [Google Scholar] [CrossRef]
- World Anti-Doping Code International Standard Prohibited List. 2024. Available online: https://www.wada-ama.org/sites/default/files/2023-09/2024list_en_final_22_september_2023.pdf (accessed on 21 January 2025).
- Pugh, J.; Pugh, C. Neurostimulation, doping, and the spirit of sport. Neuroethics 2020, 14, 141–158. [Google Scholar] [CrossRef]
- Davis, N.J. Neurodoping: Brain stimulation as a performance-enhancing measure. Sports Med. 2013, 43, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, E.; Nunneley, C.E.; Hsu, S.; Kass, J.S. Cutaneous adverse effects of neurologic medications. CNS Drugs 2016, 30, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Sports dermatology. In Braun Falco’s Dermatology, 4th ed.; Plewig, G., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1599–1607. [Google Scholar] [CrossRef]
- Emre, M.; Bernabei, R.; Blesa, R.; Bullock, R.; Cunha, L.; Daniëls, H.; Dziadulewicz, E.; Förstl, H.; Frölich, L.; Gabryelewicz, T.; et al. Drug profile: Transdermal rivastigmine patch in the treatment of Alzheimer’s Disease. CNS Neurosci. Ther. 2010, 16, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Greenspoon, J.; Herrmann, N.; Adam, D.N. Transdermal rivastigmine: Management of cutaneous adverse events and review of the literature. CNS Drugs 2011, 25, 575–583. [Google Scholar] [CrossRef]
- Lefèvre, G.; Sedek, G.; Jhee, S.S.; Leibowitz, M.T.; Huang, H.-L.; Enz, A.; Maton, S.; Ereshefsky, L.; Pommier, F.; Schmidli, H.; et al. Pharmacokinetics and pharmacodynamics of the novel daily rivastigmine transdermal patch compared with twice-daily capsules in Alzheimer’s Disease patients. Clin. Pharmacol. Ther. 2008, 83, 106–114. [Google Scholar] [CrossRef]
- Park, J.; Oh, J.-P.; Ku, K.; Jin, Y.; Kim, E.J.; Lee, J.-H. Preventing donepezil-induced adverse effects through N-acetylcysteine Co-administration. J. Alzheimer’s Dis. 2024, 101, 1281–1292. [Google Scholar] [CrossRef]
- Gomolin, I.H.; Smith, C.; Jeitner, T.M. Donepezil dosing strategies: Pharmacokinetic considerations. J. Am. Med. Dir. Assoc. 2011, 12, 606–608. [Google Scholar] [CrossRef]
- Jain, K.K. Evaluation of Memantine for neuroprotection in dementia. Expert Opin. Investig. Drugs 2000, 9, 1397–1406. [Google Scholar] [CrossRef]
- Reed, T.; Kadosh, R.C. Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. J. Inherit. Metab. Dis. 2018, 41, 1123–1130. [Google Scholar] [CrossRef]
- Debelee, T.G. Skin lesion classification and detection using machine learning techniques: A systematic review. Diagnostics 2023, 13, 3147. [Google Scholar] [CrossRef]
| Article | Article Design | Year | Group (Male/Female, Age) | Type of Skin Lesion and Localization | Time Elapsed Since the Onset of Skins Lesions | Pharmaceutical /Non-Pharmaceutical Method |
|---|---|---|---|---|---|---|
| 1. Navarro-Triviño F.J.N and Ruiz-Villaverde R. [7] | Case report | 2020 | Male, 84 | Multiple round erythematous–edematous lesions on the chest | 3 months | Rivastigmine patch 9.5 mg/24 h |
| 2. Golüke et al. [8] | Case report | 2014 | Male, 74 | Pruritus and allergic rash on left flank | 1 year | Rivastigmine patch 9.5 mg/24 h |
| 3. Imbernón-Moya et al. [9] | Case report | 2016 | Male, 80 | Progressive asymptomatic hair growth on forearms | 3 months | Rivastigmine oral −3 mg/12 h |
| 4. Grieco et al. [10] | Case report | 2011 | Male, 75 | Erythematous–edematous lesions on chest and arms | 15 days | Rivastigmine patch 9.5 mg/24 h |
| 5. Makris et al. [11] | Case report | 2010 | Female, 85 | Maculopapular itchy rash, hypersensitivity | 8 weeks | Rivastigmine patch 4.6 mg/24 h |
| Maculopapular eruption on the anterior and posterior chest, arms with progressive extension | The second day | Rivastigmine oral solution 6 mg/24 h | ||||
| 6. Allain-Veyrac et al. [12] | Case report | 2011 | Male, 88 | Itchy, symmetrical, pure-purple erythematous rashes in the axilla and groin (SDRIFE or else named Baboon Syndrome) | 4 months | Rivastigmine 1.5 mg × 2/24 h |
| 7. Alva et al. [13] | Original article | 2015 | ACTION TRIAL Male and Female > 50 years old | Erythema on application site reaction (12.5%) Site dermatitis (8.4%) | 24 weeks | Rivastigmine patch 13.3 mg/24 versus 4.6 mg/24 h |
| OPTIMA TRIAL Male and Female > 50 years old | Erythema on application site reaction | 72–96 weeks | Rivastigmine patch 9.5 mg/24 h versus 13.3 mg/24 h | |||
| IOL (initial open-labeled) | 11.6% IOL | 24–48 weeks | ||||
| DB (randomized double-dummy) | 6.0% DB | 48 weeks | 2 patches—one placebo and one active | |||
| Site dermatitis 0.9% IOL 0% DB | ||||||
| 8. Naharci and Tasci [14] | Case report | 2018 | Female, 84 | Lower lip and tongue swollen Mild swelling of upper and lower eyelids | 1 month and 3 days with the 9.5 mg patch | Rivastigmine patch 4.6 mg/24 h to 9.5 mg/24 h |
| 9. Chouchana et al. [15] | Case report | 2024 | Male, 79 | Pruritic erythematous maculopapular eruption at patch application site | 3 months 1 month | Rivastigmine 4.6 mg/24 h Rivastigmine 9.5 mg/24 h |
| Spread of the rash over 70% of the body surface, including arms, legs, chest and abdomen | 2 weeks | Rivastigmine oral 1.5 mg × 2/24 h | ||||
| 10. Mancano [16] | Case report | 2018 | Male, 79 | Erythematous eruptions and papules on trunk and extremities | 2 months | Memantine |
| 11. Saito et al. [17] | Case report | 2017 | Male, 89 | Erythematous eruptions on chest and extremities, mainly on flexures | 2 months | Memantine |
| 12. Sabbagh et al. [18] | Original article | 2023 | 256 participants (male and female) > 40 years old | Mild skin irritation at the application site | Day 22 (3 weeks) | Donepezil transdermal delivery system (TDS) |
| 13. Hussian et al. [19] | Case report | 2012 | Female, 84 | Non-squamous erythematous papules, round lesions and vesicles on the trunk, arms, and legs—linear Ig A disease | 4 weeks | Donepezil |
| 14. Kortteenniemi et al. [20] | Case report | 2019 | Females—18 years old and | Skin erythema and nodular itchy lesion | After 2 and 6 days | Transcranial direct current stimulation |
| Female—19 years old | Non-itchy and non-lumpy lesion | After 2 days | ||||
| 15. Lu and Lam [21] | Case report | 2019 | 1 male and 2 females—mean age 69 years old | Mild redness of the skin | Right after the first session | Transcranial direct current stimulation (cathodal electrode) |
| Skin burns | The fourth or fifth session | |||||
| 16. Frank et al. [22] | Case report | 2010 | 3 patients | Mild skin burns | After the fourth session | Transcranial direct current stimulation (anodal electrode) |
| 17. Maas et al. [23] | Case report | 2021 | Male—47 years old | 2 targeted areas of skin burned in the tattoo below the electrode’s corners | After the tenth session | Transcranial direct current stimulation (cathodal electrode) |
| 18. Wang et al. [24] | Case report | 2015 | Male—25 years old | Skin burns on frontal region | After a single session | Transcranial direct current stimulation (cathodal electrode) |
| Method Implicated | Most Frequent Skin Lesions | Studies |
|---|---|---|
| Rivastigmine | Itchy rashes | [7,8,9,10,11,12,13,14,15] |
| Donepezil | Non-itchy lesions (papules, vesicles, skin irritation) | [18,19] |
| Memantine | Erythematous eruptions | [16,17] |
| Transcranial direct current stimulation | Skin burns | [20,21,22,23,24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, S.-C.; Popescu, R.; Voiculescu, V.; Negrei, C. Skin Lesions as Signs of Neuroenhancement in Sport. Brain Sci. 2025, 15, 315. https://doi.org/10.3390/brainsci15030315
Popescu S-C, Popescu R, Voiculescu V, Negrei C. Skin Lesions as Signs of Neuroenhancement in Sport. Brain Sciences. 2025; 15(3):315. https://doi.org/10.3390/brainsci15030315
Chicago/Turabian StylePopescu, Sorana-Cristiana, Roman Popescu, Vlad Voiculescu, and Carolina Negrei. 2025. "Skin Lesions as Signs of Neuroenhancement in Sport" Brain Sciences 15, no. 3: 315. https://doi.org/10.3390/brainsci15030315
APA StylePopescu, S.-C., Popescu, R., Voiculescu, V., & Negrei, C. (2025). Skin Lesions as Signs of Neuroenhancement in Sport. Brain Sciences, 15(3), 315. https://doi.org/10.3390/brainsci15030315






